Abstract
Background
Eosinophilic otitis media (EOM) is often associated with comorbid asthma. The middle ear cavity is part of the upper airway. Therefore, EOM and asthma can be considered to be a crucial part of the “one airway, one disease” phenomenon. Based on the concept of one airway, one disease in the context of allergic rhinitis and asthma, optimal level of inhalation therapy for better asthma control leads to improvement in allergic rhinitis.
Objective
We conducted a pilot study to determine whether appropriate strengthening of inhalation therapy for asthma is effective for EOM.
Methods
Fifteen patients with EOM and comorbid asthma were enrolled in this study. Eight patients were randomly selected and administered appropriately strengthened inhalation therapy for asthma (strengthened group). The effect of the therapy on EOM was assessed by comparing a questionnaire for ear symptoms, clinical characteristic score, pure tone audiometry, blood tests and temporal bone computed tomography (CT) examination before and after the therapy. Seven other EOM + asthma patients without the above mentioned therapy were included as controls.
Results
In the strengthened group, the score of ear symptoms, clinical characteristics score, peripheral blood eosinophil count, CT score, and air conduction hearing level improved significantly after strengthening the inhalation therapy, but not in the control group. The lung function tests (forced vital capacity [%predicted], forced expiratory volume in 1 second [FEV1] [L], and FEV1 [%predicted]) significantly increased in the strengthened group after the therapy, but not in the control group.
Conclusion
In this study we demonstrated that EOM improved along with improved lung function when appropriately optimal inhalation therapy was implemented in patients with EOM and asthma. Administration of optimizing therapy for asthma might be effective for concomitant EOM.
Keywords: Eosinophilic otitis media; One airway, one disease; Optimized asthma treatment; Asthma
Introduction
Eosinophilic otitis media (EOM) is an intractable otitis media of the upper airway characterized by eosinophil-dominant effusion. Patients with EOM are highly susceptible to developing asthma (in the lower airway) [1], and, conversely, asthmatics can develop EOM in direct proportion to the severity of their asthma [2]. In EOM (as in asthma) there is local production of helper T type-2 (Th2) cytokines, such as interleukin-5 (IL-5) [3]. The middle ear cavity is connected with the nasopharynx via the Eustachian tube, making it an extension of the upper respiratory tract. EOM and asthma have a mutual pathologic relationship similar to that between allergic rhinitis (another upper airway disease) and asthma [4]. The latter relationship has been considered to be ‘one airway, one disease,’ because nasal provocation affects asthma in the lower airway, while bronchial provocation in the lower airway affects allergic rhinitis in the upper airway [5,6]. Allergic rhinitis patients improved in response to orally inhaled steroids [7]. Indeed, inhaled steroids decreased nasal brush eosinophils and nasal lavage fluid levels of eosinophil cationic protein [7]. Most findings for ‘one airway, one disease’ indicate a systemic link between the upper and lower airways that involves the bloodstream and bone marrow [8,9].
We previously reported that appropriate strengthening of inhaled therapy for asthma in patients with EOM complicated by asthma also improved the EOM [10]. We hypothesized that optimizing asthma therapy could improve EOM in patients with asthma, and this study aimed to clarify whether optimally strengthened inhaled therapy for asthma could be a treatment for EOM.
Materials and Methods
Materials
From April 2010 through December 2016, we treated 15 EOM patients who also had asthma in our Otolaryngology Department at Tokyo Women's Medical University Hospital (Table 1). EOM was diagnosed using the diagnostic criteria for EOM [1]. All patients were being treated for asthma at the time when they were first examined in the Otolaryngology Department. Control of the asthma in all patients was evaluated by a respiratory physician in accordance with the Global Initiative for Asthma (GINA) guideline [11].
Table 1. Patient baseline characteristics.
| Characteristic | Control group (n = 7) | Strengthened group (n = 8) | p value | |
|---|---|---|---|---|
| Sex (n) | ||||
| Male:female | 3:4 | 2:6 | NS | |
| Age (yr) | 62.3 ± 8.0 | 55.9 ± 11.4 | NS | |
| Asthma severity (n) | ||||
| Moderate:severe | 3:4 | 2:6 | NS | |
| Associated diseases, yes (%) | ||||
| Aspirin intolerance | 33.3 | 7.7 | NS | |
| Chronic sinusitis | 66.7 | 69.2 | NS | |
| Allergic rhinitis | 50.0 | 53.8 | NS | |
| Baseline mean scores | ||||
| Ear symptom score | 8.4 ± 5.2 | 14.1 ± 6.7 | NS | |
| Clinical characteristic score | 3.1 ± 2.7 | 3.4 ± 2.0 | NS | |
| Temporal bone CT score | 7.0 ± 2.7 | 7.3 ± 2.5 | NS | |
| Eosinophil count in PB (/µL) | 706.3 ± 515.0 | 1,070.4 ± 1,266.1 | NS | |
| Total serum IgE level (IU/mL) | 172.1 ± 139.9 | 582.9 ± 517.6 | NS | |
| Baseline treatments | ||||
| Intratympanic TA, yes | 1 (14.3) | 0 (0) | NS | |
| Systemic corticosteroids, yes | 2 (28.6) | 1 (12.5) | NS | |
| Systemic antibiotics, yes | 0 (0) | 1 (12.5) | NS | |
| ICS dose (µg/day) | 494.3 ± 92.9 | 336.3 ± 208.0 | NS | |
| LABA dose (µg/day) | 79.1 ± 36.0 | 54.5 ± 49.1 | NS | |
| LAMA, yes | 1 (14.3) | 1 (12.5) | NS | |
| Leukotriene receptor antagonist, yes | 6 (85.7) | 4 (50.0) | NS | |
| Lung function tests | ||||
| FVC (%pred) | 102.0 ± 14.6 | 101.9 ± 14.5 | NS | |
| FEV1 (L) | 1.79 ± 0.50 | 2.17 ± 0.90 | NS | |
| FEV1 (%pred) | 76.5 ± 20.0 | 83.3 ± 22.3 | NS | |
| FEV1/FVC (%) | 62.1 ± 15.0 | 70.4 ± 12.7 | NS | |
| DLco (mL/min/mmHg) | 20.6 ± 4.2 | 19.0 ± 4.1 | NS | |
| DLco (%pred) | 90.9 ±19.1 | 86.2 ± 15.1 | NS | |
| DLco/VA (%pred) | 124.6 ± 14.7 | 117.9 ± 19.0 | NS | |
| Reversibility of FEV1 (mL) | 60.8 ± 78.7 | 49.6 ± 54.4 | NS | |
| Reversibility of FEV1 (% increase) | 29.3 ± 96.1 | -10.0 ± 36.5 | NS | |
Values are presented as mean ± standard deviation or number (%) unless otherwise indicated.
CT, computed tomography; PB, peripheral blood; TA, triamcinolone acetonide; ICS, inhaled corticosteroid (ICS dose was converted to beclomethasone equivalents); LABA, long-acting β2 agonist; LAMA, long-acting muscarinic antagonist; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; FEV1 (%pred), FEV1% of the patient divided by the average FEV1% in the population for any person of similar age, sex, and body composition; DLco, diffusing capacity of the lung carbon monoxide [CO]; DLco/VA, diffusing capacity of the lung CO/ventilation of the alveoli; Reversibility of FEV1, change in FEV1 value after bronchodilator administration compared to baseline value; Reversibility of FEV1 (% increase), rate of improvement; NS, not significant.
Design
All 15 patients entered in the study at the time they were diagnosed with EOM in the Otolaryngology Department of Tokyo Women's Medical University Hospital. At that same time, various examinations, including lung function tests, were conducted by a respiratory physician, while diagnosis for asthma was performed and extant treatments were evaluated. Table 1 shows the lung function tests that were carried out. During the following week, a questionnaire for ear symptoms was administered [12], various clinical characteristic scores were determined [12], and pure tone audiometry, blood tests and a temporal bone computed tomography (CT) examination were performed. Eight patients were randomly selected by envelop method for strengthening of their inhalation therapy for asthma (strengthened group). The remaining 7 patients served as a control group. In the strengthened group, 4 patients were using fluticasone propionate/salmeterolxinafoate (FP/SM), 2 patients were using budesonide/formoterol fumarate hydrate (BUD/FM), and 1 patient was using beclometasone dipronate (BDP). One patient was decided not using inhaled corticosteroid because he was only using FP/SM temporarily. In the control group, 5 patients were using FP/SM, 2 patients were using BUD/FM.
Based on the results of asthma severity in accordance with the GINA guideline, the dosage (μg/day) of inhaled drug in the strengthened group was increased to the optimal dose for treating each patient’s asthma (Tables 1, 2). Inhaled corticosteroid/long-acting beta 2 agonist (ICS/LABA) was used to strengthen the inhalation therapy in all strengthened group patients. Other drugs for asthma and EOM that were being used at the baseline were continued during this study for all 15 patients.
Table 2. ICS dosages (μg/day) at entry (baseline) in the 2 groups and after strengthening in the strengthening group (converted to beclomethasone equivalents).
| Case No. | Control group | Strengthened group | |
|---|---|---|---|
| Baseline | Baseline | After strengthening | |
| 1 | 500 | 500 | 1,000 |
| 2 | 320 | 500 | 1,000 |
| 3 | 500 | 500 | 1,000 |
| 4 | 500 | 50 | 500 |
| 5 | 500 | 0 | 1,000 |
| 6 | 500 | 320 | 520 |
| 7 | 640 | 500 | 1,000 |
| 8 | - | 500 | 1,000 |
Some of the patients had experienced asthma exacerbations and taken corticosteroids, in addition to inhaled therapy. The basic therapy for EOM was intratympanic instillation of triamcinolone acetonide (TA), and oral antibiotics were administered when a bacterial infection was suspected based on a finding of middle ear effusion. Table 1 summarizes the basic treatments for asthma and EOM that were being administered at the start of the study. One year after enrollment in this study, all 15 patients (i.e., both groups) were again subjected to the same examinations as at the baseline: a questionnaire for ear symptoms, clinical characteristic score, pure tone audiometry, blood tests, and temporal bone CT. The examination results were compared with those at the time of enrollment. In addition, the respiratory physician again performed lung function tests for all patients, and the results were compared with those at entry.
This study was approved by the Ethics Committee of Tokyo Women’s Medical University Hospital (approval number: 3769), and written informed consent was obtained from each patient.
Evaluation of hearing
The air conduction and bone conduction hearing functions of EOM patients were evaluated using the pure tone hearing test. When the hearing exceeded the measurement limit at each frequency, the measurement limit + 5 dB was assigned as the hearing level at that frequency.
Evaluation of temporal bone CT scanning
The CT shadow of each portion of the ear (Eustachian tube, tympanum, and mastoid) was assigned a score of 0, no abnormality; 1, partial opacification; or 2, total opacification. The temporal bone CT scans were evaluated by one of the authors who was familiar with their reading and was blinded to the patient information.
Evaluation of ear symptoms and clinical characteristics
All 15 patients were evaluated for 8 ear symptoms (echo in the ear, tinnitus, otorrhea, dizziness, breathing sound in the ear, autophony, aural fullness, and otalgia) by assigning scores of 0 to 5 (none, 1, rare; 2, sometimes; 3, often; 4, usually; 5, worst) in accordance with a questionnaire created by Iino et al. [12]. In addition, 5 clinical characteristics (quantity of middle ear effusion/otorrhea, condition of the middle ear mucosa, frequency of intratympanic administration of corticosteroid, frequency of systemic administration of corticosteroid, frequency of systemic administration of antibiotics) were evaluated by assigning scores of 0 to 2 points depending on the quantity, condition or frequency [12]. The evaluations were performed at entry and after 1 year.
Statistical analyses
Wilcoxon signed rank test was used to compare the results between at entry and 1 year later for both the strengthened group and the control group. The Mann-Whitney U test and the chi-square test were used to compare the results between at entry and 1 year later in both the strengthened group and the control group. A p value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
All the patients had moderate or severe asthma. The evaluation of the asthma control found that it was inadequate (partially controlled or uncontrolled) in all of the patients. Table 1 shows the characteristics of the patients in the control group and strengthened group at entry. The 2 groups showed no statistically significant differences in regard to the number of patients, sex ratio, age, asthma severity, percentage of patients with aspirin intolerance, percentage of patients with chronic sinusitis, percentage of patients with allergic rhinitis, peripheral blood eosinophil count, or the total IgE level. At entry, there were no significant differences between the 2 groups in terms of use of TA, systemic corticosteroids, antibiotics, ICS dose (converted to beclomethasone [BDP conversion]), LABA dose, long-acting muscarinic antagonist or leukotriene receptor antagonist (Table 1). The 2 groups showed no significant differences in the results of any of the lung function tests at entry (Table 1).
Ear symptom score
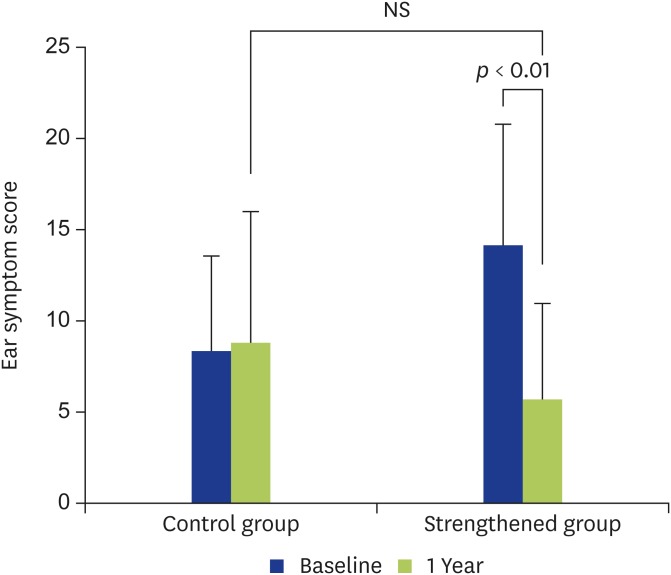
In the control group, no significant improvement was seen in the ear symptom score (Fig. 1). On the other hand, in the strengthened group, statistically significant improvement was seen in the score after strengthening compared to before strengthening (i.e., at entry) (Fig. 1). Statistically significant improvement was especially seen for echo in the ear (p < 0.05), otorrhea (p < 0.01), breathing sound in the ear (p < 0.05), and aural fullness (p < 0.05) (Table 3).
Fig. 1.
Changes in the ear symptom scores are shown. In the control group, the ear symptom score showed no significant (NS) change at 1 year compared with at entry. In the strengthened group, the ear symptom score had improved significantly at 1 year after strengthening compared with at entry (p < 0.01).
Table 3. Ear symptom score and clinical characteristic score at entry (baseline) and after strengthening in the strengthened group.
| Score | Baseline | After strengthening | p value | |
|---|---|---|---|---|
| Ear symptom score | ||||
| Echo in the ear | 2.0 ± 0.0 | 0.7 ± 1.1 | <0.01 | |
| Tinnitus | 3.1 ± 1.9 | 2.7 ± 1.8 | NS | |
| Otorrhea | 2.4 ± 1.9 | 0.3 ± 0.7 | <0.01 | |
| Dizziness | 0.6 ± 1.7 | 0.1 ± 0.3 | NS | |
| Breathing sound in the ear | 2.8 ± 2.3 | 0.3 ± 0.7 | <0.05 | |
| Autophony | 2.0 ± 1.9 | 0.4 ± 0.7 | NS | |
| Aural fullness | 3.6 ± 1.5 | 1.4 ± 1.9 | <0.05 | |
| Otalgia | 1.7 ± 1.9 | 0.4 ± 0.7 | NS | |
| Clinical characteristic score | ||||
| Quantity of otorrhea | 2.7 ± 1.2 | 0.8 ± 0.8 | <0.01 | |
| Condition of middle ear mucosa | 0.2 ± 0.7 | 0.1 ± 0.3 | NS | |
| Frequency of intratympanic administration of corticosteroids | 0.4 ± 0.9 | 0.7 ± 1.4 | NS | |
| Frequency of systemic administration of corticosteroids | 0.2 ± 0.7 | 0.2 ± 0.7 | NS | |
| Frequency of systemic administration of antibiotics | 0.4 ± 0.9 | 0.2 ± 0.7 | NS | |
Values are presented as mean ± standard deviation.
NS, not significant.
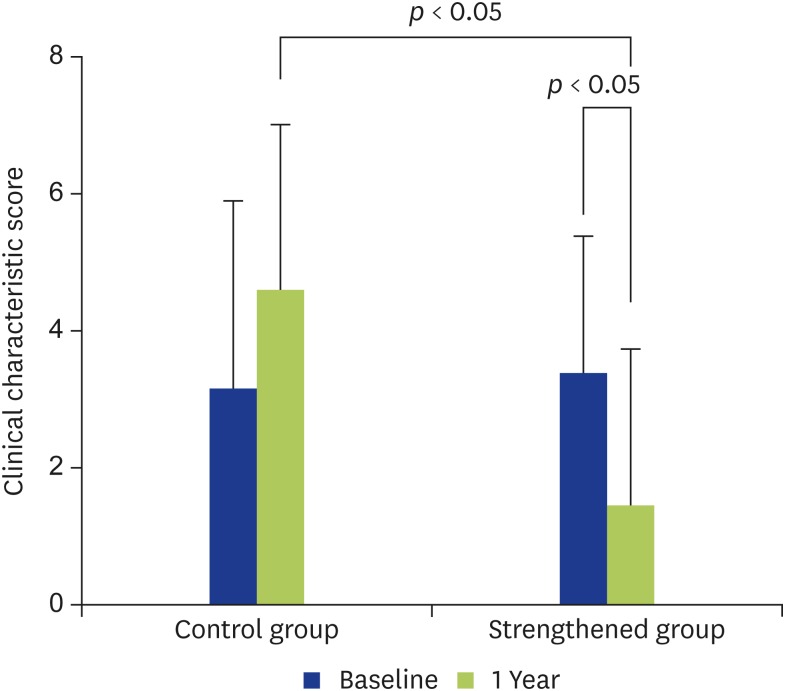
Clinical characteristic score
There was no improvement in the clinical characteristic score in the control group (Fig. 2), whereas it showed statistically significant improvement in the strengthened group (Fig. 2). However, within the strengthened group, the score significantly improved only for the otorrhea status (p < 0.01) (Table 3). No significant differences were found between the 2 groups in regard to the frequency of intratympanic instillation of TA, the frequency of use of systemic steroids, or the frequency of use of systemic antibiotics (Table 3).
Fig. 2.
Changes in the clinical characteristic scores are shown. In the control group, the clinical characteristic score showed no significant change at 1 year compared with at entry. In the strengthened group, the clinical characteristic score had improved significantly at 1 year after strengthening compared with at entry (p < 0.05). Significant difference was also seen between the 2 groups at 1 year (p < 0.05).
Eosinophil count in peripheral blood (Table 1)
There was no significant improvement in the eosinophil count in the control group: 706.3 ± 515.0 (/μL) at entry and 583.3 ± 562.0 (/μL) after 1 year. Conversely, the eosinophil count in the strengthened group improved markedly: from 1,070.4 ± 1,266.1 (/μL) before strengthening to 279.9 ± 161.8 (/μL) after strengthening (p < 0.01).
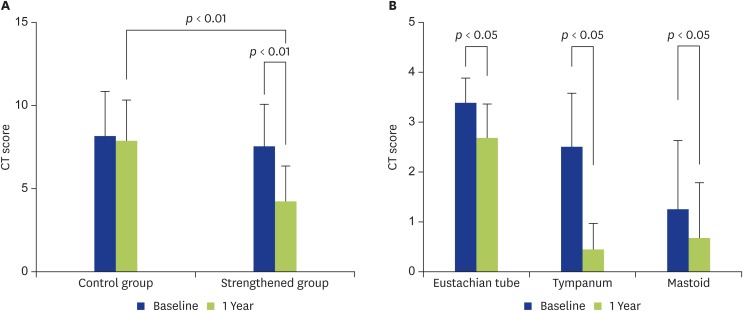
Temporal bone CT score
The CT score also did not show significant improvement in the control group, but significant improvement was seen in the strengthened group (Fig. 3A). In addition, when different sites were compared in the strengthened group, improvement was found in each of the Eustachian tube, tympanum and mastoid, with the improvement in the tympanum being most striking (p < 0.01) (Fig. 3B).
Fig. 3.
Temporal bone computed tomography (CT) scores are shown. (A) In the control group, the temporal bone CT score showed no significant change at 1 year compared with at entry. In the strengthened group, the CT score had improved significantly at 1 year after strengthening compared with at entry (p < 0.01). The significant difference was also seen between the 2 groups at 1 year (p < 0.01). (B) The changes in the temporal bone CT scores are compared by site in the strengthened group. The temporal bone CT score had improved significantly at 1 year after strengthening compared with at entry for each of the Eustachian tube (p < 0.05), tympanum (p < 0.01), and mastoid (p < 0.05). The improvement for the tympanum was most striking.
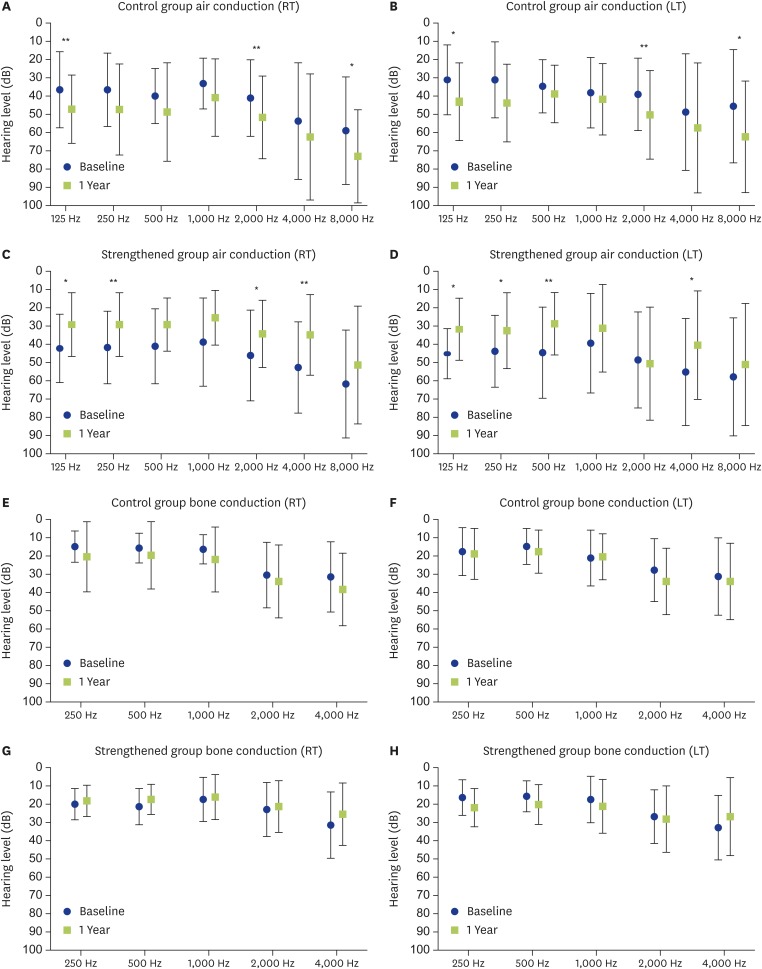
Air and bone conduction hearing levels
Fig. 4 shows the results of the air and bone conduction hearing tests. In the control group the air-conduction hearing level tended to become worse at most frequencies, and statistically significant worsening was seen at 125 Hz, 2,000 Hz, and 8,000 Hz in both ears (Fig. 4A, B). On the other hand, in the strengthened group, statistically significant improvement was seen at 125 Hz, 250 Hz, 2,000 Hz, and 4,000 Hz for the right ear and at 125 Hz, 250 Hz, 500 Hz, and 4,000 Hz for the left ear after strengthening the asthma therapy compared with before (i.e., the baseline at entry) (Fig. 4C, D). The bone conduction hearing level did not change significantly at any of the tested frequencies in either group from entry until 1 year later (Fig. 4E–H).
Fig. 4.
Air and bone conduction hearing levels are shown. (A, B) Changes in the air conduction hearing levels (RT/LT) in the control group. In the control group, the hearing levels showed a tendency to have worsened at 1 year compared with at entry. The hearing level on both sides had worsened significantly at 125 Hz, 2,000 Hz, and 8,000 Hz. *p < 0.05. **p < 0.02. (C, D) Changes in the air conduction hearing levels (RT/LT) in the strengthened group. In the strengthened group, the hearing levels showed a tendency to have improved at 1 year after strengthening compared with at entry. Significant improvement was seen for the right ear at 125 Hz, 250 Hz, 2,000 Hz, and 4,000 Hz, and for the left ear at 125 Hz, 250 Hz, 500 Hz, and 4,000 Hz. *p < 0.05. **p < 0.02. (E, F) Changes in the bone conduction hearing levels (RT/LT) in the control group. In the control group, the hearing levels had not changed significantly at 1 year compared with at entry. (G, H) Changes in the bone conduction hearing levels (RT/LT) in the strengthened group. In the strengthened group, the hearing levels had not changed significantly at 1 year after strengthening compared with at entry. RT, right ear; LT, left ear.
Lung function tests (Table 1)
The results of the lung function tests were as follows. The results in the strengthened group improved significantly after strengthening of the asthma therapy: from forced vital capacity (FVC) (%predicted) 101.9 ± 14.5, forced expiratory volume in 1 second (FEV1) (L) 2.17 ± 0.90 and FEV1 (%predicted) 83.3 ± 22.2 before, to FVC (%predicted) 113.5 ± 9.1 (p < 0.01), FEV1 (L) 2.45 ± 0.74 (p < 0.05), and FEV1 (%predicted) 104.7 ± 12.0 (p < 0.05) after. None of those test parameters changed significantly in the control group. FEV1/FVC (%), DLco (mL/min/mmHg), DLco (%predicted), DLco/VA (%predicted), and reversibility of FEV1 did not change significantly in either group.
Discussion
EOM is an intractable otitis media characterized by marked eosinophil infiltration of the middle ear. EOM is refractory to such conventional treatments for otitis media as myringotomy (which is effective for otitis media with effusion [OME]) and antibiotics. Administration of systemic and topical corticosteroids is the only current effective treatment for EOM [12]. All 15 asthma patients with EOM enrolled in this pilot study had inadequately controlled (partially controlled or uncontrolled) asthma. Those who were assigned to the strengthened group showed better asthma control in response to the optimized inhaled ICS/LABA therapy, resulting in improving lung function tests and blood eosinophilia. Those changes led to improvement in the EOM. Optimized inhaled asthma therapy thus may be useful for treatment of EOM in asthmatics.
Asthma is present in 90% of EOM patients, and EOM is present in 10% of asthma patients. In addition, EOM was reported to complicate 11% of eosinophilic sinusitis cases, while eosinophilic sinusitis complicates 64% of EOM cases [13]. The middle ear is connected to the nasopharynx via the Eustachian tube. And the middle ear mucosa is composed of glandular epithelial cells similar to those of the bronchial mucosa and paranasal mucosa, so it can be considered part of the upper respiratory tract.
Also, if the extent of the paranasal sinus shadow in patients with eosinophilic sinusitis is scored by CT and the severity is classified, the CT score correlates positively with the eosinophil count in both the blood and sputum [14]. The number of eosinophils in sputum is, like the number of eosinophils in the blood, an indicator of the severity of asthma. It is thought that the more severe asthma is, the more severe eosinophilic sinusitis is. Similar to the case of eosinophilic sinusitis, there are reports that EOM is more likely to develop as asthma becomes more severe [2], and asthma and EOM are also increasingly considered to bear a close relationship. Therefore, EOM and eosinophilic sinusitis of the upper respiratory tract, and asthma, which is a common lower respiratory complication, are coming to be considered to have a “one airway, one disease” relationship.
The concept of “one airway, one disease” has come to be established and accepted due to the following thinking. Allergic rhinitis and asthma show a high rate of concurrence, and they are thought to be Th2 type (eosinophilic) inflammatory pathologies that occur in one organ, i.e., the “respiratory tract,” comprised of the upper and lower respiratory tracts. When steroid inhalation therapy was administered for asthma, which is a lower respiratory tract disease, upper respiratory tract lesions improved, and when topical nasal glucocorticosteroid treatment was administered for allergic rhinitis, which is an upper respiratory disease, lower respiratory tract lesions improved [15]. Therefore, we hypothesized that, for EOM, as well, which is thought to have a one airway, one disease relationship with asthma, the inhalation therapy for copresent asthma should be evaluated, and that appropriate strengthening of that therapy in the case of poor asthma control would lead to improvement of the EOM. Indeed, the inhalation therapy for asthma was inadequate in all of the patients enrolled in this study, and when we appropriately strengthened the treatment, the lung function and blood eosinophilia improved, and so did the EOM.
As in the case of asthma, EOM has been found to be a Th2 type inflammation in which helper T type-2 cytokines such as IL-5 are produced locally [3]. In Th2-type inflammation in the respiratory tract, cytokines (thymic stromal lymphopoietin [TSLP], IL-33, IL-25, etc.) produced mainly by epithelial cells are thought to be important for subsequent induction of Th2-type inflammation. Indeed, expression of these cytokines was elevated in airway mucosal epithelial cells in allergic rhinitis, eosinophilic sinusitis and asthma [16,17,18,19]. When antigens, etc., stimulate airway mucosal epithelial cells, the epithelial cells produce TSLP, IL-33, IL-25, etc. Those cytokines then induce production of Th2 cytokines (IL-4, IL-5, IL-13, etc.), and Th2 type inflammation manifests [20,21].
This results in elevated expression of IL-5 throughout the body, and it induces eosinophil differentiation in the bone marrow and is also involved in migration of eosinophils from the bone marrow into the blood [22]. Chemokines (eotaxin, etc.) produced by epithelial cells and fibroblasts are considered important for the recruitment and migration of eosinophils into the airway mucosa [23]. As a result, in airway Th2-type inflammation, including EOM, eosinophils not only infiltrate into the airway mucosa but also increase in the blood.
For allergic rhinitis, asthma and other airway Th2-type inflammatory diseases, use of a topical glucocorticosteroid is the treatment of first choice, and that is true for EOM, as well. Directly suppressing cytokine production by the epithelial cells responsible for airway Th2-type inflammation via the use of a topical glucocorticosteroid is a reasonable modality of treatment. In this study, all the enrolled patients inhaled the inhalant for asthma and we instructed them to then exhale it from the mouth. Therefore, it is unlikely that the inhaled asthma drug entered the middle ear via the Eustachian tube during expiration and downregulated the middle ear mucosal epithelial inflammation, leading to improvement in the EOM [10].
There are 2 possible mechanisms by which strengthening of inhalation therapy for asthma is effective in improving EOM. One is as follows. ICS/LABA suppresses production of cytokines such as TSLP that are produced by lower respiratory mucosal epithelial cells and induce Th2 type inflammation. That cytokine suppression leads in turn to suppression of Th2 cytokine production in the lower respiratory mucosa, systemic suppression of IL-5 expression, and also to suppression of production of eosinophils in the bone marrow and their migration into the blood. These events may have contributed to reduced eosinophil infiltration of the middle ear mucosa.
The second possible mechanism involves the ICS systemic exposure due to ICS/LABA. Although ICS exerts its anti-inflammatory effect locally in the respiratory tract, when it exceeds a certain dosage, systemic exposure leads to adverse effects such as adrenal suppression [24]. Twenty percent cortisol suppression is used as an indicator of the effect of ICS on the hypothalamic-pituitary-adrenal axis. Since the ICS/LABA used in this pilot study was FP/SM DPI or BUD/FM DPI, their 20% cortisol suppression levels were set at 900 μg/day with FP/SM DPI and 600 μg/day with BUD/FM DPI [24]. The ICS dosage after strengthening of inhalation therapy was increased to 1,000 μg/day in 6 of 8 patients, so the inhibitory effect on Th2-type (eosinophilic) inflammation in the blood and bone marrow due to systemic exposure to ICS inhalation therapy cannot be ignored.
Interestingly, all the asthma patients with EOM enrolled in this study had inadequate asthma control. It was reported that 55% of randomly chosen asthma patients had persistent asthma, and that 48% of those persistent asthma patients had received inappropriate treatment that did not adequately control their asthma [25]. Asthma patients tend to believe that their asthma symptoms were well controlled even if they had partly or uncontrolled asthma [26]. In the concept of “one airway, one disease,” as already noted, the inflammatory states of the upper airway and the lower airway were reported to show a positive correlation. Therefore, the possibility of EOM developing in patients with asthma cannot be ruled out because the asthma treatment is often inadequate. For treatment of patients with EOM, we think it is important to evaluate asthma, which is said to be present in 90% of EOM patients, and to then implement appropriate inhalation therapy. To that end, in facilities that have the services of a respiratory physician, the extant asthma therapy must be evaluated using objective examinations.
Fractional exhaled nitric oxide concentration (FeNO) is being used as an objective test of asthma. In asthma, inducible nitric oxide synthase is expressed due to eosinophilic inflammation [27] and its production is accelerated, so FeNO rises. In fact, FeNO correlated with the degree of eosinophil infiltration of the airway mucosa [28] and the eosinophil ratio in bronchoalveolar lavage fluid [29]. In this way, since FeNO reflects the state of control of lower respiratory eosinophilic inflammation in asthma and can be conveniently measured, we can judge whether or not the administered asthma therapy is optimal [30]. It has been shown that appropriate treatment of asthma based on FeNO may aid in treatment of EOM.
Systemic and topical corticosteroids have been the main treatments for EOM because of their effectiveness. Recently, recombinant humanized monoclonal anti-IgE antibody therapy (omalizumab) [31] and pegylated interferon [32] have been reported to be effective for EOM. Omalizumab improves the subjective symptoms and clinical characteristics, and pegylated interferon also improves the clinical findings. In the present study we demonstrated that optimal inhaled ICS/LABA therapy for asthma, not only improving the subjective symptoms and clinical characteristics of EOM but also the temporal bone CT findings and pure tone audiometric findings. Our results suggest that this strengthened therapy is potentially a new modality of treatment for EOM in those with comorbid asthma, although randomized controlled studies are needed to further confirm these findings.
Footnotes
Author Contributions: Conceptualization: Manabu Nonaka. Data curation: Manabu Nonaka. Formal analysis: Yukako Seo. Funding acquisition: Manabu Nonaka. Investigation: Manabu Nonaka. Project administration: Yukie Yamamura, Etsuko Tagaya. Resources: Yukie Yamamura, Etsuko Tagaya. Supervision: Pawankar Ruby. Validation: Yukako Seo. Writing - original draft: Manabu Nonaka. Writing - review & editing: Manabu Nonaka.
References
- 1.Iino Y, Tomioka-Matsutani S, Matsubara A, Nakagawa T, Nonaka M. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx. 2011;38:456–461. doi: 10.1016/j.anl.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Seo Y, Nonaka M, Tagaya E, Tamaoki J, Yoshihara T. Eosinophilic otitis media is associated with asthma severity and smoking history. ORL J Otorhinolaryngol Relat Spec. 2015;77:1–9. doi: 10.1159/000370122. [DOI] [PubMed] [Google Scholar]
- 3.Nonaka M, Fukumoto A, Ozu C, Mokuno E, Baba S, Pawankar R, Yagi T. IL-5 and eotaxin levels in middle ear effusion and blood from asthmatics with otitis media with effusion. Acta Otolaryngol. 2003;123:383–387. doi: 10.1080/0036554021000028117. [DOI] [PubMed] [Google Scholar]
- 4.Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000;106(5 Suppl):S201–S205. doi: 10.1067/mai.2000.110151. [DOI] [PubMed] [Google Scholar]
- 5.Corren J, Adinoff AD, Irvin CG. Changes in bronchial responsiveness following nasal provocation with allergen. J Allergy Clin Immunol. 1992;89:611–618. doi: 10.1016/0091-6749(92)90329-z. [DOI] [PubMed] [Google Scholar]
- 6.Braunstahl GJ, Kleinjan A, Overbeek SE, Prins JB, Hoogsteden HC, Fokkens WJ. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. 2000;161:2051–2057. doi: 10.1164/ajrccm.161.6.9906121. [DOI] [PubMed] [Google Scholar]
- 7.Greiff L, Andersson M, Svensson C, Linden M, Wollmer P, Brattsand R, Persson CG. Effects of orally inhaled budesonide in seasonal allergic rhinitis. Eur Respir J. 1998;11:1268–1273. doi: 10.1183/09031936.98.11061268. [DOI] [PubMed] [Google Scholar]
- 8.Pawankar R. Allergic rhinitis and asthma: are they manifestations of one syndrome? Clin Exp Allergy. 2006;36:1–4. doi: 10.1111/j.1365-2222.2006.02420.x. [DOI] [PubMed] [Google Scholar]
- 9.Braunstahl GJ, Hellings PW. Allergic rhinitis and asthma: the link further unraveled. Curr Opin Pulm Med. 2003;9:46–51. doi: 10.1097/00063198-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Nonaka M, Yamamura Y, Tagaya E, Pawankar R, Yoshihara T. Improvement of eosinophilic otitis media by optimized asthma treatment. Allergy Asthma Immunol Res. 2013;5:175–178. doi: 10.4168/aair.2013.5.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global Initiative for Asthma. GINA report, global strategy for asthma management and prevention - updated 2010. The Global Initiative for Asthma; 2010. [Google Scholar]
- 12.Iino Y, Nagamine H, Kakizaki K, Komiya T, Katano H, Saruya S, Kodera K. Effectiveness of instillation of triamcinolone acetonide into the middle ear for eosinophilic otitis media associated with bronchial asthma. Ann Allergy Asthma Immunol. 2006;97:761–766. doi: 10.1016/S1081-1206(10)60967-2. [DOI] [PubMed] [Google Scholar]
- 13.Ishitoya J, Sakuma Y. Eosinophilic chronic rhinosinusitis and eosinophilic otitis media. Arerugi. 2011;60:535–545. [PubMed] [Google Scholar]
- 14.ten Brinke A, Grootendorst DC, Schmidt JT, De Bruïne FT, van Buchem MA, Sterk PJ, Rabe KF, Bel EH. Chronic sinusitis in severe asthma is related to sputum eosinophilia. J Allergy Clin Immunol. 2002;109:621–626. doi: 10.1067/mai.2002.122458. [DOI] [PubMed] [Google Scholar]
- 15.Watson WT, Becker AB, Simons FE. Treatment of allergic rhinitis with intranasal corticosteroids in patients with mild asthma: effect on lower airway responsiveness. J Allergy Clin Immunol. 1993;91(1 Pt 1):97–101. doi: 10.1016/0091-6749(93)90301-u. [DOI] [PubMed] [Google Scholar]
- 16.Zhu DD, Zhu XW, Jiang XD, Dong Z. Thymic stromal lymphopoietin expression is increased in nasal epithelial cells of patients with mugwort pollen sensitive-seasonal allergic rhinitis. Chin Med J (Engl) 2009;122:2303–2307. [PubMed] [Google Scholar]
- 17.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, Suh LA, Norton J, Harris KE, Grammer LC, Chandra RK, Conley DB, Kern RC, Schleimer RP, Kato A. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600.e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iinuma T, Okamoto Y, Yamamoto H, Inamine-Sasaki A, Ohki Y, Sakurai T, Funakoshi U, Yonekura S, Sakurai D, Hirahara K, Nakayama T. Interleukin-25 and mucosal T cells in noneosinophilic and eosinophilic chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2015;114:289–298. doi: 10.1016/j.anai.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Buc M, Dzurilla M, Vrlik M, Bucova M. Immunopathogenesis of bronchial asthma. Arch Immunol Ther Exp (Warsz) 2009;57:331–344. doi: 10.1007/s00005-009-0039-4. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 22.Simson L, Foster PS. Chemokine and cytokine cooperativity: eosinophil migration in the asthmatic response. Immunol Cell Biol. 2000;78:415–422. doi: 10.1046/j.1440-1711.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- 23.Yao T, Kojima Y, Koyanagi A, Yokoi H, Saito T, Kawano K, Furukawa M, Kusunoki T, Ikeda K. Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope. 2009;119:1053–1059. doi: 10.1002/lary.20191. [DOI] [PubMed] [Google Scholar]
- 24.Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80:372–380. doi: 10.1111/bcp.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Marco R, Cazzoletti L, Cerveri I, Corsico A, Bugiani M, Accordini S, Carrozzi L, Dallari R, De Togni A, Marinoni A, Pirina P, Janson C ISAYA Study Group. Are the asthma guideline goals achieved in daily practice? A population-based study on treatment adequacy and the control of asthma. Int Arch Allergy Immunol. 2005;138:225–234. doi: 10.1159/000088723. [DOI] [PubMed] [Google Scholar]
- 26.Lai CK, De Guia TS, Kim YY, Kuo SH, Mukhopadhyay A, Soriano JB, Trung PL, Zhong NS, Zainudin N, Zainudin BM Asthma Insights and Reality in Asia-Pacific Steering Committee. Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol. 2003;111:263–268. doi: 10.1067/mai.2003.30. [DOI] [PubMed] [Google Scholar]
- 27.Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P, Redington A, Bousquet J, Godard P, Holgate S, Polak JM. Induction of nitric oxide synthase in asthma. Lancet. 1993;342:1510–1513. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 28.Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1376–1381. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 29.Warke TJ, Fitch PS, Brown V, Taylor R, Lyons JD, Ennis M, Shields MD. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57:383–387. doi: 10.1136/thorax.57.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syk J, Malinovschi A, Johansson G, Undén AL, Andreasson A, Lekander M, Alving K. Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a randomized, controlled trial. J Allergy Clin Immunol Pract. 2013;1:639–648. 648.e1–648.e8. doi: 10.1016/j.jaip.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Iino Y, Hara M, Hasegawa M, Matsuzawa S, Shinnabe A, Kanazawa H, Yoshida N. Clinical efficacy of anti-IgE therapy for eosinophilic otitis media. Otol Neurotol. 2012;33:1218–1224. doi: 10.1097/MAO.0b013e318263d5b8. [DOI] [PubMed] [Google Scholar]
- 32.Neff BA, Voss SG, Carlson ML, O'Brien EK, Butterfield JH. Treatment of eosinophilic otitis media with pegylated interferon-α 2a and 2b. Laryngoscope. 2017;127:1208–1216. doi: 10.1002/lary.26303. [DOI] [PubMed] [Google Scholar]