Abstract
Background
The basophil activation test (BAT) is a promising tool for monitoring allergen-specific immunotherapy responses.
Objective
We aimed to investigate the changes in basophil activation in response to the inhalant allergens of house dust mite (HDM) and mugwort pollen during immunotherapy in patients with allergic rhinitis.
Methods
We enrolled patients with allergic rhinitis who were to receive subcutaneous immunotherapy for the inhalant allergens HDM or mugwort. A BAT was performed to assess CD63 upregulation in response to allergen stimulation using peripheral blood collected from the patients prior to immunotherapy and at 3, 6, 12, and 24 months after beginning immunotherapy. Rhinitis symptoms were evaluated using the rhinitis quality of life questionnaire (RQLQ) at 1-year intervals.
Results
Seventeen patients (10 with HDM sensitivity, 3 with mugwort sensitivity, and 4 with sensitivity to both HDM and mugwort) were enrolled in the study. Basophil reactivity to HDM did not change significantly during 24 months of immunotherapy. However, a significant reduction in basophil reactivity to mugwort was observed at 24-month follow-up. There was no significant association between the change in clinical symptoms by RQLQ and the change in basophil reactivity to either allergen. The change in allergen-specific basophil reactivity to HDM was well correlated with the change in nonspecific basophil activation induced by anti-FcεRI antibody, although basophil reactivity to anti-FcεRI antibody was not significantly reduced during immunotherapy.
Conclusion
Suppression of CD63 upregulation in the BAT was only observed with mugwort at 2-year follow-up. However, the basophil response did not reflect the clinical response to immunotherapy.
Keywords: Allergen immunotherapy, Basophil activation test, House dust mite, Mugwort
INTRODUCTION
Allergen-specific immunotherapy (AIT) is the only disease-modifying allergy treatment targeting the underlying immunologic mechanisms [1]. In contrast to pharmacologic treatment, AIT can lead to immunological tolerance to allergens by reorienting the inappropriate T helper (Th)2 immune response to the allergen toward a Th1 and regulatory T-cell response and inducing antigen-specific IgG4-blocking antibodies [2]. Although the mechanism of AIT is not yet fully understood, a potential long-term curative effect is expected with AIT in patients with allergic disease.
The clinical efficacy of AIT for various allergic diseases, including allergic rhinitis, allergic conjunctivitis, allergic asthma, and insect venom hypersensitivity, has been well documented [3]. Despite its proven clinical efficacy, biomarkers efficiently monitoring the associated immunological changes and reflecting clinical responses have not yet been identified. Several potential surrogate markers for AIT responses have been suggested, including in vivo skin test reactivity; in vitro immunological parameters such as cytokines, lymphocyte subsets, and allergen-specific IgE and IgG antibodies; and local and systemic inflammatory markers. However, all these markers have limitations in terms of their diagnostic and predictive value, reproducibility, and clinical availability [4,5]. Thus, ideal biomarkers for predicting and monitoring clinical and immunological responses to AIT are needed.
The basophil activation test (BAT) is a flow cytometry-based assay used to assess the degree of basophil activation by measuring the expression of basophil cell surface markers after exposure to stimuli [6]. Peripheral blood basophils, as well as tissue mast cells, are the primary effector cells in IgE-mediated allergic reactions, and basophils express unique surface markers, such CD63 and CD203c, during activation and degranulation. The BAT is a reliable tool for detecting basophil activation status in vitro and is a promising tool for the diagnosis and monitoring of allergic disease [6]. After the BAT was developed and established, many studies have explored its utility for monitoring AIT responses. Several studies have reported that basophil activation is reduced after immunotherapy to bee venom and tree and grass pollen allergens [7,8,9]. However, the utility of BAT for monitoring immunotherapy to other allergens, such house dust mites (HDMs) and weed pollens have not been evaluated. In the present study, we investigated the changes in basophil activation in response to the inhalant allergens HDM and mugwort pollen during immunotherapy in patients with allergic rhinitis.
MATERIALS AND METHODS
Patients
We enrolled patients with allergic rhinitis who were to receive allergen immunotherapy to control their symptoms of rhinoconjunctivitis. The enrolled patients showed a positive skin test to one or more inhalant allergens, including HDM or mugwort, and had experienced persistent symptoms of rhinorrhea, nasal itching, sneezing, and obstruction, perennially or seasonally despite medication. This study was approved by the Institutional Review Board of the investigating hospital, and informed consent was obtained from all study subjects.
AIT and study protocol
All study subjects were administered allergen-specific subcutaneous immunotherapy (SCIT) for the inhalant allergens deemed relevant to their allergic symptoms. The allergens for immunotherapy were selected based on the results of a skin prick test and the clinical history of the conditions aggravating allergic symptoms. Immunotherapy was conducted according to a conventional build-up schedule with two kinds of allergen extracts, Novo Helison Depot from Allergopharma (Reinback, Germany) or Tyrosin S from Allergy Therapeutics Ltd. (West Sussex, United Kingdom). In the initial build-up phase, the allergen concentration was increased every week according to the patient’s allergen sensitivity over a period of 4–6 months. Once the patient reached a maintenance dose, the maintenance dose was administered at 4-week intervals. Peripheral blood was collected for the BAT prior to immunotherapy and at 3, 6, 12, and 24 months after beginning immunotherapy. In addition, rhinitis symptom status and allergen-specific IgG4 were evaluated using the rhinitis quality of life questionnaire (RQLQ) [10] and ImmunoCAP analysis at intervals of 1 year.
Basophil activation test
The BAT was performed using Flow CAST kit (Bühlmann Laboratories AG, Schönenbuch, Switzerland) according to the manufacturer’s instructions to detect the expression of the basophil activation surface marker CD63 in peripheral blood. Briefly, anticoagulated blood samples were gently homogenized by inverting the tube several times. Then, basophils were stimulated with 50 µL of each allergen diluted in stimulation buffer according to the manufacturer’s instructions. Allergens for the BAT (Dermatophagoides pteronyssinus [Dp], Dermatophagoides farine [Df], and mugwort) were purchased from Bühlmann Laboratories. For positive controls, both a monoclonal anti-FcεRI antibody and N-formyl-methionyl-leucyl-phenylalanine (fMLP; 2 µM) were used. For the negative background control, 50 µL of stimulation buffer was used. To each tube contained 100 µL of stimulation buffer, 50 µL of patient blood, and 20 µL of staining reagent (containing anti-CD63-FITC and anti-CCR3-PD monoclonal antibodies) were added. The tubes were incubated at 37°C for 25 minutes, and then stimulation was stopped by adding 2 mL of lysis buffer. After centrifugation for 5 minutes at 500 × g, the supernatant was decanted, and 300 µL of washing buffer was added to each tube. The cells were resuspended by gently vortexing before flow cytometry analysis. Flow cytometry analysis was performed using a BD FACSCalibur (BD Biosciences, San Jose, CA, USA), and the data were analyzed using BD Cell-Quest software (BD Biosciences). Upregulation of the activation marker CD63 was calculated as the percentage of CD63-positive cells compared with the total number of basophils.
Statistical analysis
To compare the differences in the basophil activation response across multiple follow-up time points, the Friedman test was used for nonparametric statistical analysis. The Wilcoxon signed rank test was used to compare basophil reactivity before and after immunotherapy. Spearman’s correlation test was used for nonparametric analysis of correlation between 2 variables. Statistical analyses were performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). A p value less than 0.05 was considered statistically significant.
RESULTS
Clinical characteristic of patients
Seventeen patients were enrolled in this study. The mean age was 38.5 ± 9.9 years. Eight patients (47.1%) were male, and 9 patients were female. The types of allergic rhinitis were perennial type, which showed persistent symptoms all year around, in 13 patients (76.5%) and seasonal type, which showed rhinitis symptoms only in specific seasons, in 4 patients. Thirteen patients also had ocular conjunctivitis, and 4 patients had asthma. Fourteen patients were sensitive to HDM and received immunotherapy with HDM extract. Seven mugwort pollen-sensitive patients and five birch pollen-sensitive patients who received immunotherapy with the relevant allergens were also enrolled in the study. All patients were followed up for at least 12 months, and 11 patients were followed up for 24 months (Table 1).
Table 1. Baseline characteristics of study subjects.
| Subject No. | Age | Sex | Type of AR | Comorbidities | Allergens for SCIT & BAT |
|---|---|---|---|---|---|
| P1 | 35 | F | PARC | - | Dp, Df, grass-mix, birch, hazel, oak |
| P2 | 34 | F | SAR | - | Ragweed, mugwort |
| P3 | 37 | M | PAR | Asthma | Dp, Df, mugwort |
| P4 | 61 | F | PAR | Asthma | DP, Df |
| P5 | 36 | M | PARC | - | DP, Df |
| P6 | 38 | M | PARC | - | Dp, Df, mugwort |
| P7 | 52 | M | SARC | - | DP, Df, birch, hazel |
| P8 | 24 | M | PAR | - | Dp, Df |
| P9 | 29 | F | PARC | - | DP, Df, birch, hazel |
| P10 | 33 | F | PARC | - | Dp,Df |
| P11 | 54 | F | SARC | - | Birch, hazel, ragweed, mugwort |
| P12 | 29 | F | PARC | - | Dp, Df |
| P13 | 36 | M | PARC | - | Dp, Df, mugwort |
| P14 | 43 | M | PARC | Chronic urticaria | Dp, Df |
| P15 | 48 | M | SARC | Food allergy | Birch, popula, ragweed, mugwort |
| P16 | 32 | F | PARC | Asthma, NSAIDs hypersensitivity | Dp, Df, mugwort |
| P17 | 33 | F | PARC | Asthma | Dp, Df, cat |
AR, allergic rhinitis; SCIT, subcutaneous immunotherapy; BAT, basophil activation test; PAR(C), perennial allergic rhinitis (rhinoconjunctivitis); SAR(C), seasonal allergic rhinitis (rhinoconjunctivitis); NSAIDs, nonsteroidal anti-inflammatory drugs; Dp, Dermatophagoides pteronyssinus; Df, Dermatophagoides farine.
Changes in basophil reactivity to HDM allergen stimulation during immunotherapy
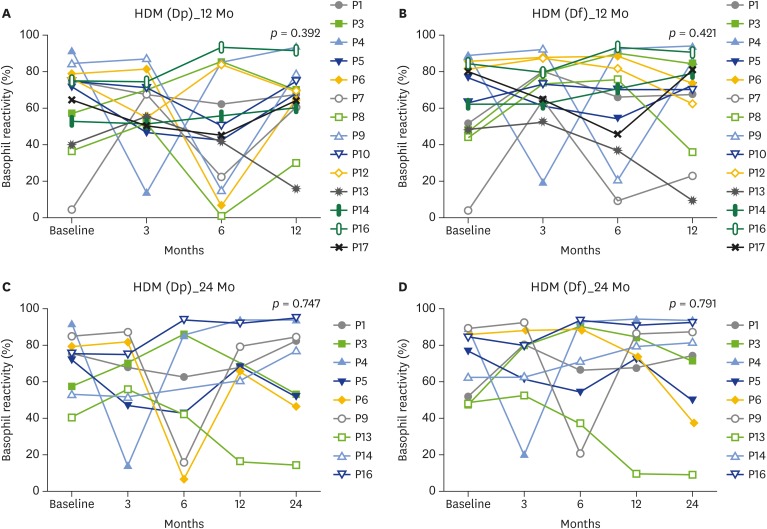
Basophil reactivity to the Dp and Df allergens did not change significantly after 12 or 24 months of immunotherapy. Some patients showed fluctuations in basophil responses; however, the final BAT results at 12 and 24 months after initiation of immunotherapy showed that basophil activation was not significantly reduced (Fig. 1A–D). Some patients who were followed up for 24 months showed a gradual decrease in basophil reactivity to Df (56.5% decrease in P6 and 81.6% decrease in P13). However, other patients showed enhanced basophil activation (43.3% increase in P1, 50.8% increase in P3, and 9.3% increase in P16; Fig. 1D).
Fig. 1.
Changes of CD63 basophil reactivity to house dust mite (HDM) allergens in patients with house dust mite immunotherapy, basophil reactivity to Dp allergen during 12-month follow-up (A), basophil reactivity to Df allergen during 12-month follow-up (B), basophil reactivity to Dp allergen during 24-month follow-up (C), basophil reactivity to Df allergen during 24-month follow-up (D). Dp, Dermatophagoides pteronyssinus; Df, Dermatophagoides farine.
Changes in basophil reactivity to mugwort allergen stimulation during immunotherapy
Basophil reactivity to mugwort allergen did not change significantly after 12 months of immunotherapy (Fig. 2A, B). However, there was a significant reduction in basophil reactivity after 24 months of immunotherapy (p = 0.048 by Friedman test). A reduction in basophil reactivity was observed in most patients treated with mugwort immunotherapy who completed 24 months of follow-up (Fig. 2C). Basophil reactivity was significantly reduced by 72.1%, from 52.6 ± 22.6 at baseline to 14.7 ± 12.8 at 24 months (Fig. 2D).
Fig. 2.
Changes of CD63 basophil reactivity to mugwort allergen in patients with mugwort immunotherapy, basophil reactivity to mugwort during 12-month follow-up (A), comparison of basophil reactivity to mugwort before immunotherapy vs. 12 following immunotherapy (B), basophil reactivity to mugwort during 24-month follow-up (C), comparison of basophil reactivity to mugwort before immunotherapy vs. 24 following immunotherapy (D).
Change of rhinitis quality of life, IgG4, and basophil activation
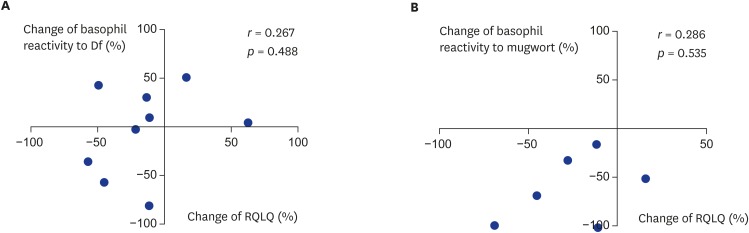
There was no significant association between the change in clinical symptoms, as measured by the RQLQ, and the change in basophil reactivity to Df. In fact, 3 out of 9 patients showed a discrepancy, with increased basophil reactivity and improved RQLQ scores after immunotherapy (Fig. 3A). In addition, the change in basophil reactivity to mugwort was not significantly correlated with the change in RQLQ score. Four out of 6 patients showed a positive correlation between the change in RQLQ and the change in basophil reactivity; however, the remaining 2 patients did not show a correlation (Fig. 3B).
Fig. 3.
Correlation between changes of basophil reactivity to allergen and change of clinical response parameter (RQLQ), Df allergen (A), mugwort allergen (B). Dp, Dermatophagoides pteronyssinus; Df, Dermatophagoides farine.
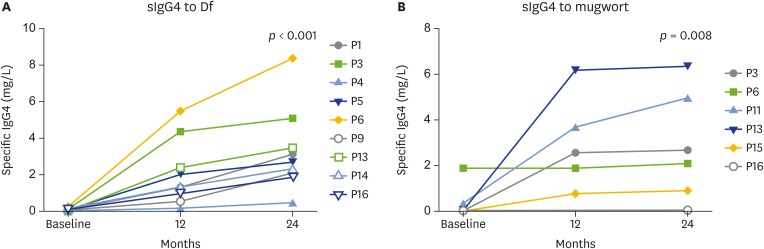
Allergen-specific IgG4 to house-dust mite and mugwort were significantly increased during immunotherapy in 12 months and 24 months of follow-up. The up-regulations of allergen-specific IgG4 levels were observed in most of patients for both Df and mugwort (Fig. 4A, B). However, the change of IgG4 to Df or mugwort was not significantly correlated with the change of basophil activation (data not shown).
Fig. 4.
Levels of allergen-specific IgG4 (sIgG4) during immunotherapy, Df allergen (A), mugwort allergen (B). Df, Dermatophagoides farine.
Nonspecific basophil activation during immunotherapy
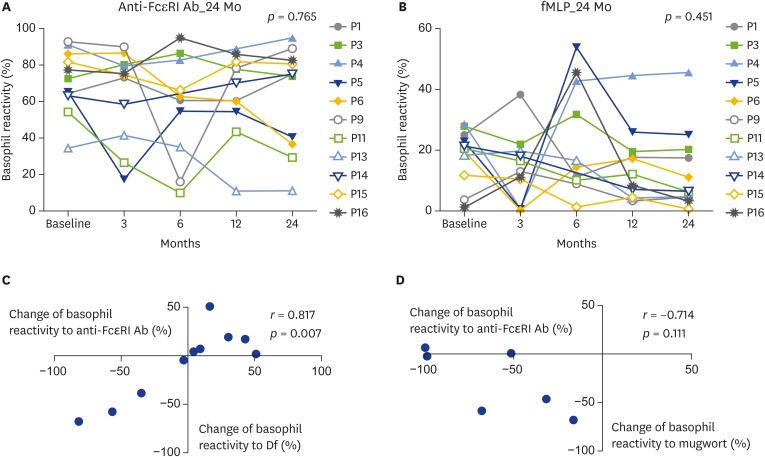
We also analyzed basophil reactivity to anti-FcεRI antibody (IgE-mediated basophil activation) and fMLP (non-IgE-mediated basophil activation) to evaluate whether nonspecific basophil activation changes occur during immunotherapy. There were no significant changes in basophil activation induced by either nonspecific basophil stimulator during 24 months of follow-up (Fig. 5A, B). Interestingly, the change in allergen-specific basophil reactivity to Df was well correlated with the change in nonspecific basophil activation induced by anti-FcεRI antibody (Fig. 5C). However, there was no correlation between the change in basophil reactivity to mugwort and the change in nonspecific basophil activation induced by anti-FcεRI antibody (Fig. 5D). In addition, there were no significant correlations between the changes in allergen-specific basophil reactivity and the changes in nonspecific basophil activation induced by fMLP (data not shown).
Fig. 5.
Changes of CD63 basophil reactivity to nonspefic basophil stimulator during 24 months of immunothrapy, basophil reactivity to anti-FcεRI antibody (Ab) (A), basophil reactivity to N-formyl-methionyl-leucyl-phenylalanine (fMLP) (B), correlation between changes of basophil reactivity to Df allergen and change of nonspecific basophil response to anti-FcεRI antibody (C), correlation between changes of basophil reactivity to mugowort allergen and change of nonspecific basophil response to anti-FcεRI antibody (D). Df, Dermatophagoides farine.
DISCUSSION
Our study showed that the changes in basophil activation during immunotherapy differed according to the allergen administered. While basophil activation in response to HDM did not change significantly, basophil activation in response to mugwort was reduced after immunotherapy, and a significant change was observed after 2 years of immunotherapy. However, the change in the basophil response to the allergen failed to reflect the change in clinical symptoms and allergen-specific IgG4 levels for both HDM and mugwort. To the best of our knowledge, our study is the first study to investigate the change in basophil response to HDM and mugwort during subcutaneous AIT.
After the BAT was introduced to detect basophil activation status after allergen challenge, many researchers have investigated its utility for monitoring and predicting immunotherapy responses, primarily for pollen and venom allergy immunotherapy. Japanese researchers studied CD203c expression as a biomarker during rush immunotherapy for Japanese cedar pollinosis [11]. Although symptom improvement was observed in all patients, CD203c expression showed a variable response. Some showed a reduction in CD203c expression, whereas other patients did not. Nonresponders, who did not show increased CD203c expression in response to both Japanese cedar pollen extract and anti-IgE antibody, were also found [11]. Decreased basophil activation was also demonstrated in patients treated with modified birch pollen extract immunotherapy [12] and olive pollen-specific preseasonal allergoid immunotherapy [8]. Recently, long-term follow-up basophil activation results were reported for grass pollen SCIT [9]. The study showed that CD63 expression in basophils in response to 1 µg/mL of grass pollen was reduced after the up-dosing phase, before the first pollen season after beginning SCIT and at 1 or 2 years after completion of 3–5 years of SCIT. In contrast, CD63 expression in basophils in response to 10-µg/mL grass pollen was significantly reduced only at 1 or 2 years after completion of SCIT. The basophil response, as measured as the area the under curve (AUC), was 55% lower at 1–2 years after completion of SCIT [9].
The clinical utility of BAT for venom immunotherapy was first investigated by Erdmann et al. [13]. They demonstrated that quantification of CD63 expression could be a valuable tool for the diagnosis of hymenopteran venom allergy, but fail to show the usefulness of BAT for monitoring successful immunotherapy after 6 months of venom immunotherapy [13]. Around the same time, another report also indicated the uninformative performance of BAT for short-term (3 months) monitoring of immunotherapy response [14]. However, intermediate and long-term follow-up studies showed different results. A study evaluating the utility of the BAT after completion of bee venom immunotherapy for more than 4 years showed that CD63 basophil activation was significantly reduced and was associated with tolerance to sting challenge [7]. Interestingly, this response was observed at submaximal stimulating allergen concentrations. Recently, Rodríguez Trabado et al. [15] studied the short, intermediate, and long-term changes in basophil reactivity for up to 5 years after starting bee venom immunotherapy. An early decrease in basophil activation was observed during the first 3 months of treatment. However, this decrease was not maintained at 6–18 months, but was observed again after 2 years of treatment. This study suggests BAT results may differ depending on the timing of assessment after immunotherapy [15]. Based on these reports, basophil activation seems to decrease in the early time points after immunotherapy, then increases, and finally decreases again after long-term maintenance treatment (at least 2 years). The results of the BAT to mugwort in our study are consistent with those of previous reports.
Several studies have suggested that allergen desensitization of basophils after AIT is nonspecific and may involve a common intracellular signaling pathway [16,17]. Witting Christensen et al. [17] showed reduced CD63 expression in basophils from multiallergic subjects desensitized with grass allergen, following unrelated challenge with birch, rDer p2, or anti-IgE, which was similar to that observed after challenge with grass pollen. The desensitization reduced p38 MAPK phosphorylation, which was correlated with decreased CD63 expression. Suppression of nonspecific basophil activation was also noted in another study. Upregulation of CD63 via the IgE receptor, either specifically with peanut allergen or nonspecifically with anti-IgE antibody, was suppressed by active oral peanut immunotherapy. In contrast, the basophil response to fMLP was not suppressed by immunotherapy [5]. In our study, suppression of the nonspecific CD63 basophil response to anti-FcεRI antibody or fMLP was not observed during 24 months of immunotherapy. However, the change in basophil reactivity to anti-FcεRI antibody was well correlated with the change in basophil reactivity to HDM allergen. Although discrepant results were observed for basophil reactivity to mugwort, our data support previous results, indicating that the change in allergen-specific basophil reactivity during AIT is associated with desensitization of a nonspecific pathway involving IgE receptor. Further study is needed to confirm this finding and determine the detailed pathway.
Our study has some limitations. First, the BAT was performed with only one concentration of allergen. The concentration of the stimulating allergen in BAT may affect the sensitivity of test for detecting changes in the basophil response to allergen during AIT. Previous reports showed a greater reduction in basophil reactivity after AIT with submaximal allergen concentrations. The basophil response can be assessed as either basophil sensitivity or basophil reactivity using the allergen concentration at which the half-maximal response occurs or as the AUC with serial concentrations of allergen [18]. Using several concentrations of allergen in the BAT might permit a better understanding of the basophil response during AIT. Second, other clinical and immunologic markers, such as skin test reactivity, T-cell subsets, and cytokines, were not evaluated in this study. A comparison of BAT with other biomarkers is an intriguing research topic, and may provide useful tools for monitoring AIT. Lastly, the number of patients who completed long-term follow-up was small. Therefore, longer follow-up of a larger study population is needed in the further studies. Nonetheless, our study provides valuable clinical information as the first study of basophil response to HDM and mugwort in patients with subcutaneous AIT. Suppression of CD63 upregulation in BAT was observed only in the mugwort allergen and this change was detected only in the 2 years of long-term follow-up. Despite significant change of basophil response to mugwort, basophil response was not useful for reflecting clinical response of AIT. Clear correlation between basophil reactivity to anti-FcεRI antibody and HDM suggest nonspecfic desensitization in downstream pathway of IgE receptor might occur in the AIT. Long-term follow-up study of BAT with several different concentration of allergen is needed in the future for better understanding of basophil response during AIT.
Footnotes
Funding: This study was supported by a grant from Seoul National University Bundang Hospital Research Fund (grant number: 02-2011-054).
Author Contributions: Conceptualization: Sae-Hoon Kim, Yoon-Seok Chang. Data curation: Soon-Hee Kim, Soo-Jie Chung, Jung-Hyun Kim, Suh-Young Lee, Byung-Keun Kim, Kyung-Whan Lim. Formal analysis: Sae-Hoon Kim, Soo-Jie Chung, Kyung-Whan Lim. Funding acquisition: Sae-Hoon Kim. Investigation: Sae-Hoon Kim, Soon-Hee Kim, Soo-Jie Chung, Jung-Hyun Kim, Suh-Young Lee, Byung-Keun Kim, Kyung-Whan Lim, Yoon-Seok Chang. Project administration: Sae-Hoon Kim, Yoon-Seok Chang. Resources: Sae-Hoon Kim, Yoon-Seok Chang. Supervision: Sae-Hoon Kim, Yoon-Seok Chang. Validation: Sae-Hoon Kim. Writing - original draft: Sae-Hoon Kim, Yoon-Seok Chang. Writing - review & editing: Sae-Hoon Kim, Yoon-Seok Chang.
References
- 1.Nelson HS. Some highlights of the first century of immunotherapy. Ann Allergy Asthma Immunol. 2011;107:417–421. doi: 10.1016/j.anai.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120(3 Suppl):S25–S85. doi: 10.1016/j.jaci.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Moingeon P. Biomarkers for allergen immunotherapy: a “panoromic” view. Immunol Allergy Clin North Am. 2016;36:161–179. doi: 10.1016/j.iac.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Senna G, Calderon M, Makatsori M, Ridolo E, Passalacqua G. An evidence-based appraisal of the surrogate markers of efficacy of allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2011;11:375–380. doi: 10.1097/ACI.0b013e328348a7cd. [DOI] [PubMed] [Google Scholar]
- 6.McGowan EC, Saini S. Update on the performance and application of basophil activation tests. Curr Allergy Asthma Rep. 2013;13:101–109. doi: 10.1007/s11882-012-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eržen R, Košnik M, Silar M, Korošec P. Basophil response and the induction of a tolerance in venom immunotherapy: a long-term sting challenge study. Allergy. 2012;67:822–830. doi: 10.1111/j.1398-9995.2012.02817.x. [DOI] [PubMed] [Google Scholar]
- 8.Gokmen NM, Ersoy R, Gulbahar O, Ardeniz O, Sin A, Unsel M, Kokuludag A. Desensitization effect of preseasonal seven-injection allergoid immunotherapy with olive pollen on basophil activation: the efficacy of olive pollen-specific preseasonal allergoid immunotherapy on basophils. Int Arch Allergy Immunol. 2012;159:75–82. doi: 10.1159/000335251. [DOI] [PubMed] [Google Scholar]
- 9.Zidarn M, Košnik M, Šilar M, Bajrović N, Korošec P. Sustained effect of grass pollen subcutaneous immunotherapy on suppression of allergen-specific basophil response; a real-life, nonrandomized controlled study. Allergy. 2015;70:547–555. doi: 10.1111/all.12581. [DOI] [PubMed] [Google Scholar]
- 10.Park KH, Cho JS, Lee KH, Shin SY, Moon JH, Cha CI. Rhinoconjunctivitis quality of life questionnaire (RQLQ) as an evaluator of perennial allergic rhinitis patients-the first report. Korean J Otolaryngol-Head Neck Surg. 2002;45:254–262. [Google Scholar]
- 11.Nagao M, Hiraguchi Y, Hosoki K, Tokuda R, Usui T, Masuda S, Yamaguchi M, Fujisawa T. Allergen-induced basophil CD203c expression as a biomarker for rush immunotherapy in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2008;146(Suppl 1):47–53. doi: 10.1159/000126061. [DOI] [PubMed] [Google Scholar]
- 12.Ceuppens JL, Bullens D, Kleinjans H, van der Werf J PURETHAL Birch Efficacy Study Group. Immunotherapy with a modified birch pollen extract in allergic rhinoconjunctivitis: clinical and immunological effects. Clin Exp Allergy. 2009;39:1903–1909. doi: 10.1111/j.1365-2222.2009.03379.x. [DOI] [PubMed] [Google Scholar]
- 13.Erdmann SM, Sachs B, Kwiecien R, Moll-Slodowy S, Sauer I, Merk HF. The basophil activation test in wasp venom allergy: sensitivity, specificity and monitoring specific immunotherapy. Allergy. 2004;59:1102–1109. doi: 10.1111/j.1398-9995.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown SG, Haas MA, Black JA, Parameswaran A, Woods GM, Heddle RJ. In vitro testing to diagnose venom allergy and monitor immunotherapy: a placebo-controlled, crossover trial. Clin Exp Allergy. 2004;34:792–800. doi: 10.1111/j.1365-2222.2004.01949.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez Trabado A, Cámara Hijón C, Ramos Cantariño A, Romero-Chala S, García-Trujillo JA, Fernández Pereira LM. Short-, intermediate-, and long-term changes in basophil reactivity induced by venom immunotherapy. Allergy Asthma Immunol Res. 2016;8:412–420. doi: 10.4168/aair.2016.8.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thyagarajan A, Jones SM, Calatroni A, Pons L, Kulis M, Woo CS, Kamalakannan M, Vickery BP, Scurlock AM, Wesley Burks A, Shreffler WG. Evidence of pathway-specific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clin Exp Allergy. 2012;42:1197–1205. doi: 10.1111/j.1365-2222.2012.04028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witting Christensen SK, Kortekaas Krohn I, Thuraiaiyah J, Skjold T, Schmid JM, Hoffmann HJ. Sequential allergen desensitization of basophils is non-specific and may involve p38 MAPK. Allergy. 2014;69:1343–1349. doi: 10.1111/all.12482. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann HJ, Santos AF, Mayorga C, Nopp A, Eberlein B, Ferrer M, Rouzaire P, Ebo DG, Sabato V, Sanz ML, Pecaric-Petkovic T, Patil SU, Hausmann OV, Shreffler WG, Korosec P, Knol EF. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015;70:1393–1405. doi: 10.1111/all.12698. [DOI] [PubMed] [Google Scholar]