Abstract
PURPOSE
Mother’s Day (May) is a holiday with substantial demand for flowers, associated with heightened flower production and escalated pesticide use. The effect of spray seasons on pesticide exposures of children living in agricultural communities but who do not work in agriculture is poorly understood. In this study, we estimated the association of time after Mother’s Day harvest with children’s acetylcholinesterase (AChE) activity. AChE is a physiological marker of organophosphate/carbamate pesticide exposures that may take up to 3 months to normalize after its inhibition.
METHODS
We examined 308 children, aged 4–9 years, in Ecuadorian agricultural communities during a low flower-production season but within 63–100 days (mean: 81.5 days, SD: 10.9) after Mother’s Day harvest. We quantified AChE activity (mean: 3.14 U/mL, SD: 0.49) from a single finger-stick sample.
RESULTS
We observed positive linear associations between time after the harvest and AChE among participants living near plantations. The associations were strongest among participants living within 233m (0.15 U/mL [95%CI: 0.02, 0.28]), slightly weaker among participants living within 234–532m (0.11 U/mL [0.00, 0.23]), and not associated among participants at greater distances. Similar findings were observed across categories of areas of flower plantations within 500 m of homes.
CONCLUSIONS
These cross-sectional findings suggest that a peak pesticide use period can decrease AChE activity of children living near plantations. These seasonal pesticide exposures could induce short- and long-term developmental alterations in children. Studies assessing exposures at multiple times in relation to pesticide spray seasons among children who do not work in agriculture are needed.
Keywords: pesticides, organophosphate, cholinesterase, agriculture, children, Mother’s Day
Introduction
The seasonal nature of agricultural work may bring populations in rural settings into increased contact with pesticides during peak pesticide-use periods. Studies among agricultural workers have found that pesticide spray periods are associated with increased pesticide exposure biomarkers and inhibition of acetylcholinesterase (AChE) activity (Peiris-John et al. 2005; Crane et al. 2013; Strelitz et al. 2014; Krenz et al. 2015; Singleton et al. 2015; Quandt et al. 2015), a physiological biomarker of exposure to cholinesterase inhibitor pesticides (i.e. organophosphate and carbamate pesticides) (Makhaeva et al. 2009).
Children and other non-agricultural workers living in agricultural communities may also have an elevated risk of exposure to pesticides during pesticide spray seasons; however, very few studies to date have assessed this in children who do not work in agriculture (Crane et al. 2013; Thompson et al. 2014; Galea et al. 2015) and, to our knowledge, only one study with a limited number of such children has assessed AChE activity as an outcome (Crane et al. 2013). In the latter study elevated urinary markers of organophosphate and cholinesterase depressions were observed during the pesticide spray season compared to baseline (Crane et al. 2013). In the former studies, high spray seasons, compared to low spray seasons, were associated with greater urinary metabolite concentrations of organophosphates among children living with agricultural workers (Thompson et al. 2014), whereas children not living with agricultural workers but living within agricultural communities were not found to have differences in urinary pesticide metabolites concentrations between the low and high pesticide spray seasons (Thompson et al. 2014; Galea et al. 2015). The limited number of studies on this topic highlights the importance of conducting these studies in general populations of children living in agricultural settings.
Some routes of exposure of children who do not work in agriculture include off-target drift from pesticide application sites and take-home pathways from farm workers. This has been shown in multiple US studies that have measured pesticide levels in house dust, urine and skin swabs in relation to residential proximity to plantations and presence of farmworkers at home (Simcox et al. 1995; Loewenherz et al. 1997; Lu et al. 2000; McCauley et al. 2001; Curl et al. 2002; Hogenkamp et al. 2004; Quandt et al. 2004; Curwin et al. 2005; Ward et al. 2006; Weppner et al. 2006; Ramaprasad et al. 2009).
Many organophosphate pesticides irreversibly inhibit AChE activity, resulting in build-up of the neurotransmitter, acetylcholine, active in the autonomic nervous system, at neuromuscular junctions, and as a neuromodulator in the brain. Irreversible inhibition of erythrocytic AChE can last for approximately 80 days (Mason 2000), until a sufficient number of erythrocytes have been turned over to return activity to pre-exposure levels. Exposures to cholinesterase inhibitor pesticides have been consistently associated with cognitive and neurobehavioral deficits in children in various studies (Kofman et al. 2006; Eskenazi et al. 2007, 2014; Rauh et al. 2011; Suarez-Lopez et al. 2013).
In the present study, we evaluated AChE activity in relation to time after the Mother’s Day flower harvest among children who participated in the Secondary Exposure to Pesticides among Children and Adolescents (ESPINA: Exposición Secundaria a Plaguicidas en Niños y Adolescentes [Spanish]) study. Children of this study did not work in agriculture but lived in agricultural communities in Pedro Moncayo county, Ecuador. We hypothesized that, as a result of pesticide exposures, AChE activity would be lower in children examined sooner after Mother’s Day compared to children examined later.
Production of roses and other flowers in Pedro Moncayo county increases substantially between October and May to meet the heightened demand of flowers for holidays including Christmas (December 25), Valentine’s Day (February 14), Easter (March/April) and Mother’s Day (May). After the Mother’s Day harvest, production slows substantially during the summer months. Because there are strict no-tolerance policies for the importation of crops with pests in many countries including the US (U.S. Department of Agriculture 2012), the use of pesticides in floriculture continues until soon before the harvest (Narvaez et al. 2002; Harari 2004). The floriculture industry in Pedro Moncayo County employs 21% of adults (Suarez-Lopez et al. 2012) and occupies 5.8% (1800 hectares) of the land (Gobierno Municipal del Canton Pedro Moncayo 2011). The Ecuadorian floriculture industry uses many different pesticides, including fungicides, herbicides and various classes of insecticides, which are sprayed by workers using hand sprayers. The pesticide use reports of 7 flower plantations in Pedro Moncayo County in 2008, as required for their yearly permit of operation, included 23 different insecticides, including the following cholinesterase inhibitor pesticides: carbamates (methiocarb, carbofuran, aldicarb) and organophosphates (diazinon, dimethoate, acephate, chlorpyrifos). Other reported pesticides included over 50 different fungicides.
Methods
In 2008 as part of the ESPINA study, we examined 313 4- to 9-year-old children and interviewed their parents or guardians. Most children (73%) were recruited from past participation in a large and representative survey (2004 Survey of Access and Demand of Health Services in Pedro Moncayo County) conducted by Fundacion Cimas del Ecuador in collaboration with Pedro Moncayo County communities. The remaining 27% of children were newly recruited participants. The ESPINA study includes participants from all 5 parishes of Pedro Moncayo county and has similar socio-economic and racial distributions as the general population of children there. Detailed participant recruitment information has been described elsewhere (Suarez-Lopez et al. 2012). Parents provided informed consent for themselves and for their children. Participants, at least 7 years old, provided assent for participation in the study. The present analyses include information of 308 children who had complete co-variate data for this study. This study was approved by the Institutional Review Boards of Fundación Cimas del Ecuador, the University of Minnesota, the University of California San Diego, Universidad San Francisco de Quito and the Ministry of Public Health of Ecuador. The main objective of the ESPINA study is to understand the effects of pesticide exposure on children’s growth and neurobehavioral outcomes.
Participants were examined once within 100 days of the Mother’s Day harvest in 2008 (range: 63 – 100 days, mean: 84.5 days, SD: 10.8 days. AChE measurements were conducted on 20 different days with an average of 15 participants being examined each day. Exams were conducted in 7 schools distributed across the 5 parishes that make-up Pedro Moncayo County: Malchinguí, Tocachi, La Esperanza, Tabacundo and Tupigachi. Parents or guardians were interviewed at home to obtain information on socio-economic status, health, demographics and pesticide exposure histories of members of the household.
Children’s height was measured using a stadiometer to the nearest mm following standard procedures. Height-for-age z-scores were calculated using the World Health Organization normative sample (WHO Multicentre Growth Reference Study Group 2006). AChE activity and hemoglobin concentration were assessed using the EQM Test-mate ChE Cholinesterase Test System 400 (EQM research, Cincinnati, OH) from a finger-stick blood sample, following standard procedures (EQM Research 2003).
The distance from each participant’s home to the nearest flower plantation was calculated. Geographical coordinates of participant homes were collected in 2004, 2006 and 2010 by Fundacion Cimas del Ecuador as part of the System of Local and Community Information (Sistema de Información Local y Comunitario), using portable global positioning receivers. Plantation edges (areal polygons) were created by measuring the coordinates of each corner of each plantation’s perimeter. The distance of homes to the nearest 1 m segment of flower plantation edge and areas of flower plantation within a given radius from a home were calculated using ArcGIS 9.3. (Esri, Redlands, CA). We also calculated the areas of flower plantations within 500 m buffers around each participant’s home.
Statistical Analysis
For greater precision, we calculated the number of days between the approximate end of the Mother’s Day harvest (5/08/2008, 00:00 am) and the date and time of the beginning of the examination. The associations between time after the Mother’s Day harvest and AChE activity were analyzed using two multiple linear regression models defined a-priori: model 1 adjusted for a minimal number of potential confounders including age, sex, and race. In this cohort we observed that age was positively associated with AChE activity (Suarez-Lopez et al. 2012). Model 2 further accounted for chronic nutritional status and additional potential sources of pesticide exposure: model 1 + height-for-age z-score, income, distance to the nearest plantation edge and flower worker cohabitation status. In this cohort, children living with floricultural workers were found to have lower AChE activity compared to children living with non-agricultural workers (Suarez-Lopez et al. 2012). We also considered residential distance to plantations and flower worker cohabitation to be potential effect modifiers as described below. All models were further adjusted for hemoglobin concentration considering that the main outcome was erythrocytic AChE activity. It is important to account for varying red blood cell compositions of blood, as these variations can alter the values of erythrocytic AChE activity (EQM Research 2003).
We assessed effect modification by flower worker cohabitation status, sex and by 2 constructs of potential drift of pesticides from plantations onto homes: a) home distance to the nearest flower plantation, and b) areas of flower plantations within 500 m of participants’ homes. We tested statistical significance of interaction terms (Xβpredictor * Xβeffect modifier) within models 1 and 2. We then analyzed the associations stratified by categories of the significant effect modifiers. For instance, we analyzed and plotted the associations stratified by tertiles of residential distance to the nearest flower plantation, and by a 3-category variable of areas of flower plantations within 500 m of participants’ homes divided as follows: a) zero m2, b) lower median split of non-zero values, c) upper median split of non-zero values. We selected this 3-category variable over tertiles considering the substantial proportion of participants with values of zero m2 (38%). The plots consisted of adjusted means of AChE activity across time since Mother’s Day harvest, and the model 2 linear function (association) of time since Mother’s Day harvest and AChE activity. Analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC)
To include most children in multivariable analyses, we imputed missing information for race, income and residential distance to the nearest flower plantation. Monthly household income was imputed for 17 children according to 3 variables significantly associated with income: maternal education, type of housing in 2004 (i.e. house, apartment, room, shack) and building materials in 2004 (i.e. brick, adobe, wood). For children not examined in 2004, income (n=4) and residential distance to the nearest flower plantation (n=3) were imputed from a random selection of values generated from a random normal distribution based on the concurrent ESPINA mean ± standard deviation (SD) values of the corresponding variable. We created a “missing” race category to account for 14 children with missing information. Because only 5 children in this study were white and 2 were black, we incorporated these 7 children in the mestizo (mix of white and indigenous) category to improve model stability when adjusting for race.
Sensitivity Analysis
Because children examined earlier in the exam period were younger than children examined later (Table 1), we considered the possibility of potential for residual confounding of age after its adjustment in our statistical models. In our participants, we previously observed positive associations between age and AChE activity (Suarez-Lopez et al. 2012). We tested all exposure-outcome associations among a randomly-selected subset of participants with an equal distribution of age (in quartiles) across quartiles of time after Mother’s Day harvest as sensitivity analyses, using SAS 9.4 (‘surveyselect’ procedure). A total of 64 participants (21%) were excluded to achieve this “age-balanced subgroup” (n=244). The random selection of participants was conducted using SAS 9.4 (‘surveyselect’ procedure). A total of 64 participants (21%) were excluded to achieve this “age-balanced subgroup” (n=244).
Table 1.
Participant characteristics by exam date. N=308
| Days after Mother’s Day harvest (quartiles) | |||||
|---|---|---|---|---|---|
|
|
|||||
| 1st | 2nd | 3rd | 4th | P-trend | |
| N | 77 | 77 | 77 | 77 | |
| Range (days) | 63.4–76.5 | 76.6–84.5 | 84.6–95.4 | 95.5–99.6 | |
| Demographic and socio-economic status | |||||
| Age, years | 6.3 (1.5) | 6.5 (1.4) | 6.8 (1.7) | 6.8 (1.7) | 0.01 |
| Sex, male | 49% | 52% | 55% | 49% | 0.93 |
| Race, mestizo | 86% | 63% | 59% | 96% | 0.87 |
| Race, indigenous | 11% | 36% | 37% | 4% | 0.79 |
| Monthly household income a | 3.1 (0.9) | 3.6 (0.7) | 3.0 (0.6) | 2.7 (0.8) | <0.01 |
| Home distance to nearest flower plantation, m | 584.0 (458) | 293.6 (207) | 402.0 (299) | 501.5 (288) | 0.18 |
| Area of flower plantation within 500m of residence (m2) | 1880 (0, 24650) | 3239(1056, 15190) | 1797(0, 14264) | 0(0, 1470) | 0.22 |
| Flower worker cohabitation | 53% | 71% | 30% | 43% | 0.01 |
| Anthropometry | |||||
| Height-for-age z-score, SD | −1.06 (0.75) | −1.45 (1.04) | −1.38 (0.94) | −1.07 (1.03) | 0.98 |
| Hemoglobin, g/L | 12.19 (1.26) | 12.71 (.96) | 12.62 (1.19) | 13.06 (0.04) | <0.01 |
Table entries are percentage, mean (SD) or median (25th, 75th percentile)
Monthly income categories (USD): 1=0–50, 2=51–150, 3=151–300, 4=301–500, 5=501–1000, 6=>1000
Results
Participant characteristics
The mean age of children at the time of assessment was 6.6 years (SD = 1.6); 51% were male, 76% mestizo, 22% indigenous, and 49% lived concurrently with at least one floricultural worker. The overall mean height-for-age z-score was substantially lower than the WHO normative sample’s: −1.25 (SD: 0.98). The mean AChE activity was 3.14 U/mL (SD: 0.49) and the mean hemoglobin concentration was 12.6 g/L (SD: 1.16).
Children examined sooner after the Mother’s Day harvest were younger (p-trend= 0.01), had greater household income (p<0.01), were more likely to live with a floricultural worker (p=0.01), and had lower hemoglobin concentrations (p<0.01) than children examined later (Table 1). The age-balanced subgroup did achieve a similar distribution of age across quartiles of time after Mother’s Day (p=0.59), while the distributions of other covariates remained relatively unchanged compared to the full sample (Table 2).
Table 2.
Participant characteristics by exam date among a random subset of participants with similar distributions of age across days after Mother’s Day harvest. N=244
| Days after Mother’s Day harvest (quartiles) | |||||
|---|---|---|---|---|---|
|
|
|||||
| 1st | 2nd | 3rd | 4th | P-trend | |
| N | 61 | 61 | 61 | 61 | |
| Range (days) | 63.4–76.5 | 76.6–84.7 | 84.8–95.4 | 95.5–99.6 | |
| Demographic and socio-economic status | |||||
| Age, years | 6.5 (1.5) | 6.5 (1.6) | 6.9 (1.7) | 6.4 (1.5) | 0.59 |
| Sex, male | 43% | 46% | 54% | 43% | 0.96 |
| Race, mestizo | 85% | 57% | 61% | 95% | 0.93 |
| Race, indigenous | 13% | 42% | 35% | 5% | 0.97 |
| Monthly household income a | 3.2 (0.9) | 3.5 (0.6) | 3.0 (0.6) | 2.8 (0.9) | 0.03 |
| Home distance to nearest flower plantation, m | 586 (466) | 297 (217) | 405 (327) | 529 (288) | 0.45 |
| Area of flower plantation within 500m of residence (m2) | 6089 (0, 50058) | 3239(1012, 15190) | 2562(0, 14443) | 0(0, 1092) | 0.08 |
| Flower worker cohabitation | 51% | 84% | 26% | 46% | 0.02 |
| Anthropometry | |||||
| Height-for-age z-score, SD | −1.06 (0.77) | −1.60 (1.13) | −1.36 (0.88) | −1.07 (1.02) | 0.70 |
| Hemoglobin, g/L | 12.13 (1.19) | 12.79 (1.03) | 12.64 (1.26) | 13.03 (0.91) | <0.01 |
Table entries are percentage or mean (SD)
Monthly income categories (USD): 1=0–50, 2=51–150, 3=151–300, 4=301–500, 5=501–1000, 6=>1000
Time after harvest and AChE activity
The AChE difference per SD (10.8 days) of time after Mother’s Day harvest (β) was non-significant in either the complete study sample (β [95% CI]: model 1= 0.02 U/ml [−0.02, 0.06]; R2=0.42); model 2 = 0.03 U/mL [−0.02, 0.07]; R2=0.44) or the age-balanced subgroup (model 1= 0.02 U/ml [−0.03, 0.06]; R2=0.38); model 2 = 0.02 U/mL [−0.02, 0.07]; R2=0.39).
Time after harvest and AChE activity: interactions with residential distance to flower plantation
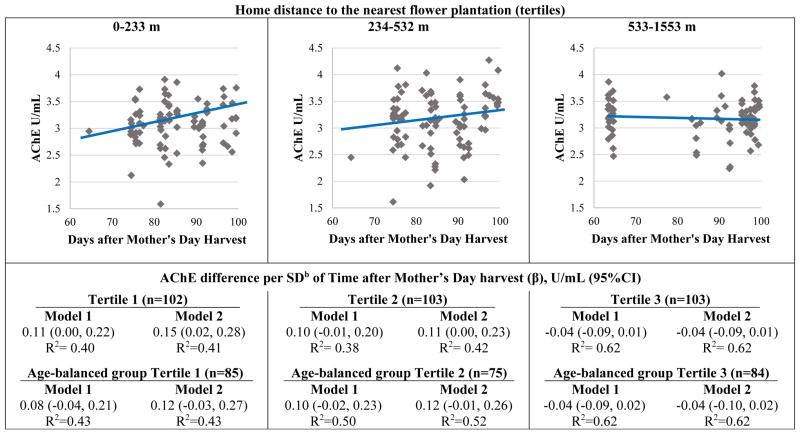
We observed significant effect modification by home distance to the nearest flower plantation in the association between time after Mother’s Day harvest and AChE activity (model 1: p=0.007, R2=0.45, model 2: pinteraction=0.002, R2= 0.46). In Table 3, we present the estimates for all covariates of model 2 including the interaction term. In models 1 and 2, AChE activity had positive linear associations with time after Mother’s Day harvest among participants living within 532 m (tertiles 1 and 2) of a flower plantation (Figure 1). The associations were strongest among participants living within 233m (tertile 1, β [95% CI]: model 2= 0.15 U/mL [0.02, 0.28]), slightly weaker among participants living within 234–532m (tertile 2: model 2= 0.11 U/mL [0.00, 0.23]), and were not significantly related among participants living at distances of 533m and greater (tertile 3, model 2= −0.04 U/mL [−0.09, 0.01]). These associations in the age-balanced subgroup were very similar.
Table 3.
Linear regression estimates of model 2 covariates in relation to AChE activity, including the interaction term between time after the Mother’s Day harvest and distances of homes to the nearest flower plantation.
| Estimate (β) | 95% CI | |
|---|---|---|
| Time after Mother’s Day (per 10.8 days) | 0.156 | (0.066, 0.247) |
| Residential distance to the nearest flower plantation (per 100 m) | 0.159 | (0.071, 0.247) |
| Time after Mother’s Day * Residential distance to nearest flower plantation (interaction term) | −0.019 | (−0.030, −0.007) |
| Male gender | 0.054 | (−0.028, 0.137) |
| Cohabitation with a flower worker (vs not) | −0.021 | (−0.107, 0.064) |
| Age (per year) | 0.037 | (0.010, 0.065) |
| Height-for-age z-score (per SD) | 0.028 | (−0.017, 0.074) |
| Income (per category a) | 0.041 | (−0.014, 0.095) |
| Indigenous race (vs not) | 0.042 | (−0.061, 0.145) |
| Hemoglobin (per mg/dL) | 0.252 | (0.213, 0.291) |
Monthly income categories (USD): 1=0–50, 2=51–150, 3=151–300, 4=301–500, 5=501–1000, 6=>1000
Intercept: −1.696 (95% CI: −2.584, −0.807)
Equation: .
Figure 1.
Children’s acetylcholinesterase (AChE) activity in relation to Mother’s Day harvest, by tertiles of home distance to the nearest flower plantationa.
The depicted regression lines correspond to model 2 including all participants stratified by tertiles. Diamonds are adjusted means of AChE activity
aInteraction with home distance to the nearest flower plantation: Pmodel 2=0.002
bSD: 10.8 days
Model 1 adjustments: age, sex, race and hemoglobin concentration.
Model 2 adjustments: age, sex, race, hemoglobin concentration, height-for-age z-score, income and flower worker cohabitation status.
Time after harvest and AChE activity: interactions with areas of flower plantations within 500 m of homes
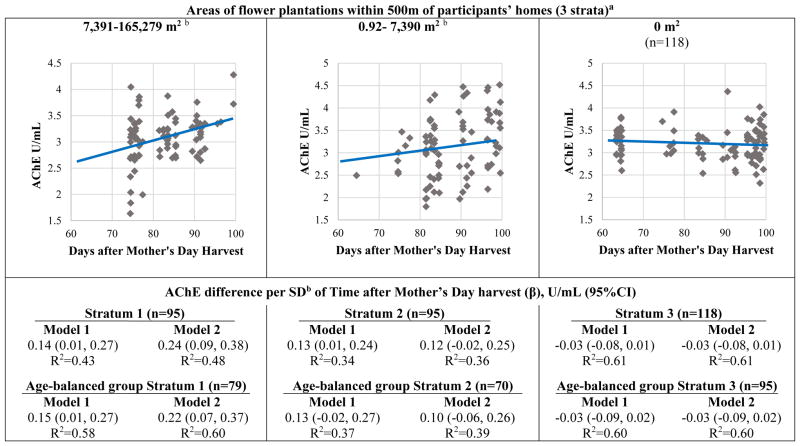
We did not observe a statistically significant interaction by areas of flower plantations within 500 m of participant’s homes in the association between time after Mother’s Day harvest and AChE activity (model 2: pinteraction=0.14; R2=0.44). Given the notable correlation between home distances to plantations and plantation areas within 500 m of homes (r=0.41) and the theoretical construct of drift of pesticides from plantations to homes, we stratified the associations of areas of flower plantations within 500 m of participant’s homes (Figure 2). We observed that children who lived in homes with the highest areas of flower plantations within 500 m of their homes (upper median split of non-zero values: 7,319–165,279 m2) had significant positive associations between AChE activity and time after the harvest (β [95% CI] model 2: 0.24 U/mL [0.09, 0.38]). The associations among children in the lower median split of non-zero values (0.92–7,390 m2) were also positive and reached statistical significance in model 1 and were borderline non-significant in model 2. No associations were observed among children with no flower plantations within 500 m of their homes. These associations were very similar in the age-balanced subgroup.
Figure 2.
Children’s acetylcholinesterase (AChE) activity in relation to Mother’s Day harvest, by categories of areas of flower plantations within 500m of participants’ homesa.
The depicted regression lines correspond to model 2 including all participants stratified by 3 categories. Diamonds are adjusted means of AChE activity.
aInteraction with areas of flower plantations within 500m of homes: Pmodel 2=0.14
bMedian split of non-zero values
cSD: 10.8 days
Model 1 adjustments: age, sex, race and hemoglobin concentration.
Model 2 adjustments: age, sex, race, hemoglobin concentration, height-for-age z-score, income and flower worker cohabitation status.
Time after harvest and AChE activity: interactions with sex and flower worker cohabitation
We observed no effect modification by sex or by flower worker cohabitation in the association between time after Mother’s Day harvest and AChE activity.
Discussion
Our findings suggest that major episodic use of sprayed pesticides of a large floriculture industry tied to global demand for cut flowers, may have measurable impacts on children who live in agricultural communities nearby. These children come into contact with pesticides predominantly through off-target drift from plantations and parental occupational take-home pathways. Pesticide drift associated with closer residential proximity to plantations seemed of importance, as the associations were only observed among children living within 532 m of a flower plantation (and especially within 233 m), and were strongest with larger areas of flower plantations within 500 m of their homes. The results of this study support the hypothesis that children living in agricultural settings are exposed a greater amount of cholinesterase inhibitor pesticides during high production periods, which in turn may affect their physiological and/or developmental processes. Within this study population, we recently reported that children who were examined sooner after the Mother’s Day flower harvest had lower performance in neurobehavioral domains including attention/inhibitory control, visuospatial processing and sensorimotor (Suarez-Lopez et al. 2017).
The observed associations in the present cross-sectional analyses had important parallels with a detailed 10-month longitudinal study of 95 adolescent agricultural workers in Egypt, which included measures during high and low exposure seasons. Participants had multiple measurements of AChE and butyrylcholinesterase (BChE) activity, and of 3,5,6-trichloro-2-pyridinol (TCPy), a urinary metabolite of the organophosphate chlorpyrifos during the study period (Crane et al. 2013). TCPy levels increased during the pesticide application season and decreased shortly thereafter. Consistent with these findings, AChE and BChE activity decreased during the pesticide application season, and increased after the end of the application period. The recovery of AChE and BChE activity continued through 180 days after the end of the application period. This association remained for longer than the average time of the normalization of AChE levels after irreversible inhibition with organophosphate exposures previously described among agricultural workers (mean 82 days, 95% confidence interval: 72–98 days) (Mason 2000). This may be explained by small renewing (background) pesticide exposures present in the environment. In our study, the positive association between time and AChE activity continued through 100 days after the harvest among participants living within 532 m of a plantation. A longer examination window in our study may have been needed to observe a plateau in the association which would mark the end of the apparent AChE “recovery” (we are not able to assess true recovery with cross-sectional data).
There are few existing studies that have assessed the effects of high pesticide spray seasons on the exposures of children who do not work in agriculture. Among non-agricultural worker adolescents in Egypt (n=38), elevated urinary markers of organophosphate and depressions of AChE and BChE activity were observed during the pesticide spray season compared to baseline (Crane et al. 2013). A study of children living in an agricultural area in Washington State, USA, observed greater urinary metabolite concentrations of organophosphates during high-spray compared to low-spray seasons among children who lived with an agricultural worker. (Thompson et al. 2014). Both of these studies concur with our findings. However, in the latter study, these associations were not observed among children who did not live with agricultural workers. Contrary to our expectation, in our study, we did not observe differences in the associations between time after Mother’s Day harvest and AChE activity between children living with a flower plantation worker (n=152) compared to children living with non-agricultural workers (n=156). This suggests that the rate of introduction of pesticides into the homes by flower plantation worker (e.g. bringing pesticide contaminated clothing, tools, etc. into the home) does not increase during peak exposure periods in amounts to affect AChE activity. Also, discrepant with our findings, a study of non-agricultural worker adults and children living near agricultural farms in the United Kingdom did not find differences in urinary pesticide metabolites between the low and high pesticide spray seasons (Galea et al. 2015). Differing findings likely reflect differences in the selection of exposure assessment used (urinary metabolites vs AChE), and agricultural pesticide use and practices amongst different crops and countries.
Proximity of homes to plantations and greater areas of flower plantations near participants’ homes were important pathways of children’s exposure in relation to time after the spray season. Greater proximity of homes to agricultural crops has been positively associated with greater pesticide exposures among adults and children not directly involved in agriculture (Keifer et al. 1996; Ward et al. 2006; Coronado et al. 2011). Within our study, the associations across strata of areas of flower plantations within 500 m of participants’ homes mirrored those across strata of home distances to the nearest flower plantations. The congruence of the findings across strata of these related but different constructs of potential drift of pesticides from plantations strengthens our findings. Area of flower plantations within 500 m of homes is likely a better predictor of drift than home distances to the nearest flower plantation because it implicitly accounts for areas of plantations that could be potentially fumigated with pesticides. The associations between home distances to flower plantations and areas of flower plantations in relation to AChE activity within our cohort is a separate topic of analysis.
While children in our study did not work in agriculture, our findings are coherent with various studies of agricultural workers (mostly adult) demonstrating that pesticide spray periods are associated with increased pesticide exposure biomarkers, including cholinesterase depression (Peiris-John et al. 2005; Crane et al. 2013; Strelitz et al. 2014; Krenz et al. 2015; Singleton et al. 2015; Quandt et al. 2015).
The main limitation of this study is that participants were only examined once, which precludes us from assessing actual change in AChE activity between pre-pesticide application, application and post-application periods. Conducting exposure assessments at all three periods is difficult in this population because the heightened pesticide use periods start at approximately October/November and remain high until early May. The cross-sectional design of our study allowed us to estimate the associations of time after the harvest with AChE activity while avoiding to inflict multiple blood draws to this group of young children. Additionally, we do not have quantification of pesticide levels from bio-specimens to determine which specific cholinesterase inhibitors or other pesticides are entering children’s bodies. Yet, the carbamate and organophosphate pesticides reported to be used by flower plantations in Pedro Moncayo are reasonably some of the cholinesterase inhibitors we would expect to find in children of our study. As compared to measurements of pesticides or pesticide metabolites in body fluids, AChE activity is less sensitive to low-level exposures. However, it is a low-cost well-established marker of exposure that reflects a physiological change associated with cholinesterase inhibitor exposures. It is also a more stable (lower within individual variability) indicator of cholinesterase inhibitor exposure (Lefkowitz et al. 2007; Griffith et al. 2011; Bradman et al. 2013) and provides a much wider exposure window than bio-specimen quantification. Within the ESPINA study, we have found that children’s AChE activity was inversely associated with cohabitation with floricultural workers, duration of cohabitation and with greater number of pesticide “take-home” pathways, which provides some evidence of the use of AChE to assess low-dose pesticide exposures (Suarez-Lopez et al. 2012).
This is one of the largest studies to date to estimate pesticide exposures in children in relation to a known pesticide exposure period. Our sample size allowed us to detect effect modification by distances of homes to plantations in this context for the first time. The high participation rates and fast recruitment and examination of participants of our study was possible due to the well-established partnerships and ongoing community-based participatory practices between our investigators at Fundacion Cimas del Ecuador and communities of Pedro Moncayo County. Although this study was not designed to specifically assess AChE change after a known heightened exposure period, our approach allowed us to obtain AChE values at 20 points in time within 100 days from Mother’s Day harvest, averaging 15 participants per time point. This allowed us to have detailed approximations of seasonal changes in AChE activity, albeit with cross-sectional data. The limited number of investigations that have assessed the effects of pesticide spray seasons on the exposure of non-agricultural worker children living in agricultural communities raises the need to conduct more investigations assessing exposures at multiple points in time before and after the spray seasons of various types of crops.
Conclusion
This study provides additional evidence that heightened pesticide usage periods can increase pesticide exposures, as reflected by physiological alterations in AChE activity, among children who do not work in agriculture but live within approximately 500m of a flower plantation. Our study, though based on cross-sectional information, contrasted the AChE activity of a relatively large sample of children across a 1-month exposure window, within 100 days after the Mother’s Day harvest. The findings of our study highlights the potential impact of globalization and trade on exposures to people living near flower plantations. Mother’s Day is a time to acknowledge the importance of mothers in our societies, and the gift of flowers has become an important component of these celebrations. The heightened demand of flowers is important for the floriculture industry; unfortunately, it is also an important source of heightened pesticide exposures affecting not only its workers, but also children living near plantations. These heightened seasonal pesticide exposures are of concern as they can decrease the neurobehavioral performance of children, and may induce short- and long-term physiological and developmental alterations.
Acknowledgments
We thank Dr. Jose Suarez Torres, Dolores Lopez Paredes and Fundación Cimas del Ecuador for providing the infrastructure, logistical support, access to the Local and Community Information System and their long history of collaboration with Pedro Moncayo County communities; all of which were key to the success of this study. We also thank the Tabacundo Health Center of the Ministry of Public Health of Ecuador, for their assistance, and especially the people of Pedro Moncayo County and their local governments for their collaboration and support of this project.
Funding Sources: Research reported in this publication was supported by the National Institute of Occupational Safety and Health (1R36OH009402-01), and the National Institute of Environmental Health Sciences of the National Institutes of Health (grants R01ES025792-01, R21ES026084-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Institute of Occupational Safety and Health
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Bradman A, Kogut K, Eisen Ea, et al. Variability of organophosphorous pesticide metabolite levels in spot and 24-hr urine samples collected from young children during 1 week. Environ Health Perspect. 2013;121:118–24. doi: 10.1289/ehp.1104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado GD, Holte S, Vigoren E, et al. Organophosphate pesticide exposure and residential proximity to nearby fields: evidence for the drift pathway. J Occup Environ Med. 2011;53:884–91. doi: 10.1097/JOM.0b013e318222f03a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane AL, Abdel Rasoul G, Ismail AA, et al. Longitudinal assessment of chlorpyrifos exposure and effect biomarkers in adolescent Egyptian agricultural workers. J Expo Sci Environ Epidemiol. 2013;23:356–62. doi: 10.1038/jes.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl CL, Fenske RA, Kissel JC, et al. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ Health Perspect. 2002;110:A787–92. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin BD, Hein MJ, Sanderson WT, et al. Urinary and hand wipe pesticide levels among farmers and nonfarmers in Iowa. J Expo Anal Environ Epidemiol. 2005;15:500–8. doi: 10.1038/sj.jea.7500428. [DOI] [PubMed] [Google Scholar]
- EQM Research. Test-mate ChE Cholinesterase Test System (Model 400) Instruction Manual. 2003 http://www.eqmresearch.com/Manual-E.pdf.
- Eskenazi B, Kogut K, Huen K, et al. Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environ Res. 2014;134C:149–157. doi: 10.1016/j.envres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea KS, MacCalman L, Jones K, et al. Urinary biomarker concentrations of captan, chlormequat, chlorpyrifos and cypermethrin in UK adults and children living near agricultural land. J Expo Sci Environ Epidemiol. 2015;25:623–31. doi: 10.1038/jes.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobierno Municipal del Canton Pedro Moncayo. El Cantón. 2011 http://www.pedromoncayo.gob.ec.
- Griffith W, Curl CL, Fenske Ra, et al. Organophosphate pesticide metabolite levels in pre-school children in an agricultural community: within- and between-child variability in a longitudinal study. Environ Res. 2011;111:751–6. doi: 10.1016/j.envres.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari R. Seguridad, salud y ambiente en la floricultura. IFA, PROMSA; Quito: 2004. [Google Scholar]
- Hogenkamp A, Vaal M, Heederik D. Pesticide exposure in dwellings near bulb growing fields in The Netherlands: an explorative study. Ann Agric Environ Med. 2004;11:149–53. [PubMed] [Google Scholar]
- Keifer M, Rivas F, Moon JD, Checkoway H. Symptoms and cholinesterase activity among rural residents living near cotton fields in Nicaragua. Occup Environ Med. 1996;53:726–9. doi: 10.1136/oem.53.11.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofman O, Berger A, Massarwa A, et al. Motor inhibition and learning impairments in school-aged children following exposure to organophosphate pesticides in infancy. Pediatr Res. 2006;60:88–92. doi: 10.1203/01.pdr.0000219467.47013.35. [DOI] [PubMed] [Google Scholar]
- Krenz JE, Hofmann JN, Smith TR, et al. Determinants of butyrylcholinesterase inhibition among agricultural pesticide handlers in Washington State: an update. Ann Occup Hyg. 2015;59:25–40. doi: 10.1093/annhyg/meu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz LJ, Kupina JM, Hirth NL, et al. Intraindividual stability of human erythrocyte cholinesterase activity. Clin Chem. 2007;53:1358–63. doi: 10.1373/clinchem.2006.085258. [DOI] [PubMed] [Google Scholar]
- Loewenherz C, Fenske Ra, Simcox NJ, et al. Biological monitoring of organophosphorus pesticide exposure among children of agricultural workers in central Washington State. Environ Health Perspect. 1997;105:1344–53. doi: 10.1289/ehp.971051344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide exposure of children in an agricultural community: evidence of household proximity to farmland and take home exposure pathways. Env Res. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- Makhaeva GF, Aksinenko AY, Sokolov VB, et al. Synthesis of organophosphates with fluorine-containing leaving groups as serine esterase inhibitors with potential for Alzheimer disease therapeutics. Bioorg Med Chem Lett. 2009;19:5528–30. doi: 10.1016/j.bmcl.2009.08.065. [DOI] [PubMed] [Google Scholar]
- Mason HJ. The recovery of plasma cholinesterase and erythrocyte acetylcholinesterase activity in workers after over-exposure to dichlorvos. Occup Med (Lond) 2000;50:343–7. doi: 10.1093/occmed/50.5.343. [DOI] [PubMed] [Google Scholar]
- McCauley LA, Lasarev MR, Higgins G, et al. Work characteristics and pesticide exposures among migrant agricultural families: a community-based research approach. Environ Health Perspect. 2001;109:533–538. doi: 10.1289/ehp.01109533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez A, Betancourt O, Vera B, et al. Línea de Base Prevención y eliminación progresiva del trabajo infantil en la floricultura en los cantones Cayambe y Pedro Moncayo. Organizacion Internacional de Trabajo; Lima: 2002. [Google Scholar]
- Peiris-John RJ, Ruberu DK, Wickremasinghe AR, van-der-Hoek W. Low-level exposure to organophosphate pesticides leads to restrictive lung dysfunction. Respir Med. 2005;99:1319–24. doi: 10.1016/j.rmed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Quandt SA, Arcury TA, Rao P, et al. Agricultural and residential pesticides in wipe samples from farmworker family residences in North Carolina and Virginia. Environ Health Perspect. 2004;112:382–7. doi: 10.1289/ehp.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt SA, Pope CN, Chen H, et al. Longitudinal Assessment of Blood Cholinesterase Activities Over 2 Consecutive Years Among Latino Nonfarmworkers and Pesticide-Exposed Farmworkers in North Carolina. J Occup Environ Med. 2015;57:851–7. doi: 10.1097/JOM.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaprasad J, Tsai MG-Y, Fenske RA, et al. Children’s inhalation exposure to methamidophos from sprayed potato fields in Washington State: exploring the use of probabilistic modeling of meteorological data in exposure assessment. J Expo Sci Environ Epidemiol. 2009;19:613–23. doi: 10.1038/jes.2008.66. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119:1196–201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox NJ, Fenske RA, Wolz Sa, et al. Pesticides in household dust and soil: exposure pathways for children of agricultural families. Env Heal Perspect. 1995;103:1126–1134. doi: 10.1289/ehp.951031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton ST, Lein PJ, Dadson OA, et al. Longitudinal assessment of occupational exposures to the organophosphorous insecticides chlorpyrifos and profenofos in Egyptian cotton field workers. Int J Hyg Environ Health. 2015;218:203–11. doi: 10.1016/j.ijheh.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelitz J, Engel LS, Keifer MC. Blood acetylcholinesterase and butyrylcholinesterase as biomarkers of cholinesterase depression among pesticide handlers. Occup Environ Med. 2014;71:842–7. doi: 10.1136/oemed-2014-102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Checkoway H, Jacobs DR, et al. Potential short-term neurobehavioral alterations in children associated with a peak pesticide spray season: The Mother’s Day flower harvest in Ecuador. Neurotoxicology. 2017 doi: 10.1016/j.neuro.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Jacobs DR, Himes JH, et al. Lower acetylcholinesterase activity among children living with flower plantation workers. Environ Res. 2012;114:53–9. doi: 10.1016/j.envres.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Jacobs DR, Himes JH, Alexander BH. Acetylcholinesterase Activity, Cohabitation with Floricultural Workers, and Blood Pressure in Ecuadorian Children. Environ Health Perspect. 2013;121:619–624. doi: 10.1289/ehp.1205431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Griffith WC, Barr DB, et al. Variability in the take-home pathway: farmworkers and non-farmworkers and their children. J Expo Sci Environ Epidemiol. 2014;24:522–31. doi: 10.1038/jes.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. Cut Flowers and Greenery Import Manual. Washington, D.C: 2012. [Google Scholar]
- Ward MH, Lubin J, Giglierano J, et al. Proximity to crops and residential exposure to agricultural herbicides in iowa. Environ Health Perspect. 2006;114:893–7. doi: 10.1289/ehp.8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weppner S, Elgethun K, Lu C, et al. The Washington aerial spray drift study: children’s exposure to methamidophos in an agricultural community following fixed-wing aircraft applications. J Expo Sci Environ Epidemiol. 2006;16:387–96. doi: 10.1038/sj.jea.7500461. [DOI] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]