Abstract
It is increasingly evident that alcohol-induced, gut-mediated peripheral endotoxemia plays a significant role in glial cell activation and neuro-inflammation. Using a mouse model of chronic alcohol feeding, we examined the causal role of endotoxin- and cytokine-responsive Pde4 subfamily b (Pde4b) expression in alcohol-induced neuro-inflammation. Both pharmacologic and genetic approaches were used to determine the regulatory role of Pde4b.
In C57Bl/6 wild type (WT) alcohol fed (WT-AF) animals, alcohol significantly induced peripheral endotoxemia and Pde4b expression in brain tissue, accompanied by a decrease in cAMP levels. Further, along with Pde4b, there was a robust activation of astrocytes and microglia accompanied by significant increases in the inflammatory cytokines (Tnfα, Il-1β, Mcp-1 and Il-17) and the generalized inflammatory marker Cox-2. At the cellular level, alcohol and inflammatory mediators, particularly LPS, Tnfα and Hmgb1 significantly activated microglial cells (Iba-1 expression) and selectively induced Pde4b expression with a minimal to no change in Pde4a and d isoforms. In comparison, the alcohol-induced decrease in brain cAMP levels was completely inhibited in WT mice treated with the Pde4 specific pharmacologic inhibitor rolipram and in Pde4b−/− mice. Moreover, all the observed markers of alcohol-induced brain inflammation were markedly attenuated. Importantly, glial cell activation induced by systemic endotoxemia (LPS administration) was also markedly decreased in Pde4b−/− mice. Taken together, these findings strongly support the notion that Pde4b plays a critical role in coordinating alcohol-induced, peripheral endotoxemia mediated neuro-inflammation and could serve as a significant therapeutic target.
Keywords: Alcohol, neuroinflammation, cAMP, Pde4b, microglial activation
1. Introduction
The adverse effects of alcohol consumption on the brain include severe alcohol-related neuro-inflammation and brain damage that may contribute to cognitive decline and neurodegeneration (Vetreno and Crews, 2014). In both human and animal studies, alcohol abuse causes glial cell activation leading to neuro-inflammation and injury (Montesinos et al., 2016). Direct and indirect inflammatory effects of alcohol on glial cells are substantially mediated by toll-like receptor 4 (Tlr4) signaling (Montesinos et al., 2016; Szabo and Lippai, 2014). With regards to direct effects, Guerri’s group showed that alcohol exposure induces Tlr4-mediated activation of NFκB and MAPK pathway resulting in inflammatory cytokine production by microglial cells (Blanco and Guerri, 2007; Fernandez-Lizarbe et al., 2009). The critical role of Tlr4 signaling in alcohol-mediated inflammatory responses in microglial cells was further confirmed by studies using Tlr4−/− mice (Alfonso-Loeches et al., 2010; Pascual et al., 2011). Moreover, seminal work by Crew’s group showed that alcohol-induced neuro-inflammation and injury results in release of damage-associated molecular patterns (DAMPs), such as high mobility group box 1 (Hmgb1), that can act as ligands and activate Tlr4 signaling in glial cells (Crews et al., 2015; Szabo and Lippai, 2014). In relation to indirect effects, there is increasing evidence that alcohol-induced peripheral inflammation contributes to the development of neuro-inflammation (Qin et al., 2007). Work done by us and others shows that chronic alcohol exposure causes gut dysbiosis, as well as intestinal barrier dysfunction and increased permeability, resulting in peripheral endotoxemia (Bull-Otterson et al., 2013; Forsyth et al., 2009; Keshavarzian et al., 2001; Mutlu et al., 2009; Rao et al., 2004; Yan et al., 2011). Importantly, peripheral endotoxemia is the major cause of peripheral inflammatory cytokine production which in turn can strongly influence neuro-inflammation and injury (Crews and Vetreno, 2016). Although Tlr4-mediated signaling is implicated, the mechanism(s) by which alcohol regulates glial cell activation and neuro-inflammation are not completely understood.
We have demonstrated that the alcohol-induced increase in PDE4 expression and consequent decrease in intracellular cAMP plays a critical role in alcohol “priming” effect and exaggerated response of peripheral monocytes/macrophages to LPS (Gobejishvili et al., 2008; Gobejishvili et al., 2006). Moreover, studies using Pde4b−/− mice demonstrated that Pde4b is critical for LPS/Tlr4 signaling and is essential for LPS-induced Tnfα production (Jin and Conti, 2002; Jin et al., 2005). Importantly, chronic ethanol consumption significantly increases peripheral/liver as well as brain Tlr4 expression and downstream inflammatory gene expression contributing to ethanol induced peripheral as well as neuro-inflammation (Crews et al., 2013; Fernandez-Lizarbe et al., 2009; Floreani et al., 2010; Kirpich et al., 2012). Further, besides Tlr4 expression, increased brain Pde4b mRNA expression has also been observed in mouse models with high degree of alcohol preference (Mulligan et al., 2006).
The importance of alcohol-mediated peripheral endotoxemia and inflammation, along with Pde4b expression in brain inflammation, is supported by the findings that a single intraperitoneal administration of endotoxin significantly upregulates Pde4b expression in activated glial cells along with the CNS expression of inflammatory mediators such as Cox-2, Il-1β, Tnfα, and Vcam-1 (Johansson et al., 2011; Reyes-Irisarri et al., 2008). Further, in relevance to a significant contribution of peripheral Tnfα in alcohol-induced neuro-inflammation (Qin et al., 2007), the Tnfα-mediated increase in Pde4b expression regulates inflammatory responses in microglia cells (Ghosh et al., 2015; Ghosh et al., 2012). Taken together, there is a strong rationale for the causal role of Pde4b expression in mediating the direct and indirect effects of alcohol leading to glial cell activation and neuro-inflammation. Nevertheless, the role of Pde4b in integrating the direct and indirect neuro-inflammatory effects of alcohol has not been examined and is an important mechanistic focus of this work. The results of this study show that alcohol increases brain Pde4b expression leading to a decrease in cAMP levels, activation of glial cells, and neuro-inflammation. We show that pharmacological and genetic inhibition of Pde4b markedly attenuates the neuro-inflammatory effects of chronic alcohol consumption.
2. Materials and Methods
2.1. Animal Model
12 week old male C57BL/6 (wild type-WT) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). A breeding pair of Pde4b−/− mice generated on C57Bl/6J background was a kind gift from Prof. Marco Conti (UCSF). All mice were housed in a pathogen-free, temperature-controlled animal facility with 12-hour light /12-hour dark cycles. All experiments were carried out according to the criteria outlined in the Guide for Care and Use of Laboratory Animals and with approval of the University of Louisville Institutional Animal Care and Use (IACUC) and Institutional Biosafety (IBC) Committees.
To assess the effects of chronic alcohol consumption on the development of brain inflammation, we employed a well-established model of alcohol feeding that shows changes in the gut-liver axis involving intestinal barrier dysfunction and systemic endotoxemia and innate immune activation and hepatic inflammation (Bull-Otterson et al., 2013; Kirpich et al., 2012). C57Bl/6 and Pde4b−/− male mice were pair-fed the Lieber-DeCarli liquid diet (Lieber-DeCarli type, Bioserv, Frenchtown, NJ). Alcohol was gradually increased over a period of one week and then mice were fed the ethanol diet [5% (w/v)] ad libitum for another 2 weeks (AF). The Lieber-DeCarli liquid diet contained 15.1% kcal as protein, 35.9% kcal as fat, 13.5% kcal as carbohydrates and 35.5% kcal as either ethanol [AF; ethanol 5% (w/v)] or maltose dextrin [PF - isocaloric control]. Additional groups of AF and PF animals were treated with the Pde4 specific inhibitor rolipram (5 mg/kg) 3 times/week. Rolipram (C16H21NO3) (Biomol, Enzo Life Sciences, Farmingdale, NY) was dissolved in sterile DMSO and diluted with sterile phosphate buffered saline (PBS) just before injection. Food consumption was carefully monitored and blood alcohol levels were measured. No significant differences in either food consumption or blood alcohol levels were found between the groups (Avila et al., 2016). Mice were sacrificed 1 and 2 weeks after starting 5% (w/v) alcohol. At sacrifice, mice were anesthetized with an intraperitoneal injection of Nembutal, 80 mg/kg. There were 5–7 mice in each experimental group. Whole blood was collected and plasma was stored at −80°C for analysis.
2.2. LPS treatment
Wild type C57BL/6J and Pde4b−/− male mice were intraperitoneally (i.p.) injected with a single dose of sterile PBS) or 5 mg/kg LPS (Escherichia coli 0111: B4, Difco, Detroit, MI) for 6 hours. Before use, LPS was dissolved in sterile, pyrogen-free water, sonicated, and diluted with sterile PBS.
2.3. Primary microglial isolation and treatment
Microglial cells were obtained from postnatal day 4 (P4) mice pups according to a slightly modified protocol (Cole and de Vellis, 2001; Saura et al., 2003). Primary microglial cells were plated at a density of 0.3~0.5 million cells/ml and treated with ethanol (25–50 mM) in serum-free DMEM supplemented with N-2 (ref# 17502-048, Life Technologies, Waltham, MA). Cells were plated at the same density in 10% (vol/vol) fetal bovine serum (Invitrogen, Carlsbad, CA) containing DMEM and subsequently treated with LPS, recombinant Hmgb1 and Tnfα. Mouse Hmgb1 recombinant protein was purchased from eBiosciences (cat# 34-8401, eBioscience, Inc., San Diego, CA). Recombinant murine Tnfα protein (cat# 510-RT-050/CF) was purchased from R@D Systems Inc. (Minneapolis, MN). Recombinant Tnfα and Hmgb1 proteins were tested for LPS contamination before using in microglia experiments.
2.4. Western blot analysis
Brain tissues were lysed using RIPA buffer containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitors. Proteins (25 μg) were analyzed by SDS-polyacrylamide gel electrophoresis as described previously (Avila et al., 2016). Quantification was performed with Image LabTMSoftware (BioRad, Life Science Research, Hercules, CA). Gfap and Gapdh antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX), and anti-Pde4b2 was a gift from Dr. Marco Conti’s laboratory.
2.5. RNA isolation and real-time PCR analysis
Total RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA) and qRT-PCR was performed as described previously (Avila et al., 2016). The specific primers were purchased from integrated DNA technologies (IDT, Coralville, Iowa) (Avila et al., 2016; Gobejishvili et al., 2011). The relative gene expression was analyzed using 2−ΔΔCt method by normalizing β-actin and 18S gene expression in all the experiments.
2.6. Immunohistochemistry
Brains were harvested from mice transcardially perfused with 20 ml PBS, followed by 15 ml of 4% paraformaldehyde (PFA) in PBS, pH 7.4. Brains were dissected and additionally fixed overnight at 4°C in 4% PFA. Following fixation, brains were transferred to 30% sucrose solution and stored for 3 days at 4°C. The tissue was embedded in freezing media (Triangle Biomedical Sciences, Durham, NC), sectioned coronally at 30 μm on a cryostat, then mounted on microscope slides (Fisher Scientific, Pittsburgh, PA) and stored at −80°C. Slides were warmed at 37°C for 20 minutes, and the tissue was blocked in TBS + 0.1% Triton X-100, 0.5% BSA, and 10% normal donkey serum for 1 hour at room temperature and then incubated overnight at 4°C with primary antibodies in blocking buffer, followed by incubation in secondary antibodies at room temperature for 1 hour. TRITC (1:200)-, FITC (1:200)-, CY5 (1:200)-, or AMCA (1:100)-conjugated secondary antibody F(ab’) fragments (all from donkey) and normal donkey serum (017-000-121) were purchased from Jackson Immunoresearch (West Grove, PA). Negative controls included appropriate species-specific, non-immune IgGs instead of primary antibodies.
All images were captured with a Nikon TE 300 inverted microscope equipped with a Spot CCD camera using identical exposure settings. Elements software (Nikon, Melville, NY) was used to threshold baseline brightness and contrast identically for each image. Immunostaining was carried out for detecting the presence of activated astrocytes (Gfap; ab4674; Abcam; Cambridge, MA), microglia (Iba-1; 019,19741; Wako; Richmond, VA), neurons (NeuN; ABN90P; Millipore; Temecula, CA) and the pro-inflammatory cytokine cyclooxygenase-2 (Cox-2; 160126; Cayman Chemical; Ann Arbor, MI). All images were captured with a Nikon TE 300 inverted microscope equipped with a Spot CCD camera using identical exposure settings. For immunohistochemical quantitation, at least five 20 μm hippocampal sections per brain were stained and photographed with a 10X objective. Elements software was used to threshold baseline brightness and contrast identically for each image for all quantitative object measurements. The positively stained areas within the hippocampus were quantified by a scorer blinded to genotype or treatment group. To assess the presence of inflammatory markers, the total area within the hippocampus positive for either Gfap, Cox-2, or Iba-1 was quantified by digital image analysis using the basic densitometric thresholding features of Elements software, similar to methods previously reported (Donnelly et al., 2009). Threshold values were obtained and set for each marker and held constant for each image quantified. Measurements of brain cAMP levels. Brain tissues were homogenized in 0.1N HCl and assayed according to the manufacturer instructions. cAMP ELISA kit was purchased from Enzo Life Sciences (Farmingdale, NY).
2.7. Cytokine measurement
Brain tissues were homogenized in a buffer containing 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L, PMSF, 0.05% Tween-20, and a cocktail of protease inhibitors (Sigma) (Fox et al., 2005). Il-1β, MCP-1, Il-17, and Tnfα levels were quantified using customized Milliplex® MAP Kit (Cat # MPXMCYTO-70K, Millipore) and Luminex100 reader (Luminex Corp., Austin, TX 78727, USA) Endotoxin assay. Serum endotoxin levels were measured using Limulus Amebocyte Lysate test kit (Lonza, Walkersville, MD) according to the manufacturer’s instructions.
2.8. Soluble CD14 ELISA
Serum samples were collected after centrifugation, and quantified using sCD14 ELISA (R&D systems, Minneapolis, MN) kit in accordance with the manufacturer’s instructions.
3. Statistical analysis
Statistical analysis was performed using GraphPad Prism SoftwareStatistical significance was calculated using one-way ANOVA followed by Bonferroni’s post-test and the Student t test. For IHC quantitation, a one-way ANOVA assuming unequal variance was performed followed by post-hoc Tukey HSD t-tests. Spearman correlation analysis was used to assess the correlations between endotoxin levels and brain inflammatory cytokines. Data are presented as the mean ± standard deviation (SD). P<0.05 was considered significant.
4. Results
4.1. Alcohol consumption induces systemic endotoxemia and brain inflammation
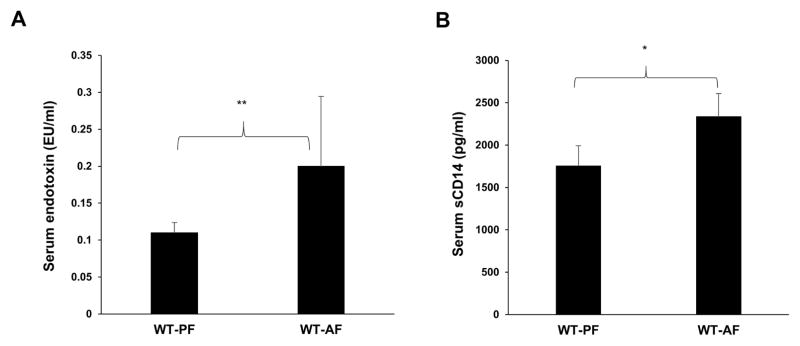
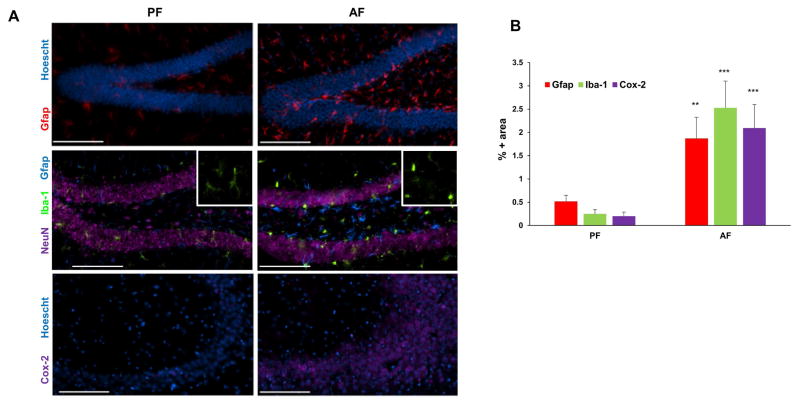
We examined serum endotoxin levels for assessment of gut barrier function and soluble CD14 levels for gut microbial translocation and systemic monocyte/macrophage activation (Pilakka-Kanthikeel et al., 2012) in a well-established mouse model of chronic alcohol feeding (Bull-Otterson et al., 2013; Kirpich et al., 2012). In comparison to control animals pair-fed with isocaloric maltose dextrin (PF), alcohol feeding (ethanol 5% w/v) for 1 week led to a significant increase in serum endotoxin levels (Fig. 1A). Further, following the increase in endotoxin, an increase in serum sCD14 levels indicative of gut barrier dysfunction and systemic immune activation, was observed after 2 weeks of chronic alcohol feeding (Fig. 1B). Concomitant brain inflammation was assessed by examining glial cell activation utilizing immunohistochemical (IHC) analysis. A robust activation of astrocytes and microglia was observed in the hippocampal dentate as indicated by Gfap and Iba-1 expression, respectively. This was accompanied by morphological changes from resting, ramified microglia to condensed and activated microglia (Fig. 2A, inset). We further evaluated the effect of alcohol on Cox-2 expression, a generalized marker for brain inflammation. Concomitant with cell activation, there was a significant upregulation of Cox-2 expression in the hippocampal CA3 region of AF-mice compared to PF mice (Fig. 2). These differences were representative of an increase in these inflammatory markers throughout the brain (data not shown). These data suggest that chronic alcohol consumption-mediated gut/systemic changes are linked with the development of brain inflammation.
Figure 1.
Alcohol increases systemic endotoxin and sCD14 levels in mice. A, Serum endotoxin levels were measured using LAL assay 1 week after starting 5% alcohol and B, Serum sCD14 levels were measured using sCD14 ELISA kit after 2 weeks of starting 5% alcohol. Data are presented as means and S.D. (n=5–7 in each group). *P < 0.05, **P<0.01.
Figure 2.
Alcohol induces glial activation and neuro-inflammation. A, Representative images of the inflammatory state of brains of their respective treatment groups: astrocytic activation marker Gfap (red, hippocampus-dentate gyrus), microglial marker Iba-1 (green, hippocampus—dentate gyrus) and inflammatory marker Cox-2 (purple, hippocampus-CA3 region), in the brains of PF and AF mice after two weeks of feeding. Hoescht (blue) and NeuN (purple) staining are used as nuclear and neuronal markers, respectively. Bar = 100 μm. B, Quantitation of IHC staining. The percentage of image area positive for Gfap, Iba-1, and Cox-2 are significantly higher (**p<0.01, ***p<0.001) in alcohol-fed mice (AF) relative to control pair-fed animals (PF). Data are means ± SD (n = 5–7 in each group).
4.2. Alcohol consumption increases Pde4b expression and decreases cAMP levels in the brain
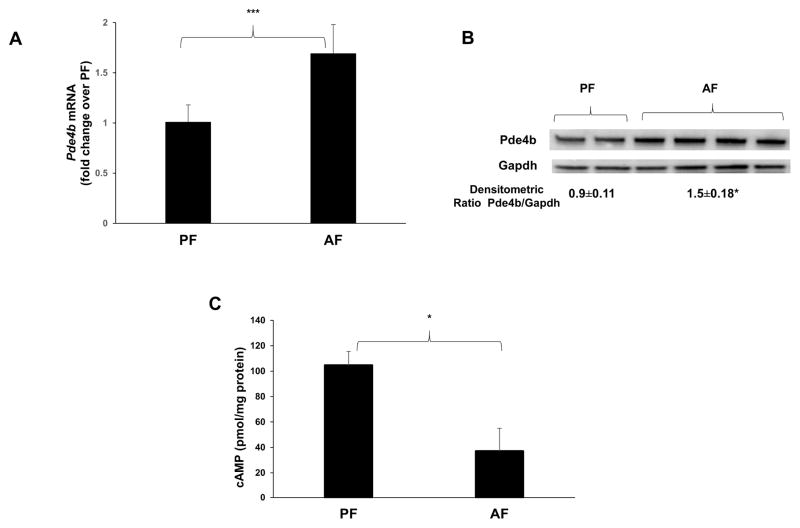
Elevated systemic endotoxin in mice and rats significantly increases Pde4b expression in brain and is characterized by an increase in Cox-2 expression (Johansson et al., 2011; Reyes-Irisarri et al., 2008). Real time qPCR and Western blot analyses of brain tissue showed a significant increase in Pde4b expression in alcohol fed mice as compared to PF mice (Fig. 3A, B). Importantly, the increase in Pde4b was accompanied by a significant decline in cAMP levels in the brain homogenates of AF mice (Fig. 3C).
Figure 3.
Alcohol increases Pde4b expression and decreases cAMP levels in the brain. A, mRNA levels of brain Pde4b quantified by real time qPCR and B, Immunoblot analysis of brain homogenates from WT mice that were PF and AF for 1 week. (n = 5–7 in each group). C, brain cAMP levels measured by using cAMP ELISA kit were normalized by protein content. Data are presented as means ± SD (n = 5–7 in each group). *P < 0.05.
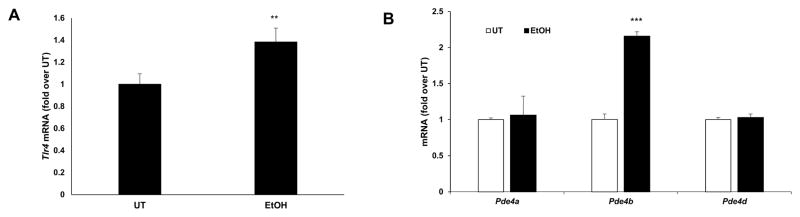
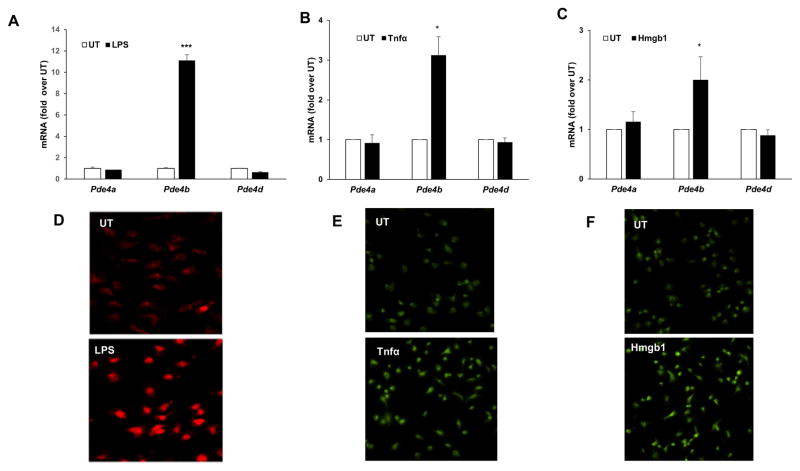
Our earlier work has demonstrated that alcohol and LPS-inducible inflammatory cytokine production in peripheral monocyte/macrophages is critically regulated by increased Pde4 expression and the consequent decrease in cellular cAMP (Gobejishvili et al., 2008; Gobejishvili et al., 2006). Microglia are the brain resident macrophages and play a major role in the development of brain inflammation and pathology (Ransohoff, 2016; Sasaki, 2016). Accordingly, the effect of inflammatory mediators relevant to alcohol-induced brain inflammation, ethanol, LPS, Tnfα and Hmgb1, on microglial Pde4 expression was examined. Neonatal cortical microglial cells were treated with ethanol (25 mM; 48 h), LPS (100 ng/ml; 4 h), Tnfα (20 ng/ml, 90 minutes) and Hmgb1 (10 μg/ml, 90 minutes). Ethanol treatment of primary microglia cells increased Tlr4 and Pde4b mRNA expression (Fig. 4). More importantly, all other inflammatory mediators also resulted in a significant induction of Pde4b mRNA expression with a minimal to no change in Pde4a and d (Fig. 5A–C). Further, induction of Pde4b protein expression in microglial cells was also confirmed by IHC analyses (Fig. 5D–F). Taken together, these data suggest that Pde4b is the predominant Pde4 subfamily member involved in brain cAMP metabolism and inflammation that occurs in response to chronic alcohol consumption and peripheral endotoxemia and inflammatory changes.
Figure 4.
Ethanol-mediated increase of Tlr4 and Pde4b mRNA expression in primary microglial cells. mRNA levels of Tlr4, Pde4a, b and d were quantified by real time qPCR in primary microglia cells treated with 50 mM ethanol (EtOH) for 24 hours. Data are presented as mean ± SD from 3 independent experiments, ** P<0.01 and *** P<0.001 compared UT.
Figure 5.
Increase of Pde4b expression in primary microglial cells in response to LPS, Tnfα and Hmgb1. mRNA levels of Pde4a, b and d were quantified by real time qPCR in A, cells treated with LPS, 100 ng/ml for 90 minutes. B, cells treated with recombinant Tnfα, 20 ng/ml for 90 minutes. C, cells treated with recombinant Hmgb1, 10 μg/ml for 90 minutes. Data are presented as mean ± SD from 3 independent experiments, *P<0.05, and *** P<0.001 compared UT. Representative images of IHC for Pde4b protein in D, cells treated with LPS, 100 ng/ml for 3 hours, E, in cells treated with Tnfα, 20 ng/ml for 3 hours, F, in cells treated with Hmgb1, 10 μg/ml for 3 hours.
4.3. Pharmacological inhibition or genetic knockout of Pde4b abrogates alcohol-induced glial activation and inflammation in the brain
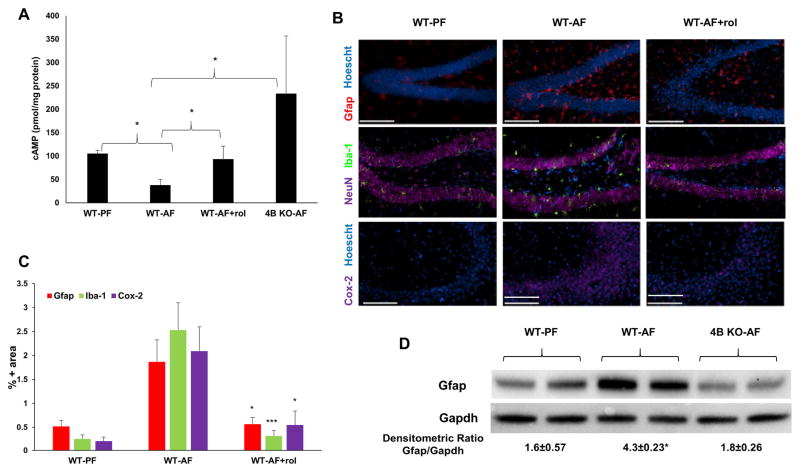
The causal role of alcohol-induced brain Pde4b expression in glial cell activation and brain inflammation was examined by employing both pharmacological and genetic approaches that inhibit Pde4b. For the pharmacological inhibition of Pde4b, the blood brain barrier (BBB) permeable Pde4-specific inhibitor rolipram was administered i.p. (5 mg/kg; 3 times/week), along with chronic alcohol feeding. The pathogenic role of Pde4b was also determined by examining the effect of chronic alcohol administration in Pde4b−/− mice. Neither rolipram treatment nor genetic ablation of Pde4b had any significant effect on consumption of alcohol-containing diet and blood alcohol levels (Avila et al., 2016). The efficacy of Pde4 inhibition by both approaches was assessed by evaluating their effect on the brain cAMP levels. The alcohol-induced decrease in brain cAMP levels was prevented in Pde4b−/− as well as WT animals treated with rolipram (Fig. 6A). These data strongly support the notion that alcohol-induced Pde4b expression plays a contributory role in the decrease of brain cAMP levels.
Figure 6.
Pde4 inhibition prevents alcohol-induced effects on cAMP and inflammation in the brain. A, Brains from PF and AF mice were lysed, and cellular cAMP levels were measured. Obtained cAMP values were normalized by protein content. Data are presented as means ± SD (n=5–7 in each group). *P < 0.05. B, Cox-2, Gfap and Iba-1 expression in mouse brains. Images are representative of the inflammatory state of the whole brain of their respective treatment groups. (n = 5–7 in each group). C, Quantitation of IHC staining. The percentage of image area positive for Gfap, Iba-1, and Cox-2 are significantly lower (*p<0.05, ***p<0.001) in rolipram-treated alcohol-fed mice (WT-AF+rol) relative to alcohol-fed animals (WT-AF). (n = 5–7 in each group). Bar = 100 μm. Data are means ± S.D. D, Immunoblot analysis of brain Gfap of WT and Pde4b−/− mice fed alcohol for 2 weeks. *p<0.05 compared to WT-PF and 4B KO-AF.
Further, in the context of the regulatory role of Pde4 expression in glial cell activation, IHC analysis was performed on the brain tissue obtained from alcohol-fed animals with and without rolipram treatment. Significantly, there was a marked attenuation of alcohol-induced Gfap, Cox-2 and Iba-1 expression by rolipram (Fig. 6B). Additionally, Western blot analysis of brain homogenates from WT and Pde4b−/− animals showed a complete inhibition of alcohol-induced Gfap expression that was seen in WT animals (Fig. 6C). Overall, these data strongly support the causal role of Pde4b expression in glial cell activation induced by chronic alcohol consumption.
4.4. Pde4b−/− mice do not exhibit glial activation in response to endotoxin administration
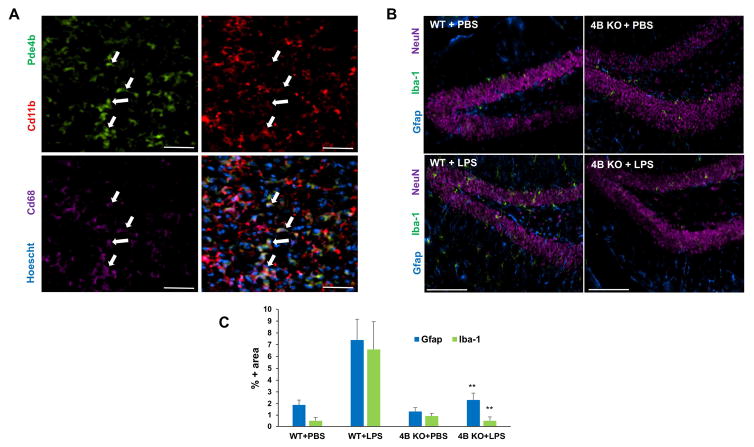
To validate the causal relationship between systemic endotoxemia in alcohol-induced brain Pde4b expression and inflammation, we examined the effect of intraperitoneal administration of LPS in WT and Pde4b−/− mice. WT and Pde4b−/− mice were injected with 5 mg/kg LPS and brain tissues were examined at 6 h post LPS injection for Pde4b expression and glial cell activation by IHC analysis. Immunohistochemical staining for Pde4b (green), Cd11b (red) and Cd68 (violet) of brain tissues of WT mice after LPS administration showed that Pde4b is localized in activated macrophage/microglia cells (Fig. 7A, bottom right corner). Compared to WT mice, LPS mediated activation of glial cells was markedly attenuated in Pde4b−/− mice, as indicated by Gfap+ astrocytes and Iba1+ microglia (Fig. 7B). These data demonstrate that systemic endotoxin induced glial activation and brain inflammation is mediated by Pde4b expression. Further, these data also strongly support the notion that chronic alcohol-induced systemic endotoxemia drives Pde4b expression which critically regulates astrocyte and microglia activation and brain inflammation.
Figure 7.
Pde4b−/− mice are protected from endotoxin-induced glial activation. A, IHC staining for Pde4b (green), Cd11b (red) and Cd68 (violet) demonstrating the localization of Pde4b in macrophage/microglia of WT mice 6 h after LPS administration. Bar = 50 μm. B, Expression of CD11b/c, Gfap, and NeuN in the dentate gyrus. C, Iba-1 immunostaining is up-regulated within 6 hours of LPS exposure. Images are representative of the inflammatory state of the whole brains of their respective treatment groups. C, Quantitation of IHC staining. The percentage of image area positive for Iba-1 and Gfap are significantly lower (**p<0.01) in LPS-injected Pde4b−/− mice (4B KO+LPS, n=4) relative to LPS-injected WT mice (WT+LPS, n=4). Data are means ± S.D.
4.5. Pde4 inhibition attenuates the alcohol-induced pro-inflammatory cytokine production in the brain parenchyma
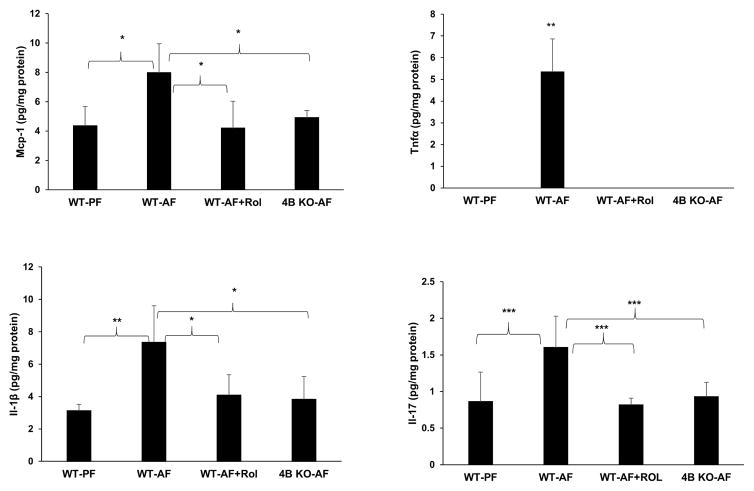
To further assess the contributory of role of Pde4b in alcohol-induced inflammatory response in the brain, the effect of Pde4b inhibition on pro-inflammatory cytokine production in the brain parenchyma was examined. Commensurate with the increased activation of glial markers, there was a significant increase in inflammatory cytokines Tnfα, Il-1β, Il-17 and inflammatory chemokine Mcp-1 in the brain tissue of WT alcohol fed mice (Fig. 8). Additionally, in view of the potential contribution of alcohol-induced systemic endotoxemia, we examined the correlation of serum endotoxin levels and brain inflammatory cytokines using Spearman correlation analysis. This correlation analysis that measures the strength and direction of association between two ranked variables showed a strong positive correlation between endotoxin levels and two out of four inflammatory cytokines Mcp-1 and Il-17 in alcohol fed mice (r=0.857, p=0.024 for Mcp-1 and r=0.821, p=0.034 for Il-17). These analyses indicate the potential role of alcohol-induced endotoxemia in inducing brain inflammatory cytokines. Notably, in contrast, alcohol fed Pde4b−/− and rolipram-treated mice showed a marked attenuation of all the inflammatory cytokines and chemokines, supporting the role of Pde4b in regulating alcohol-induced brain inflammation (Fig. 8).
Figure 8.
Pde4 inhibition prevents alcohol-induced increases in neuro-inflammatory cytokines/chemokines. Mcp-1, Tnfα, Il-1β and Il-17 were measured in brain homogenates of wild type PF, AF, AF with rolipram treatment and Pde4b−/- mice. Obtained values were normalized by protein content. Data are presented as mean ± S.D (n = 5–7 in each group). *P<0.05, **P<0.01, ***P<0.01.
5. Discussion
Significant advances have been made in understanding the deleterious effects of alcohol consumption on individual organ systems such as brain, liver, immune, cardiovascular, and endocrine systems. However, the pathogenic links between different organ systems in the presence of alcohol are only beginning to be investigated. Emerging data suggest that these interactions could provide important insights into the mechanism(s) by which alcohol-induced pathology in one organ influences the function of other organs. This study examined the development of brain inflammation in the context of chronic alcohol consumption and systemic endotoxemia-associated events.
Present results showed that alcohol feeding significantly increased serum endotoxin levels. More importantly, this rise in endotoxin levels was accompanied by elevated serum sCD14 (Fig. 1). Increased release of sCD14 from monocytes is observed in response to stimulation with endotoxin and other microbial antigens and therefore is considered as a marker for activation of monocytes/macrophages (Bazil and Strominger, 1991; Bufler et al., 1995; Dauphinee and Karsan, 2006; Durieux et al., 1994; Shive et al., 2015). These results indicate that alcohol feeding led to gut barrier dysfunction and translocation of microbial products resulting in activation of the systemic innate immune response.
Concomitant with increased endotoxemia and activation of innate responses, alcohol induced brain inflammatory changes as demonstrated by glial cell activation (Fig. 2). These findings suggest that chronic alcohol-induced peripheral endotoxemia and inflammation are linked to the subsequent development of brain inflammation. Concerning the systemic inflammatory changes induced by alcohol, our earlier data showed that alcohol induces Pde4 expression in circulating, as well as hepatic, monocytes/macrophages (Gobejishvili et al., 2008). Moreover, increased Pde4 expression plays a critical role in the priming effects of alcohol leading to induction and exacerbation of LPS-inducible inflammatory cytokine expression. Analogous to the peripheral inflammatory responses and cytokine production, alcohol induces Tlr4 and Pde4b expression in microglial cells as well as total brain homogenates (Figs. 3, 4). Interestingly, alcohol treatment specifically affected the expression of Pde4b, with minimal to no effect on Pde4a and d sub-family members. These findings are particularly relevant in the context of the direct effects of alcohol on glial cell activation. Significantly, these data are also in agreement with the direct activating effects of alcohol on glial cells that occur via TLR4 signaling, as observed previously (Alfonso-Loeches et al., 2010; Blanco and Guerri, 2007; Fernandez-Lizarbe et al., 2009; Pascual et al., 2011). Although, LPS is a strong inducer of Pde4b expression in peripheral monocytes/macrophages (Banks and Robinson, 2010; Gobejishvili et al., 2011; Jin and Conti, 2002; Jin et al., 2005), very little LPS gains access to the brain due to the poor passage through the BBB. Hence, the alcohol-induced systemic endotoxemia is likely to play an indirect role in inducing brain Pde4b expression and inflammation via triggering of systemic cytokines such as Tnfα. Indeed, Tnfα and other cytokines induce Pde4b expression and inflammatory changes in glial cells (Ghosh et al., 2012). In view of this, peripheral TNF could initiate Pde4b and cytokine expression in the glial cells. Moreover, TNF and cytokines produced by glial cells could further induce and sustain brain Pde4b expression perpetrating alcohol-induced brain inflammation. Besides cytokines, Pde4b expression could be induced via activation of Tlrs, particularly Tlr4 in glial cells. In this regard, our results show that alcohol increases Tlr4 expression in microglial cells and this increase is accompanied by an elevation of Pde4b expression (Fig. 4). Activation of Tlr4 on glial cells and induction of Pde4b expression could also be driven by localized DAMPs such as Hmgb1, which are produced during alcohol-induced neuro-inflammation and injury and can act as ligands and activate Tlr4 signaling (Bianchi, 2009; Crews et al., 2012; Lippai et al., 2013). Taken together, in the context of brain inflammatory changes, these data suggest that alcohol along with gut-derived endotoxemia and systemic inflammation likely initiate the induction of Pde4b expression in the brain parenchyma which can be further sustained by glial cell activation and neuronal injury in the alcohol fed animals.
The functional consequence of alcohol-induced brain Pde4b expression is demonstrated by a substantial decline in cAMP levels (Fig. 3B). cAMP helps to maintain immune homeostasis by suppressing the release of pro-inflammatory mediators (e.g., Tnfα, Il-17, and Ifnγ) and promoting the release of anti-inflammatory mediators (e.g., Il-10) by immune cells (Zidek, 1999). Hence, similar to our observations related to systemic/hepatic inflammatory responses (Gobejishvili et al., 2008; Gobejishvili et al., 2006), the induction of Pde4 expression and the subsequent decrease in cAMP levels occurring in response to chronic alcohol consumption likely plays a key regulatory role in glial cell activation and inflammatory cytokine expression in the brain. Notably, the causal role of Pde4b expression/activity in alcohol-induced glial cell activation and brain inflammation was strongly supported by using the pharmacologic inhibitor, rolipram, as well as Pde4b−/− mice. Specifically, the lack of Pde4b expression in Pde4b−/− mice as well as the inhibition of its activity by rolipram prevented the alcohol-induced decrease in brain cAMP levels. These data strongly suggest that alcohol-induced alterations and brain cAMP homeostasis are predominantly regulated by Pde4b expression. Significantly, inhibition of Pde4 expression/activity and prevention of the decline in cAMP levels also markedly attenuated glial cell activation and brain inflammatory cytokine production (Fig. 8). The attenuating effect of Pde4 inhibition on alcohol-induced brain inflammation could be occurring (i) indirectly, via suppression of systemic inflammation as well as (ii) directly, via suppression of inflammatory cytokine expression by activated glial cells. Moreover, since inflammatory cytokines can further activate and sustain Pde4b expression in glial cells, Pde4 inhibition could also impact the autocrine loop of Pde4b activation, inflammatory signaling and cytokine production.
6. Conclusions
Current data identifies and establishes that an alcohol-mediated increase in Pde4b expression plays a critical pathogenic role in alcohol-induced neuro-inflammation. In relevance to clinical applications, these data not only delineate the mechanistic role of Pde4b, but also demonstrate that it is a significant therapeutic target for alcohol-induced neuro-inflammation and neurologic diseases.
Highlights.
Chronic alcohol consumption induces peripheral endotoxemia and inflammation.
Chronic alcohol increases brain Pde4b expression and decreases cAMP levels.
Chronic alcohol induces activation of glial cells and neuro-inflammation.
Pharmacologic inhibition of Pde4b and Pde4b gene knockout, markedly attenuate alcohol-induced glial cell activation and brain inflammatory cytokine expression.
First report on the critical role of cAMP-specific Pde4b in alcohol-induced neuro-inflammation.
Targeting Pde4b expression/activity is a potential therapeutic strategy to mitigate alcohol-induced neuro-inflammation.
Acknowledgments
This work was supported by grants from National Institutes of Health: U01AA022618 (SB), R21AA022189 (LG), U01AA021901 (CJM), P50AA024337 (CJM), P20GM113226 (CJM), and GM103507 (SRW).
Footnotes
Conflict of Interest: The authors declare no competing financial interests
Chemical compounds
Rolipram (C16H21NO3)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila DV, Barker DF, Zhang J, McClain CJ, Barve S, Gobejishvili L. Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis. J Pathol. 2016;240:96–107. doi: 10.1002/path.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav Immun. 2010;24:102–109. doi: 10.1016/j.bbi.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazil V, Strominger JL. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–1574. [PubMed] [Google Scholar]
- Bianchi ME. HMGB1 loves company. J Leukoc Biol. 2009;86:573–576. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–2630. doi: 10.2741/2259. [DOI] [PubMed] [Google Scholar]
- Bufler P, Stiegler G, Schuchmann M, Hess S, Kruger C, Stelter F, Eckerskorn C, Schutt C, Engelmann H. Soluble lipopolysaccharide receptor (CD14) is released via two different mechanisms from human monocytes and CD14 transfectants. Eur J Immunol. 1995;25:604–610. doi: 10.1002/eji.1830250244. [DOI] [PubMed] [Google Scholar]
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, Kong M, Barker D, McClain C, Barve S. Metagenomic Analyses of Alcohol Induced Pathogenic Alterations in the Intestinal Microbiome and the Effect of Lactobacillus rhamnosus GG Treatment. PLoS One. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R, de Vellis J. Preparation of Astrocyte, Oligodendrocyte, and Microglia Cultures from Primary Rat Cerebral Cultures. In: Fedoroff S, Richardson A, editors. Protocols for Neural Cell Culture. Humana Press, Inc; Totowa, NJ: 2001. pp. 117–127. [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High Mobility Group Box 1/Toll-like Receptor Danger Signaling Increases Brain Neuroimmune Activation in Alcohol Dependence. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Sarkar DK, Qin L, Zou J, Boyadjieva N, Vetreno RP. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol Res. 2015;37:331–341. 344–351. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl) 2016;233:1543–1557. doi: 10.1007/s00213-015-3906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods. 2009;181:36–44. doi: 10.1016/j.jneumeth.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux JJ, Vita N, Popescu O, Guette F, Calzada-Wack J, Munker R, Schmidt RE, Lupker J, Ferrara P, Ziegler-Heitbrock HW, et al. The two soluble forms of the lipopolysaccharide receptor, CD14: characterization and release by normal human monocytes. Eur J Immunol. 1994;24:2006–2012. doi: 10.1002/eji.1830240911. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Floreani NA, Rump TJ, Abdul Muneer PM, Alikunju S, Morsey BM, Brodie MR, Persidsky Y, Haorah J. Alcohol-induced interactive phosphorylation of Src and toll-like receptor regulates the secretion of inflammatory mediators by human astrocytes. J Neuroimmune Pharmacol. 2010;5:533–545. doi: 10.1007/s11481-010-9213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, Ferriero DM, Vexler ZS. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25:1138–1149. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Aguirre V, Wai K, Felfly H, Dietrich WD, Pearse DD. The interplay between cyclic AMP, MAPK, and NF-kappaB pathways in response to proinflammatory signals in microglia. Biomed Res Int. 2015;2015:308461. doi: 10.1155/2015/308461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Garcia-Castillo D, Aguirre V, Golshani R, Atkins CM, Bramlett HM, Dietrich WD, Pearse DD. Proinflammatory cytokine regulation of cyclic AMP-phosphodiesterase 4 signaling in microglia in vitro and following CNS injury. Glia. 2012;60:1839–1859. doi: 10.1002/glia.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobejishvili L, Avila DV, Barker DF, Ghare S, Henderson D, Brock GN, Kirpich IA, Joshi-Barve S, Mokshagundam SP, McClain CJ, Barve S. S-adenosylmethionine decreases lipopolysaccharide-induced phosphodiesterase 4B2 and attenuates tumor necrosis factor expression via cAMP/protein kinase A pathway. J Pharmacol Exp Ther. 2011;337:433–443. doi: 10.1124/jpet.110.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobejishvili L, Barve S, Joshi-Barve S, McClain C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2008;295:G718–724. doi: 10.1152/ajpgi.90232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2006;291:G681–688. doi: 10.1152/ajpgi.00098.2006. [DOI] [PubMed] [Google Scholar]
- Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175:1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- Johansson EM, Sanabra C, Cortes R, Vilaro MT, Mengod G. Lipopolysaccharide administration in vivo induces differential expression of cAMP-specific phosphodiesterase 4B mRNA splice variants in the mouse brain. J Neurosci Res. 2011;89:1761–1772. doi: 10.1002/jnr.22707. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013;94:171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S, Guerri C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol Clin Exp Res. 2016;40:2260–2270. doi: 10.1111/acer.13208. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun. 2011;25(Suppl 1):S80–91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Pilakka-Kanthikeel S, Huang S, Fenton T, Borkowsky W, Cunningham CK, Pahwa S. Increased gut microbial translocation in HIV-infected children persists in virologic responders and virologic failures after antiretroviral therapy. Pediatr Infect Dis J. 2012;31:583–591. doi: 10.1097/INF.0b013e31824da0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- Reyes-Irisarri E, Perez-Torres S, Miro X, Martinez E, Puigdomenech P, Palacios JM, Mengod G. Differential distribution of PDE4B splice variant mRNAs in rat brain and the effects of systemic administration of LPS in their expression. Synapse. 2008;62:74–79. doi: 10.1002/syn.20459. [DOI] [PubMed] [Google Scholar]
- Sasaki A. Microglia and brain macrophages: An update. Neuropathology. 2016 doi: 10.1111/neup.12354. [DOI] [PubMed] [Google Scholar]
- Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS. 2015;29:1263–1265. doi: 10.1097/QAD.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Lippai D. Converging actions of alcohol on liver and brain immune signaling. Int Rev Neurobiol. 2014;118:359–380. doi: 10.1016/B978-0-12-801284-0.00011-7. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Current hypotheses on the mechanisms of alcoholism. Handb Clin Neurol. 2014;125:477–497. doi: 10.1016/B978-0-444-62619-6.00027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidek Z. Adenosine - cyclic AMP pathways and cytokine expression. Eur Cytokine Netw. 1999;10:319–328. [PubMed] [Google Scholar]