Abstract
Background
Occasionally, malignant pleural disease is only detected unexpectedly during surgery in patients with pulmonary adenocarcinoma. Previous studies mostly focused on the role of main tumor resection on patient's outcome, barely addressing the position of postoperative systemic therapy.
Methods
The medical records of 5321 non-small cell lung cancer patients who underwent thoracic surgery between January 1990 and December 2012 were reviewed. Pulmonary adenocarcinoma patients with unexpected pleural spread noted during surgery were included. The clinical and postoperative treatment variables were assessed for correlation with overall survival.
Results
In 134 patients identified, main tumor resection was performed in 87 (64.9%) patients, while 89 (66.4%) and 57 (42.5%) patients received postoperative chemotherapy and epidermal growth factor receptor- tyrosine kinase inhibitor (EGFR -TKI) therapy, respectively. Overall, the 5-year survival rate was 30.2% and median survival time was 29.3 months. Multivariate analysis showed main tumor resection and EGFR-TKI therapy were associated with better survival. Mutational status of EGFR was available in 57 patients and 43 (75.4%) had activating mutations. Resection of the main tumor conferred a better outcome in patients without EGFR mutation or with unknown EGFR mutation status and had not been treated with EGFR-TKI therapy (P = 0.003), but not in those with activating EGFR mutation and had been treated with EGFR-TKI (P = 0.857).
Conclusions
In pulmonary adenocarcinoma patients with unexpected pleural spread detected during surgery, main tumor resection and EGFR-TKI therapy correlated with better survival. Identifying EGFR mutation status before surgery can provide useful information for clinical decision during surgery.
Keywords: pulmonary adenocarcinoma, pleural spread, epidermal growth factor receptor, tyrosine kinase inhibitor, postoperative therapy
INTRODUCTION
The discovery of epidermal growth factor receptor (EGFR) mutations has resulted in a paradigm shift in the management of advanced NSCLC [1]. Numerous studies showed that in patients with advanced NSCLC carrying EGFR mutations, treatment with epidermal growth factor receptor –tyrosine kinase inhibitors (EGFR-TKIs) improved chances of survival [2–6]. Most patients with EGFR sensitizing mutations and who respond to EGFR-TKIs present with adenocarcinoma [7]. While adenocarcinoma usually presents at the lung periphery and is frequently associated with an initial malignant pleural effusion, a positive association between EGFR mutation and the presence of malignant pleural effusion has been reported [8–10].
Patients with malignant pleural disease including malignant pleural effusion and/or malignant pleural nodules are classified as stage IV (M1a) in the current staging system due to poor prognosis [11, 12]. Thus, lung adenocarcinoma patients with malignant pleural disease are considered inoperable, and are candidates for systemic therapy. However, the clinical presentation of pleural carcinomatosis has a wide range of manifestations; varying from pleural effusion large amount enough to be diagnosed by plain radiography to minimal disease which is only detected incidentally during a thoracotomy. Performing resection of the main tumor for patients with unexpected pleural spread detected during surgery is a matter of debate [13–17]. One of the limitations of previous studies was that the various confounding postoperative treatments were not taking into account. Since there have been remarkable changes in systemic therapy for advanced pulmonary adenocarcinoma, resulting in significant improvement in survival outcomes [18, 19]. In this study, we decided to further persuade the prognostic factors, particularly postoperative systemic therapy (POST), in pulmonary adenocarcinoma patients who had unexpected pleural spread detected during thoracic surgery.
RESULTS
Patient characteristics
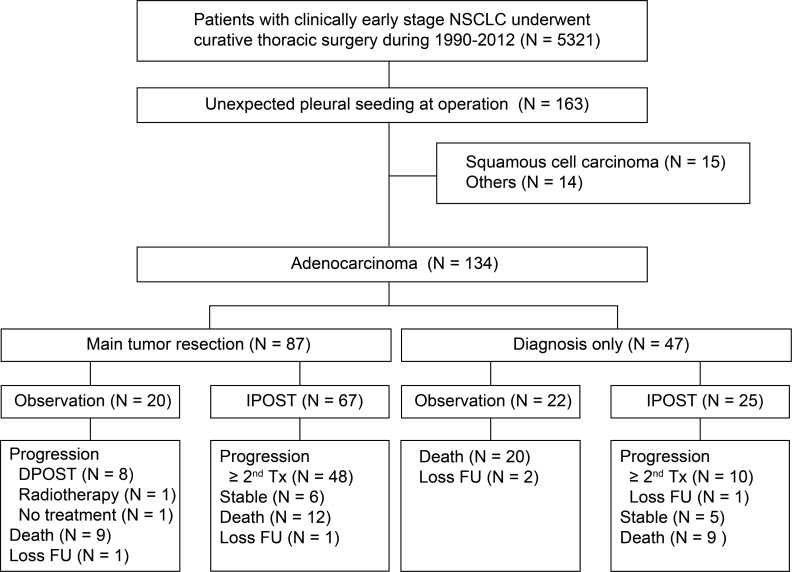
Between January 1990 and December 2012, 5321 patients with a clinical diagnosis of lung cancer underwent thoracic surgery in our institute. A total of 163 (3.1%) patients, mostly adenocarcinomas (n = 134, 82.2%) had unexpected pleural spread detected during surgery (Figure 1). In patients with pulmonary adenocarcinoma, the median age was 63.3, and 55.2% of the patients were man (Table 1). Most surgeries were thoracotomies (73.9%), although video-assisted thoracoscopic surgery was the predominant surgical procedure after 2008. Intraoperative pleural effusion cytology examination was performed in 29 patients (30.2%). Main tumor resection was performed in 87 patients (64.9%); 58 patients had lobectomy and 29 patients underwent wedge resection. There was no significant difference between diagnosis by thoracotomy or by thoracotomy and the selection of following surgical procedures (as shown in Supplementary Table 1). Ninety-two (68.7%) patients received immediate POST and the median time to treatment was 1.1 months (range: 0.2–2.9 months).
Figure 1. Diagram describing recruitment strategy of study participants and follow-up.
(IPOST: immediate postoperative systemic therapy; DPOST: delayed postoperative systemic therapy; Tx: treatment).
Table 1. Demographic and clinicopathological characteristics of patients.
| Variables | Number (%) |
|---|---|
| Total | 134 (100) |
| Age, y (median) | 63.3 (32.4–81.1) |
| Sex | |
| Male | 74 (55.2) |
| Female | 60 (44.8) |
| Smoking | |
| Never smoker | 96 (71.6) |
| Ever/Current smoker | 38 (28.4) |
| Tumor location | |
| Left | 67 (50) |
| Right | 67 (50) |
| Tumor size | |
| ≤3 cm | 72 (53.7) |
| >3 cm | 56 (41.8) |
| Pathological N stage | |
| N0 | 31 (23.1) |
| N1 | 19 (14.2) |
| N2 | 38 (28.4) |
| Not examined | 46 (34.3) |
| Malignant pleural disease* | |
| MPE only | 3 (3.1) |
| MPN only | 69 (71.9) |
| MPN + MPE | 24 (25.0) |
| Surgical approach method | |
| Thoracotomy | 99 (73.9) |
| VATS convert to thoracotomy | 10 (7.4) |
| VATS | 25 (18.7) |
| Surgical procedure for main tumor | |
| Lobectomy (standard resection) | 58 (43.3) |
| Wedge resection (limited resection) | 29 (21.6) |
| No main tumor resection | 47 (35.1) |
| CEA | |
| Normal | 47 (35.1) |
| High | 45 (33.6) |
| Unknown | 42 (31.3) |
*Data available in 96 patients.
Abbreviations: MPE: malignant pleural effusion, MPN: malignant pleural nodule, VATS: video-assisted thoracoscopic surgery, CEA: carcinoembryonic antigen.
Postoperative systemic therapy
The details of the immediate POST are summarized in Table 2. Overall, 89 (66.4%) patients had received chemotherapy, out of which 73 patients were treated immediately after surgery. Fifty-seven (42.5%) patients had received EGFR-TKI therapy, out of which 19 were treated immediately after surgery. EGFR mutation testing was performed for all patients with EGFR-TKI treatment history and activating mutation were found in 43 patients (exon 19 deletion in 24, L858R in 17, and G719C and exon 19 deletion + L858R in one each).
Table 2. Immediate postoperative systemic therapy according to the EGFR mutation status.
| Group | Unknown N = 77 |
Wild type N = 14 |
Mutant N = 43 |
All N = 134 |
|---|---|---|---|---|
| Main tumor resection | 45 | 9 | 33 | 87 |
| Observation only | 12 | 0 | 0 | 12 |
| Immediate systemic therapy | 27 | 7 | 33 | 67 |
| Chemotherapy | 27 | 7 | 24 | 58 |
| EGFR-TKI | 0 | 0 | 9 | 9 |
| Systemic therapy after recurrence | 6 | 2 | 0 | 8 |
| Chemotherapy | 6 | 0 | 0 | 6 |
| EGFR-TKI | 0 | 2 | 0 | 2 |
| Diagnostic thoracotomy or thoracoscopy | 32 | 5 | 10 | 47 |
| Observation only | 22 | 0 | 0 | 22 |
| Immediate systemic therapy | 10 | 5 | 10 | 25 |
| Chemotherapy | 10 | 3 | 2 | 15 |
| EGFR-TKI | 0 | 2 | 8 | 10 |
Overall survival analysis
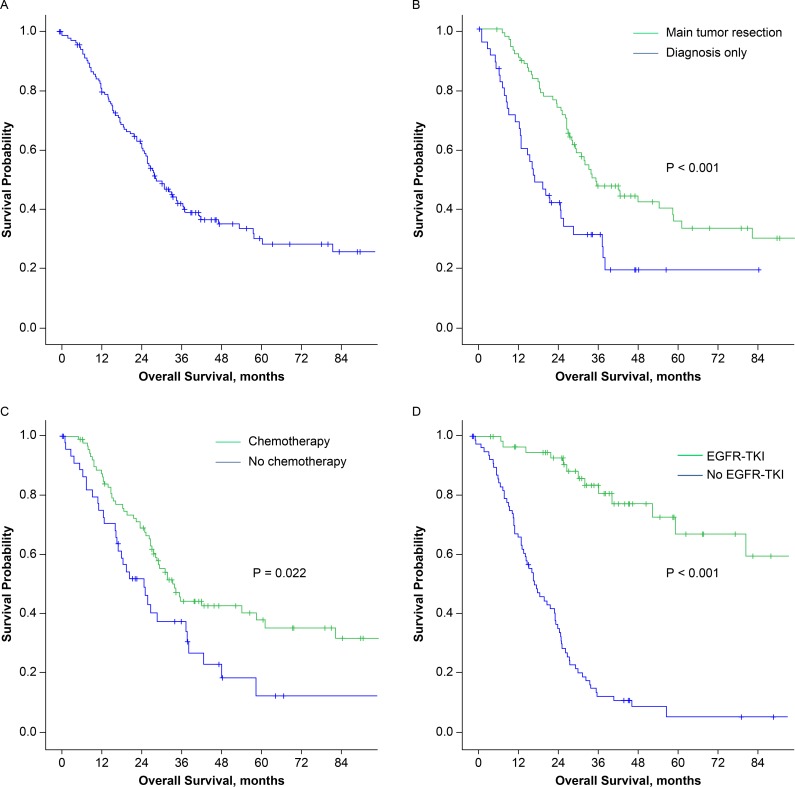
Overall, the 5-year survival rate was 30.2% and the median survival time was 29.3 months (95% CI, 23.4–35.2 months; Figure 2A). Patients who received main tumor resection had longer survival than patients who did not (median 35.3 versus 17.0 months, P < 0.001; Figure 2B). Significant survival differences were also observed between patients who underwent postoperative chemotherapy and those who did not (median, 33.8 versus 24.7 months, P = 0.022; Figure 2C) and between patients who underwent postoperative EGFR-TKI treatment and those who did not (median, 111.1 versus 18.6 months, P < 0.001; Figure 2D). The timing of the POST and tumor size were also revealed to be prognostic factors in univariate analysis. However, in multivariate analysis, only main tumor resection and EGFR-TKI therapy correlated with better survival (P = 0.013 and < 0.001, respectively, Table 3).
Figure 2.
Kaplan-Meier survival curves for (A) all 134 patients, (B) patients who received (green) or did not receive (blue) main tumor resection, (C) patients who received (green) or did not receive (blue) postoperative chemotherapy, (D) patients who received (green) or did not receive (blue) postoperative EGFR-TKI treatment. (EGFR-TKI: epidermal growth factor receptor –tyrosine kinase inhibitor).
Table 3. Univariate and multivariate analyses of prognostic factors by using Cox proportional hazards model.
| Variables | N (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P value | Hazard Ratio (95% CI) |
P value | ||
| Age | 0.183 | ||||
| <65 | 74 (55.2) | 1 | |||
| ≥65 | 60 (44.8) | 1.333 (0.873–2.037) | |||
| Sex | 0.306 | ||||
| Male | 74 (55.2) | 1 | |||
| Female | 60 (44.8) | 0.798 (0.518–1.229) | |||
| Smoking | 0.964 | ||||
| Never | 96 (71.6) | 1 | |||
| Ever | 38 (28.4) | 1.011 (0.637–1.603) | |||
| CEA level | 0.288 | ||||
| Normal | 47 (35.1) | 1 | |||
| High | 45 (33.6) | 1.373 (0.765–2.465) | |||
| Tumor size | 0.002 | 0.278 | |||
| ≤3 cm | 72 (53.7) | 1 | 1 | ||
| >3 cm | 56 (41.8) | 1.968 (1.269–3.052) | 1.29 (0.815–2.042) | ||
| Pathological N2 | 0.194 | ||||
| No | 96 (71.6) | 1 | |||
| Yes | 38 (28.4) | 1.365 (0.854–2.184) | |||
| Main tumor resection | <0.001 | 0.013 | |||
| No | 47 (35.1) | 1 | 1 | ||
| Yes | 87 (64.9) | 0.45 (0.288–0.704) | 0.484 (0.273–0.859) | ||
| Immediate postoperative therapy | <0.001 | 0.200 | |||
| No | 42 (31.3) | 1 | 1 | ||
| Yes | 92 (68.7) | 0.382 (0.249–0.587) | 0.604 (0.279–1.307) | ||
| Postoperative Chemotherapy | 0.024 | 0.190 | |||
| No | 45 (33.6) | 1 | 1 | ||
| Yes | 89 (66.4) | 0.602 (0.388–0.935) | 1.737 (0.760–3.971) | ||
| Postoperative EGFR-TKI use | <0.001 | <0.001 | |||
| No | 77 (57.5) | 1 | 1 | ||
| Yes | 57 (42.5) | 0.15 (0.087–0.257) | 0.179 (0.098–0.328) | ||
Interaction between main tumor resection and EGFR-TKI treatment
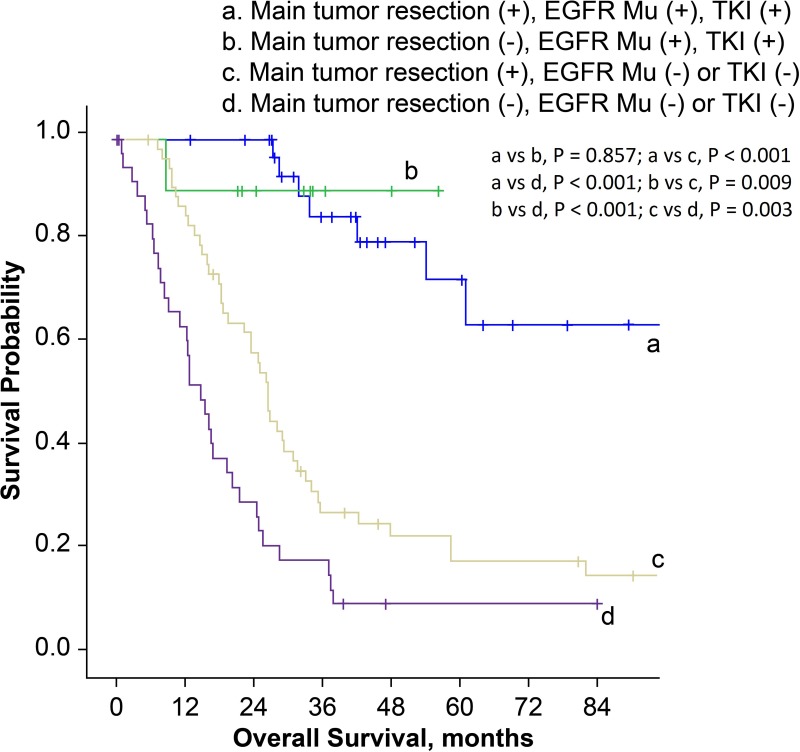
In order to establish prognostic correlation, we stratified patients according to surgical procedure and postoperative management. The median overall survival time for patients who had received main tumor resection and immediate POST was 42.1 months (95% CI, 15.3–68.9 months), which was much better compared to 18.6 months (95% CI, 10.9–26.3 months) in patients who had received main tumor resection but not immediate POST, and 16.5 months (95% CI, 7.6–25.4 months) in patients with diagnostic thoracic surgery and no instantaneous subsequent therapy (P = 0.006 and < 0.001, respectively; Figure 3). Then we stratified patients according to the surgical procedure, EGFR mutation status and history of EGFR-TKI treatment. Main tumor resection was not a prognostic factor in patients with EGFR mutations and EGFR-TKI treatment history (P = 0.857). However, in patients with EGFR wild-type, or in patients with unknown EGFR status and without EGFR-TKI treatment history, the overall survival was better for patients who received main tumor resection (P = 0.003, Figure 4).
Figure 3. Kaplan-Meier survival curves for patients stratified by surgical procedures and postoperative systemic therapy.
(IPOST: immediate postoperative systemic therapy).
Figure 4. Kaplan-Meier survival curves for patients stratified by surgical procedures, EGFR mutation status and history of EGFR-TKI treatment.
(EGFR Mu: epidermal growth factor receptor mutation; TKI: tyrosine kinase inhibitor).
DISCUSSION
In this study, when we incorporated the detailed history of postoperative therapy into our analysis, we found that the conclusion of our previous study in patients with unexpected spread at thoracotomy had to be modified according to the patients’ EGFR mutation status. Main tumor resection was still a valuable procedure for patients who were negative or unknown for EGFR mutation. However, for patients who had sensitizing EGFR mutations and had been treated by EGFR-TKI, we did not observe any survival benefit conferred by main tumor resection.
In the present study, the 5-year survival rate was 30.2% and median survival time was 29.3 months. In another study, using the Japanese lung cancer registry in 2004, Iida, T. et al. reported a very similar outcome in 313 surgical patients who had pleural carcinomatosis first detected during thoracotomy: a 5-year survival rate of 29.3% and a median survival time was of 34.0 months [20]. The result of this subgroup of stage IV disease was comparable to that in patients with stage IIB to IIIA disease [21]. Taken together with our findings, this data suggest that patients with minimal malignant pleural disease have a relatively favorable prognosis which is much different from patients, with clinically overt malignant pleural disease. Distinctive treatment strategy is thus indicated based on the nature of the disease.
In this study, main tumor resection was performed in about two-thirds of patients (64.9%), and most patients received standard surgery (lobectomy with radical lymph node dissection). We found that main tumor resection could confer survival benefit in lung adenocarcinoma patients with unexpected pleural spread detected at thoracotomy or thoracoscopy, which was in agreement with our previous results [15, 17, 20, 22]. Moreover, we found that POST also contributed to survival advantage, an aspect that has never been addressed before. In the present study, 100 patients (74.6%) received POST and 92 of them were treated immediately after surgery. In our study, patients who received POST, either as chemotherapy or as EGFR-TKI therapy had longer overall survival than those who did not. The 5-year survival rate was 43.9% in patients who received main tumor resection and immediate POST (Figure 3); this finding highlights the importance of systemic therapy after surgery in this group of patients.
Undoubtedly, with the advent of targeted therapy, the remarkable efficacy of EGFR-TKI in patients with sensitizing EGFR mutations further strengthens the value of POST in patients with unexpected pleural spread. Indeed, in patients with EGFR mutations, the effect of postoperative EGFR-TKI treatment seemed to overwhelm the influence of main tumor resection on overall survival. In agreement with other published studies, we demonstrated that main tumor resection could confer a survival benefit in patients with negative or unknown EGFR mutation status. Surprisingly, we saw no evidence to support the value of main tumor resection in extending survival outcome in patients who had sensitizing EGFR mutations with EGFR-TKI POST history. To the best of our knowledge, this is the first study to discuss the role of EGFR mutations and EGFR-TKI treatment in pulmonary adenocarcinoma patients with unexpected pleural spread detected during thoracotomy or thoracoscopy.
According to the current guideline, EGFR mutation testing is indicated at the time of diagnosis for patients with advanced NSCLC [23, 24]. Whether EGFR mutation testing should be done in clinical stage I, II or III disease remains controversial. We demonstrate here the differential survival outcomes of main tumor resection in patients with and without EGFR mutation. Our study results suggest that EGFR mutation status may assist surgeons in surgical decision making when pleural spread is found unexpectedly during thoracotomy. In addition, our results also imply that in patients with EGFR sensitizing mutations, surgery may not provide additional value over EGFR-TKI therapy, and can be avoided. This could potentially reduce surgical risks, avoid unnecessary tissue damage, and shorten time of recovery. On the other hand, we believe that in patients with negative or unknown EGFR mutation status, main tumor resection is beneficial and recommended if feasible. Thus, we advocate that EGFR mutation testing should be done at the time of diagnosis in not only patients with advanced disease but also in those with early stage disease who plan to undergo thoracic surgery. Interestingly, in other solid tumors, molecular testing is routinely performed regardless of disease stage, such as HER2 testing in invasive breast cancer [25].
This retrospective study has several limitations. First, there was inevitable surgeon bias in selecting the operative procedures for patients with unexpected pleural spread. Surgeons tend to perform diagnostic thoracotomy without main tumor resection in patients with extensive pleural involvement or poor physical fitness. Both are potential prognostic factors but are difficult to measure objectively. Therefore, it was not possible to incorporate these parameters into the regression model we used for analysis. Second, EGFR mutation testing was only performed in patients who had received EGFR-TKI therapy but not in the rest of the patients. This is related to the fact that the study period was long, and aged archived paraffin blocks are not suitable for molecular testing. Additionally, EGFR mutation per se seems not to be a prognostic factor [26, 27]. Therefore, the incompleteness of the EGFR mutation status will hardly change our study results. Third, we did not check other driver mutations, such as gene rearrangements of ALK and ROS1. However, it has been shown that patients who had driver mutations and had received matched targeted therapy did live longer but patients who had not received targeted therapy had the similar outcome to those who did not have driver mutations [28]. None of our patients had received targeted therapy other than EGFR-TKI and therefore, this missing data will not influence our results. Finally, 22 of our study subjects (16.4 %) of our study subjects did not receive systemic therapy after diagnostic thoracotomy and it may be explained, at least partly, by cultural difference. Previous reports showed a significant percentage (22.75%) of Taiwanese patients refuse treatment after lung cancer diagnosis [29]. Besides, 21 of the 22 patients (95.5%) were diagnosed to have expected pleural spread before 2000, which indicated that cytotoxic chemotherapy is the only systemic treatment at that time. (Gefitinib was reimbursed in Taiwan after Nov. 2004). Patients may refuse systemic treatment due to the concern of side effects.
In conclusion, both main tumor resection and postoperative EGFR-TKI therapy were associated with better survival in pulmonary adenocarcinoma patients with unexpected pleural spread noted during thoracic surgery. Testing for EGFR mutations should be considered in not only advanced but also in early stage lung adenocarcinoma patients. A randomized prospective study is needed to confirm the role of surgery in this subset of patients who have driver mutations detected before surgery.
METHODS
Patients
Medical records of clinical early stage lung cancer patients who had undergone thoracic surgery at this tertiary medical center were reviewed. The preoperative work-up included chest radiograph and computed tomography (CT) of chest, cardiopulmonary function evaluation, and a thorough search for distant metastases, including brain CT scan and whole body bone scan. All surgeries were performed with curative intent and the surgeon decided on the extent of surgery. The details regarding POST were obtained from the patients’ medical records. Immediate POST was defined as the systemic therapy delivered within three months post-surgery.
The definition of “unexpected pleural spread” was as follows: (1) preoperative survey indicated a resectable lung tumor and the patient was scheduled for a standard lobectomy, (2) pleural disease was first detected during surgery, (3) pleural metastasis was proved pathologically. The operative finding, the method of pulmonary resection, and the patient characteristics, like age, sex, tumor size, smoking history, and preoperative carcinoembryonic antigen level were reviewed. This study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board of Taipei Veterans General Hospital approved this study and informed consent was waived (IRB No. 2014-04-003CC).
Tumor samples and EGFR mutation testing
Tumors from patients who underwent EGFR-TKI treatment were evaluated for EGFR mutation status. Formalin-fixed, paraffin-embedded tissue was the main sample type that was used. Briefly, one of the consecutive sections was stained with hematoxylin and eosin and reviewed by pathologists to select a region which was enriched by tumor cells. The selected region was marked on a deparaffinized tissue section, and manually microdissected. Genomic DNA was extracted by PicoPure DNA extraction kit (Arcturus/Applied Biosystems, Foster City, CA, USA), and quantified by using Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA).
Two EGFR mutation testing methods were used in the study period. From 2008 to 2011, we used direct sequencing to detect mutations at exons 18 to 21. The sequence variations were confirmed by multiple independent polymerase chain reaction amplifications and repeated sequencing reactions as previously described [30]. After 2011, the majority of specimens were tested using the mutant allele-specific, real-time PCR-based, mutation detection technology [31].
Statistical analyses
Data are presented as number (percentage) or median (range) unless otherwise stated. Overall survival was defined as the interval between the date of surgery and the date of death or last follow-up (cutoff date, August 31, 2014). Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test. Multivariate Cox proportional hazard model was used to identify prognostic factors. All factors with P < 0.1 in univariate analyses were included in the Cox regression analysis using enter method. A significant difference was defined as a P < 0.05. When multiple comparisons were performed, the cutoff level of α error was reduced using Bonferroni correction. Analyses and graphing were performed using SPSS statistics software (SPSS Inc., Chicago, IL, USA).
SUPPLEMENTARY TABLE
Acknowledgments
The authors thank Editage (online.editage.com.tw) for English editing. This study was presented in part at the ESMO-ASIA Congress, Dec. 18–Dec. 21, 2015, Singapore.
Abbreviations
- CT
computed tomography
- EGFR
epidermal growth factor receptor
- EGFR-TKI
epidermal growth factor receptor –tyrosine kinase inhibitor
- NSCLC
non-small cell lung cancer
- POST
postoperative systemic therapy
- CI
confidence interval
Footnotes
Author contributions
C.H.C. and Y.C.W. are the guarantors of the paper and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. C.L.C and L.C.W contributed to study design; H.L.H and Y.C.Y contributed to the pathological analysis; C.M.T, W.H.H and T.Y.C contributed to data analysis and interpretation; and C.L.C contributed to manuscript preparation.
CONFLICTS OF INTEREST
C.H.C. and C.M.T. had received honoraria from AstraZeneca, Boehringer Ingelheim, and Roche. C.L.C. had received honoraria from Boehringer Ingelheim, and Roche. Other authors declared no conflicts of interest.
FUNDING
This work was supported by grant V104A-006 and V106A-002 from Taipei Veterans General Hospital and MOST 106-2314-B-075-031-MY3 from the Ministry of Science and Technology.
REFERENCES
- 1.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760–74. doi: 10.1038/nrc2947. https://doi.org/10.1038/nrc2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. https://doi.org/10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 3.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. https://doi.org/10.1016/s1470-2045(09)70364-x [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. https://doi.org/10.1016/s1470-2045(11)70184-x [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. https://doi.org/10.1016/s1470-2045(11)70393-x [DOI] [PubMed] [Google Scholar]
- 6.Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. https://doi.org/10.1200/jco.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 7.Tsao AS, Tang XM, Sabloff B, Xiao L, Shigematsu H, Roth J, Spitz M, Hong WK, Gazdar A, Wistuba I. Clinicopathologic characteristics of the EGFR gene mutation in non-small cell lung cancer. J Thorac Oncol. 2006;1:231–9. doi: 10.1016/s1556-0864(15)31573-2. [DOI] [PubMed] [Google Scholar]
- 8.Smits AJ, Kummer JA, Hinrichs JW, Herder GJ, Scheidel-Jacobse KC, Jiwa NM, Ruijter TE, Nooijen PT, Looijen-Salamon MG, Ligtenberg MJ, Thunnissen FB, Heideman DA, de Weger RA, et al. EGFR and KRAS mutations in lung carcinomas in the Dutch population: increased EGFR mutation frequency in malignant pleural effusion of lung adenocarcinoma. Cell Oncol (Dordr) 2012;35:189–96. doi: 10.1007/s13402-012-0078-4. https://doi.org/10.1007/s13402-012-0078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu SG, Yu CJ, Tsai MF, Liao WY, Yang CH, Jan IS, Yang PC, Shih JY. Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur Respir J. 2013;41:1409–18. doi: 10.1183/09031936.00069812. https://doi.org/10.1183/09031936.00069812 [DOI] [PubMed] [Google Scholar]
- 10.Zou J, Bella AE, Chen Z, Han X, Su C, Lei Y, Luo H. Frequency of EGFR mutations in lung adenocarcinoma with malignant pleural effusion: Implication of cancer biological behaviour regulated by EGFR mutation. J Int Med Res. 2014;42:1110–7. doi: 10.1177/0300060514539273. https://doi.org/10.1177/0300060514539273 [DOI] [PubMed] [Google Scholar]
- 11.Postmus PE, Brambilla E, Chansky K, Crowley J, Goldstraw P, Patz EF, Jr, Yokomise H. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol. 2007;2:686–93. doi: 10.1097/JTO.0b013e31811f4703. https://doi.org/10.1097/JTO.0b013e31811f4703 [DOI] [PubMed] [Google Scholar]
- 12.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. https://doi.org/10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 13.Ichinose Y, Tsuchiya R, Koike T, Kuwahara O, Nakagawa K, Yamato Y, Kobayashi K, Watanabe Y, Kase M, Yokoi K. The prognosis of patients with non-small cell lung cancer found to have carcinomatous pleuritis at thoracotomy. Surg Today. 2000;30:1062–6. doi: 10.1007/s005950070002. [DOI] [PubMed] [Google Scholar]
- 14.Fukuse T, Hirata T, Tanaka F, Wada H. The prognostic significance of malignant pleural effusion at the time of thoracotomy in patients with non-small cell lung cancer. Lung Cancer. 2001;34:75–81. doi: 10.1016/s0169-5002(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 15.Mordant P, Arame A, Foucault C, Dujon A, Le Pimpec Barthes F, Riquet M. Surgery for metastatic pleural extension of non-small-cell lung cancer. Eur J Cardiothorac Surg. 2011;40:1444–9. doi: 10.1016/j.ejcts.2011.02.076. https://doi.org/10.1016/j.ejcts.2011.02.076 [DOI] [PubMed] [Google Scholar]
- 16.Sawabata N, Matsumura A, Motohiro A, Osaka Y, Gennga K, Fukai S, Mori T. Malignant minor pleural effusion detected on thoracotomy for patients with non-small cell lung cancer: is tumor resection beneficial for prognosis? Ann Thorac Surg. 2002;73:412–5. doi: 10.1016/s0003-4975(01)03426-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang BY, Wu YC, Hung JJ, Hsu PK, Hsieh CC, Huang CS, Hsu WH. Prognosis of non-small-cell lung cancer with unexpected pleural spread at thoracotomy. J Surg Res. 2011;169:e1–5. doi: 10.1016/j.jss.2011.02.028. https://doi.org/10.1016/j.jss.2011.02.028 [DOI] [PubMed] [Google Scholar]
- 18.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. https://doi.org/10.1200/jco.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 19.Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, Tan EH, Ho JC, Chu da T, Zaatar A, Osorio Sanchez JA, Vu VV, Au JS, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595–605. doi: 10.1093/jnci/djt072. https://doi.org/10.1093/jnci/djt072 [DOI] [PubMed] [Google Scholar]
- 20.Iida T, Shiba M, Yoshino I, Miyaoka E, Asamura H, Date H, Okumura M, Tada H, Nakanishi Y, Dosaka-Akita H, Kobayashi H, Takahashi K, Inoue M, et al. Surgical Intervention for Non-Small-Cell Lung Cancer Patients with Pleural Carcinomatosis: Results From the Japanese Lung Cancer Registry in 2004. J Thorac Oncol. 2015;10:1076–82. doi: 10.1097/JTO.0000000000000554. https://doi.org/10.1097/jto.0000000000000554 [DOI] [PubMed] [Google Scholar]
- 21.Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, Goldstraw P. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. https://doi.org/10.1097/JTO.0b013e31812d05d5 [DOI] [PubMed] [Google Scholar]
- 22.Okamoto T, Iwata T, Mizobuchi T, Hoshino H, Moriya Y, Yoshida S, Yoshino I. Pulmonary resection for lung cancer with malignant pleural disease first detected at thoracotomy. Eur J Cardiothorac Surg. 2012;41:25–30. doi: 10.1016/j.ejcts.2011.04.010. https://doi.org/10.1016/j.ejcts.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, Thunnissen E, Ladanyi M. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–59. doi: 10.1097/JTO.0b013e318290868f. https://doi.org/10.1097/JTO.0b013e318290868f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Dilling TJ, Dobelbower MC, Govindan R, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw. 2015;13:515–24. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 25.Gradishar WJ, Anderson BO, Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A, Giordano SH, Goldstein LJ, Hayes DF, Hudis CA, Isakoff SJ, et al. Breast cancer version 3.2014. J Natl Compr Canc Netw. 2014;12:542–90. doi: 10.6004/jnccn.2014.0058. [DOI] [PubMed] [Google Scholar]
- 26.Lim KH, Huang MJ, Liu HC, Kuo HT, Tzen CY, Hsieh RK. Lack of prognostic value of EGFR mutations in primary resected non-small cell lung cancer. Med Oncol. 2007;24:388–93. doi: 10.1007/s12032-007-0018-8. [DOI] [PubMed] [Google Scholar]
- 27.Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol. 2009;4:22–9. doi: 10.1097/JTO.0b013e3181914111. https://doi.org/10.1097/JTO.0b013e3181914111 [DOI] [PubMed] [Google Scholar]
- 28.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, Shyr Y, Camidge DR, Sequist LV, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. https://doi.org/10.1001/jama.2014.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang HL, Kung PT, Chiu CF, Wang YH, Tsai WC. Factors associated with lung cancer patients refusing treatment and their survival: a national cohort study under a universal health insurance in Taiwan. PloS one. 2014;9:e101731. doi: 10.1371/journal.pone.0101731. https://doi.org/10.1371/journal.pone.0101731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou TY, Chiu CH, Li LH, Hsiao CY, Tzen CY, Chang KT, Chen YM, Perng RP, Tsai SF, Tsai CM. Mutation in the tyrosine kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with non-small cell lung cancer. Clin Cancer Res. 2005;11:3750–7. doi: 10.1158/1078-0432.CCR-04-1981. https://doi.org/10.1158/1078-0432.ccr-04-1981 [DOI] [PubMed] [Google Scholar]
- 31.Chiu CH, Ho HL, Chiang CL, Lin SF, Ma HH, Chuang YT, Lin KY, Tsai CM, Chou TY. Clinical characteristics and treatment outcomes of lung adenocarcinomas with discrepant EGFR mutation testing results derived from PCR-direct sequencing and real-time PCR-based assays. J Thorac Oncol. 2014;9:91–6. doi: 10.1097/JTO.0000000000000041. https://doi.org/10.1097/jto.0000000000000041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.