Abstract
Objective
To evaluate the feasibility of a brief, intensive weight loss intervention (IWL) to improve reproductive outcomes in obese subfertile women.
Design
Pilot study of IWL versus standard-of-care nutrition counseling (SCN)
Setting
Single-site, academic institution
Patients
Obese women (BMI 35 – 45 kg/m2) with anovulatory subfertility.
Interventions
Women were rigorously pre-screened to rule out secondary causes of subfertility. Eligible women were randomized to IWL or SCN. IWL consisted of 12 weeks of very-low energy diet (800 kcal/day) + 4 weeks of a low-calorie conventional food-based diet (CFD) to promote 15% weight loss. SCN consisted of 16 weeks of CFD to promote ≥ 5% weight loss. Women were transitioned to weight maintenance diets and referred back to reproductive endocrinology for ovulation induction.
Main Outcome Measures
Feasibility of recruitment, randomization, intervention implementation, and retention.
Results
Thirty-nine women were screened, 25 (64%) were eligible to participate, and 14 of those eligible (56%) agreed to be randomized - 7 in each group. One withdrew from the IWL group and 2 from the SCN group. Percent weight loss was greater in the IWL group than in the SCN group (13±5% vs. 4±4%) (p=0.01). Three of six women in the IWL group conceived and delivered term pregnancies. No pregnancies occurred in the SCN group.
Conclusions
After rigorous screening, 44% of eligible women completed the study. IWL was associated with greater percentage weight loss and improvements in insulin sensitivity.
Trial Registration
Keywords: obesity, weight loss, anovulatory, intensive dietary intervention
Introduction
In 2010, 34% of American women 20–39 years of age were obese including 17% who had body mass index (BMI) 35.0 to 39.9 and 8% who had BMI ≥40 kg/m2 (1). As the prevalence and severity of obesity have increased, so have the number of women who have obesity-related abnormalities in reproductive function including anovulation and infertility (2–7). Obesity is known to contribute to ovulatory dysfunction and to compromise ovarian response to ovulation induction agents such as clomiphene (8). Assisted reproductive technologies are less effective in overweight and obese women (4, 7, 9–10) and obese women who achieve pregnancy have higher rates of miscarriage and maternal complications associated with pregnancy (3, 11–13). Epigenetic reprogramming of the developing fetus may also have life-long, adverse health consequences for the offspring of obese women (1, 14–16). Obesity in the periconceptional period perpetuates an intergenerational cycle of obesity and insulin resistance by its deleterious impact on fat mass, insulin signaling in the liver and muscle, and hepatic fatty acid metabolism (15–16). These concerns have led to a debate regarding the appropriateness of fertility treatment for obese women (17).
Controversy exists regarding the optimal treatment of obese, subfertile women who have not previously failed ovulation induction. Guidelines from the National Institute for Health and Care Excellence (NICE) recommend that women with a BMI ≥30 kg/m2 be informed about the health benefits of losing weight before becoming pregnant for both themselves and the baby they may conceive; that health professionals advise, encourage, and help women to reduce weight before becoming pregnant using evidence-based behavior change techniques and specific dietary advice; and that health professionals offer weight-loss support programs involving diet and physical activity to their obese patients (18). Similarly, in a recent Practice Bulletin on Obesity in Pregnancy, the American College of Obstetricians and Gynecologists recommended that “optimal control of obesity begins before conception” and that motivational interviewing techniques be used to help women move through the stages of dealing with unhealthy behavior to promote weight loss, dietary modifications, and exercise (19). More detailed descriptions of how much weight to lose, how quickly to lose weight, and maintain weight loss were, however, lacking. Small studies have demonstrated that weight loss improves some reproductive outcomes (20–21), but to our knowledge, trials have not been performed to demonstrate the impact of brief, intensive weight loss interventions on ovulation, conception, or pregnancy outcomes.
There are a number of potential barriers to preconception weight loss. While recognizing the substantial risks associated with obesity in pregnancy, healthcare providers may be reluctant to recommend weight loss because of their lack of training in obesity management or concerns about the safety of weight loss in the periconception period. Patients are often hesitant to delay fertility treatments to attempt weight loss because of concerns about the limited success and the protracted time necessary to achieve weight loss, the perception that ovulation induction is a faster route to pregnancy, and the belief that the risks of pregnancy associated with obesity are small and manageable (17, 22).
In this pilot study, we assessed the feasibility of recruitment, randomization, intervention implementation, and retention and compared a brief intensive weight loss intervention (IWL) to a brief, standard-of-care nutritional counseling (SCN) intervention in severely obese, subfertile women who had not previously failed ovulation induction.
Materials and Methods
Study design
The objective of this pilot study was to examine whether a brief intensive weight loss intervention compared to brief, standard-of-care nutritional counseling was feasible and the approach acceptable to obese, subfertile women seeking ovulation induction. The study was an open-label, single site pilot study conducted within the University of Michigan (UM) Health System, Ann Arbor, Michigan. Patients were referred from the UM Center for Reproductive Medicine to the UM Weight Management Program. The study protocol was approved by the Institutional Review Board at the University of Michigan Hospital and Health Systems and all women provided written informed consent. A data safety monitoring board (DSMB) oversaw the study. The trial was registered at Clinicaltrials.gov (NCT01894074). Enrollment began in October 2013 and ended in March 2015. With the exception of the last enrollee who was followed for 6 months after the intervention, all women were followed for at least 12 months.
Participants
Women were eligible to participate if they were 18–40 years of age, had a BMI 35–45 kg/m2, had infertility (12 months of unprotected intercourse without conception), had ovulatory dysfunction (amenorrhea, irregular cycles, or progesterone level less than 10 ng/mL in the luteal phase), and had evidence of normal uterine anatomy based on prior pregnancy or had at least one patent tube documented by hysterosalpingogram or saline infusion sonogram. In addition, their partner was required to have a semen analysis demonstrating at least 20 million sperm/mL, 50% motility, and normal morphology by Kruger criteria of at least 8%. All women had ovulatory dysfunction as their diagnosis, and for some, the cause of ovulatory dysfunction was polycycstic ovarian syndrome (PCOS). Women were diagnosed with PCOS based on having at least 2 of the 3 the Rotterdam criteria: irregular menstrual cycles, hyperandrogen signs or lab findings, and polycystic ovaries on ultrasound. The medical record was reviewed to confirm these findings.
Women were excluded if they were using donor sperm, had an FSH > 10 mIU/mL, had endometriosis AFS class III or IV, were taking anti-obesity drugs or appetite suppressants within the past 2 months, had previous bariatric surgery or gastrointestinal disease, used hormonal medications within the past 2 months, had elevated prolactin, type 1 diabetes, uncorrected thyroid disease, evidence of adrenal disease, or had evidence of conditions that would complicate pregnancy (liver disease, kidney disease, autoimmune disorders such as systemic lupus erythematosus, significant anemia, history of clotting disorder, uncontrolled hypertension, heart disease, or cancer). Despite subfertility, all women were required to use an effective method of birth control during the dietary intervention. Recommended methods included oral contraceptive pills and barrier methods.
Age, race, education, employment, cardiovascular risk factors, and co-morbidities were assessed at baseline. All women also underwent anthropometric, laboratory, and behavioral testing. These included assessments at baseline and after dietary intervention of: height, weight, body mass index (calculated), blood pressure and heart rate; oral glucose tolerance testing with a 75 g oral glucose load and blood samples at 0, 30, 60, 90 and 120 minutes for estimation of insulin sensitivity (HOMA); and fasting lipid profile. Additionally, we collected information on of the number of positive LH kits from baseline and for each month during the dietary intervention using LH predictor kits supplied to the participant (unless the woman was taking oral contraceptive pills as a method of birth control during the intervention, n=2).
Depression and health-related quality-of-life (HRQOL) were assessed at baseline and after the dietary intervention. Depression was assessed with the Inventory of Depressive Symptomatology (Self-Report) (IDS-SR). HRQOL was measured with the EuroQol-5D (EQ-5D), a simple and widely used multi-attribute utility model that assesses five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression according to three levels: no problems, some problems, and extreme problems. The accompanying visual analog scale (VAS) records the patient’s self-reported health on a vertical scale where the endpoints are labeled “best imaginable health state” (100) and “worst imaginable health state” (0). The point selected on the scale provides a quantitative measure of the health outcome as judged by the individual. We have previously reported the relationship between BMI and HRQOL before and after weight loss and found that the degree of improvement in HRQOL was associated with baseline BMI, the reduction in BMI, baseline comorbidities and baseline HRQOL (23).
Eligible, consented subjects were randomized (simple randomization) by the research coordinator using sequentially numbered, opaque, sealed envelopes provided by a biostatistician who was independent of the study team. Participants were randomized 1:1 IWL or SCN.
Intensive Weight Management
Participants randomized to the IWL intervention consumed a very-low energy diet (VLED) in the form of liquid meal replacements aimed at providing 800 kcal/day (70 grams of protein and 100 grams of carbohydrate) for a period of up to twelve weeks with a weight loss goal of 15% from baseline weight. Optifast (Nestlé®, PA) provided the sachets of 800 meal replacement shakes and soups used in the trial. Following the period of VLED, participants were transitioned to a partial meal replacement plan, which they followed for two weeks. The partial meal replacement plan provided 1000 – 1200 kilocalories per day and consisted of three meal replacement products and one 400-kilocalorie conventional food meal with explicitly defined portion sizes. Over the next 2 weeks, women were transitioned to an entirely conventional food-based meal plan with an appropriate calorie intake to promote weight stability. Energy needs were calculated using the Mifflin – St Jeor equation from the Nutrition Care Manual website. Participants randomized to IWL attended counseling sessions twice per month with a registered dietitian, during which time they discussed any psychosocial stressors, mood, enablers and barriers to weight loss including hunger, cravings, other challenges and program adherence. They attended appointments with the program physician once per month for medical monitoring at which time, the physician provided encouragement, addressed health status and changes, and discussed the biology of weight homeostasis and regulation, some of the physiologic effects due to weight loss, and participant expectations. During the IWL intervention, all participants were encouraged to gradually increase their level and intensity of physical activity to 40 minutes of moderate physical activity per day and to record the number of minutes of physical activity per week on a diet and physical activity tracking sheet provided by the dietitian. They were informed that they could achieve the target number of minutes either in one single bout or divided 10-minute bouts.
Each participant had a urine pregnancy test at baseline and at completion of the study or at any time she reported a menstrual cycle was missed (if the woman had regular menses). Women who did not take oral contraceptive pills (OCPs) were provided ovulation predictor kits and asked if they had a positive test in the interval between their monthly encounters with the physician.
Standard-of-Care Nutritional Counseling
The standard-of-care nutritional counseling group followed a conventional food-based diet for 12 weeks. This treatment reflected usual care provided to obese, subfertile women seen in the University of Michigan Center for Reproductive Medicine. Caloric requirements were calculated using the Mifflin – St Jeor equation in order to promote weight loss 0.45–1.8 kg per week. Women met with a registered dietitian for a single 1.5 hour session during which they were provided with counseling and written instructions regarding weight loss calorie requirements and portion sizes using food models. They were instructed to use the “plate” method, i.e. a 10 inch plate 3/4 of which was dedicated to non-starchy vegetables and one-quarter to lean protein. They were also provided with weekly meal plans and shopping lists with calculated energy needs. They were advised to track their caloric intake on a daily basis. Suggested caloric intake was usually ~1200 kcal/day. They were provided general information about energy balance and the neuro-psychobiology of weight. Patients were offered 3 optional monthly follow-up appointments with the registered dietitian. They attended appointments with the program physician at baseline and after sixteen weeks. They were provided ovulation predictor kits and asked to report any positive tests.
Post-dietary follow-up
Once participants had completed their dietary intervention and had been transitioned to weight maintenance diets, they were referred back to CRM for ovulation induction or they tried to conceive naturally. A total of 3 cycles of ovulation induction medication were allowed to each woman who continued to have ovulatory dysfunction, determined by irregular cycles/amenorrhea or midluteal progesterone less than 10 ng/mL. Standard dosing and monitoring included starting clomiphene citrate at 50 mg on cycle days 3 – 7, use of a urinary LH kit to detect ovulation and a midluteal progesterone level 8 days after a positive LH surge. If the progesterone level was at least 10ng/mL, the dose was maintained. If the progesterone was <10 ng/mL the dose was increased by 50 mg for the subsequent cycle.
Participants’ charts were monitored for positive pregnancy tests. The patient’s reproductive and obstetrical course was reviewed and the outcomes recorded for analysis.
Assays
Glucose, insulin and lipids were measured in the Michigan Diabetes Research Center Chemistry Laboratory. The glucose assay used a hexokinase method and was run on a Randox RX Series Daytona chemistry analyzer. [Reference: Glucose Assay (GLUC-HK) Product Insert, RX Series GL 3816, Randox Laboratories Limited, United Kingdom.] The insulin assay was a double-antibody radioimmunoassay using an 125I-Human insulin tracer (Linco Research), a guinea pig anti-porcine insulin first antibody (MDRTC, 68.5% cross-reaction to human proinsulin), and a goat anti-guinea pig gamma globulin (Antibodies Inc.)-PEG second antibody and standardized against the Human Insulin International Reference Preparation (NIBSC). The cholesterol assay was an enzymatic end point method. [Reference: Cholesterol Assay (CHOL) Product Insert, RX Series CH 3810, Randox Laboratories Limited, United Kingdom.] The triglyceride assay used a GPO-PAP method and the HDL-cholesterol assay used a two-step direct method. All lipid assays were run on a Randox RX Series Daytona chemistry analyzer. [Reference: Triglyceride Assay (TRIGS) Product Insert, RX Series TR 3823, and HDL-Cholesterol Assay (HDL) Product Insert, RX Series CH 3811, Randox Laboratories Limited, United Kingdom.] Participants were provided with First Response™ Ovulation and Pregnancy Test Kits (Church & Dwight, Inc., Ewing, NJ)
Statistical analysis
The target sample size of 32 (16 subjects per treatment group) for this study was based on a desire to obtain preliminary estimates of the treatment effect. We calculated that with this sample size, there was sufficient (≥80%) power to detect an absolute treatment difference of 50% (i.e. 60% of women in the IWL arm and 10% of the women in the standard-of-care arm would achieve clinical pregnancy) or greater with a two-sided Type I error of 5%. This sample size also provided sufficient power to detect large effect sizes (1.02 or greater) for continuous outcomes, such as changes in weight or blood pressure between the treatment groups. Descriptive statistics were used to explore the distribution, central tendency (mean, median) and variation (standard deviation, interquartile range, and range) of each measurement for each group. T-tests and Fisher’s exact tests were used for discrete outcomes. The nominal significance level was 0.05 with no adjustments for multiplicity.
Results
We screened 39 women of whom 25 were found to be eligible and 14 agreed to participate (see Figure 1). Our inability to achieve a target sample size of 32 was due to the extremely restrictive eligibility criteria and reluctance of eligible women to delay ovulation induction. Participants had a mean age of 32 ± 4 years and a mean BMI of 41 ± 3 kg/m2. After the baseline oral glucose tolerance test, participants were randomly allocated to their treatments. Seven participants were randomized to each group. One participant withdrew from the IWL intervention after one week of starting the dietary intervention and two participants withdrew from SCN group prior to the dietary intervention. Reasons for withdrawal are given in Figure 1. No participant reported any serious adverse events during the dietary interventions.
Figure 1. CONSORT Flow Diagram of Study Population.
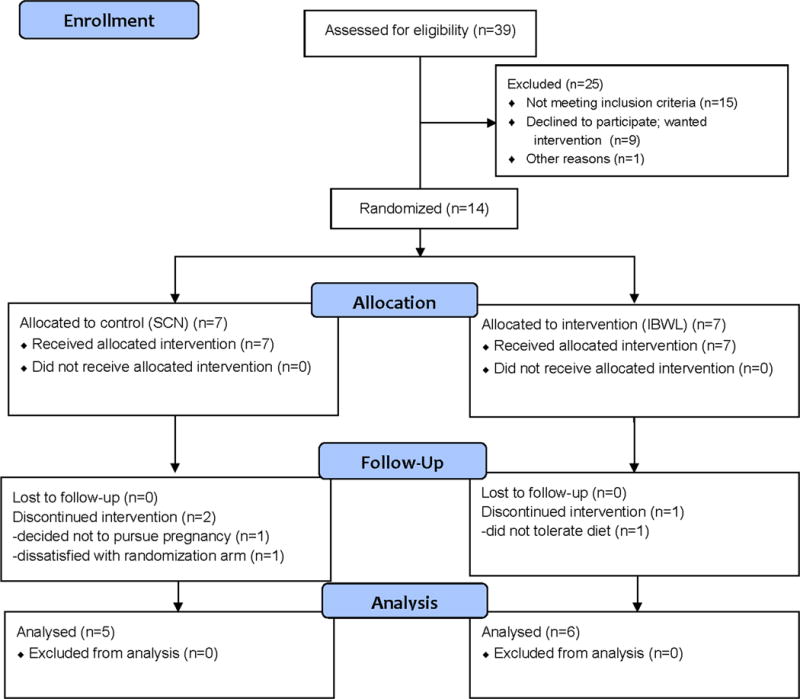
SCN = Standard-of-care nutrition counseling
IBWL = Intensive behavioral weight loss
Baseline demographics and obstetrical history are shown in Table 1. There were no statistically significant differences between completers and drop-outs in baseline measures. There were no differences between groups for marital status, level of education, alcohol consumption, tobacco use or illicit drug use. Five of eleven of subjects had been having unprotected intercourse for 2 years. Three had been having unprotected intercourse for >2 years. Only two reported having unprotected intercourse for <2 years. The median years of subfertility were slightly greater in the SCN group (median 2.0 years) than in the IWL group (median 1.2 years) although women in the IWL group tended to be older (median age 35 years) compared to those in the SCN group (median age 29 years). Five of 6 in the IWL group had previous pregnancies and 3 of 5 in the SCN group had previous pregnancies (Table 1). At baseline, there were no differences in glycemic indexes (fasting glucose, fasting insulin, glucose tolerance or HOMA-IR) (Table 1).
Table 1.
Baseline characteristics and obstetrical history
| Characteristics | IWL Completers N=6 |
IWL Withdrawn N=1 |
SCN Completers N=5 |
SCN Withdrawn N=2 |
|---|---|---|---|---|
| Age (yrs) | 33 ± 5 | 28 | 30 ± 4 | 33 ± 2 |
| Weight (kg) | 108 ± 10 | 96.3 | 107 ± 14 | 117 ± 5 |
| BMI (kg/m2) | 41 ± 4 | 40 | 41 ± 4 | 42 ± 0 |
| Waist circumference (cm) | 118 ± 8 | 109 | 117 ± 9 | 118 ± 21 |
| Hip circumference (cm) | 126 ± 7 | 120 | 126 ± 7 | 133 ± 3 |
| Systolic Blood Pressure (mmHg) | 122 ± 15 | 110 | 122 ± 12 | 126 ± 1 |
| Diastolic Blood Pressure (mmHg) | 63 ± 6 | 64 | 65 ± 9 | 72 ± 11 |
| Lipids | ||||
| Total Cholesterol (mg/dl) | 158 ± 25 | N/A | 177 ± 18 | 235 ± 35 |
| Triglycerides (mg/dl) | 172 ± 44 | N/A | 151 ± 95 | 157 ± 55 |
| HDL (mg/dl) | 40 ± 12 | N/A | 42 ± 5 | 57 ± 1 |
| LDL (mg/dl) | 87 ± 15 | N/A | 105 ± 8 | 147 ± 23 |
| Fasting glucose (mg/dL) | 99 ± 6 | 87 | 95 ± 9 | 85 ± 2 |
| Insulin (μU/mL) | 267 ± 5 | N/A | 33 ± 3 | N/A |
| HOMA-IR | 7 ± 1 | N/A | 8 ± 1 | N/A |
| EQ-5D Index | 0.87 ± 0.06 | 1 | 0.89 ± 0.09 | 1 ± 0 |
| EQ-5D Visual Analog Scale | 70 ± 11 | 100 | 66 ± 16 | 65 ± 7 |
| IDS-SR | 16 ± 3 | 15 | 18 ± 12 | 12 ± 12 |
| Obstetrical History | ||||
| No. of participants with previous pregnancy | 5 | 0 | 2 | 1 |
| Total # of pregnancies | 7 | 0 | 2 | 1 |
| No. of participants with spontaneous abortion (Ab) or ectopic pregnancy | 1 | 0 | 2 | 0 |
| Total # of Ab or ectopic pregnancies | 3 (1 subject) | 0 | 2 | 0 |
| No. of participants with previous live births | 4 | 0 | 0 | 1 |
| Total # of previous live births | 7 | 0 | 0 | 1 |
| Median years of infertility | 1.6 | 1 | 2 | 4.5 |
| Birth Control Method during Intervention | ||||
| Condoms | 4 | N/A | 2 | N/A |
| Hormonal | 2 | N/A | 0 | N/A |
| Abstinence | 0 | N/A | 3 | N/A |
N/A = not applicable
Mean ± standard deviation reported for continuous outcomes
BMI, body mass index; HDL, high-density lipoproteins; LDL, low-density lipoproteins; HOMA-IR, homeostatic model assessment-insulin resistance; EQ-5D, EuroQol-5D; IDS-SR, Inventory of Depressive Symptomatology (Self-Report)
At follow-up, both treatment groups lost weight, but the mean weight loss at 12 weeks was significantly greater in the IWL group (Table 2). Participants assigned to the IWL lost 14 ± 6 kg (13% of initial weight), range: 4 to 21 kg compared to 5 ± 5 kg (4% of initial weight), range: 0 to 10 kg for participants in the standard group (p<0.05). BMI changed by 5 ± 2 units in the IWL group compared to 2 ± 2 units in the SCN (p<0.02). In the IWL group, waist circumference decreased by 10±7 cm (p<0.05) and hip circumference decreased by 11 cm (p<0.01) (data not shown). Five of the 6 participants in the IWL group moved from very severely obese to severe or moderately obese. Fasting glucose, fasting insulin and HOMA-IR (for all measures, p<0.05) improved in the IWL group relative to the SCN group (Table 2).
Table 2.
Within group and between group changes in anthropometric, laboratory, and quality-of-life outcomes.
| Parameter | IWL N=6 |
p-value[1] | SCN N=5 |
p-value[1] | Group Difference | p-value[2] |
|---|---|---|---|---|---|---|
| Weight (kg) | −14 ± 6 | 0.00 | −5 ± 5 | 0.09 | 10 | 0.02 |
| Change in weight (%) | 13 ± 5 | 0.00 | 4 ± 4 | 0.07 | 9 | 0.01 |
| BMI (kg/m2) | −5 ± 2 | 0.00 | −2 ± 2 | 0.09 | 4 | 0.02 |
| Systolic Blood Pressure (mmHg) | −2 ± 6 | 0.41 | −6 ± 6 | 0.08 | 4 | 0.34 |
| Diastolic Blood Pressure (mmHg) | −5 ± 4 | 0.05 | 0 ± 11 | 1.00 | 5 | 0.39 |
| Lipids | ||||||
| Total Cholesterol (mg/dl) | −7 ± 17 | 0.36 | 9 ± 19 | 0.38 | 15 | 0.19 |
| Triglycerides (mg/dl) | −27 ± 55 | 0.29 | 31 ± 62 | 0.33 | 57 | 0.14 |
| HDL (mg/dl) | −1 ± 6 | 0.71 | 2 ± 5 | 0.34 | 3 | 0.36 |
| LDL (mg/dl) | −4 ± 9 | 0.30 | 1 ± 11 | 0.91 | 5 | 0.44 |
| Fasting glucose (mg/dL) | −9 ± 6 | 0.01 | 1 ± 9 | 0.83 | 10 | 0.05 |
| Insulin (μU/mL) | −10 ± 4 | 0.00 | 4 ± 14 | 0.64 | 14 | 0.05 |
| HOMA-IR | −3 ± 1 | 0.00 | 1 ± 4 | 0.55 | 4 | 0.03 |
| EQ-5D Index | 0.05 ± 0.08 | 0.21 | −0.01 ± 0.07 | 0.84 | 0.06 | 0.26 |
| EQ-5D Health Score | 13 ± 14 | 0.07 | −3 ± 12 | 0.63 | 16 | 0.07 |
| IDS-SR | −5 ± 4 | 0.02 | 2 ± 7 | 0.60 | 7 | 0.07 |
p-value based on paired t-test
p-value based on two-sample t-test
Mean ± standard deviation reported
BMI, body mass index; HDL, high-density lipoproteins; LDL, low-density lipoproteins; HOMA-IR, homeostatic model assessment-insulin resistance; EQ-5D, EuroQol-5D; IDS-SR, Inventory of Depressive Symptomatology (Self-Report)
Table 3 shows the number of women reporting positive LH predictor kit tests, the number of medication-induced cycles, pregnancy rates, live births, miscarriages (none), gestational diabetes, hypertensive disorders of pregnancy and preeclampsia. Women in the IWL group required fewer medication cycles (1±1) compared to those in the SCN group (3±0) (p<0.05).
Table 3.
Obstetrical Outcomes by treatment group
| IWL N=6 |
SCN N=5 |
p-value[1.2] | |
|---|---|---|---|
|
| |||
| Participants with LH surge (non-hormonal users) | 3 (out of 4) | 1 | 0.21 |
|
| |||
| Ovulation induction | 5/6 | 3/5 | 0.14 |
|
| |||
| Average number of cycles of ovulation induction medication | 1 | 3 | 0.02 |
|
| |||
| Confirmed pregnancies | 3 | 0 | 0.18 |
|
| |||
| Gestational diabetes | 2 | N/A | N/A |
| -diet controlled | 1 | ||
| -metformin monotherapy | 1 | ||
|
| |||
| Hypertension in pregnancy | 0 | N/A | N/A |
|
| |||
| Preeclampsia | 0 | N/A | N/A |
|
| |||
| Singleton live birth | 3 | 0 | 1.00 |
N/A = not applicable
p-value based on Fisher’s Exact Test
p-value based on two sample t-test
Women in the IWL group had improvements in measures of HRQOL: mean EQ-5D index increased from 0.87 to 0.92 (0.05±0.08, with a change of +0.03 considered to be clinically significant) whereas there was worsening or no change in the SCN group. Health-related quality-of-life scores also increased in the IWL group by 13 ±14 although not statistically significant with worsening in the SCN group with a change of −3±12 (treatment difference of 16 and p=NS). There were improvements in depressive symptomatology as measured by the IDS-SR in the IWL group but no improvements in depressive symptomatology for the SCN group.
Weight loss, ovulation and conception tended to be greater in the IWL group compared with the SCN group but neither ovulation nor conception reached statistical significance (3/6 women ovulated and conceived in the IWL group vs 0/5 in the SCN group). When these pregnancies were followed, there were 3 live births in the IWL group. All births were at term.
Discussion
This pilot study demonstrated that a brief intensive weight loss intervention in severely obese, subfertile women was feasible and resulted in significant reductions in weight and improvements in metabolic and ovulatory outcomes.
Although most obese women are not infertile, obesity has a negative impact on fecundity (2, 4, 13). Obese women are more likely to have subfertility than women of normal body weight. Obese women experience impaired fecundity both in natural and assisted conception cycles (6, 13, 24–26). Studies have shown an increased risk of anovulatory subfertility in obese women (odds ratio 2 to 3) (1) by mechanisms that include hyperandrogenism and PCOS (5, 27). In addition, obesity has an impact on psychosocial factors. Obese people may not have sexual intercourse as frequently as thinner people (28). Obese women are more likely to experience sexual dysfunction (28, 29). A decrease in coital activity may serve to prolong the period to pregnancy. Reduction in weight is likely to augment self-esteem in issues of intimacy and sexual health. Three large retrospective population-based studies have shown lower pregnancy rates in obese women (2, 6, 25). Obesity is associated with a linear reduction in fecundity from the moderately obese to the very obese (7). Obesity induces a state of insulin resistance and compensatory hyperinsulinemia. Weight loss of 5% or more in obese women results in increased insulin sensitivity, an increase in sex-hormone binding globulin and trends toward normalization of reproductive hormonal profiles favoring restoration of menstrual cyclicity in women with and without PCOS (4, 20–21, 27, 30–31).
Studies in both animals and humans have shown that pre-pregnancy weight is associated with adverse reproductive, maternal, gestational and fetal risks and that weight loss can ameliorate or reverse some of these effects. Indeed, surgical approaches have emerged as an effective albeit complex strategy to promote durable weight loss and remission of type 2 diabetes in severely obese women. A number of studies have found a decreased prevalence of obesity in the offspring of mothers who underwent maternal surgical weight loss (32).
Therefore, we hypothesized that a lifestyle intervention that resulted in short-term weight loss similar to the immediate weight loss observed after bariatric surgery (33, 34), but without the costs, potential complications, and long-term nutritional deficiencies associated with bariatric surgery might be of benefit.
McMillen and her colleagues have demonstrated that energy restriction in the periconceptual period may result in both benefits and adverse consequences. They studied the metabolic and endocrine effects of dietary interventions during the periconceptual period in an animal model. They defined the periconceptual period as including oocyte maturation, follicular development, conception, and embryo/blastocyst growth up until implantation. They suggested that when maternal nutritional interventions extend beyond implantation to include early placentation, the more appropriate description is “early gestation”. Non-pregnant ewes were either over-nourished or normally nourished for at least 4 months before artificial insemination. In two subgroups of non-pregnant ewes, the investigators imposed a 70% restriction of normal energy intake for 4 weeks before and one week after conception and showed ablation of the adverse effects of high maternal prepregnancy weight on offspring adiposity and glucose tolerance. However, this dietary restriction was also associated with enhanced cortisol response to stress in female lambs at 3–4 months of age. The adrenal gland was also bigger at 4 months in the male and female lambs exposed to the dietary restriction and there was a decrease in the adrenal expression of the insulin-like growth factor 2 (35).
In our study, we imposed a ~40% energy restriction to reduce weight by ~15% and allowed for a period of weight stability on a 1200–1500 kcal/day weight maintenance diet before referring participants back to the reproductive endocrinologist. Although participants’ energy restriction was less severe and pre-dated ovulation and conception, uncertainty exists to persistence of some of the metabolic costs seen in the study of an animal model.
This pilot study showed that over one-third of obese, subfertile women presenting to an academic center for reproductive medicine had secondary causes of subfertility or conditions other than obesity that would represent relative contraindications to pregnancy. Of eligible women, nearly half (44%) declined to be randomized to brief dietary interventions because of concerns related to the time necessary to achieve weight loss, concerns about the limited success of weight loss, a perception that ovulation induction is a faster route to pregnancy, and a belief that the risks of pregnancy associated with obesity are small and manageable. Once enrolled, in the brief weight loss interventions, over one in seven withdrew for reasons including both disappointment at randomization to standard of care therapy and inability to adhere to the intensive weight loss intervention.
Like us, Phelan et al., reported difficulties recruiting participants, although unlike us, they were studying a low-intensity behavioral intervention (partially mail-based) to prevent excessive weight gain during pregnancy, not before pregnancy. Of the 1499 potential participants approached, only 401 (26%) were ultimately eligible, available, consented and randomized, and only 320 of them (82%) completed the final 6-month post-partum assessment. The intervention reduced excessive weight gain in normal weight, but not overweight or obese women (36).
Despite a 21% attrition in the present study, the IWL appeared to be both effective and safe in the short-term. With IWL, substantial weight loss was achieved in a short period of time with significant improvements in diastolic blood pressure, HOMA-IR, and emotional health. None of these improvements were manifest in the SCN group. Mean weight loss among the IWL group was 14 ± 6 kg from baseline. Participants’ insulin sensitivity improved in the IWL group as shown by the change in HOMA-IR by −3 ± 1 vs 1 ± 4 (p<0.05).
Three of six (50%) participants in the IWL group became pregnant within six months following completion of the IWL intervention. Two conceived after 1 cycle of clomiphene and one conceived without ovulation induction. None of the participants from the SCN group conceived with the opportunity of 3 cycles of clomiphene.
Our findings suggest that a brief, 16-week, intensive weight loss intervention for women with pre-pregnancy BMI of 35–45 kg/m2, with a goal or reducing initial body weight by 15% may be more effective than standard-of-care nutritional counseling. Our findings differ from those of a previous pilot study conducted in Australia using VLED for 27–41 days immediately before in vitro fertilization as opposed to ovulation induction. That study found that half of participants reported no fertilization despite significant weight loss. However, four of 10 women withdrew from the study after only two weeks and the percentage weight loss was only 6% in the remaining 6 participants as compared to the 13% weight loss observed in our study. The authors reported that IVF treatment outcomes were not assessed definitively because the study was underpowered given small sample size, low percentage of weight loss and the large number of drop-outs (37).
Trials of intensive lifestyle management in at risk populations have shown prevention and control of diabetes and cardiovascular risk factors (38–39). There are compelling reasons to undergo more intensive weight loss particularly in motivated and more severely obese patients. VLED programs are used when rapid weight loss is necessary because of an obesity-related disease. In other patients with obesity, it is an alternative to other conservative approaches for treatment of obesity. In type 2 diabetes it may improve long-term glucose metabolism better than conventional weight reducing diets. Further, VLEDs have no serious harmful effects and can safely be used in patients with various chronic diseases (40–41). Nackers et al. showed that middle-aged, moderately to severely obese women who lost weight rapidly compared to moderately or slowly were no more susceptible to weight regain than in the other 2 groups. Indeed, the women who were “fast” losers were more motivated and had better short and long-term weight loss (42). In our study, we imposed a ~40% energy restriction to reduce weight and allowed for a period of weight stability on a 1200–1500 kcal/day weight maintenance diet before referring participants back to the reproductive endocrinologist. Although participants’ energy restriction was less severe and pre-dated ovulation and conception, uncertainty exists as to the potential adverse effects seen in the neonate as seen in the animal model.
Our results show a relatively high rate of ineligibility for IWL, a reluctance to be randomized, and a high initial drop-out rate. At the same time, individuals undertaking IWL demonstrated greater absolute weight loss and percentage change from baseline weight, positive changes in metabolic parameters and health-related quality-of-life, and a short interval to ovulation and conception. Limitations to our study include its small sample size, baseline imbalance in obstetrical histories, lack of a direct measure of resting metabolic rate or use of more sophisticated tool for calculating energy requirements, lack of quantitative assessment of dietary intake and physical activity.
Given the small sample size, there is uncertainty around the point estimates of the percentage of pregnancies in the IWL and SCN groups. We reviewed a number of hypothetical pregnancy estimates supported by the pilot study. Using a conservative estimate of the IWL effect, the 80% lower confidence bound of 20% and 0% for IWL and SCN, respectively, a future similar study would require 63 subjects who were eligible and willing to be randomized. However, if we use the upper confidence bound of 80% and 37% for IWL and SCN, respectively, we would require only 34 subjects who were eligible and willing to be randomized (similar to our projected sample recruitment) (total sample size to achieve at least 80% power with two-sided type I error of 5% (East 6, Cytel Corporation, Cambridge, MA; two-sample test of proportions).
Finally, a strength of our study was that non-obesity related causes of infertility were ruled out. Based on the promising results of this pilot study, we believe that the brief intervention that we tested will be acceptable to women concerned about delaying fertility therapy, especially in light of the increased risk of pregnancy-related complications that may be improved and the emerging evidence of adverse epigenetic effects of obesity on offspring.
A larger, multi-site trial comparing these interventions that includes following women through delivery, obtaining hormonal measures, quantifiable dietary assessment in parallel with high throughput metabolite profiling may help to identify women who will respond to aggressive weight loss with improved conception and pregnancy and provide greater understanding of the interactions between diet, metabolites and clinical outcomes in order to provide greater precision and tailoring to our patients.
Capsule.
This is the feasibility of a brief, intensive weight loss intervention to improve reproductive outcomes in obese, subfertile women: a pilot study.
Acknowledgments
The study was supported by a grant from the Michigan Institute for Clinical Research (Grant Number U040012 PI: Rothberg) and the core services of the Michigan Nutrition Obesity Research Center (Grant Number DK089503) and the Michigan Center for Diabetes Research (Grant Number P30DK020572). We would like to thank Nestle®, PA for providing the meal replacement product. We would like to thank the members of the DSMB for their oversight. We would like to thank Dr. Spino for her statistical support.
Dr. Rothberg reports non-financial support from Nestle during the conduct of the study.
Footnotes
Competing interests
Drs. Rothberg, Lanham, Randolph, and Smith and Ms. Fowler and Miller have nothing to disclose. The WMP has received non-financial support from Optifast (Nestle®).
Authors’ contributions
AR was responsible for study design, data collection, data analysis, data interpretation, the literature search, and writing the manuscript. NM was responsible for data analysis and generation of the tables and figure. ML was responsible for data interpretation. CF was responsible for data collection and data interpretation. JR was responsible for data interpretation. YR was responsible for study design and data interpretation. All authors were involved in writing the manuscript and had final approval of the submitted and published versions.
References
- 1.Flegal KM. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):49–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5(2):247–50. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Lake JK, Power C, Cole TJ. Women’s reproductive health: the role of body mass index in early and adult life. Int J Obes Relat Metab Disord. 1997;21(6):432–8. doi: 10.1038/sj.ijo.0800424. [DOI] [PubMed] [Google Scholar]
- 4.Norman RJ, Clark AM. Obesity and reproductive disorders: a review. Reprod Fertil Dev. 1998;10(1):55–63. doi: 10.1071/r98010. [DOI] [PubMed] [Google Scholar]
- 5.Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171–7. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 6.Yogev Y, Visser GH. Obesity, gestational diabetes and pregnancy outcome. Semin Fetal Neonatal Med. 2009;14(2):77–84. doi: 10.1016/j.siny.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306(6876):484–7. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobo RA, Gysler M, March CM, Goebelsmann U, Mishell DR., Jr Clinical and laboratory predictors of clomiphene response. Fertil Steril. 1982;37(2):168–74. [PubMed] [Google Scholar]
- 9.Grodstein F, Goldman MB, Ryan L, Cramer DW. Self-reported use of pharmaceuticals and primary ovulatory infertility. Epidemiology. 1993;4(2):151–6. doi: 10.1097/00001648-199303000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Norman RJ, Chura LR, Robker RL. Effects of obesity on assisted reproductive technology outcomes. Fertil Steril. 2008;89(6):1611–2. doi: 10.1016/j.fertnstert.2007.02.065. [DOI] [PubMed] [Google Scholar]
- 11.Aviram A, Hod M, Yogev Y. Maternal obesity: implications for pregnancy outcome and long-term risks-a link to maternal nutrition. Int J Gynaecol Obstet. 2011;115(Suppl 1):S6–10. doi: 10.1016/S0020-7292(11)60004-0. [DOI] [PubMed] [Google Scholar]
- 12.Tamer Erel C, Senturk LM. The impact of body mass index on assisted reproduction. Curr Opin Obstet Gynecol. 2009;21(3):228–35. doi: 10.1097/GCO.0b013e32832aee96. [DOI] [PubMed] [Google Scholar]
- 13.Zain MM, Norman RJ. Impact of obesity on female fertility and fertility treatment. Womens Health (Lond Engl) 2008;4(2):183–94. doi: 10.2217/17455057.4.2.183. [DOI] [PubMed] [Google Scholar]
- 14.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587(Pt 4):905–15. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholas LM, MOrrison JL, Rattanatray L, Zhang S, Ozanne SE, McMillen IC. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes (Lond) 2016;40(2):229–38. doi: 10.1038/ijo.2015.178. [DOI] [PubMed] [Google Scholar]
- 16.Lillycrop KA, Burdge GC. Epigenetic changes in early life and future risk of obesity. Int J Obes (Lond) 2011;35(1):72–83. doi: 10.1038/ijo.2010.122. [DOI] [PubMed] [Google Scholar]
- 17.Vahratian A, Smith YR. Should access to fertility-related services be conditional on body mass index? Hum Reprod. 2009;24(7):1532–7. doi: 10.1093/humrep/dep057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NICE. Fertility: assessment and treatment for people with fertility problems. London: NICE; 2013. p. CG156. http://www.nice.org.uk/CG156 (accessed 18 Nov 2013) [Google Scholar]
- 19.Obstetrics & Gynecology Issue. 2015 Dec;126(6):e112–e126. doi: 10.1097/AOG.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 20.Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update. 2004;10(3):267–80. doi: 10.1093/humupd/dmh018. [DOI] [PubMed] [Google Scholar]
- 21.Clark AM, Ledger W, Galletly C, Tomlinson L, Blaney F, Wang X, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod. 1995;10(10):2705–12. doi: 10.1093/oxfordjournals.humrep.a135772. [DOI] [PubMed] [Google Scholar]
- 22.Pandey S, Maheshwari A, Bhattacharya S. Should access to fertility treatment be determined by female body mass index? Hum Reprod. 2010;25(4):815–20. doi: 10.1093/humrep/deq013. [DOI] [PubMed] [Google Scholar]
- 23.Rothberg AE, McEwen LN, Kraftson AT, Neshewat GM, Fowler CE, Burant CF, Herman WH. The impact of weight loss on health-related quality-of-life: implications for cost-effective analyses. Qual Life Res. 2014;23(4):1371–6. doi: 10.1007/s11136-013-0557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81(2):384–92. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140(3):347–64. doi: 10.1530/REP-09-0568. [DOI] [PubMed] [Google Scholar]
- 26.Wang JX, Davies M, Norman RJ. Body mass and probability of pregnancy during assisted reproduction treatment: retrospective study. BMJ. 2000;321(7272):1320–1. doi: 10.1136/bmj.321.7272.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates GW, Whitworth NS. Effect of body weight reduction on plasma androgens in obese, infertile women. Fertil Steril. 1982;38(4):406–9. [PubMed] [Google Scholar]
- 28.Brody S. Slimness is associated with greater intercourse and lesser masturbation frequency. J Sex Marital Ther. 2004;30(4):251–61. doi: 10.1080/00926230490422368. [DOI] [PubMed] [Google Scholar]
- 29.Trischitta V. Relationship between obesity-related metabolic abnormalities and sexual function. J Endocrinol Invest. 2003;26(3 Suppl):62–4. [PubMed] [Google Scholar]
- 30.Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502–5. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- 31.Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1992;36(1):105–11. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 32.Patti ME. Reducing maternal weight improves offspring metabolism and alters (or modulates) methylation. PNAS. 2013;110(32):12589–12860. doi: 10.1073/pnas.1309724110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackness C, Karmally W, Febres G, Conwell I, Ahmed L, Bessler M, McMahon DJ, Korner J. Very Low-Calorie Diet Mimcs the Eearly BEneficial Effect of Roux-en-Y Gastric Bypass on Insulin Sensitivity and β-Cell Funciton in Type 2 Diabetes Patients. Diabetes. 2013;62:3027–3032. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothberg AE, McEwen LN, Kraftson AT, Fowler CE, Herman WH. Very-low-energy diet for type 2 diabetes: An underutilizzed therapy? J Diabetes Complications. 2014;28:506–510. doi: 10.1016/j.jdiacomp.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Rattanatray L, Morrison JL, Nicholas LM, Lie S, McMillen IC. Maternal obesity and the early origins of childhood obesity: weighing up the benefits and costs of maternal weight loss in the periconceptional period for the offspring. Exp Diabetes Res. 2011;2011:585749. doi: 10.1155/2011/585749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. AJCN. 2011;93(4):772–9. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsagareli V, Noakes M, Norman RJ. Effect of a very-low-calorie diet on in vitro fertilization outcomes. Fertil Steril. 2006;86(1):227–9. doi: 10.1016/j.fertnstert.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 38.The Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. NEJM. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Look AHEAD Research Group. The Look AHEAD Study: A Description of the Lifestyle Intervention and the Evidence Supporting It. Obesity. 2006;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mustajoki P, Pekkarinen T. Very low energy diets in the treatment of obesity. Obes Rev. 2001;1:61–72. doi: 10.1046/j.1467-789x.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 41.Delbridge E, Porietto J. State of the science: VLED (Very Low Energy Diet) for obesity. Asia Pac J Clin Nutr. 2006;15(Suppl):49–54. [PubMed] [Google Scholar]
- 42.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;13:161–7. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


