Abstract
T helper 17 (Th17) cells have recently been implicated in depression, which adds to the list of several other diseases of the central nervous system (CNS) that are already known to involve Th17 cells. In CNS diseases, it is thought that the signature cytokine produced by Th17 cells, interleukin-17A (IL-17A), mediates the detrimental effects of Th17 cells. In depression, although Th17 cells increase, the lack of a correlation between depression severity and blood IL-17A levels suggests that Th17 cells promote depressive symptoms, which may not be entirely dependent on IL-17. However, little is known about the mechanism of action of Th17 cells or the source of CNS Th17 cells in depression. It is likely that Th17 cells promote neuroinflammation and activation of microglia and astrocytes, actions that may contribute to neuronal damage. A source of Th17 cells is the small intestine where they are regulated by the composition of the microbiome. It remains to be determined through what mechanisms of action Th17 cells affect depression and if Th17 cells can be considered a novel therapeutic target in depression.
1. Overview of Th17 cells
The immune system is divided into two arms: the innate and adaptive immune systems. The innate system, comprising antigen presenting cells (APC) such as monocytes/macrophages and dendritic cells, is thought to be rapidly activated to induce an inflammatory response. If the insult or infection is not rapidly cleared by the innate system, the innate immune system recruits the adaptive immune system to promote the resolution of the infection or insult. The adaptive immune system is comprised of B and T cells. Amongst T cells, the CD4 + T cells differentiate into various T helper (Th) cell subtypes following antigen recognition through APC presentation, co-stimulation and a cocktail of cytokines. The cocktail of cytokines required to differentiate a Th cell varies depending on the Th subtype that is being induced. Th17 cells are a subpopulation of CD4+ T cells of the adaptive immune system that are characterized by the production of the inflammatory cytokine interleukin (IL)-17 (IL-17A and IL-17F) (Harrington et al., 2005), and which also produce IL-21 and IL-22 (Gaffen et al., 2014). Since the discovery in 2005 of the requirement for IL-6 and transforming growth factor β (TGF) for the differentiation of Th17 cells, a variety of other cytokines have been shown to promote Th17 cell differentiation in vitro, such as tumor necrosis factor (TNF)α, IL-1β, IL-21, or IL-23. Besides activation by antigen recognition and by co-stimulatory signals, Th17 cells require activation of the master transcription factor, retinoic acid receptor-related orphan receptor (ROR)γT, to differentiate (Ivanov et al., 2006). However, other transcription factors are also implicated in contributing to Th17 cell differentiation, such as basic leucine zipper transcription factor ATF-like (BATF), Runt-related transcription factor-1 (Runx1), aryl hydrocarbon receptor (Ahr), interferon (IFN) regulatory factor-4 (IRF4), signal transducer and activator of transcription-3 (STAT3), and STAT5 (Yang et al., 2014). It is important to point out that Th17 cells are plastic cells as there are apparently a number of variations in differentiated Th17 cells, and Th17 cells can convert to Th1 cells or T regulatory (Treg) cells (Muranski and Restifo, 2013). Th1 cells are proinflammatory CD4+ T cells characterized by the production of IFNγ and require IL-12 to differentiate. Treg cells are anti-inflammatory CD4+ T cells expressing the transcription factor Foxp3 and require TGFβ to differentiate. Bacteria are one of the signals that trigger Th17 cells differentiation, Th17 cells are constitutively present in a part of the gut, the lamina propria of the small intestines, due to a specific population of bacteria present there (segmented filamentous bacteria), where they ensure immune surveillance and proper gut function and are quasi absent in other organs such as lung or liver (Ivanov et al., 2008). Infections or other conditions that increase IL-6 and TGFβ can increase the the Th17 Th17 cell cell population, and once activated Th17 cells promote the eradication of extracellular bacteria and fungal infections, such as infection by Candida Albicans (Hernandez-Santos and Gaffen, 2012). Th17 cells can also be major pathological contributors to a variety of autoimmune diseases, such as multiple sclerosis, in which Th17 cells are autoreactive T cells with pathogenic properties that exacerbate autoimmunity (Lee et al., 2012).
2. Th17 cells and depression
Depression is a prevalent, yet undertreated disease (Belmaker and Agam, 2008). One novel exploratory way to improve the understanding of depression relies on the discovery that depression is associated with the elevation of proinflammatory markers (Miller and Raison, 2016). Many rodent models have been developed to study the role of inflammation in depressive-like behaviors (Dantzer et al 2008). Even though depression is not recapitulated in rodents and caution should be taken when interpreting behavioral results in rodents, converging measurements of various rodent behaviors associated with depression in humans have proven useful in uncovering the role of inflammation in depression. Thus, the role of Th17 cells in depression has only recently been investigated and originated from the growing evidence that inflammation promotes depressive symptoms. Indeed, many proinflammatory cytokines, including IL-6, TNFα and IL-1β, are elevated in the blood of depressed patients. Since these same cytokines are known to promote Th17 cell differentiation, it was hypothesized that Th17 cells would be elevated in the blood of depressed patients. Indeed, one study found that Th17 cells were increased in the blood of depressed patients (Chen et al., 2011; Davami et al., 2016) and two studies found IL-17A, the signature cytokine produced by Th17 cells, to be elevated in the blood of depressed patients (Chen et al., 2011; Davami et al., 2016). Moreover, antidepressants maintain the balance between anti-inflammatory Treg cells and proinflammatory Th17 cells in depressive patients (Zhang et al., 2013b). However, little is known about the potential role of Th17 cells in depression, and blood levels of IL-17A, the signature cytokine produced by Th17 cells, do not always correlate with depression (Kim et al., 2013; Liu et al., 2012). Nevertheless, patients with psoriasis who have elevated levels of IL-17A also have increased risk for depression and anxiety disorders (Kurd et al., 2010), similarly to multiple sclerosis and arthritis patients. These findings suggest that IL-17A production by Th17 cells in itself may not be sufficient to promote depression, but rather that a pathogenic sub-population of Th17 cells has additional actions or is differentially localized in order to promote depression. The percent of Th17 cells was increased 3-fold in the brain of mice exhibiting depressive-like behavior compared to mice that do not exhibit depressive-like behavior (Beurel et al., 2013). Similarly, the Th17 cells level increased in the brain of mice subjected to chronic restraint stress compared to unstressed mice (Beurel et al., 2013). Adoptive transfer of Th17 cells is sufficient to promote susceptibility to depressive-like behavior (Beurel et al., 2013). And blockage of the master transcription factor RORγT, required for the differentiation of Th17 cells (Ivanov et al., 2006), using either a knockout model or a small inhibitor, was sufficient to render mice resistant to the induction of learned helplessness, as did neutralization of IL-17A (Beurel et al., 2013). Furthermore, administration of IL-17A promotes depressive-like behaviors (Nadeem et al., 2017). The small number of studies on the subject precludes making a definitive conclusion about the role of Th17 cells in depression. However, it is suspected that Th17 cells have a negative impact on the susceptibility to depression (Kurd et al 2010). Based on the knowledge of the characteristics of the Th17 cells in multiple sclerosis and psoriasis, it seems likely Th17 cells associated with depression develop a specific pathogenic phenotype, but clearly further investigations are needed to determine the role of Th17 cells in depression, including studies of their origin, whether they are released from their peripheral niche (the gut) or if they differentiate in situ in the brain. Another important question to answer is to determine if Th17 cells are bystanders of inflammation, as has been described in other CNS pathologies associated with Th17 cells (see section 4). Further studies may also determine if Th17 cells can be used as either biomarkers or as a therapeutic target in depression, since Th17 cells are normally only present in the intestine in healthy individuals and appear in the bloodstream after disease induction.
In the remaining portion of this review, we review the roles of Th17 cells in the brain that have been shown for other diseases and the properties of these cells that might be of significance for depression.
3. CD4+ T cells actions in the brain
A. Infiltration
Although the precise definition remains a matter of debate, the brain is thought of as immune privileged because the expression of MHC class II molecules is low in the brain parenchyma, the entry of peripheral immune cells occurs only at certain interfaces such as the blood-cerebrospinal fluid (CSF) barrier, the CSF-brain barrier, and the blood-brain barrier (Ransohoff and Engelhardt, 2012). And maybe the most important feature is that the antigen repertoire of the CNS may not be totally represented by peripheral immune cells and in particular, numerous antigens that are expressed by the CNS might not be recognized by the adaptive immune system.
Recently, the discovery of lymphatic vessels in the CNS, which help clear the brain interstitial fluid into the CSF, supports the concept that the adaptive immune system develops tolerance to CNS antigens rather than immunity (Louveau et al., 2015). However, it is part of the physiologic immune surveillance function of T cells to patrol the meninges and choroid plexus. CD4+ or CD8+ T cells have often been reported to be in the CNS parenchyma after stress, infection, injury, and in certain neurological diseases, including autoimmune diseases such as multiple sclerosis and neurodegenerative diseases like Alzheimer disease. In the case of infections, some of the CD8+ T or CD4+ T cells become tissue-resident and provide memory function to protect the CNS from re-infection (Korn and Kallies, 2017). Although the mechanism of infiltration of T cells into the brain in non-pathological conditions is not known, it has been hypothesized that CD4+ T cells, with the help of IFNγ, can enter into the CNS through the choroid plexus (Baruch et al., 2016), which might also be the mechanism whereby Th17 cells infiltrate the CNS in depression, although the number of infiltrated Th17 cells is lower than in CNS related autoimmune diseases such as multiple sclerosis, for example. In neuropathological diseases in contrast, a massive infiltration of leukocytes has been reported. Thus, in experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis, it has been shown that Th17 cells enter the CNS in the absence of the lectin and very late antigen-4 (VLA4), at the level of the brain stem and cerebellum rather than the spinal cord, whereas Th1 cells require VLA4 expression to enter the CNS parenchyma (Rothhammer et al., 2011). It is important to note that in EAE, the majority of the pathogenic Th17 cells co-express IFNγ and IL-17A (Hirota et al., 2011), and IFNγ might confer the signal for Th17 cells to infiltrate the CNS. However, the main attractive signal of Th17 cells to enter the CNS during autoimmune diseases such as EAE remains the chemokine gradient. In particular, the presence of C-C chemokine receptor type 6 (CCR6) at the surface of pathogenic Th17 cells is thought to be critical for the promotion of EAE (Yamazaki et al., 2008), even though other chemokine receptors are also involved. CD4+ T cells have also been shown to promote entry of antibodies to the CNS. Altogether, in neurodegenerative diseases CD4+ T cells infiltrate the brain mainly via the choroid plexus. Nonetheless, the mode of entry of Th17 cells into the CNS needs to be determined in depression to understand the mechanism whereby the proportion of Th17 cells relative to CD4+ T cells increases in the brain of depressed mice (Beurel et al. 2013).
B. Th17 cell pathogenicity
Overall, Th17 cells found outside the intestine and especially within the CNS are thought to be pathogenic. This is particularly true in EAE where both autoreactive Th1 and Th17 cells have been considered pathogenic, and Th17 cells expressing IFNγ have been considered to contribute to the exacerbation of EAE. Pathogenicity of Th17 cells has also been associated with increased expression of CCR6, IL-23R, and granulocyte macrophage colony-stimulating factor (GM-CSF). There are multiple pathways whereby Th17 cells become encephalitogenic, and these pathways integrate signals from a variety of transcription factors that control Th17 cell specialization, such as BATF, IRF4 and STAT3. Moreover, recent fate-mapping approaches have uncovered pathways that maintain or disrupt the proinflammatory features of Th17 cells in vivo (Ciofani et al., 2012). Thus for example, expression of the Th1 transcription factor, T-bet, is required for Th17 cells to acquire destructive properties in the CNS, consistent with the expression of IFNγ in pathogenic Th17 cells, whereas in the gut T-bet-deficient Th17 cells are pathogenic (Krausgruber et al., 2016). Besides transcription factors, microRNAs and RNA–binding proteins have also been shown to modulate the pathogenicity of Th17 cells (Ichiyama et al., 2016). Th17 cells are highly plastic cells and it is likely that many subpopulations of Th17 cells exist in different diseases. In EAE, the use of single-cell RNA sequencing uncovered unexpected candidates that modulate the pathogenic state of Th17cells, such as CD5L (Davis et al., 2015). Of particular interest, Th17 cells can also reprogram towards other lineages such proinflammatory Th1 cells or anti-inflammatory Treg cells. In the case of reprogramming towards the Treg phenotype, Th17 cells can adopt an IL-10 producing Treg type 1 (Tr1) phenotype and contribute to the resolution of inflammation in the CNS (Jankovic et al., 2010). However, because this reprogramming also occurs with other subsets of CD4+ cells, it has been hypothesized that this might be the end-stage of the inflammatory T effector cells (Jankovic et al., 2010). It has also been proposed that interaction with the antigen presenting cells (APCs) and B cells leads to a fine-tuning of the Th17 cell response, and therefore clarifying the location and the recruitment of APCs and B cells in the CNS might help to understand the roles of the different Th17 subsets. Altogether, although it remains to be determined what governs the pathogenic profiles of Th17 cells, it is clear that cytokines, TCR activation, co-stimulatory signals, and environmental and metabolic signals regulate the profiles of Th17 cells. Altogether this suggests that Th17 cells associated with depression might also be a subpopulation of Th17 cells with characteristics specific to depression. Of particular interest for depression, since it is a chronic disease with recurrent episodes, uncovering the role of memory T cells within or outside the Th17 lineage might help explain the recurrence of the depressive episodes.
4. IL-17A and other CNS diseases
Th17 cells and/or IL-17A have been found in the brain in many CNS diseases. We briefly consider the potential involvement of Th17 and/or IL-17A in multiple sclerosis, autism spectrum disorders, epilepsy, and Alzheimer disease, as these diseases have already been extensively reviewed elsewhere.
A. Multiple sclerosis
The roles of Th17 cells in the CNS mainly has been described in the context of EAE and multiple sclerosis, where there is a massive infiltration of Th17 cells. These CNS Th17 cells are thought to promote demyelinating pathology by inducing apoptosis of oligodendrocytes through IL-17A production. Associated with the demyelination, Th17 cells also induce neuronal cell death by directly interacting with neurons and inducing elevation of Ca2+ levels, creating neuronal toxicity (Siffrin et al., 2010). Th17 cell infiltration in EAE is also associated with the recruitment of neutrophils or other immune cells into the CNS, which leads to further recruitment of Th17 cells and damage to the CNS.
B. Autism Spectrum disorders
IL-17A has recently been implicated in autism spectrum disorders (Kugelberg, 2016). IL-17A was found increased in the blood of children with autism (Al-Ayadhi and Mostafa, 2012), and the IL-17A gene is enriched in a genome-wide copy number variant in autistic patients (Kugelberg, 2016). In the mouse model of maternal immune activation, a mouse model for autism spectrum disorders, IL-17A production is increased in CD4+ T cells isolated from offspring of infected compared to non-infected mothers, and recently Th17 cells were shown to be required for the maternal immune activation induction of autistic behaviors in mice (Choi et al., 2016).
C. Epilepsy
IL-17A was found to be elevated in the CSF of patients with epilepsy, and seizure frequency and severity was associated with interictal levels of IL-17A (Bautista et al., 2003; Taylor et al., 1971), suggesting that neuronal induction of the IL-17A pathway may contribute to the pathology of seizure disorders (Vezzani, 2005).
D. Alzheimer disease
In Alzheimer disease, Th17 cells were shown to infiltrate the brain parenchyma, and promote both neuroinflammation and neuronal cell death by activation of the Fas pathway (Zhang et al., 2013a). Th17 cells found in Alzheimer disease seem to be antigen specific for Aβ1–42, and reduction of these cells improved disease outcome in a rat model (Zhang et al., 2013a).
Th17 cells are also increased in the brain after stroke and infection. This suggests that the presence of Th17 cells in the brain, independently of the age of the individual, has detrimental consequences for the brain, and the disease outcomes depend on the critical window period in which Th17 cells infiltrate the brain. These findings suggest that for the developing brain, Th17 cells promote autistic traits, whereas Th17 cells in an aging brain can promote, for example, Alzheimer disease features. Therefore, depending on the time and duration of the induction of Th17 cells, the disease outcomes and severity might differ.
5. Peripheral versus central source of Th17 cells
A. Mechanisms of action of IL-17A in the brain
The mechanisms of action of IL-17A in the brain can be direct or indirect effects depending on which cell types produce IL-17A and on which cell types IL-17A targets. IL-17A has been shown to impair the brain blood barrier integrity and to activate astrocytes and microglia (Zimmermann et al., 2013), which might result in an exacerbation of neuroinflammation and synaptic dysfunction. In the CNS, astrocytes, neurons and microglia produce IL-17A in pathological conditions (Chen et al., 2017; Hu et al., 2014), and IL-17RA and IL-17RC are expressed on astrocytes, microglia, and endothelial cells. Once activated, these receptors induce the production of many cytokines and chemokines, and antimicrobial peptides (Gaffen, 2009). The overall effects of IL-17A produced by CNS resident cells seem to be local (Moynes et al., 2014) and to involve changes in the tuning of synapse function, but at high concentration Th17 cells are often interpreted as inducing damage to the CNS through neuronal cell death, astrogliosis, and microglia activation. In rare circumstances, IL-17A has been shown to have a positive effect on neuronal regeneration by promoting the recruitment to the site of injury of neutrophils and platelets, which promote neurite outgrowth through the secretion of VEGF (Moynes et al., 2014).
As described in the previous section, the source of IL-17A in the brain is more likely to be from brain resident cells than circulating Th17 cells, except for pathologies like multiple sclerosis, psoriasis, and arthritis where the level of circulating Th17 cells is elevated which could affect the brain, as exemplified by the high comorbidity of multiple sclerosis, psoriasis, and arthritis with depression. It is also possible that surveying CD4+ T cells can differentiate in situ upon receiving signals from the neuroinflammatory milieu. However, because Th17 cells infiltrate the brain parenchyma without requiring VLA4 signals, it can also be hypothesized that Th17 cells may reach the brain if present in the peripheral circulation. Indeed, Th17 cells are mainly present in the gut of healthy individuals, and they are not detectable in the blood (Gaffen, 2009; Shen et al., 2009). Therefore, one possible source of peripheral Th17 cells outside of Th17 cells associated with autoimmune diseases is the lamina propria of the small intestine.
B. Th17 cells and microbiome
The microbiome is a consortium of commensal bacteria, shaped throughout the lifetime of an individual, and is highly dynamic and surprisingly personalized. It is controlled by factors such as genetics, age, dietary input, individual metabolism, stress, and geography, and while a third of the gut microbiota is identical across humans, the other two thirds display a diversity unique to the individual (Macedo et al., 2017; Rieder et al., 2017). As part of normal function, the gut is in perpetual physiological inflammation: there is a constant turnover of lymphocytes and other immune cells, as well as a continuous survey of the intestinal environment including the multitude of antigens associated with food ingestion. The gut hosts the largest concentration of immune cells in the body, which must lay restrained yet ready to defend the mucosal barrier from bacterial invaders. The bacteria residing in the gut take advantage of immune tolerance to enhance their survival by communicating with immune cells defending the mucosal barrier as well as with the intestinal epithelium and enteric nervous system (Powell et al., 2017). It is believed that this communication between gut microbiota and the immune system is bidirectional, complementary, and symbiotic, beginning in early life and continuing throughout the lifetime of the individual (Rieder et al., 2017). In addition, immune cells of the gut are able to communicate with the CNS through trafficking of liberated immune cells to the brain, or by the use of cytokines that are able to cross the blood-brain barrier or enter through the brain lymphatic system (Powell et al., 2017; Wu et al., 2010). Afferent sensory fibers and enteric neurons provide additional lines of communication to the brain while bacterial metabolites or immunostimulants such as lipopolysaccharide acting on microglial or neuron toll-like receptors represent humoral methods of dialogue along the gut-brain axis (Fung et al., 2017). The brain is able to respond to communication through efferent autonomic innervation (vagus nerve) as well as the neuroendocrine hypothalamic-pituitary-adrenal axis (Rieder et al., 2017), which have the effect of altering gut permeability, gut motility, mucus production, and cytokine secretion by immune cells, particularly T cells, ultimately contributing to changes in gut bacterial composition (Fung et al., 2017).
The human intestine harbors ~100 trillion bacteria that are crucial for health. The composition of the gut microbiota varies among individuals as mentioned above, but it is estimated to consist of ~1000 species and >7000 strains. Bacteriodetes and Firmicutes are predominant, while Proteobacteria, Actinobacteria, Fusobacteria, Verrucomicrobiota and others are also present in smaller amounts. Particular members of the microbiome are required to develop specific components of the adaptive and innate immune systems. Th17 cell responses, in particular, in the lamina propria are also shaped by bacteria. Thus, without colonization by a member of the intestinal epithelium-adhering group of segmented filamentous bacteria (SFB), mice do not have proper development of Th17 cells in the small intestine and therefore are unable to mount an effective Th17 immune response (Powell et al., 2017). Compared to normal mice, after induction of EAE germ-free mice display reduced inflammation, disease scores, and number of Th17 cells (Fung et al., 2017), but recolonization of germ-free mice with SFB alone is able to restore susceptibility to EAE (Colpitts and Kasper, 2017). It is thought that SFB induces a two steps process to induce the differentiation of Th17 cells. First, colonization of SFB by gavage primes CD4+ T cells in the draining lymph nodes to polarize CD4+ T cells towards Th17 cells, and secondly, promotes the production by the ileum epithelial cells of serum amyloid A proteins: SAA1 and SAA2, which promotes local production of IL-17 in RORγT+ T cells (Sano et al., 2015). IL-22 produced by type 3 innate lymphoid cells (ILC3) upon SFB gavage was responsible for inducing the production of SAA by epithelial cells (Sano et al., 2015), showing a collaboration between SFB, microenvironment and Th17 cell induction.
Consistent with the bidirectional communication between the gut, brain, and immune system, stress-induced behavioral changes are modulated by changes of the microbiome. Previous work with germ-free mice clarified the impact that the gut microbiota has within the gut-brain axis to alter neurological function in behavioral disorders and other conditions of the CNS. Germ-free mice demonstrate attenuated symptoms of anxiety (Rieder et al., 2017), and reconstitution of gut microbiota of germ-free mice in early stages of life, but not during adult life, partially restores anxious behaviors (Macedo et al., 2017). Additionally, germ-free mice exhibit a volumetric expansion of the amygdala and hippocampus, demonstrating that microbiota structurally influences the CNS (Powell et al., 2017). Germ-free mice display defective production of stress- and anxiety-associated cytokines, including IL-6, TNF, and IL-1β. Humans with MDD have been shown to possess a distinct microbiota with a lower degree of diversity compared to healthy controls, and fecal transfer of human MDD microbiota into germ-free mice is sufficient to induce depressive-like behaviors and anxiety in the recipient mice (Fung et al., 2017). Altogether this suggests that depression might result from change in microbiome in response to stress that, for example, might change the intestinal Th17 cell population, which in turn might affect brain homeostasis.
6. Concluding Remarks
Th17 cells are potent regulators of brain function as they target various brain resident cells including neurons, astrocytes, and microglia and participate in neuroinflammation. Recently, they have been implicated in depression both in humans and rodents. Although their role in depression remains to be more fully defined, it provides a potential new therapeutic strategy for depression. Many molecules targeting the Th17/IL-17A pathway have been developed and are in phase III of clinical trials for psoriasis or other autoimmune diseases (Bartlett and Million, 2015). Moreover, uncovering the source of Th17 cells in depression might help to understand the brain-gut axis that has emerged as a potential new target for depression.
Figure.
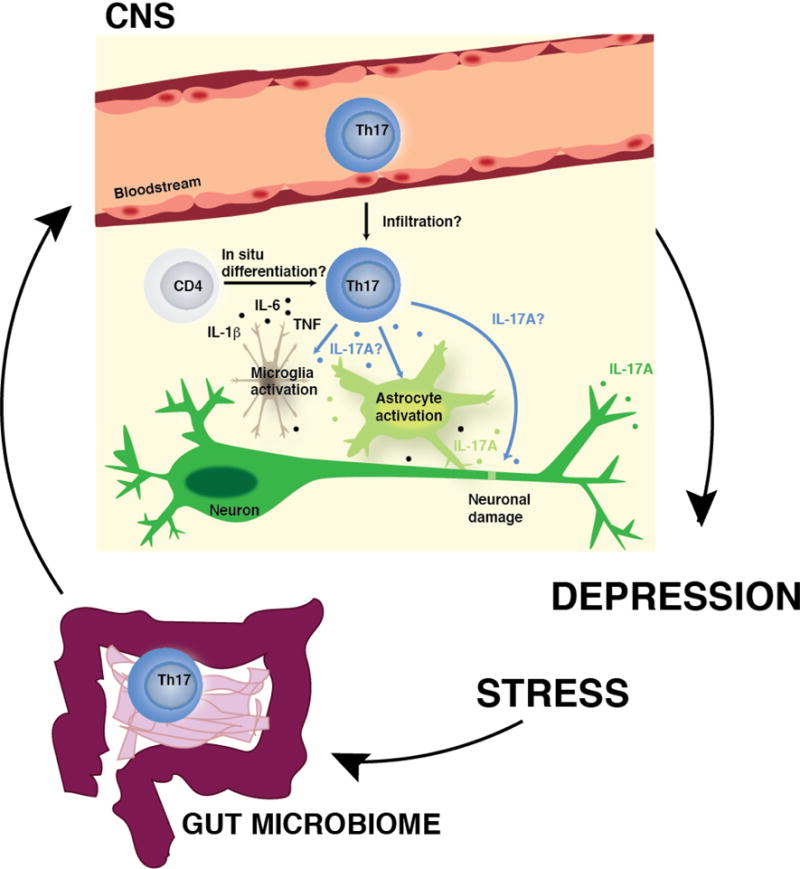
Potential mechanisms of action of Th17 cells in the brain during depression. In response to stress, peripheral Th17 cells (possibly released from the lamina propria of the small intestine) may infiltrate the brain parenchyma, or surveying CD4+ T cells may differentiate into Th17 cells in situ after receiving signals from proinflammatory cytokines (e.g. IL-6, IL-1β, TNF) generated during the neuroinflammatory response to stress. Once in the brain parenchyma, Th17 cells may exert direct or indirect effects on the brain via production of IL-17A or other cytokines or factors produced by Th17 cells by either inducing activation of astrocytes and microglia, and/or by inducing neuronal damage, enhancing the neuroinflammatory response (including the production of IL-17A by CNS resident cells) and leading to increased susceptibility to depressive-like behaviors.
Highlights.
Th17 cells are increased in the blood of depressed patients
Th17 cells promote depressive-like behaviors in mice
Th17 cells are pathogenic in most CNS diseases
Th17 cells accumulate in the brain of mice exhibiting depressive-like behavior
Acknowledgments
We would like to thank the NIH for supporting this research, and Dr. Richard Jope for his constructive comments on the manuscript.
Grant support
Research in the authors’ laboratory was supported by grants from the NIMH (MH104656, MH110415).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no financial or conflict of interest.
References
- Al-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. J Neuroinflammation. 2012;9:158. doi: 10.1186/1742-2094-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett HS, Million RP. Targeting the IL-17-T(H)17 pathway. Nat Rev Drug Discov. 2015;14:11–12. doi: 10.1038/nrd4518. [DOI] [PubMed] [Google Scholar]
- Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, Kertser A, David E, Amit I, Schwartz M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med. 2016;22:135–137. doi: 10.1038/nm.4022. [DOI] [PubMed] [Google Scholar]
- Bautista JF, Foldvary-Schaefer N, Bingaman WE, Luders HO. Focal cortical dysplasia and intractable epilepsy in adults: clinical, EEG, imaging, and surgical features. Epilepsy Res. 2003;55:131–136. doi: 10.1016/s0920-1211(03)00118-9. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry. 2013;73:622–630. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Itakura E, Nelson GM, Sheng M, Laurent P, Fenk LA, Butcher RA, Hegde RS, de Bono M. IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature. 2017;542:43–48. doi: 10.1038/nature20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, Sun Y. Emerging tendency towards autoimmune process in major depressive patients: a novel insight from Th17 cells. Psychiatry Res. 2011;188:224–230. doi: 10.1016/j.psychres.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkhurst CN, Muratet M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpitts SL, Kasper LH. Influence of the Gut Microbiome on Autoimmunity in the Central Nervous System. J Immunol. 2017;198:596–604. doi: 10.4049/jimmunol.1601438. [DOI] [PubMed] [Google Scholar]
- Davami MH, Baharlou R, Ahmadi Vasmehjani A, Ghanizadeh A, Keshtkar M, Dezhkam I, Atashzar MR. Elevated IL-17 and TGF-beta Serum Levels: A Positive Correlation between T-helper 17 Cell-Related Pro-Inflammatory Responses with Major Depressive Disorder. Basic Clin Neurosci. 2016;7:137–142. doi: 10.15412/J.BCN.03070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FP, Kanno Y, O’Shea JJ. A Metabolic Switch for Th17 Pathogenicity. Cell. 2015;163:1308–1310. doi: 10.1016/j.cell.2015.11.033. [DOI] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hernandez-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell host & microbe. 2012;11:425–435. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MH, Zheng QF, Jia XZ, Li Y, Dong YC, Wang CY, Lin QY, Zhang FY, Zhao RB, Xu HW, et al. Neuroprotection effect of interleukin (IL)-17 secreted by reactive astrocytes is emerged from a high-level IL-17-containing environment during acute neuroinflammation. Clin Exp Immunol. 2014;175:268–284. doi: 10.1111/cei.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama K, Gonzalez-Martin A, Kim BS, Jin HY, Jin W, Xu W, Sabouri-Ghomi M, Xu S, Zheng P, Xiao C, et al. The MicroRNA-183–96–182 Cluster Promotes T Helper 17 Cell Pathogenicity by Negatively Regulating Transcription Factor Foxo1 Expression. Immunity. 2016;44:1284–1298. doi: 10.1016/j.immuni.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jankovic D, Kugler DG, Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 2010;3:239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Kim YK, Hwang JA, Yoon HK, Ko YH, Han C, Lee HJ, Ham BJ, Lee HS. Plasma Levels of IL-23 and IL-17 before and after Antidepressant Treatment in Patients with Major Depressive Disorder. Psychiatry Investig. 2013;10:294–299. doi: 10.4306/pi.2013.10.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Kallies A. T cell responses in the central nervous system. Nat Rev Immunol. 2017;17:179–194. doi: 10.1038/nri.2016.144. [DOI] [PubMed] [Google Scholar]
- Krausgruber T, Schiering C, Adelmann K, Harrison OJ, Chomka A, Pearson C, Ahern PP, Shale M, Oukka M, Powrie F. T-bet is a key modulator of IL-23-driven pathogenic CD4(+) T cell responses in the intestine. Nat Commun. 2016;7:11627. doi: 10.1038/ncomms11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E. Neuroimmunology: IL-17A mediates a path to autism. Nat Rev Immunol. 2016;16:205. doi: 10.1038/nri.2016.35. [DOI] [PubMed] [Google Scholar]
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A. The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis. 2012;15:183–187. doi: 10.1111/j.1756-185X.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo D, Filho AJ, Soares de Sousa CN, Quevedo J, Barichello T, Junior HV, Freitas de Lucena D. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord. 2017;208:22–32. doi: 10.1016/j.jad.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynes DM, Vanner SJ, Lomax AE. Participation of interleukin 17A in neuroimmune interactions. Brain Behav Immun. 2014;41:1–9. doi: 10.1016/j.bbi.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A, Ahmad SF, Al-Harbi NO, Fardan AS, El-Sherbeeny AM, Ibrahim KE, Attia SM. IL-17A causes depression-like symptoms via NFkappaB and p38MAPK signaling pathways in mice: Implications for psoriasis associated depression. Cytokine. 2017;97:14–24. doi: 10.1016/j.cyto.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol. 2017;14:143–159. doi: 10.1038/nrgastro.2016.191. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Rieder R, Wisniewski PJ, Alderman BL, Campbell SC. Microbes and mental health: A review. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Rothhammer V, Heink S, Petermann F, Srivastava R, Claussen MC, Hemmer B, Korn T. Th17 lymphocytes traffic to the central nervous system independently of alpha4 integrin expression during EAE. J Exp Med. 2011;208:2465–2476. doi: 10.1084/jem.20110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee JY, Ziel JW, Miraldi ER, Domingos AI, et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell. 2015;163:381–393. doi: 10.1016/j.cell.2015.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, et al. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33:424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–387. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A. Inflammation and epilepsy. Epilepsy Curr. 2005;5:1–6. doi: 10.1111/j.1535-7597.2005.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sundrud MS, Skepner J, Yamagata T. Targeting Th17 cells in autoimmune diseases. Trends Pharmacol Sci. 2014;35:493–500. doi: 10.1016/j.tips.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ke KF, Liu Z, Qiu YH, Peng YP. Th17 cell-mediated neuroinflammation is involved in neurodegeneration of abeta1–42-induced Alzheimer’s disease model rats. PLoS One. 2013a;8:e75786. doi: 10.1371/journal.pone.0075786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhen H, Yao W, Bian F, Mao X, Yang X, Jin S. Antidepressant drug, desipramine, alleviates allergic rhinitis by regulating Treg and Th17 cells. Int J Immunopathol Pharmacol. 2013b;26:107–115. doi: 10.1177/039463201302600110. [DOI] [PubMed] [Google Scholar]
- Zimmermann J, Krauthausen M, Hofer MJ, Heneka MT, Campbell IL, Muller M. CNS-targeted production of IL-17A induces glial activation, microvascular pathology and enhances the neuroinflammatory response to systemic endotoxemia. PLoS One. 2013;8:e57307. doi: 10.1371/journal.pone.0057307. [DOI] [PMC free article] [PubMed] [Google Scholar]


