Abstract
Fungal gluco-amylase is required for the production of sugars from starchy substrates. Commercially available fungal gluco-amylase is quite costly which makes the process uneconomical. This study was undertaken to standardize physico-chemical parameters for optimum production of gluco-amylases from Aspergillus spp. Two fungal cultures, i.e., Aspergillus niger and Aspergillus terreus, were compared for gluco-amylase activity both under stationary and shake flask conditions. Among two fungal cultures, maximum gluco-amylase activity was shown by A. niger (243.09 U/ml) under stationary conditions as compared to A. terreus (126.34 U/ml). Gluco-amylase activity of A. niger increases by 42.48% from 243.09 to 346.35 U/ml after optimization using response surface methodology, whereby a substrate concentration of 7%, yeast extract 0.25%, temperature 32.5 °C and pH 5.5 were found to be optimum for gluco-amylase production. Crude enzyme was compared with commercial enzyme and it was found that when 500 U of Glucoamylase ex. Rhizopus were inoculated into starch-supplemented minimal media (SSMM) liquefied using 2 g of fungal diastase, it increases the reducing sugar concentration from 2.19 to 21.15 mg/ml and a saccharification efficiency of 77.7% was achieved, whereas 1.5 ml of crude enzyme (extracted from A. niger) was able to produce 14.46 mg/ml of reducing sugars with a saccharification efficiency of 53.2%.
Keywords: Corn, Gluco-amylase, Aspergillus spp., Response surface methodology (RSM), Saccharification
Introduction
Starch from various crops is well thought out as a potential source for the industrial production of bioethanol as it is easily available and has low market price. On a percent dry basis, agricultural products like sorghum, corn, wheat and other cereal grains contain around 60–75% (w/w) starch, which is hydrolysable to glucose with a considerable weight increase and is used as a good resource in many fermentation processes (Kunamneni and Singh 2005). During this procedure, starch is solubilised and after that subjected to two enzymatic treatments keeping in mind the end goal to acquire fermentable sugars (Pervez et al. 2014). The most important use of liquefied starch is for the glucose production, which is then used to produce dextrose, dextrose syrup or high fructose corn syrup (HFCS). Alternatively, the fermentation of glucose can be carried out for making other by-products such as ethanol, organic acids or amino acids.
Maize or corn acts as a basic raw material to thousands of commercial products including oil, alcoholic beverages, starch, pharmaceuticals, enzyme production, food sweeteners, cosmetics, gum, textile, package and paper industries. The bioconversion of starch into ethanol includes liquefaction and saccharification, which involves conversion of starch into sugar by amylolytic enzymes followed by fermentation, which converts sugar into ethanol using an ethanol producing microorganism such as yeast, Saccharomyces cerevisiae (Sahni and Goel 2015). In India, after wheat and rice, maize is the most important food cash crop. India ranks sixth in global maize production, contributing to 1% of world production. Its average annual production is 22.26 million metric tons in 2015. Starch is a polysaccharide composed of 20–25% amylose and 75–80% amylopectin. Gluco-amylases (1,4-α-d-glucanglucohydrolase, EC 3.2.1.3) are exo-amylases which hydrolyse α-1,4 and α-1,6-glycosidic linkages in oligosaccharides (Bagheri et al. 2014). Gluco-amylases produced by fungi and yeasts are highly thermolabile and it remains active at wide range of acidic pH (Vihinen and Mantsala 1989). Gluco-amylases of Aspergillus niger, A. awamori and Rhizopus oryzae have great industrial value.
Materials and methods
Maize varieties
Three different maize varieties viz. PMH-1, PMH-2 and Parkash were procured from Maize Section of Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana. The grains were collected and stored in air tight containers for further use.
Organism
The amylolytic fungus A. niger and Aspergillus terreus (obtained from Department of Microbiology, PAU, Ludhiana) were grown on potato dextrose agar (PDA) plates and maintained by periodic subculturing. Two fungal cultures were compared for gluco-amylase production periodically and culture producing maximum gluco-amylase was selected for further studies.
Proximate analysis of maize varieties
Different maize varieties were oven dried at 65 °C to bring the moisture content of the grains at 8–12% for milling in a cemotec 1090 laboratory grinder mill and thereafter were sieved through a 2.0 mm sieve (Mangat et al. 2010). Proximate analysis of all the three varieties of corn flour was done for estimating starch (Clegg 1956), crude protein, crude fibre, moisture content and ash content (AACC 2000).
Quantitative gluco-amylase production studies of fungal cultures
Substrate for gluco-amylase production
Corn flour was obtained by milling different varieties of corn grains and was used as the substrate for gluco-amylase production. Composition of starch-supplemented minimal media (SSMM) broth (g/l) used for amylase production was corn flour (5%), K2HPO4 (0.7%), KH2PO4 (0.3%), Na2SO4 (0.1%), (NH4)SO4 (0.1%), MgSO4 (0.1%) and sodium citrate (0.05%).
Inoculum preparation
The fungal cultures (A. niger and A. terreus) were grown on potato dextrose agar medium at 28 and 40 °C, respectively, for 7 days. A spore suspension having 106 spores/ml was prepared and used for inoculation into the starch-supplemented minimal media broth.
Production of gluco-amylases
Periodic gluco-amylase activity of A. niger was estimated on different maize varieties. The gluco-amylase production in fungal cultures was studied under shake flask as well as stationary conditions at 28 °C. Flasks (250 ml capacity) in duplicate were dispensed with 100 ml of production medium and inoculated with 1 ml of spore suspension @ 106 spores/ml. The flasks were incubated under shake flask as well as stationary conditions. The samples were drawn periodically for 7 days and used for the estimation of reducing sugars (Miller 1959) and gluco-amylase activity (Bernfeld 1955).
One unit of gluco-amylase activity was defined as the µg of reducing sugar in terms of maltose equivalents produced per min per ml of crude enzyme:
Standardization of process parameters for saccharification of corn starch
Effect of various nitrogen sources on gluco-amylase production
Effect of different organic and inorganic nitrogen sources, i.e., yeast extract, soybean meal, urea and ammonium sulphate on gluco-amylase production was studied by supplementing ammonium sulphate in starch-supplemented minimal media. Different concentrations of nitrogen sources were added so as to keep the percent nitrogen constant as in original minimal media (2.1%). Ammonium sulphate (0.1%) was substituted with urea (0.045%), yeast extract (0.2%) and soybean meal (0.27%). The medium containing different nitrogen sources was inoculated with fungal cultures and checked periodically for gluco-amylase activity.
Optimization of process parameters using response surface methodology (RSM)
For optimization, the analysis of variance (ANOVA) for the overall effect of four factor variables, i.e., substrate concentration (1.0–5.0%), yeast extract (0.1–0.4%), temperature (25–40 °C) and pH (4.0–7.0) on the response variable, i.e., gluco-amylase activity according to the fitted model was done using software Statgraphics Centurian XVI.I and the least significant factor affecting the response variable was selected. The fungal culture was inoculated in SSMM broth containing PMH-1 as a substrate at varying concentrations and different conditions of temperature and pH. The three-dimensional contour plots according to the fitted model were drawn using the software. Response Surface Methodology was used for regression analysis of the experiment. 26 sets of various combinations (Table 6) were obtained, under which experiments were performed for determining the gluco-amylase activity (U) as response variable.
Table 6.
Experimental response profile for optimization of process parameters for gluco-amylase activity using Aspergillus niger
| Run | Substrate concentration (%) | Yeast extract (%) | Temperature (°C) | pH | Observed amylase activity (U/ml) | Observed desirability | Predicted desirability |
|---|---|---|---|---|---|---|---|
| 1 | 1.0 | 0.1 | 25.0 | 4.0 | 110.63 | 0.34 | 0.34 |
| 2 | 5.0 | 0.1 | 25.0 | 4.0 | 180.36 | 0.56 | 0.57 |
| 3 | 1.0 | 0.4 | 25.0 | 4.0 | 97.47 | 0.30 | 0.31 |
| 4 | 5.0 | 0.4 | 25.0 | 4.0 | 172.52 | 0.54 | 0.53 |
| 5 | 1.0 | 0.1 | 40.0 | 4.0 | 112.5 | 0.35 | 0.35 |
| 6 | 5.0 | 0.1 | 40.0 | 4.0 | 163.57 | 0.51 | 0.56 |
| 7 | 1.0 | 0.4 | 40.0 | 4.0 | 97.07 | 0.30 | 0.29 |
| 8 | 5.0 | 0.4 | 40.0 | 4.0 | 143.04 | 0.45 | 0.48 |
| 9 | 1.0 | 0.1 | 25.0 | 7.0 | 27.25 | 0.08 | 0.07 |
| 10 | 5.0 | 0.1 | 25.0 | 7.0 | 197.31 | 0.62 | 0.68 |
| 11 | 1.0 | 0.4 | 25.0 | 7.0 | 8.77 | 0.02 | 0.02 |
| 12 | 5.0 | 0.4 | 25.0 | 7.0 | 187.48 | 0.59 | 0.61 |
| 13 | 1.0 | 0.1 | 40.0 | 7.0 | 13.63 | 0.04 | 0.09 |
| 14 | 5.0 | 0.1 | 40.0 | 7.0 | 212.6 | 0.67 | 0.67 |
| 15 | 1.0 | 0.4 | 40.0 | 7.0 | 1.54 | 0.0 | 0.01 |
| 16 | 5.0 | 0.4 | 40.0 | 7.0 | 166.51 | 0.52 | 0.57 |
| 17 | 0.5 | 0.25 | 32.5 | 5.5 | 30.83 | 0.09 | 0.09 |
| 18 | 7.0 | 0.25 | 32.5 | 5.5 | 346.35 | 1.0 | 0.99 |
| 19 | 3.0 | 0.1 | 32.5 | 5.5 | 219.77 | 0.69 | 0.64 |
| 20 | 3.0 | 0.55 | 32.5 | 5.5 | 165.6 | 0.52 | 0.50 |
| 21 | 3.0 | 0.25 | 17.5 | 5.5 | 10.86 | 0.03 | 0.03 |
| 22 | 3.0 | 0.25 | 47.5 | 5.5 | 22.05 | 0.06 | 0.001 |
| 23 | 3.0 | 0.25 | 32.5 | 2.5 | 173.59 | 0.54 | 0.54 |
| 24 | 3.0 | 0.25 | 32.5 | 8.5 | 140.04 | 0.44 | 0.37 |
| 25 | 3.0 | 0.25 | 32.5 | 5.5 | 318.28 | 0.98 | 0.99 |
| 26 | 3.0 | 0.25 | 32.5 | 5.5 | 313.32 | 0.98 | 0.99 |
Comparative amylase production studies of intrinsic isolates with commercial enzymes
Selected isolate was used for gluco-amylase production at optimized conditions and crude enzyme was compared with standard enzyme available in the market for the efficiency of saccharification of liquefied corn grains. Fungal Diastase (Enzyme Bioscience Pvt. Ltd.) was used as a standard for α-amylase and Gluco-amylase ex. Rhizopus (SRL Pvt. Ltd.) was used as a standard for gluco-amylase. SSMM (pH 5.5) was autoclaved at 121 °C and 15 psi for 15 min and inoculated with 2 g of fungal diastase and incubated at 90 °C in shaking water bath at 150 rpm for 90 min and checked periodically for liquefaction. For saccharification, the periodic estimation was done by incubating reaction mixture having gluco-amylase at 60 °C and percent saccharification was calculated as:
Results and discussion
Proximate analysis of maize varieties
Results depicted in Table 1 revealed the comparative starch content, crude protein, crude fibre, moisture and ash content of all three varieties on percent dry weight basis. PMH-2 was found to have maximum starch content (70.03%) followed by PMH-1 (68.97%) and Parkash (65.08%). Crude protein content was also maximum for PMH-2 (13.09%) followed by Parkash (12.18%) and PMH-1 (11.85%). Crude fibre (%) of all three varieties was found to differ statistically from 2.77 to 3.17%, being maximum for Parkash. Moisture and ash content of three varieties have been found to vary between 11.08–12.02 and 1.37–1.63%, respectively. Mangat et al. (2010) reported the composition of two maize varieties viz. PMH-1 and PMH-2. The total starch, crude protein, crude fat and moisture content of maize samples on percent dry basis were 69, 11, 5 and 12 for PMH-1 and 70, 9, 5.25 and 11.8 for PMH-2, respectively.
Table 1.
Comparative proximate analysis of local varieties of corn (Zea mays)
| S. no | Componenta (% db) | Maize variety | CD@5% | ||
|---|---|---|---|---|---|
| PMH-1 | PMH-2 | Parkash | |||
| 1. | Starch | 68.97 ± 0.05 | 70.03 ± 0.04 | 65.08 ± 0.06 | 0.184 |
| 2. | Crude protein | 11.85 ± 0.01 | 13.09 ± 0.03 | 12.18 ± 0.05 | 0.113 |
| 3. | Crude fibre | 2.93 ± 0.03 | 2.77 ± 0.03 | 3.17 ± 0.03 | 0.115 |
| 4. | Moisture | 12.02 ± 0.02 | 11.73 ± 0.04 | 11.08 ± 0.08 | 0.19 |
| 5. | Ash | 1.43 ± 0.03 | 1.63 ± 0.02 | 1.37 ± 0.02 | 0.081 |
aValues indicate mean of three replicates ± standard error
Comparative gluco-amylase production studies of fungal cultures
Two different fungal cultures viz. A. niger and A. terreus were procured from Department of Microbiology, Punjab Agricultural University, Ludhiana. Gluco-amylase activity of A. niger was determined on three maize varieties and it was found that the maximum activity (115.54 U/ml) was shown on 4th day of incubation at 28 °C using PMH-1 as a substrate and concentration of reducing sugars increases from 0.44 to 2.26 mg/ml (Table 2); thus, PMH-1 was selected as suitable maize variety for further production of gluco-amylase. The maximum gluco-amylase activity of A. niger (243.09 U/ml) was observed after 4 days of incubation at 28 °C and that of A. terreus (126.34 U/ml) was observed after 5 days of incubation at optimum growth temperature (40 °C) (Gao et al. 2008) (Table 3). The enzyme activity was observed both under shake flask as well as stationary conditions of incubation and it was found that the maximum activity was observed under stationary conditions, i.e., 228.37 U/ml (Table 4). Ayodeji and Ajele (2016) selected six soil fungal species including Aspergillus sp. and screened for gluco-amylase production by submerged fermentation. Among the different species, A. niger gave the highest yield (activity of 32.5 U/ml) followed by A. fumigatus with an enzyme activity of 25.7 U/ml.
Table 2.
Periodic estimation of gluco-amylase activity of Aspergillus niger on different maize varieties
| S. no | Incubation period (days) | Gluco-amylase activity (U/ml) | Reducing sugars produced (mg/ml) | ||||
|---|---|---|---|---|---|---|---|
| PMH-1 | PMH-2 | Parkash | PMH-1 | PMH-2 | Parkash | ||
| 1. | 3 | 115.54 ± 0.08 | 104.17 ± 0.07 | 98.48 ± 0.03 | 1.3 ± 0.04 | 1.22 ± 0.05 | 1.18 ± 0.07 |
| 2. | 4 | 243.06 ± 0.04 | 237.27 ± 0.04 | 214.36 ± 0.05 | 2.26 ± 0.05 | 2.22 ± 0.04 | 2.05 ± 0.04 |
| 3. | 5 | 149.38 ± 0.07 | 136.14 ± 0.05 | 108.61 ± 0.04 | 1.56 ± 0.03 | 1.46 ± 0.07 | 1.26 ± 0.08 |
| CD@5% | 0.25 | 0.203 | 0.177 | 0.538 | 0.41 | 0.29 | |
Incubated at 28 °C under stationary conditions
Table 3.
Periodic estimation of gluco-amylase activity of Aspergillus spp. on PMH-1
| S. no | Incubation period (days) | Gluco-amylase activity (U/ml) | Reducing sugars produced (mg/ml) | ||
|---|---|---|---|---|---|
| A. niger a | A. terreus b | A. niger a | A. terreus b | ||
| 1. | 3 | 115.69 ± 0.08 | 23.13 ± 0.04 | 1.30 ± 0.05 | 0.61 ± 0.24 |
| 2. | 4 | 243.09 ± 0.04 | 74.37 ± 0.05 | 2.26 ± 0.06 | 0.99 ± 0.03 |
| 3. | 5 | 149.36 ± 0.06 | 126.34 ± 0.03 | 1.56 ± 0.04 | 1.38 ± 0.05 |
| 4. | 6 | 139.07 ± 0.05 | 102.54 ± 0.24 | 1.48 ± 0.08 | 1.24 ± 0.04 |
| CD@5% | 0.118 | 0.403 | 0.387 | 0.511 | |
aIncubated at 28 °C under stationary conditions
bIncubated at 40 °C under stationary conditions
Table 4.
Comparative gluco-amylase activity of A. niger under shake flask and stationary conditions
| S. no | Conditions | Gluco-amylase activity (U/ml) | Reducing sugars produced (mg/ml) |
|---|---|---|---|
| 1. | Shake flask | 144.72 ± 0.04 | 1.52 ± 0.04 |
| 2. | Stationary | 228.37 ± 0.07 | 2.15 ± 0.06 |
| CD@5% | 0.2 | 0.308 |
Effect of various nitrogen sources on gluco-amylase production by A. niger
It was observed that yeast extract has a significant effect on gluco-amylase activity of A. niger. Gluco-amylase activity increases from 241 to 304 U/ml by substituting ammonium sulphate in the medium with yeast extract (0.2%) (Table 5). Nwagu and Okolo (2011) also reported an increase in amylase production by Fusarium sp. by supplementing soybean meal with potassium nitrate (1880 U/ml) and ammonium chloride (2097 U/ml), whereas sodium nitrate had an inhibitory effect observed by the reduction in the extracellular amylase production.
Table 5.
Effect of different nitrogen source on gluco-amylase production of A. niger
| S. no | Nitrogen Source | Incubation period (days) | Gluco-amylase production (U/ml) |
|---|---|---|---|
| 1. | Ammonium sulphate (0.1%) | 3 | 115.56 ± 0.04 |
| 4 | 241.01 ± 0.07 | ||
| 5 | 149.52 ± 0.07 | ||
| 2. | Urea (0.05%) | 3 | 14.01 ± 0.02 |
| 4 | 112.46 ± 0.03 | ||
| 5 | 53.16 ± 0.08 | ||
| 3. | Yeast extract (0.2%) | 3 | 139.65 ± 0.06 |
| 4 | 304.01 ± 0.02 | ||
| 5 | 147.30 ± 0.05 | ||
| 4. | Soybean meal (0.27%) | 3 | 51.00 ± 0.01 |
| 4 | 96.38 ± 0.03 | ||
| 5 | 100.98 ± 0.02 |
CD@5% after 4 days of incubation = 0.118
Optimization of cultural conditions for starch liquefaction and saccharification by response surface methodology (RSM)
Statgraphics Centurian XVI.I software was used for the optimization of different cultural conditions for gluco-amylase production by A. niger using corn flour as a substrate. Fermentation was carried out under stationary conditions using 100 ml of corn flour-supplemented minimal media for each of 26 runs according to RSM plan and the response was studied in terms of amylase activity (U/ml). Runs suggested by RSM were performed and their results are shown in Table 6 for enzyme production from corn flour using A. niger.
Saccharification of starch using A. niger
Results from Table 6 showed that in SSMM broth inoculated with A. niger, maximum gluco-amylase activity (346.35 U/ml) was found at 7% substrate concentration, 0.25% yeast extract concentration, 32.5 °C temperature and pH 5.5 after an incubation of 4 days.
The regression equation of the fitted model for gluco-amylase is:
where X1 is the substrate concentration, X2 is the yeast extract concentration, X3 is the temperature, and X4 is the pH
Effect of combined cultural conditions on enzyme production by A. niger
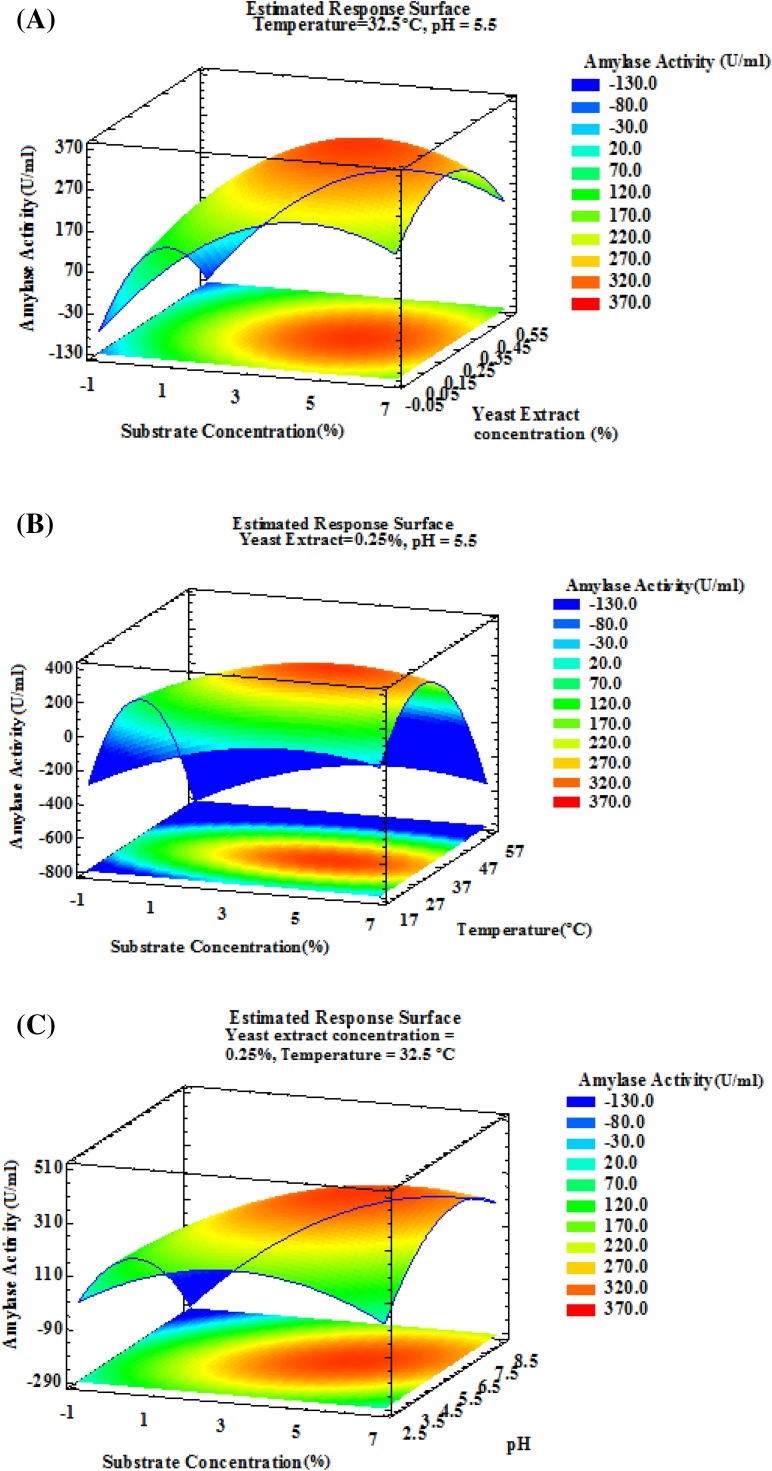
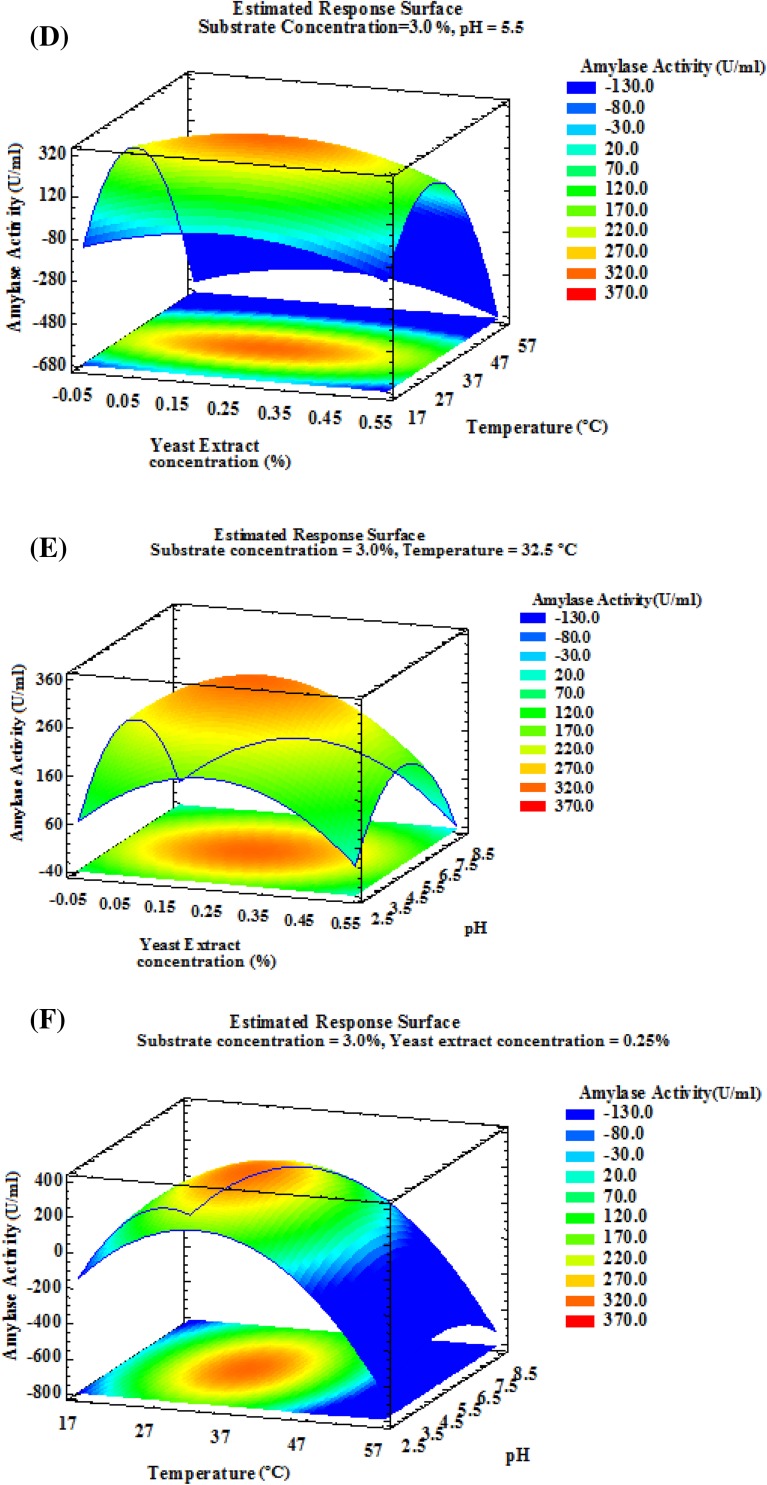
The contour plots for different interactions of any two independent variables, while holding the third and fourth variable constant, on enzyme production were generated using software, Statgraphics Centurian XVI.I. The optimization was done with the target of maximum gluco-amylase activity. Estimated response surface plots showing the effect of different parameters on gluco-amylase activity were studied. Results (Fig. 1) showed that the maximum gluco-amylase activity falls in the range of 240–347 U/ml at varying substrate concentration (1–5%), yeast extract concentration (0.1–0.4%), temperature (25–40 °C) and pH (4.0–7.0).
Fig. 1.
3D response surface plots representing interaction between significant cultural parameters on gluco-amylase activity (U/ml) using Aspergillus niger
Gluco-amylase activity of A. niger increases by 42.48% from 243.09 to 346.35 U/ml after optimization using response surface methodology at 7.0% substrate concentration, 0.25% yeast extract, 32.5 °C temperature and 5.5 pH. Our results are similar with that obtained by Malik et al. (2013) who developed a medium containing 10% each of starch and lactose together with some inorganic nutrients and found a maximum gluco-amylase production of 25.08 U/ml/min by mutant A. niger under optimum conditions of pH, inoculums concentration, agitation speed, temperature and incubation period of 5, 4%, 200 rpm, 30 °C and 48 h, respectively. Mustafa et al. (2016) determined the optimal fermentation conditions for maximum production of crude amylase enzyme by A. flavus NSH9 employing RSM. The maximum amylase activity was recorded as 1.055 ± 0.03 U/ml after 4 days of fermentation with 100% (v/v) moisture holding capacity, pH 6.5 and temperature 28 °C.
Comparative saccharification studies of intrinsic isolate with commercial enzyme
Using different incubation period for liquefaction, it was observed that considerable liquefaction started from 60 min and maximizes at 75 min where residual starch concentration decreases from 49.20 to 24.50 mg/ml with 50.1% liquefaction (Table 7). On comparing different units of commercial enzymes (20–100 U), it was found that with increase in enzyme concentration there is an increase in the amount of reducing sugars (mg/ml) produced from 3.31 to 11.89 mg/ml after incubation of 2.0 h (Table 8). For comparison of commercial enzyme with our isolate, 100 and 500 U of enzyme were used. When 500 U of Gluco-amylase ex. Rhizopus were inoculated into SSMM liquefied using 2 g of Fungal Diastase, it increases the reducing sugar concentration from 2.19 to 21.15 mg/ml, whereas 1.5 ml of crude enzyme (extracted from A. niger) was able to produce 14.46 mg/ml of reducing sugars in comparison to that of commercial enzyme (Table 9).
Table 7.
Periodic liquefaction of corn starch using fungal diastase
| S. No. | Time (min) | Residual starch (mg/ml) | % Liquefaction |
|---|---|---|---|
| 1. | 0 | 49.20 ± 0.07 | Nil |
| 2. | 15 | 48.48 ± 0.05 | 1.46 ± 0.02 |
| 3. | 30 | 45.81 ± 0.18 | 6.9 ± 0.09 |
| 4. | 45 | 43.95 ± 0.09 | 10.67 ± 0.08 |
| 5. | 60 | 31.45 ± 0.02 | 36.08 ± 0.03 |
| 6. | 75 | 24.55 ± 0.02 | 50.1 ± 0.04 |
| 7. | 90 | 25.27 ± 0.40 | 48.6 ± 0.04 |
| CD@5% | 0.523 | 0.177 |
Table 8.
Periodic saccharification of liquefied corn starch using different concentrations of commercial enzyme
| S. no. | Time (h) | Gluco-amylase ex. Rhizopus (in terms of reducing sugars mg/ml) | |||||
|---|---|---|---|---|---|---|---|
| 20 U | 40 U | 60 U | 80 U | 100 U | 500 U | ||
| 1. | 0.5 | 2.36 ± 0.05 | 2.61 ± 0.07 | 2.52 ± 0.02 | 2.64 ± 0.06 | 2.60 ± 0.07 | 3.2 ± 0.54 |
| 2. | 1.0 | 2.52 ± 0.02 | 2.74 ± 0.05 | 3.10 ± 0.03 | 3.19 ± 0.06 | 4.10 ± 0.04 | 6.26 ± 0.05 |
| 3. | 1.5 | 2.99 ± 0.03 | 2.97 ± 0.06 | 4.20 ± 0.04 | 4.28 ± 0.09 | 7.42 ± 0.07 | 10.62 ± 0.18 |
| 4. | 2.0 | 3.31 ± 0.04 | 3.32 ± 0.05 | 6.76 ± 0.06 | 9.86 ± 0.04 | 11.89 ± 0.06 | 13.91 ± 0.07 |
| 5. | 2.5 | 3.26 ± 0.02 | 2.98 ± 0.06 | 7.03 ± 0.04 | 9.79 ± 0.04 | 11.86 ± 0.07 | 14.46 ± 0.04 |
| CD@5% | 0.112 | 0.177 | 0.126 | 0.189 | 0.193 | 0.421 | |
Incubation temperature—60 °C
Table 9.
Comparative amylase production studies of crude enzyme with commercial enzyme
| S. no | Time (h) | Reducing sugars (mg/ml) | Saccharification efficiency (%) | ||
|---|---|---|---|---|---|
| Crude enzyme (1.5 ml) | Gluco-amylase ex. Rhizopus (500 U) | Crude enzyme (1.5 ml) | Gluco-amylase ex. Rhizopus (500 U) | ||
| 1. | 0.5 | 3.2 ± 0.54 | 3.2 ± 0.03 | 12.19 ± 0.04 | 11.85 ± 0.03 |
| 2. | 1.0 | 6.26 ± 0.05 | 6.48 ± 0.06 | 23.79 ± 0.05 | 23.65 ± 0.09 |
| 3. | 1.5 | 10.62 ± 0.18 | 11.52 ± 0.03 | 40.31 ± 0.02 | 42.38 ± 0.06 |
| 4. | 2.0 | 13.91 ± 0.07 | 21.15 ± 0.17 | 52.63 ± 0.12 | 77.7 ± 0.06 |
| 5. | 2.5 | 14.46 ± 0.04 | 21.10 ± 0.03 | 53.20 ± 0.07 | 77.43 ± 0.05 |
| 6. | 3.0 | 13.61 ± 0.12 | 21.13 ± 0.09 | 47.78 ± 0.11 | 77.50 ± 0.06 |
| CD@5% | 0.563 | 0.421 | 0.237 | 0.187 | |
| Control (mg/ml) | After liquefaction (mg/ml) |
|---|---|
| Starch concentration—49.20 | Starch concentration—24.50 |
| Reducing sugars—0.44 | Reducing sugars—2.19 |
Using starch-supplemented minimal media (SSMM)
Jagatee et al. (2015) studied dextrinisation and saccharification of sweet potato starch by two amylase enzymes Palkolase (α-amylase) and Palkodex (gluco-amylase), respectively. The study revealed that the optimised parameters for dextrinisation and saccharification are incubation time of 45 min and 24 h, pH of 5.5 and 4.5, temperature of 90 and 65 °C, enzyme concentration of 20 and 224 μl, respectively. The maximum sugar of 423 and 876 mg/g was released after dextrinization and saccharification which is 26.71 and 64.61% more than the initial concentration, respectively. Studies by Hudeckova et al. (2017) observed the liquefaction and saccharification of waste bread using commercial enzymes BAN 240L (α-amylase from B. amyloliquefaciens) and AMG 300L (gluco-amylase from A. niger) by Novozymes. Optimal conditions for the liquefaction were pH 6.0 and 80 °C. The final concentration of total sugars was 67.08 g/l. The time of liquefaction was about 180 min. After liquefaction, gluco-amylase was added for saccharification. Optimal conditions for saccharification were pH 4.2 and temperature 60 °C and the time of saccharification was 90 min resulting in total sugar concentration of 70.75 g/l.
Conclusion
The optimized conditions for maximum gluco-amylase production by A. niger were incubation temperature 32.5 °C, pH 5.5, substrate concentration 7 and 0.25% yeast extract concentration. It was found that when 500 U of Glucoamylase ex. Rhizopus were inoculated into starch-supplemented minimal media (SSMM) liquefied using 2 g of Fungal Diastase, it increases the reducing sugar concentration from 2.19 to 21.15 mg/ml and a saccharification efficiency of 77.7% was achieved, whereas 1.5 ml of crude enzyme (extracted from A. niger) was able to produce 14.46 mg/ml of reducing sugars with a saccharification efficiency of 53.2%. Percent conversion on the basis of reducing sugars produced per g of dry substrate was calculated on the basis of starch (68.97%) and it was found that reducing sugars produced were 535.9 mg/g of dry substrate by commercial enzyme and were 366.9 mg/g of dry substrate by crude enzyme.
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
References
- AACC . Approved methods of American association of cereal chemists. 10. St. Paul: MN American Association of Cereal Chemists; 2000. [Google Scholar]
- Ayodeji AO, Ajele JO. Optimization of culture parameters for production of raw starch degrading amylase from isolated soil fungal species in Akure, Nigeria. Proc Biochem. 2016;28:170–177. [Google Scholar]
- Bagheri A, Khodarahmi R, Mostafaie A. Purification and biochemical characterisation of glucoamylase from a newly isolated Aspergillus niger: relation to starch processing. J Food Chem. 2014;161:270–278. doi: 10.1016/j.foodchem.2014.03.095. [DOI] [PubMed] [Google Scholar]
- Bernfeld P. Amylases, alpha and beta. Methods Enzymol. 1955;1:149–158. doi: 10.1016/0076-6879(55)01021-5. [DOI] [Google Scholar]
- Clegg KM. The application of anthrone reagent to the estimation of starch in cereals. J Sci Food Agric. 1956;7:40–44. doi: 10.1002/jsfa.2740070108. [DOI] [Google Scholar]
- Gao J, Weng H, Zhu D, Yuan M, Guan F, Xi Y. Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Biores Technol. 2008;99:7623–7629. doi: 10.1016/j.biortech.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Hudeckova H, Supinova P, Babak L. Optimization of enzymatic hydrolysis of waste bread before fermentation. Appl Microbiol. 2017;65:35–40. doi: 10.1111/lam.12742. [DOI] [Google Scholar]
- Jagatee S, Pradhan C, Dash PK, Sahoo S, Mohanty RC. Optimization for saccharification of sweet potato (Ipomoea batatas L) flour for enhanced ethanol production. Int J Sci Technol Manag. 2015;4:67–76. [Google Scholar]
- Kunamneni A, Singh S. Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production. Biochem Eng J. 2005;27:179–190. doi: 10.1016/j.bej.2005.08.027. [DOI] [Google Scholar]
- Malik S, Iftikhar T, Haq I, Khattak MI. Process optimization for amylo-glucosidase by a mutant strain of Aspergillus niger in stirred fermenter. Pak J Bot. 2013;45:663–666. [Google Scholar]
- Mangat M, Kalra KL, Kocher GS, Phutela R, Sharma S. Comparative ethanol production from two corn varieties by commercial enzymes. Starch/Starke. 2010;62:647–651. doi: 10.1002/star.200900253. [DOI] [Google Scholar]
- Miller GJ. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mustafa SR, Husaini A, Hipolito CN, Suhaili N, Roslan HA. Application of response surface methodology for optimizing process parameters in the production of amylase by Aspergillus flavus NSH9 under solid state fermentation. Braz Arch Biol Technol. 2016;59:615–632. doi: 10.1590/1678-4324-2016150632. [DOI] [Google Scholar]
- Nwagu AT, Okolo BN. Extracellular amylase production of a thermotolerant Fusarium sp. isolated from eastern nigerian soil. Proc Biochem. 2011;54:649–658. [Google Scholar]
- Pervez S, Aman A, Iqbal S, Siddiqui N, Qader S. Saccharification and liquefaction of cassava starch: an alternative source for the production of bioethanol using amylolytic enzymes by double fermentation process. J Biotechnol. 2014;14:49–52. doi: 10.1186/1472-6750-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni TK, Goel A. Microbial enzymes with special reference to α-amylase. Bio Evol. 2015;2:19–25. [Google Scholar]
- Vihinen M, Mantsala P. Microbial amylolytic enzymes. Crit Rev Biochem Mol Biol. 1989;24:329–419. doi: 10.3109/10409238909082556. [DOI] [PubMed] [Google Scholar]