Abstract
Background/Aims
The efficacy of anti-tumor necrosis factor α (anti-TNFα) antibodies for postoperative Crohn's disease (CD) in patients who were treated with these agents prior to surgery is largely unknown.
Methods
CD patients who underwent intestinal resection and received anti-TNFα agents after surgery were divided into 2 groups according to the presence or absence of preoperative anti-TNFα treatment: anti-TNFα restart group or anti-TNFα naïve group. Endoscopic recurrence after surgery was examined according to the preoperative conditions, including administration of anti-TNFα agents before surgery.
Results
Thirty-six patients received anti-TNFα antibody after surgery: 22 in the anti-TNFα restart group and 14 in the anti-TNFα naïve group. Endoscopic recurrence after surgery was more frequently observed in the anti-TNFα restart group than in the anti-TNFα naïve group (68% vs. 14%, P<0.001). Multivariate analysis revealed the following significant risk factors of endoscopic recurrence after surgery: anti-TNF restart group (odds ratio [OR], 28.10; 95% CI, 3.08–722.00), age at diagnosis <23 years (OR, 24.30; 95% CI, 1.67–1,312.00), serum albumin concentration at surgery <3.3 g/dL (OR, 34.10; 95% CI, 1.72–2,804.00), and presence of inflammation outside of the surgical site (OR, 21.40; 95% CI, 1.02–2,150.00). Treatment intensification for patients with endoscopic recurrence in the anti-TNFα restart group showed limited responses, with only 1 of 12 patients achieving endoscopic remission.
Conclusions
The efficacy of restarting anti-TNFα antibody treatment after surgery was limited, and treatment intensification or a change to different classes of biologics should be considered for those patients.
Keywords: Crohn disease, Anti-tumor necrosis factor α, Surgery
INTRODUCTION
Crohn's disease (CD) involves chronic and progressive disease courses characterized by periods of remission and clinical recurrences. Intestinal damage gradually accumulates after disease onset of CD, and patients often require surgery during their long disease course. In addition, a considerable number of patients who undergo surgery require additional surgeries, despite medical treatment after initial surgery.1,2,3 A recent systematic review and meta-analysis showed the following ratios of requirement of second intestinal resection in CD patients: 24.2% within 5 years and 35.0% within 10 years after the initial surgery.4 In this context, early postoperative endoscopic recurrence rates were reportedly to be 14%–38% from the database with data compiled from different countries.5
Anti-tumor necrosis factor α (anti-TNFα) antibodies have been used as a potent therapy for CD. These particular agents are effective not only for CD patients who have never undergone surgery but also for those who have received surgery. In fact, fewer endoscopic recurrences were observed in patients who received anti-TNF agents after surgery than in those who did not.6 In the meanwhile, a report suggested that no significant difference in the endoscopic recurrence rate was observed between biological and conventional therapy after ileocecal resections in CD patients. In this context, the efficacy of anti-TNF agents for postoperative CD patients have been evaluated in patients who had no history of undergoing anti-TNFα antibody treatment prior to surgery. Because primary nonresponses or secondary loss of responses can frequently occur in CD patients who are administered anti-TNF agents, a substantial portion of patients who require anti-TNF agents after surgery receive the agents also prior to surgery in clinical practice.
Nevertheless, it has been largely unknown whether anti-TNF agents are also effective for postoperative CD patients who had received the agents prior to surgery. More importantly, little is known whether the elimination of the affected bowel regions due to surgery raises the sensitivity to the anti-TNF agents in CD patients who are refractory to the agents. In addition to the issue of preoperative anti-TNFα antibody administration, knowledge regarding other preoperative conditions that may affect the efficacy of postoperative anti-TNFα antibody treatment is also limited.
In this study, therefore, we investigated the patients who received anti-TNFα antibodies after intestinal surgery. The presence or absence of endoscopic recurrence was examined according to the preoperative conditions of the patients including administration of anti-TNF agents prior to surgery.
METHODS
1. Patients
A retrospective chart review of CD patients who underwent surgery including intestinal resection from July 2005 to January 2016 at Okayama University Hospital was performed. The diagnosis of CD was based on a combination of conventional criteria that included clinical symptoms, endoscopy, histopathology, and/or radiographic findings.1 Patients who underwent surgical resection for intestinal lesions and were administered anti-TNFα agents after surgery were considered eligible. The exclusion criteria were surgery without anastomosis (e.g., colostomy, closure surgery, and anal fistula surgery), follow-up period less than 1 year after surgery, and no administration of anti-TNFα therapy after surgery.
The eligible patients were divided into 2 groups: patients who had received anti-TNFα agents prior to surgery (the anti-TNFα restart group) and patients who had not received the agents prior to surgery (the anti-TNFα naïve group). The efficacy of post-surgical treatment with anti-TNFα antibody was assessed with endoscopic findings and compared between the 2 groups. In addition, clinical factors associated with preoperative conditions that may predict endoscopic recurrence after surgery in patients of both groups were evaluated. The evaluated factors were the clinical background, duration of anti-TNFα therapy prior to surgery, BMI at surgery, laboratory data at surgery, concomitant medications at surgery, and indication for surgery. In addition, the presence or absence of residual inflammation outside the surgical site was examined.
This retrospective analysis was approved by the Institutional Review Board (IRB No. 1506-046) of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences and informed consents were waived.
2. Treatment and Follow-up Policy for CD after Surgery
All CD patients were treated based on the guidelines published by the British Society of Gastroenterology, the European Crohn's and Colitis Organization, and the American College of Gastroenterology.7,8,9,10
With regard to the policy of anti-TNFα agent administration after surgery, patients who had received anti-TNFα agents before surgery were always scheduled to receive anti-TNFα antibody after surgical intervention. In patients who had not received anti-TNFα agents prior to surgery, patients with penetrating-type CD, those with extensive resection of the small intestine, and those with residual inflammation outside the surgical site were proactively considered to be indicated for anti-TNFα therapy after surgery. The anti-TNFα agents were started as soon as the infection and inflammation related surgery were controllable.
The anti-TNFα agents used were infliximab (IFX) or adalimumab (ADA), because these 2 agents alone were approved for CD in Japan. In principle, the agent selected after surgery was similar to that administered prior to surgery. If patients had received both agents prior to surgery, IFX was restarted after surgery except for those with a history of intolerance to IFX.
CD patients with intestinal resection were recommended to receive an endoscopy around 12 months after surgery even during clinical remissions. Patients with suspected flare-up of CD, when they showed worsening clinical symptoms or increased levels of CRP, also underwent colonoscopy. Mucosal status was evaluated according to the Rutgeerts score11 by endoscopists during the procedure. Endoscopic recurrence was defined as the findings with a Rutgeerts score ≥i2 (>5 aphthous lesions or larger lesions confined) from the ileum to the rectum including anastomosis.
3. Statistical Analyses
The patient characteristics were compared using the chi-square test, Fisher exact test, and the Mann-Whitney U-test. Multivariate analyses using a logistic regression model were conducted to identify variables associated with risk of endoscopic recurrence. Variables with P-values below 0.20 in a univariate analysis were further tested in a multivariate analysis. The OR with the 95% CI was calculated. A statistical comparison was performed by using the log-rank test. P-values <0.05 were considered statistically significant. All of the statistical analyses were performed using the JMP Pro software program version 12 (SAS Institute Inc., Cary, NC, USA).
RESULTS
1. Patient Characteristics
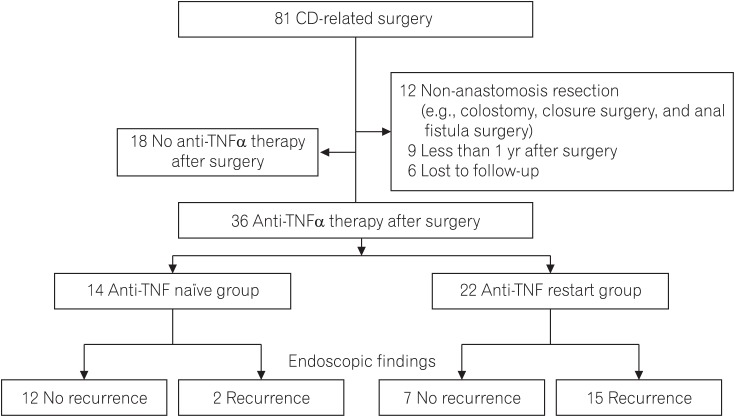
A total of 81 CD patients underwent intestinal resection in our hospital during the study period. Of these, 36 patients received anti-TNFα antibody therapy post-surgery: 22 had received preoperative anti-TNFα antibody therapy (the anti-TNFα restart group) and 15 had never received (the anti-TNFα naïve group) (Fig. 1). The interval between surgery and the start of the anti-TNFα agent was 19 days (range, 12–40 days), which did not differ significantly between the anti-TNFα restart group and the anti-TNFα naïve group.
Fig. 1. Flow chart of the present study. TNF, tumor necrosis factor.
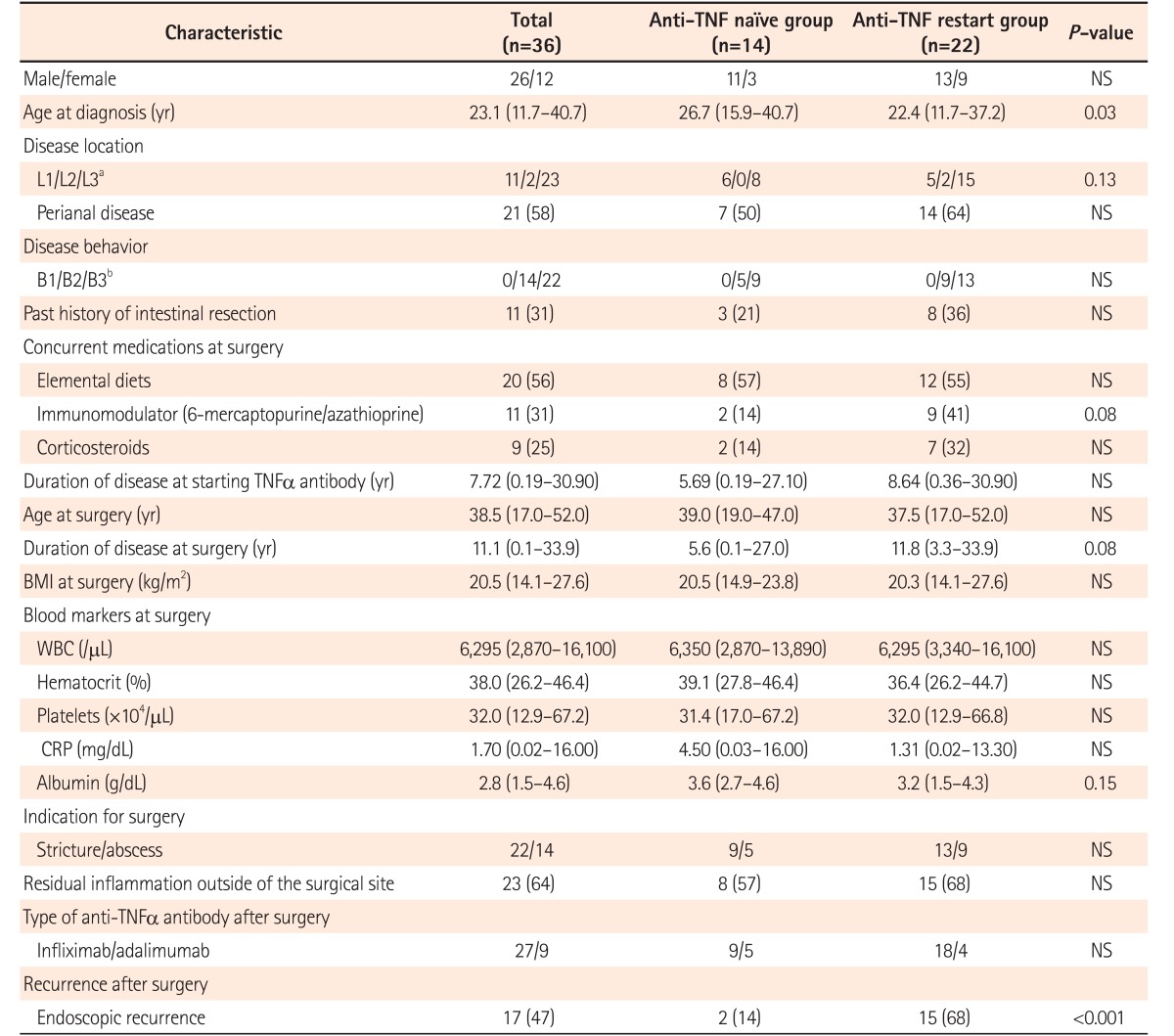
The clinical characteristics of the analyzed patients are summarized in Table 1. The age at CD diagnosis was lower in patients in the anti-TNFα restart group than in patients in the anti-TNFα naïve group (22.4 [11.7–37.2] years vs. 26.7 [15.9–40.7] years, P=0.03), while other preoperative clinical factors, including Montreal classification, concurrent medications at the time of surgery, and indication for surgery did not differ significantly between the 2 groups. Of 36 patients 27 (75%) received IFX, and 9 (25%) received ADA. No patients switched the type of anti-TNFα agents on the grounds of surgery. Endoscopic recurrence after surgery was more frequently observed in patients in the anti-TNFα restart group than in patients in the anti-TNFα naïve group (15/22 [68%] vs. 2/14 [14%], P<0.001) (Table 1).
Table 1. Characteristics of the Patients in the Present Study.
Values are presented as number (%) or median (range).
aL1, ileal; L2, colonic; L3, ileocolonic.
bB1, inflammation; B2, structuring; B3, penetrating.
TNF, tumor necrosis factor; NS, not significant; WBC, white blood cell.
2. Risk Factors of Endoscopic Recurrence in Patients Receiving Anti-TNFα Antibody Therapy after Surgery
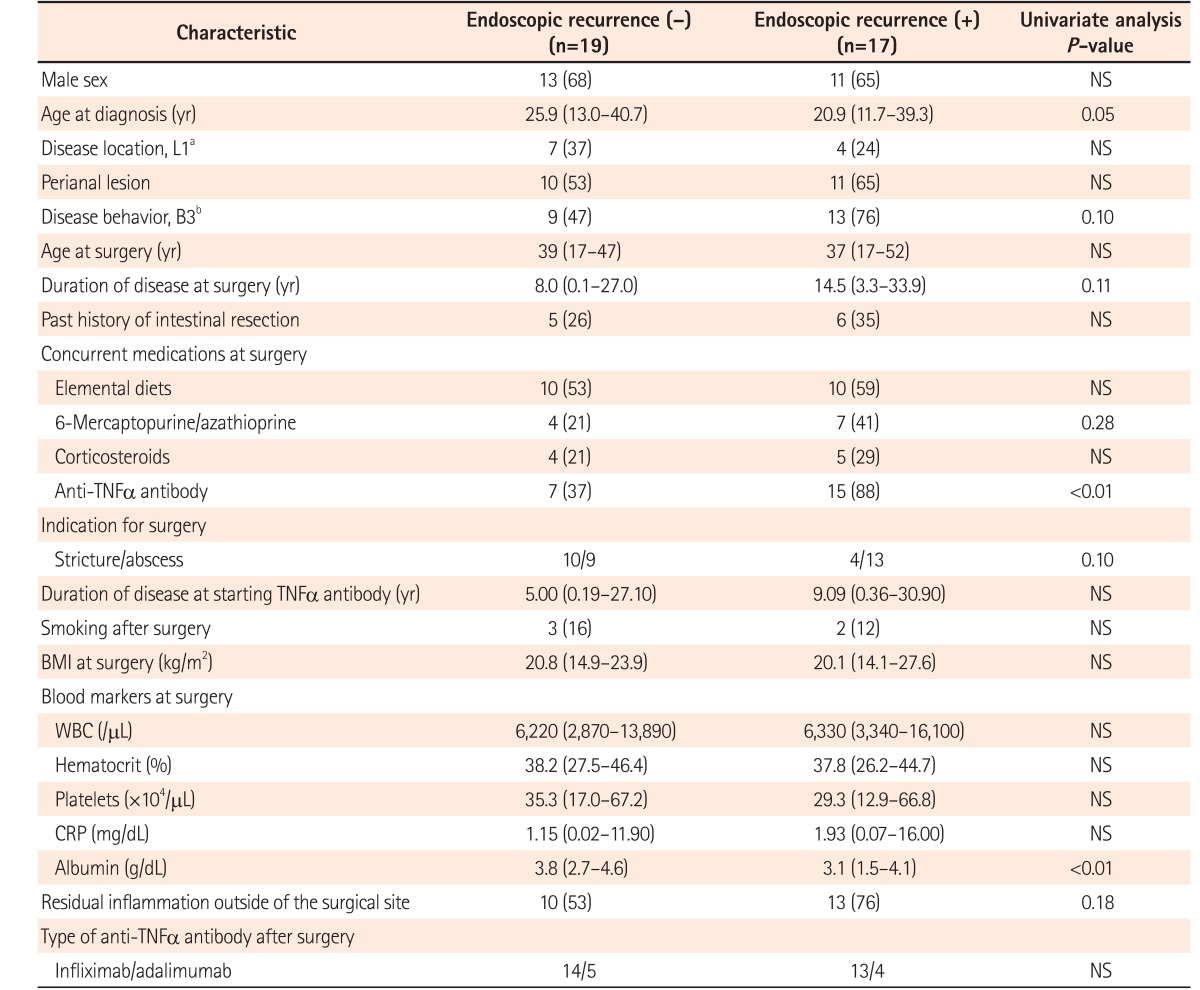
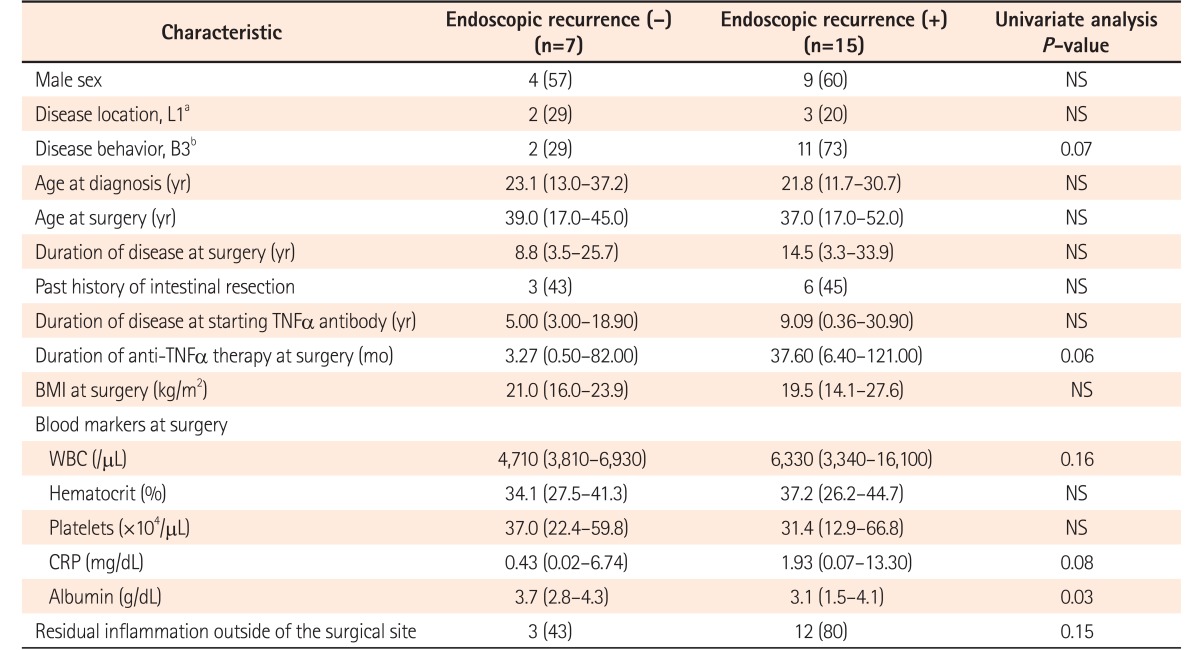
Characteristics were compared between patients with and without endoscopic recurrence (n=17 vs. n=19) after surgery (Table 2). In addition to the higher frequency of preoperative anti-TNFα agent administration in patients with postoperative endoscopic recurrence, only serum albumin at the time of surgery showed a significant difference between patients with and without postoperative endoscopic recurrence (3.1 [1.5–4.1] g/dL vs. 3.8 [2.7–4.6] g/dL, P=0.007). No significant difference in endoscopic recurrence after surgery was observed between patients with IFX or those with ADA (13/27 vs. 4/9, P=1.00).
Table 2. Characteristics of Patients with Endoscopic Recurrence after Surgery.
Values are presented as number (%) or median (range).
aL1, ileal.
bB3, penetrating.
NS, not significant; TNF, tumor necrosis factor; WBC, white blood cell.
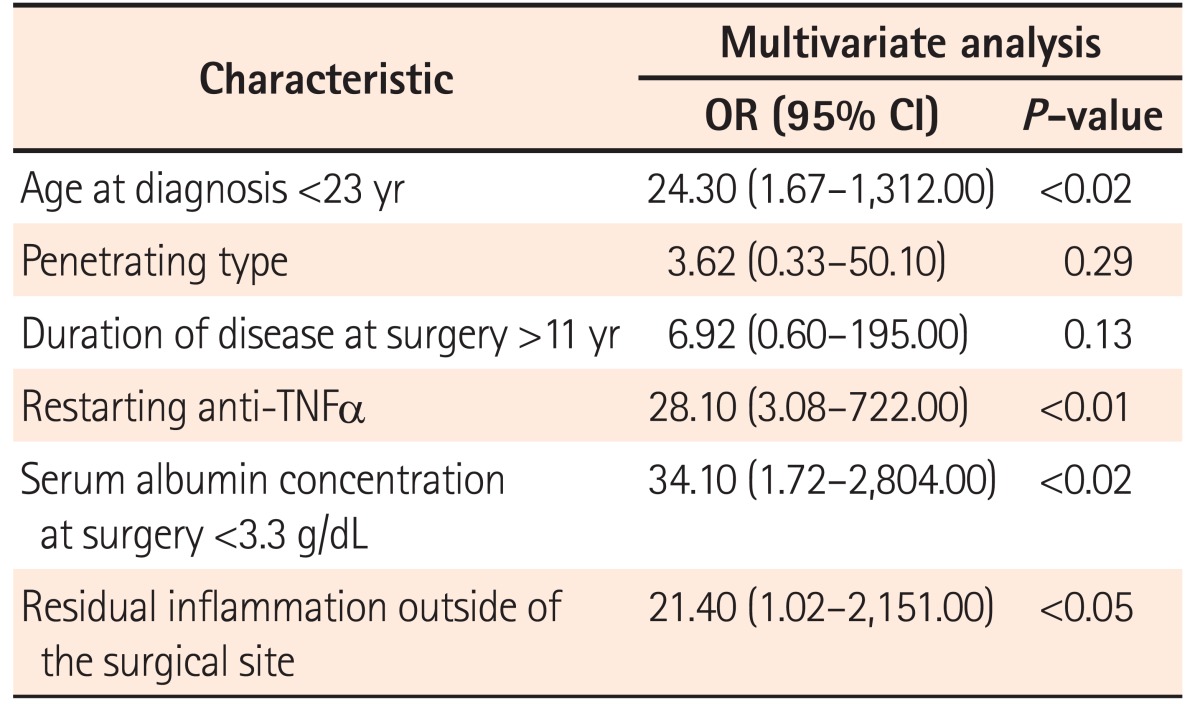
Multivariate analysis revealed that the risk factors for endoscopic recurrence after surgery were anti-TNFα antibody administration prior to surgery (OR, 28.10; 95% CI, 3.08–722.00; P<0.002), age at diagnosis <23 years (OR, 24.30; 95% CI, 1.67–1,312.00; P=0.017), serum albumin concentration at surgery <3.3 g/dL (OR, 34.10; 95% CI, 1.72–2,804.00; P=0.018), and the presence of inflammation outside the surgical site (OR, 21.40; 95% CI, 1.02–2,151.00; P=0.048) (Table 3).
Table 3. Risk Factors of Endoscopic Recurrence for Patients with Anti-TNFα Therapy after Surgery.
TNF, tumor necrosis factor.
3. Risk Factors of Endoscopic Recurrence in Patients Who Had Received Preoperative Anti-TNFα Antibody Therapy
As the patients who had received preoperative anti-TNFα antibody administration were more likely to show postoperative endoscopic recurrence, the risk factors of endoscopic recurrence were examined among only patients with preoperative anti-TNFα antibody administration (Table 4). Serum albumin concentration at the time of surgery was significantly lower in patients with endoscopic recurrence than in patients without endoscopic recurrence (3.1 [1.5–4.1] g/dL vs. 3.7 [2.7–4.3] g/dL, P=0.03). Those with endoscopic recurrence were more likely to have the penetrating type of disease or be treated with a longer duration of anti-TNFα antibody therapy before surgery, although the significant differences were marginal (P=0.07 and P=0.06, respectively).
Table 4. Risk Factors of Endoscopic Recurrence for Patients Who Were Administrated Anti-TNFα Therapy Refractory before Surgery.
Values are presented as number (%) or median (range).
aL1, ileal.
bB3, penetrating.
TNF, tumor necrosis factor; NS, not significant; WBC, white blood cell.
All 15 patients with endoscopic recurrence had their treatment regimen intensified, including dose intensification of anti-TNFα agents (n=10), switching of anti-TNFα agents (n=4), dose modification of immunomodulatory drugs (n=6), and addition of corticosteroids (n=3). Of the 12 patients who were followed up for 1 year or more after intensification of treatment, 6 (50%) showed clinical responses. Only 1 patient achieved endoscopic remission. Two of 6 patients without a clinical response to treatment intensification underwent another surgery.
DISCUSSION
The current study demonstrated several preoperative risk factors that may affect the efficacy of postoperative anti-TNFα antibody treatment in CD patients: younger age at diagnosis, anti-TNFα antibody treatment prior to surgery, and residual inflammation outside the surgical site. The most striking result of this study is that patients who had received preoperative anti-TNFα agents were more likely to develop endoscopic recurrence after surgery despite restarting anti-TNFα antibody treatment. In addition, dose intensification of anti-TNFα agents and/or addition of immunomodulatory drugs showed only limited efficacy for those patients. Thus, the efficacy of restarting anti-TNFα agents after surgery was not considered prominent, and elimination of affected intestinal lesions by surgery did not induce the efficacy of anti-TNFα agents sufficiently.
The main reason for the requirement of surgery, despite anti-TNFα antibody treatment, appears to be primary ineffectiveness or secondary loss of response to the agent(s). It is known that most cases with a secondary loss of response are associated with the development of anti-drug antibodies.12,13 The anti-drug antibodies that developed prior to surgery probably work against any anti-TNFα antibody treatment given after surgery. Therefore, those who initially responded to anti-TNFα agents but subsequently lost the response are expected to show recurrence despite postoperative anti-TNFα antibody treatment. This concept is consistent with the result of the subanalysis performed that patients with endoscopic recurrence after surgery had a marginally longer duration of preoperative anti-TNFα antibody treatment (Table 4).
The requirement for surgery early after initiation of anti-TNFα antibody treatment may not indicate primary ineffectiveness to the agent but insufficient effectiveness owing to the presence of intestinal complications, as the presence of intestinal complications is known to be one of the risk factors of the lower efficacy of anti-TNFα agents.14,15,16 Therefore, in these patients, removal of intestinal complications by surgery might reconstitute the efficacy of anti-TNFα agents. This concept is also consistent with the result that patients who did not demonstrate endoscopic recurrence after surgery had a shorter duration of preoperative anti-TNFα antibody treatment. Thus, although intestinal resection for the recovery of anti-TNFα agents was not shown to be effective in the current study, early surgical intervention after primary non-responsiveness or surgical intervention prior to the initial start of anti-TNFα agents may be effective for patients with intestinal lesions applicable to surgery.
Lower serum albumin concentrations were found to be significantly correlated with endoscopic recurrence in postoperative CD patients receiving anti-TNFα antibody therapy. Low serum albumin reflects the long process of ineffective treatment and/or the presence of intestinal failure. Such ineffective treatment may facilitate not only the progression of intestinal complications but also production of anti-drug antibodies. Moreover, low serum albumin concentrations have been shown to be significantly correlated with higher IFX clearance, and therefore, patients with low albumin levels may be primary non-responders or they may develop anti-drug antibodies more rapidly than other patients.17 Early surgical interventions, particularly before serum albumin levels decrease, should be considered for patients who show resistance to anti-TNFα agents.
Younger age at diagnosis and residual inflammation outside the surgical site were identified as risk factors for endoscopic recurrence after surgery in patients with postoperative administration of anti-TNFα agents. These results are logical because CD patients with younger onset are likely to have aggressive and disabling disease courses,18 and because the presence of multiple inflammatory regions indicates an extensive disease phenotype. These factors should also be considered when a treatment strategy is developed after surgery.
The lower efficacy of restarting anti-TNFα agents after surgery raises the question about whether intensification of treatment post-surgery could increase the efficacy of postoperative anti-TNFα antibody treatment. In the current study, half of the patients who showed endoscopic recurrence after surgery did indeed respond to intensification of treatment, although endoscopic remission was not obtained in most of the patients and some required additional surgery. In this context, simultaneous treatment intensification with restarting of anti-TNFα agents after surgery may improve prognosis of patients with preoperative anti-TNFα antibody administration, and the efficacy of such a treatment strategy should be verified in the future.
Recently, biological agents of different classes have become available for CD patients. Both vedolizumab, an anti-integrin antibody, and ustekinumab, an interleukin 12/23 antibody, have shown efficacy for anti-TNFα antibody-refractory CD patients.19,20 These agents may be considered preferentially as postoperative treatment for patients who have risks for recurrence after surgery, including preoperative anti-TNFα agent administration. Further investigations are also required to address this important issue.
This study has some limitations. Firstly, this is a retrospective study with a relatively small number of patients conducted in a single-center. Secondly, the timing of endoscopic follow-up after surgery differed between patients. Lastly, the anti-drug antibodies, which may be one of the reasons of the ineffectiveness of postoperative anti-TNFα antibody treatment, could not be measured in this study. Although these are drawbacks, the strength of our findings remain in that the postoperative treatment strategy for CD patients have been suggested according to the preoperative conditions including history of anti-TNFα agent administration prior to surgery.
In conclusion, the efficacy of anti-TNFα antibody treatment for postoperative CD patients differed according to several preoperative patient conditions. In particular, patients who had received anti-TNFα agents prior to surgery were likely to develop endoscopic recurrence. Because biologics of new classes have been introduced into clinical practice, the treatment strategy for postoperative CD patients should be carefully considered with the preoperative conditions in mind.
Footnotes
FINANCIAL SUPPORT: The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION: S.H. and J.K., design and conduct of clinical trials contributing data to these analyses; S.T., Y.K., T.I., Y.S., M.T., S.K., K.H., H.O., data collection and analysis. All authors provided critical content review and final ap proval of this manuscript.
References
- 1.Steinhart AH, Girgrah N, McLeod RS. Reliability of a Crohn's disease clinical classification scheme based on disease behavior. Inflamm Bowel Dis. 1998;4:228–234. doi: 10.1097/00054725-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Rutgeerts P. Review article: recurrence of Crohn's disease after surgery-the need for treatment of new lesions. Aliment Pharmacol Ther. 2006;24(Suppl 3):29–32. doi: 10.1111/j.1365-2036.2006.03056.x. [DOI] [PubMed] [Google Scholar]
- 3.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV., Jr Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn's disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol. 2014;109:1739–1748. doi: 10.1038/ajg.2014.297. [DOI] [PubMed] [Google Scholar]
- 5.Kotze PG, Saad-Hossne R, Spinelli A. Endoscopic postoperative recurrence rates in Crohn's disease in Korea: the beginning of a new approach? Intest Res. 2014;12:258–259. doi: 10.5217/ir.2014.12.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV., Jr Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn's disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015;148:64–76.e2. doi: 10.1053/j.gastro.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotze PG, Spinelli A, da Silva RN, et al. Conventional versus biological therapy for prevention of postoperative endoscopic recurrence in patients with Crohn's disease: an international, multicenter, and observational study. Intest Res. 2015;13:259–265. doi: 10.5217/ir.2015.13.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Hanauer SB, Sandborn WJ Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009;104:465–483. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 10.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 12.Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn's disease. Am J Gastroenterol. 2008;103:944–948. doi: 10.1111/j.1572-0241.2007.01638.x. [DOI] [PubMed] [Google Scholar]
- 13.Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimizing response to anti-TNF therapy in Crohn's disease: algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51. doi: 10.1111/apt.13445. [DOI] [PubMed] [Google Scholar]
- 14.Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010;4:355–366. doi: 10.1016/j.crohns.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Amiot A, Setakhr V, Seksik P, et al. Long-term outcome of enterocutaneous fistula in patients with Crohn's disease treated with anti-TNF therapy: a cohort study from the GETAID. Am J Gastroenterol. 2014;109:1443–1449. doi: 10.1038/ajg.2014.183. [DOI] [PubMed] [Google Scholar]
- 16.Yaari S, Benson A, Aviran E, et al. Factors associated with surgery in patients with intra-abdominal fistulizing Crohn's disease. World J Gastroenterol. 2016;22:10380–10387. doi: 10.3748/wjg.v22.i47.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. doi: 10.1097/MIB.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 18.Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn's disease. Gastroenterology. 2006;130:650–656. doi: 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627.e3. doi: 10.1053/j.gastro.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Wils P, Bouhnik Y, Michetti P, et al. Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn's disease refractory to anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol. 2016;14:242–250.e2. doi: 10.1016/j.cgh.2015.09.018. [DOI] [PubMed] [Google Scholar]