Abstract
Background
Pre-exposure prophylaxis (PrEP) for HIV has been available since 2012. Even so, PrEP has not been widely accepted among healthcare providers and MSM some of whom are convinced that PrEP decreases condom use, and increases sexually transmitted infections (STIs).
Design
A systematic review of the state of the evidence regarding the association of PrEP with condom use, STI incidence and change in sexual risk behaviors in MSM. A structured search of databases resulted in 142 potential citations, but only ten publications met inclusion criteria and underwent data abstraction and critical appraisal.
Methods
An adapted Cochrane Collaboration domain based assessment tool was used to critically appraise the methodological components of each quantitative study, and the Mixed Methods Appraisal Tool (MMAT) was used to critically appraise qualitative and mixed-methods studies.
Results
Condom use in MSM utilizing PrEP is influenced by multiple factors. Studies indicate rates of STIs in treatment and placebo groups were high. PrEP did not significantly change STI rates between baseline and follow-up. Reporting of sexual risk improved when questionnaires were completed in private by clients. Our review found that PrEP may provide an opportunity for MSM to access sexual health care, testing, treatment and counselling services. We did not find any conclusive evidence that PrEP users increase sexual risk behaviors.
Conclusion
The perception among healthcare providers that PrEP leads to increased sexual risk behaviors has yet to be confirmed. In order to provide effective sexual health services, clinicians need to be knowledgeable about PrEP as an HIV prevention tool.
Relevance to clinical practice
In an era where HIV prevention methods are rapidly improving strategies for STI testing, treatment, counselling and prevention remain vital in order to improve health. All healthcare providers are uniquely positioned to promote sexual health through the dissemination of accurate information.
Keywords: Sexual Health, Sexuality, Health Promotion, HIV, Inequalities in Health, Risk Groups, Behavior
INTRODUCTION
The introduction of pre-exposure prophylaxis (PrEP) in 2012 for HIV in the form of Truvada® (emtricitabine + tenofovir dispoproxil fumarate) provided a new tool to help prevent HIV transmission. PrEP is a once-daily pill that is simple to use with low toxicity and good efficacy in reducing the risk of HIV acquisition (World Health Organization, 2012b). Event based dosing of PrEP—at least one pill 24 hours before and one pill 24 hours after sex—is an alternative dosing form in some countries (Molina et al., 2015). Globally, it is estimated MSM have a 19.3-fold greater odds of becoming infected with HIV compared to the general population (United Nations Population Fund, 2015). In light of this, MSM are a high risk population appropriately targeted for PrEP initiation.
The introduction of PrEP as an HIV prevention method is not without controversy and concern has been raised that it may lead MSM to think that they no longer need other preventive measures such as condoms (United Nations Population Fund, 2015; Venter, Allais, & Richter, 2014). The support for this concern is evidenced in qualitative PrEP studies which report 35%–60% of high-risk MSM believed that they would be less likely to use a condom if they were on PrEP (Brooks et al., 2012; Golub, Kowalczyk, Weinberger, & Parsons, 2010; Hoff et al., 2015)
While PrEP reduces the risk of HIV transmission it does not provide any protection against other sexually transmitted infections (STIs), which can only be prevented by consistent condom use, mutual monogamy or abstinence (World Health Organization, 2012b). STIs disproportionally affect MSM and continue to escalate in this population (World Health Organization, 2012a). The introduction of PrEP is viewed by many as fueling the recent upsurge in STIs (Kennedy 2014; World Health Organization, 2012b). STIs continue to be a major public health issue and it is estimated globally there are 357 million new cases a year (World Health Organization, 2012a). Reports of STI treatment failure rates are between 13% and 21% in MSM, and different STIs have re-emerged with a vengeance such as resistant strains of gonorrhea and chlamydia, ophthalmic and otic syphilis, and even neuro syphilis (Bissessor et al., 2015; World Health Organization, 2012a).
In many cultures, MSM are unable to access sexual health services as homosexuality is illegal and in some cases punishable by death, leaving them at high risk for HIV acquisition(World Health Organization, 2007). Even when healthcare access is not an issue, medical providers report being ill-equipped to discuss sexual health and are concerned that sexual risk behaviors, and in turn STI rates, will increase if they prescribe PrEP. Furthermore, PrEP for HIV prevention remains contentious because some medical providers fear they will be seen as condoning condomless sex (Venter et al., 2014). In order to help medical providers prescribe PrEP, the World Health Organization (WHO) has published clear guidelines that recommend the use of PrEP in conjunction with condoms (World Health Organization, 2012b).
The increase in the apparent incidence of STIs among PrEP users may be related to STI testing required by PrEP protocols in clinical practice (Cohen, Lo, Caceres, & Klausner, 2013). Historically, similar patterns occurred with the introduction of highly active antiretroviral therapy (HAART) for HIV in the mid 1990’s. As STI rates began to rise among MSM presenting for HIV treatment, some researchers hypothesized that risk compensation, rather than increased testing during the HIV treatment process, was to blame (Myers & Sepkowitz, 2013). In fact, the apparent rates of syphilis, rectal gonorrhea and chlamydia in MSM have been increasing since 2009—several years before PrEP was introduced to clinical practice—and rates continue to increase in countries were PrEP is not yet available (Bissessor et al., 2015; World Health Organization, 2012a). This suggests that PrEP alone cannot account for the observed increase in STI rates among its users. It may instead be that programs targeting MSM for HIV/STI testing and treatment are actually the cause of the reported increase in STIs. Similarly, another biomedical intervention that had biases comparable to PrEP was the oral contraceptive pill, which many assumed would lead to adverse results and an increase in sexual risk behavior (Fenton, 2010). When the pill was first brought to market, it required a woman to be married. Later, when the pill could be prescribed for any woman regardless of marital status, gonorrhea diagnoses increased and the working theory was that the unintended consequences were due to condomless sex, rather than more effective testing protocols for STIs (Myers & Sepkowitz, 2013) that were implemented at the same time. A parallel debate continues among healthcare providers, political entities and the MSM community whether PrEP is a direct link to decrease in condom use among MSM secondary to a decreased fear of being infected with HIV—just as it was feared the pill would lead to a decrease in condom use secondary to a decreased fear of becoming pregnant.
AIMS
The purpose of this study was to review the literature on the association of PrEP use with condom use, STI incidence, and change in sexual behaviors, such as anal sex and number of partners in MSM.
The research questions were:
How does PrEP use affect condom use in MSM?
How does PrEP use affect STI incidence in MSM?
How can transmission risks for HIV/STI (e.g., type of anal sex, number of partners) be assessed in MSM using PrEP?
METHODS
Design
An adapted Cochrane Collaboration domain based assessment tool was used to critically appraise the methodological components of each quantitative study (Higgins et al., 2011), and the Mixed Methods Appraisal Tool (MMAT) (Pluye, 2011) was used to critically appraise qualitative and mixed-methods studies. Qualitative and mixed-methods studies were included as they are better suited for exploring the complexities of sexual risk perception, behavior change and attitudes towards new biomedical prevention tools (Whittemore & Knafl, 2005).
Search methods
A search of current PrEP literature, which utilized medical subject headings (MeSH) and keywords to identify studies of potential interest, was constructed with the aid of an expert librarian at the University of California San Francisco. Literature searches using PubMed, Embase, Medline, PsychINFO®, Web of Science®, CINHAL and Google Scholar were performed. Initially, the following MeSH search terms were entered into the PubMed database to determine if they were appropriate: sexual partners, sexual behavior, sexually transmitted infections, sexually transmitted diseases, sexual health, gay, bisexual, homosexual, sexual risk, sexual behavior, pre-exposure prophylaxis for HIV, syphilis, gonorrhea, chlamydia, anal sex, condom use, condomless sex, biomedical HIV prevention, risk compensation, harm reduction and risk reduction. The following MeSH search string was considered most appropriate: “Sexually Transmitted Diseases”[MeSH]) AND “Pre-Exposure Prophylaxis”[MeSH]) AND “Sexual Behavior”[MeSH]) AND “Homosexuality, Male”[MeSH].” The search was expanded by including sentinel research papers and secondary analyses of the body of work identified in the database search, and a review of meeting abstracts. Inclusion criteria for studies were as follows: 1) English language; 2) PrEP for HIV prevention; 3) included MSM and transwomen; and 4) discussion of STI rates or condom use during study. Exclusion criteria were: 1) studies that only described women, children or heterosexuals; 2) studies that describe pre-exposure prophylaxis for other diseases (e.g. doxycycline for syphilis (Bolan et al., 2015); and 3) articles published prior to 2010 due to a paucity of data.
Search results
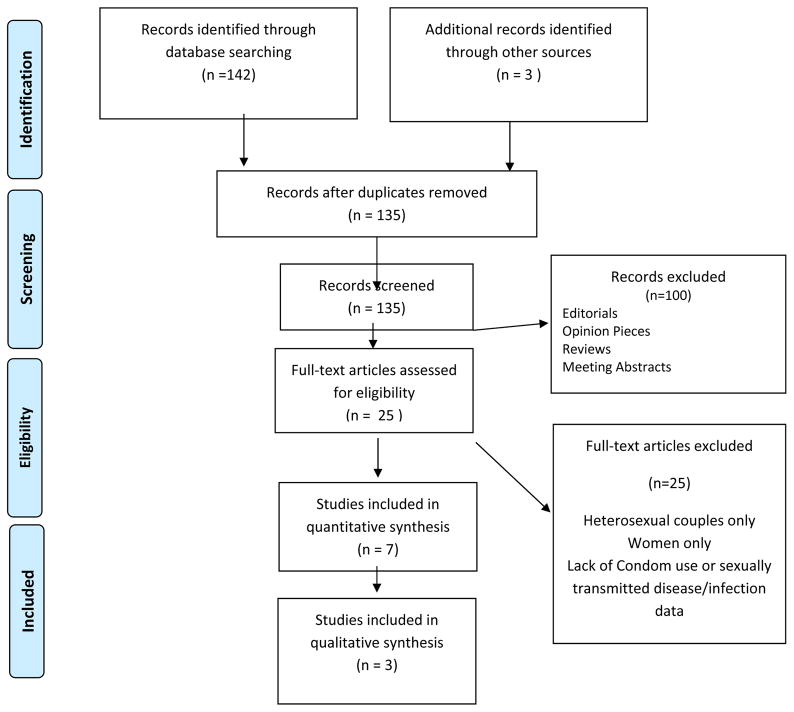
The electronic database search generated 142 potential citations, published between January 2010 and January 2016, and another 3 were identified by searching for sentinel studies (IPERGAY, PROUD, iPrEx). All titles and abstracts were screened and reviewed by the first author and Dr. David Vlahov, who has authored a body of work on HIV, risk behavior and MSM. Initially there was a disagreement regarding the inclusion of qualitative and mixed method studies as they did not include STI screening data, but upon further review it was agreed the insights they provided into PrEP related change in condom use, STIs and capturing change in sexual practices were valuable. Figure 1 presents a flow diagram of the selection and review process. After inclusion and exclusion criteria were met, and systematic reviews, practice guidelines, editorials, meeting abstracts, meta-analyses, duplicates and unrelated articles were excluded, the final number was 10.
Figure 1.
PRISMA Flow Chart
RESULTS
Characteristics of the Studies
Table 2 displays the 10 reviewed studies, which were published between 2010 and 2016; three were randomized control trials (RCTs) (Grant et al., 2010; Marcus et al., 2013; Molina et al., 2015), one began as an RCT but was changed to an open label trial (McCormack et al., 2015), one was a community open label trial (Liu et al., 2016), one was a prospective cohort study (Volk et al., 2015), one was a survey (Golub et al., 2010), two were mixed methods (Brooks et al., 2012; Hoff et al., 2015) and one was qualitative (Hojilla et al., 2015). The RCTs and open label trials had Truvada provided by Gilead at no cost to the participants (Grant et al., 2010; Liu et al., 2016; McCormack et al., 2015; Molina et al., 2015) The prospective cohort study was limited to individuals who accessed care through the Kaiser healthcare system. For that study, Truvada was not provided by Gilead and clients had a copayment toward drug cost between 30 and 50 USD (Volk et al., 2015). Daily dosing of PrEP was used in 9 studies and event based dosing was used in one study (Molina et al., 2015).
Table 2.
Overview of Studies
| Author | Grant | Golub | Brooks | Marcus | Molina | McCormack | Volk | Hojilla | Hoff | Liu |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2010 | 2012 | 2012 | 2013 | 2015 | 2015 | 2015 | 2015 | 2015 | 2016 |
| N | 2499 | 180 | 50 | 1743 | 400 | 544 | 657 | 26 | 219 | 557 |
| Design | RCT | Quantitative Survey | Mixed Methods | RCT | Double Blind RCT | Open Label RT | Prospective Cohort Study | Framework Analysis | Mixed Methods | Open Label |
| Age | 18–67 | 18–49 | 19–71 | 18–67 | 18–50+ | 29–43 | 20–68 | 21–63 | 19–71 | 18–45+ |
| Mean 27 | Mean 29 | Mean 37 | Mean 25 | Median 35 | Median 35 | Mean 37 | Median 37 | Median 36 | ||
| Participants | MSM or Trans-gender | MSM | Gay or Bisexual | MSM or Trans-gender | MSM or Trans-gender | MSM | MSM | MSM, Trans-gender | MSM | Male at Birth |
| Data Collection Methods | Baseline: CASI | ACAI | Interviewer Administered Survey | IAQ | CASI | Questionnaire | Secure Email Survey | Analysis of Counselling Notes | ACAI | IAQ |
| Follow up: In-Person Interview | Grounded Theory | Biomarkers & Physical Exam | 3-Point Testing | 3-Point Testing | 3-Point Testing | Grounded Theory | 3-Point Testing | |||
| Biomarkers & Physical Exam | Syphilis Testing | Syphilis Testing | Syphilis Testing | Syphilis Testing | ||||||
| Risk Reduction Counselling | Monthly for 6 Months, then Quarterly | n/a | n/a | Monthly | Monthly | Quarterly | Monthly or Quarterly | Monthly or Quarterly | n/a | Quarterly |
| STI Rates on PrEP | Syphilis: 13% | n/a | n/a | No Change | 41% | 50–57% | 50% | n/a | n/a | 50.9% |
| Partners | ↓ | ↔ | ↔ | ↑ | ↔ | ↑ | ↔ | ↑ ↓ | ↔ | ↓ |
| Anal Sex | ↓ | ↑ | ↑ | ↑ | ↔ | ↑ | n/a | ↑ | ↔ | ↓ |
| Condom Use | ↑ | ↓ | ↓ | ↑ | ↔ | n/a | ↔ | ↓ | ↓ | ↔ |
| Dosing | Daily | Hypothetical Daily | Hypothetical Daily | Daily | Before & After Sex | Daily | Daily | Daily | Hypothetical Daily | Daily |
3 point testing = GC/CT pharyngeal, anal and urethral. ↑ = increased, ↓ = decreased, ↔ = no change. CASI = Computer assisted structured interview.
ACAI = Audio Computer assisted Interview. IAQ = Interview Assisted Questionnaire.
Inclusion and Exclusion Criteria For The Studies
The methods and inclusion criteria varied considerably across these studies. The common elements extracted to facilitate comparisons included MSM age 18 or over who were HIV seronegative, condom use, sexual risk behavior and STI data. Definitions of sexual risk included condomless anal intercourse, (receptive or insertive) and at least one partner in the past 3, 6, or 12 months (Brooks et al., 2012; Golub et al., 2010; Grant et al., 2010; Hoff et al., 2015; Hojilla et al., 2015; Liu et al., 2016; Marcus et al., 2013). Terms used to describe the target population varied among the studies: four studies referred to MSM as men having sex with men and transgender persons(Grant et al.; Hojilla et al., 2015; Marcus et al., 2013; Molina et al., 2015), four studies only used the term MSM and did not refer to any other subgroups of people (Golub et al., 2010; Hoff et al., 2015; Volk et al., 2015), one study used the term “gay and bisexual” (Brooks et al., 2012) and three studies defined men who have sex with men as “male at birth” (Grant et al., 2010; Marcus et al., 2013; Volk et al., 2015). Definitions of sexual risk varied as follows:
Grant et al., (2010): unprotected anal receptive sex in past 12 weeks; any transactional sex in past 6 months; known HIV positive partner; any unprotected anal intercourse with partner of positive or unknown HIV status.
Golub et al., (2010): instances of substance use with at least one incident of unprotected anal intercourse (insertive or receptive) with a casual or serodiscordant male partner in last 3 months.
Hojilla et al., (2015): any condomless anal sex (insertive or receptive) with two or more male or transgender female partners in past 12 months.
Brookes et al., (2012): HIV negative MSM in a serodiscordant relationship for 12 months or longer.
Marcus et al., (2013): any condomless anal sex (insertive or receptive).
Hoff et al., (2015): serodiscordant couples engaging in any anal sex (insertive or receptive) in past 3 months.
Volk et al., (2015): risk assessed by primary care provider before referral to PrEP clinic.
Molina et al., (2015): unprotected anal sex (insertive or receptive) with at least two partners in past six months.
McCormack et al., (2015): previously attended one the 13 screening clinics and had been screened for HIV/STIs; anal intercourse (insertive or receptive) without a condom in previous 90 days and likely to have condomless anal intercourse (insertive or receptive) in next 90 days.
Liu et al., (2015): condomless receptive anal sex with at least 2 male or transwomen partners; or at least 2 episodes of condomless anal sex (insertive or receptive) with at least 1 HIV infected partner; or sex with a male or transgender partner and being diagnosed with syphilis or anal GC/CT.
Exclusion criteria included medical contraindications to PrEP (Grant et al., 2010; Hojilla et al., 2015; Liu et al., 2016; Marcus et al., 2013; McCormack et al., 2015; Molina et al., 2015; Volk et al., 2015), not meeting risk criteria (Brooks et al., 2012; Golub et al., 2010; Hoff et al., 2015), and not agreeing to or not completing follow up visits (Hojilla et al., 2015; Volk et al., 2015). Participant retention rates in studies where drug or placebo was provided were 72–78%, but over the course of all of the studies, response rates for behavioral measures decreased.
Race
Participants were recruited from multiple locations including Peru, Ecuador, Brazil, Thailand, South Africa, USA, Canada, France and the UK Race was not clearly defined in all of the studies, with some not mentioning race or ethnicity and instead merely stating the site of the trial (Grant et al., 2010; Marcus et al., 2013; Molina et al., 2015). Therefore a comparison of differences in STI rates and condom use between races/ethnicities was not an element of review in this study.
Location
All of the studies were located in urban areas with high HIV prevalence rates among MSM (25–46%), such as San Francisco, New York, Miami, London, Paris, Montreal, Rio de Janerio, Chang Mai and Cape Town (Brooks et al., 2012; Centers for Disease Control and Prevention, 2013; Golub et al., 2010; Grant et al., 2010; Hoff et al., 2015; Hojilla et al., 2015; Liu et al., 2016; Marcus et al., 2013; McCormack et al., 2015; Molina et al., 2015; Volk et al., 2015).
Socioeconomic Status
Quantifying education status and income of participants across the studies was problematic due to studies having been conducted in 10 countries with differing educational structures and levels and differing definitions of income levels (Brooks et al., 2012; Centers for Disease Control and Prevention, 2013; Golub et al., 2010; Grant et al.; Hoff et al., 2015; Hojilla et al., 2015; Liu et al., 2016; Marcus et al., 2013; McCormack et al., 2015; Molina et al., 2015; Volk et al., 2015).
Age
The age range in the quantitative studies was 18–68, three studies reported mean ages which ranged from 25–37 years old (Grant et al., 2010; Marcus et al., 2013; Volk et al., 2015), one study only provided a range of 18–45+ (Liu et al., 2016), and two studies reported a median age of 35 (McCormack et al., 2015; Molina et al., 2015). The quantitative survey study had a smaller age range of 18–49 with a mean of 29 (Golub et al., 2010). In the mixed methods studies the age range was 19–71 with a median age of 36 (Brooks et al., 2012; Hoff et al., 2015). The qualitative study that utilized a Framework Analysis had a range of 21–63 with a median age of 37 (Hojilla et al., 2015).
Substance Use
Substance use, including intravenous drug use, was not measured consistently across studies, and thus it was not possible to draw conclusions about the associations between sexual risk behavior and substance use. For example, two of the RCT studies included methamphetamine, ecstasy and gamma-hydroxybutyrate (McCormack et al., 2015; Molina et al., 2015), and another only measured alcohol use (Grant et al., 2010). The open label study included amyl nitrite (poppers), erectile dysfunction drugs and heroin (Liu et al., 2016), and the prospective cohort study included cocaine and methamphetamine. The predominant finding was that substance use did not influence adherence to PrEP but how substance use influenced condom use and sexual risk behaviors varied (Grant et al., 2010; Hojilla et al., 2015; Liu et al., 2016; McCormack et al., 2015; Molina et al., 2015; Volk et al., 2015).
Measurements of Risk Behavior
All of the studies collected individual risk behavior data by using multiple methods, including interviewer-administered questionnaires and surveys (Brooks et al., 2012; Grant et al., 2010; Liu, 2015; Marcus, 2013; McCormack et al., 2015), secure email surveys (Volk et al., 2015), computer-assisted structured interviews (Golub et al., 2010; Grant et al., 2010; Hoff et al., 2015) and data from daily diaries (McCormack et al., 2015). Questions common across all studies included number of condomless anal receptive and insertive sex episodes, number of partners (both receptive and insertive anal with or without a condom) and intended or actual use of condoms since the last visit.
In one RCT study (Grant et al., 2010), in which baseline risk behavior was captured by computer-assisted self-interview (CASI) but follow up risk behavior was captured by in-person interview, study participants predominately reported no behavior change or a decrease in risk behavior. Similarly, the studies of Liu et al. (2015) and Marcus et al. (2013), which used in-person interviews only, revealed no behavioral change or less risk taking between baseline and follow up. In contrast, McCormack et al. (2015) used questionnaires completed in private and risk behavior was reported to have increased, while Molina et al. (2015), using CASI only, found either no change or an increase in reported risk behavior between baseline visits and follow up. The mixed methods and survey studies which utilized CASI also found that there was either no change or an increase in reported risk behavior between baseline and follow up (Brooks et al., 2012; Golub et al., 2010; Hoff et al., 2015). One cohort study (Volk et al., 2015) found that participants responding to emails from healthcare providers reported no change in sexual risk, while participants responding to emails from non-healthcare providers reported increased sexual risk between baseline and follow up. Reliability for reporting risk during sexual activities has been shown to decrease over time (Kauth, 1991). To control for decreased risk reporting, daily diaries and monthly questionnaires were initiated in one RCT, but were subsequently abandoned due to a low response rate. In contrast, baseline and 12-month questionnaires, by paper and pencil or computer, in the same study yielded a higher response rate (McCormack et al., 2015).
Condom Use Change
Condom use change associated with PrEP was variable throughout the studies reviewed. The data from the blinded RCTs showed either no change (Molina et al., 2015) or increased use of condoms and only a ~4% decrease in condomless anal sex after stopping the study (Grant et al.; Marcus et al., 2013). Conversely, the open label and cohort trials reported an increase in the number of condomless receptive anal sex partners if on PrEP (Liu et al., 2016; Volk et al., 2015). In the PROUD study (McCormack et al., 2015), participants were randomized to begin PrEP either at the start of the study or after one year. Those who began on PrEP reported a larger increase in receptive anal sex without a condom with ten or more partners (21% vs. 12%; p=0.03, test for trend) at month 12 of the study. Participants also reported an increase of 14% in first time condomless receptive anal sex, however, there was no significant difference between placebo and PrEP groups, and therefore the authors did not consider this to represent a behavior change. This finding was also reflected in the secondary analysis of the iPrEx study: if a participant responded strongly believing he was taking PrEP the mean number of partners increased from 7.7 to 12.8 (p=0.04) (Marcus et al., 2013). Conversely, the open label and cohort trials reported an increase in the number of condomless receptive anal sex partners if on PrEP (Liu et al., 2016; Volk et al., 2015).
In the Golub et al. (2010) survey study the participants were told that PrEP was 80% effective against HIV and then asked what impact this would have on their condom use. Responses were dichotomized into, likely or not likely to decrease condom use on PrEP, with 35% of participants reporting that their condom use would likely decrease. In the Brooks et al. (2012) mixed methods study, in which PrEP efficacy was presented as 92% effective against HIV, thematic content analysis revealed a perception that PrEP provided an alternative to condom use for HIV prevention. Neither the quantitative survey nor the mixed methods studies discussed the importance of adherence to medication in order to provide the proposed high levels of protection against HIV which may have influenced the overall results (Brooks et al., 2012; Golub et al., 2010; Hoff et al., 2015).
Decreased condom use had interesting associations with a number of other variables. For example, in the iPrEx study participants under 25 years of age were more likely to report condomless anal receptive sex at follow up than those age 25 or older (Grant et al., 2010; Marcus et al., 2013). Conversely, in the mixed methods studies older MSM were more likely to decrease condom use (Brooks et al., 2012; Hoff et al., 2015). Situational factors, such as geographical location and place of sexual encounter also affected condom use change, with San Francisco participants reporting the greatest decrease in condom use compared to other locations (Liu et al., 2016), and encounters in bath houses more likely to involve condomless sex (Hojilla et al., 2015). Decreased condom use was associated with certain relationship factors, for example, if an HIV negative partner were using PrEP, his HIV positive partner would feel more comfortable barebacking (Hoff et al., 2015). Other variables that showed associations with decreased condom use included mental health issues, such as depression (Liu et al., 2016; Marcus et al., 2013); perception of partners being low risk (Hojilla et al., 2015); and socioeconomic factors, such as higher income and having a college degree (Golub et al., 2010; Hoff et al., 2015). Of note, substance use was found to decrease condom use (Golub et al., 2010; Hojilla et al., 2015) or have no effect on condom use change (Liu et al., 2016; McCormack et al., 2015; Volk et al., 2015).
Sexually Transmitted Infections
In order to verify whether condomless sex had occurred, participants in certain studies were tested for biomarkers of GC/CT (Liu et al., 2016; McCormack et al., 2015; Molina et al., 2015; Volk et al., 2015) and syphilis (Grant et al., 2010; Liu et al., 2016; Marcus et al., 2013; McCormack et al., 2015; Molina et al., 2015). The biomarkers used were all validated and are currently used in practice. Treatment for STIs was provided per local guidelines in all studies. There was no significant difference in STI rates between participants on PrEP and those not on PrEP in the RCT studies (Grant et al., 2010; Marcus et al., 2013; McCormack et al., 2015).
Assessment and testing for STIs was completed at baseline in six studies, but how the information was collected was not uniform across the studies as shown in Table 1. Three-point testing for GC/CT (urethral, anal and pharyngeal) and syphilis was completed in four studies at baseline and every 12 weeks (Liu et al., 2016; McCormack et al., 2015; Molina et al., 2015; Volk et al., 2015). STI incidence was high in the studies that consistently screened for anal, pharyngeal, and urethral GC/CT, and syphilis. For example, two of the RCT studies found rectal GC/CT in 32–39% of participants (McCormack et al., 2015; Molina et al., 2015) and syphilis infections ranged from 5 to 11% (Liu et al., 2016; McCormack et al., 2015; Volk et al., 2015).
Table 1.
Data collected and Methods of diagnosing STIs.
| Study | STI Data Collected | STI Diagnostic Methods |
|---|---|---|
| Grant et al., 2010 | Warts, Herpes, Syphilis, Urine leukocyte esterase positive | Physical examination, Symptoms, Partner diagnosis, Biological markers |
| Marcus et al., 2013 | Syphilis, GC/CT | Physical examination, Symptoms, Biological markers |
| McCormack et al., 2015 | Syphilis, GC/CT, HCV | Biological markers |
| Molina et al., 2015 | Syphilis, GC/CT, HCV, Herpes | Biological markers |
| Volk et al., 2015 | Syphilis, GC/CT, HCV | Biological markers |
| Liu et al., 2016 | Syphilis, GC/CT, Herpes | Biological markers |
The iPrEx study only analyzed urethral GC/CT samples if leukocytes were present in the urine and urethral screening for asymptomatic urethritis occurred every 24 weeks. There were no anal biomarker tests for GC/CT and reporting relied on exam and self-reports of symptoms or exposure making syphilis the only STI with consistent biomarker testing (Grant et al., 2010). STI rates were similar in treatment and placebo groups at all time points there were no significant between-group differences in the numbers of subjects with gonorrhea or chlamydia during follow-up. Syphilis cases decreased during follow up in both treatment arms (P trend < 0.001) (Grant et al., 2010). The PROUD study also found no significant difference in STI rates between participants on PrEP and those not on PrEP despite participants on PrEP reporting increased number of partners and an increase in receptive anal sex (McCormack et al., 2015). Liu et al. (2016) found that 51% of participants were diagnosed with one STI (CT, GC or syphilis) at baseline, with an initial decrease in rectal and pharyngeal GC/CT at 6 months followed by an increase at 12 months (p < 0.05) and a final overall STI positive rate similar to the baseline STI rate (Liu et al., 2016). Two studies did not include baseline STI data (Molina et al., 2015; Volk et al., 2015) but found increases in STIs over time at multiple follow-up visits, specifically in anal GC/CT, despite no reported changes in number of partners and similar rates of condom use throughout.
The mixed methods studies did not collect STI data however, between 30–40% of participants discussed being concerned about STI risk if they decreased condom use secondary to being on PrEP (Brooks et al., 2012; Hoff et al., 2015). Neither the survey study (Golub et al., 2010) nor the framework analysis included questions or data regarding STIs (Hojilla et al., 2015).
Impact of Risk Reduction Counselling on Behavior
All of the RCTs and open label trials provided risk reduction counselling at least on a monthly basis, as well as condoms and lubricant (Grant et al., 2010; Liu et al., 2016; McCormack et al., 2015; Molina et al., 2015). Only one study described implementing and training counsellors in the use of, a risk reduction model (RESPECT), and risk behaviors during that study did not change from baseline (Molina et al., 2015). In contrast, in the Grant et al. (2010) blinded RCT where risk reduction counselling and HIV/STI tests were provided at the same visit, risk reporting decreased. In the prospective cohort study that implemented unspecified risk reduction counselling, reports of sexual risk increased from baseline (Volk et al., 2015). Conversely, in the open label study number of anal sex partners decreased in a response to risk reduction counselling (Liu et al., 2016).
In the two mixed methods studies and the quantitative survey, participants did not receive any risk reduction counselling, condoms or lubricant because hypothetical scenarios were used (Brooks et al., 2012; Golub et al., 2010; Hoff et al., 2015). This may account for the reports of increased risk behaviors compared to the studies were risk reduction counselling was included.
In the qualitative study, participants received risk reduction counselling, condoms and lubricant at every visit. The analysis of counselling notes revealed that the risk reduction counselling provided to PrEP users included guidance on serosorting (having sex with HIV negative partners only), seroadaptation (asking sex partners their HIV status), and seropositioning (oral or insertive anal sex with HIV positive partners) and condom use. Counselling on the combination of these methods is reported to decrease sexual risk behaviors in this cohort (Hojilla et al., 2015).
DISCUSSION
Our review found that offering PrEP services provides an opportunity for MSM to access sexual health care, testing, treatment and counselling that would not be accessed otherwise. Although STI rates were high in this population, we did not find any conclusive evidence that PrEP use leads to increased sexual risk behaviors. Counselling regarding condom use and STI testing at every encounter improved compliance and should be a fundamental component of PrEP services. Adherence to a PrEP regimen, whether daily dosing or event based, is vital to preventing HIV infection. STI testing should include extra-genital testing in MSM regardless of PrEP use, in order to prevent health deficits and onward transmission. In the studies reviewed, providing privacy for MSM to complete health questionnaires improved accurate risk reporting, which allows clinicians to address behaviors that increase the risk of HIV/STIs. Event based dosing of PrEP—at least one pill 24 hours before and one pill 24 hours after sex—had a low adherence rate of 43%, and PrEP was only effective if a median of 15 or more pills per month were taken (Molina et al., 2015). Clinicians should counsel MSM that protection against HIV acquisition is dependent on adequate levels of TDF-FTC in their system.
Measuring risk behavior is complex. Our review represents the first effort, to our knowledge, to synthesize evidence regarding the association of PrEP use among MSM with changes condom use, STI incidence and sexual risk behaviors. How PrEP effects condom use in MSM is challenging to interpret from the data available. All of the trials used self-report of condom use and the response rate was low. Regardless of response rate, validity of self-report is difficult to measure and is often influenced by the individual feeling compelled to report the “correct” answer (Zenilman et al., 1995). This may explain why there was a 56 to 74% report of no change in condom use in the studies where participants were counseled to use condoms (Grant et al., 2010; Hojilla et al., 2015; Liu et al., 2016; Marcus et al., 2013; McCormack et al., 2015; Molina et al., 2015; Volk et al., 2015). Furthermore, none of the studies included questions about condom use before PrEP initiation, making it impossible to assess if “no change” meant using or not using condoms. And paradoxically, two of the trials reported an increase in condom use without any significant changes in STI rates, further suggesting that self-report of condom use is methodologically unreliable.
STI biomarker analysis is a commonly used method for quantifying sexual risk behaviors such as condom use (Hewett et al., 2008; Hotton, Gratzer, & Mehta, 2012). However, there are some behaviors for which biomarkers are ill-suited (e.g., sexual frequency or needle sharing) (Hojilla et al., 2015; Kauth, 1991; Weinhardt, Forsyth, Carey, Jaworski, & Durant, 1998). From a research perspective, relying solely on self-reports or physiologic data limits our ability to understand the full-scope of the phenomena of sexual risk behavior. Understanding sexual risk behavior necessitates a highly complex framework to conceptualize every facet of the physiological, intellectual, emotional, situational, social, cultural, legal and moral range of issues. In general, individuals consider sexual risk to equal a negative outcome, and within the MSM community for a long period of time the negative outcome was HIV acquisition. The ability to intervene clinically, with biomedical interventions such as PrEP, necessitates that healthcare providers have an understanding of how and why changes in sexual risk behaviors may occur. For example, Liu et al. (2016) noted that geographical location influenced a change in condomless receptive anal sex highlighting the need for clinicians to be aware of the accepted norms of sexual behavior in their communities when discussing PrEP.
The perception among healthcare professionals that PrEP will lead to increased risk behaviors (Venter et al., 2014; Wilton, Senn, Sharma, & Tan, 2015) has yet to be confirmed. A number of the studies reviewed here did show high overall rates of STIs in MSM on PrEP or placebo (33–57%), yet none of them reported a significant change in STI rates between baseline and follow-up. In fact one study found no change over time in rates of rectal GC/CT despite participants reporting increased number of partners and increased receptive anal sex (McCormack et al., 2015). Furthermore, while there may be a perception that it is PrEP that leads to an increase in STI rates, many of the participants had never been tested for STIs before entering a PrEP study. The emergence of PrEP as an HIV prevention tool may increase STI testing and treatments in MSM and in turn help reduce onward transmission of STIs. Moreover, the STI rates in the studies are similar to current STI rates among MSM, regardless of PrEP status, as reported by the World Health Organization (World Health Organization, 2012a), making frequent STI counselling, testing and treatment a priority worldwide to prevent health deficits.
There is some evidence within the reviewed studies that risk compensation may occur on PrEP. There was a common theme that PrEP reduced the anxiety around sexual acquisition of HIV (Brooks et al., 2012; Golub et al., 2010; Hoff et al., 2015; Hojilla et al., 2015), thus creating a perception among MSM that the need for condom use is eliminated. For example, many HIV seropositive partners expressed they would be comfortable engaging in condomless anal sex if their partner was on PrEP (Brooks et al., 2012; Golub et al., 2010; Hoff et al., 2015). However, the influence of partners’ preference not to use condoms was not measured in many of the studies, even though this has been found to have a significant influence on condom use (O’Neal & Berteau, 2015). Furthermore, a common finding was a change in sexual practice from being exclusively anally insertive, to experimenting with being anally receptive after PrEP initiation (Grant et al., 2010; Hojilla et al., 2015; McCormack et al., 2015; Molina et al., 2015; Volk et al., 2015). This willingness to experiment sexually may be attributed to a reduction in anxiety of HIV acquisition.
In order to capture risk behavior the studies made an effort to collect individual risk behavior using multiple methods that included, interviewer administered questionnaires (Liu et al., 2016; Marcus et al., 2013; McCormack et al., 2015), secure email survey (McCormack et al., 2015; Volk et al., 2015), computer-assisted self interview (Golub et al., 2010; Grant et al., 2010; Hoff et al., 2015; McCormack et al., 2015), daily diaries and paper and pencil questionnaires (McCormack et al., 2015), and review of counselling notes (Hojilla et al., 2015). In the studies reviewed herein, computer-assisted self interviews (CASIs) produced better response rates than other methods used to capture sexual risk behavior. This is consistent with the literature that has shown higher rates of risk behavior reported in CASIs than in interviewer-administered questionnaires (Macalino, Celentano, Latkin, Strathdee, & Vlahov, 2002; (Kurth et al., 2004). Participants questioned using CASI methodologies typically report number of partners and frequency of condomless sex more openly than those interviewed face-to-face, an observation that has been attributed to greater privacy for reporting socially sensitive behaviors (McAuliffe, DiFranceisco, & Reed, 2007) and socially acquiescent responses that occur with in-person interviews (Fenton, Johnson, McManus, & Erens, 2001). Behavior disclosure is often influenced by the desire to create a positive social image (Holtgraves, 2004), which may have been reflected in the results of in-person interviews. Such social desirability influence may explain why there was a decline in risk behavior reports in one RCT where the baseline behavior risk was captured by CASI but follow up risk behaviors were captured by in-person interviews (Grant et al., 2010).
The response rate for the secure email questionnaires was lower than for interviewer administered questionnaires, which may have been a consequence of the higher level of language and computer literacy required for email compared to interviewer administered questionnaires (Weinhardt et al., 1998). Where participants were required to respond to an email received from a healthcare provider the majority reported no change in sexual risk behavior (Volk et al., 2015), which may have been because this was perceived as being part of the medical record. None of the studies in this review included validity or reliability reports for the methods used to capture risk behavior. Such reports are unfortunately uncommon in sexual health research, even though suitable methodologies have been developed and tested (McAuliffe, DiFranceisco, & Reed, 2007).
Additionally, in the studies reviewed, representation of MSM under age 25, who are considered to be at greatest risk for HIV/STIs was low, 5%–30% (World Health Organization, 2012b). Data from the RCTs reflected this finding (Grant et al., 2010; Marcus et al., 2013; McCormack et al., 2015; Molina et al., 2015) with the majority of participants being in their thirties. One might speculate that this was due to decreased risk awareness or a lack of information regarding new HIV prevention tools (Underhill et al., 2015). Barriers to younger PrEP users may include inexperience negotiating healthcare systems, reluctance to discuss sexual preferences with healthcare providers, fear of parents being informed, and cost (Kubicek, Arauz-Cuadra, & Kipke, 2015). For example, in the prospective cohort study (Volk et al., 2015), which was set in a large US healthcare institution, the mean age of participants was 37. One possible explanation for this is that, while other studies provided PrEP free of charge, the Volk et al. (2015) study required a payment, which some younger MSM may not have been able to afford. Cost has been found to be a barrier to accessing PrEP and a barrier to adherence to other medications (Chakrapani et al., 2015; Eaddy, Cook, O’Day, Burch, & Cantrell, 2012; Gersh, 2014). However, there are currently no data available outside of a study context regarding the effect of cost on adherence to PrEP. Volk et al. (2015) was also the only study requiring a primary healthcare provider determination of a need for PrEP. In contrast, screening in the other studies outside of a large healthcare system at “gay-friendly” sites merely required participants to state that they wanted PrEP. However, even at such gay-friendly sites, participation by those under 25 did not increase (Grant et al., 2010; Liu et al., 2016; McCormack et al., 2015; Molina et al., 2015). All of the studies included a HIV risk component in their inclusion criteria, which may have inadvertently alienated younger MSM. The low uptake of PrEP by young MSM has been identified in other studies and may be secondary to individuals not having established a gay identity, a misconception that PrEP is synonymous with promiscuity and a lack of insight into risk behavior within communities with high HIV prevalence (Knight, Small, Carson, & Shoveller, 2016; Kubicek et al., 2015). Healthcare professionals should take these factors into consideration when interpreting the available PrEP data for younger MSM. In view of the fact that younger MSM are one of the populations in which HIV rates are increasing, this review suggests that neither an individual’s ability to pay, nor a clinician’s assessment of risk, should be prerequisites for access to PrEP.
There are methodological concerns throughout all of the studies. For instance, the mixed methods and quantitative survey studies used hypothetical scenarios with a guaranteed 80% or greater protection against HIV without addressing participants’ understanding of risk factors for HIV or STIs, making it difficult to extrapolate their findings to real world scenarios (Brooks et al., 2012; Golub et al., 2010; Hoff et al., 2015). In addition, the reporting of certain measures of sexual risk, such as frequency of anal sex, number of partners and drug use, were not consistent across the studies, making it difficult to draw inferences from these data on the effects of PrEP on risk behavior. Although all the studies included risk reduction counselling, only one study actually used a recognized risk reduction model (Molina et al., 2015).
In addition, the studies reviewed here were all potentially biased. For example, recall bias for sexual risk, which is influenced by the impact or meaning of an encounter (Grimm, 2010), was not addressed in any of the studies. It is possible that not all sexual encounters were included in participants’ reporting, and yet validity scales to adjust for recall bias during the statistical analyses were not included (Weinhardt et al., 1998). Recruitment bias is another potential problem (Collumbein, 2012). The participants recruited into the studies were all interested in PrEP as a form of protection against HIV, implying a self-awareness of risk behavior that may not be found in the general MSM population.
Notwithstanding the methodological shortcomings described above, it is always more difficult to draw inferences from a collection of studies such as those reviewed here that have different designs, and that have different variables collected from different populations, in different places.
CONCLUSION
We are presently in an optimistic period of biomedical advances to prevent HIV (Punyacharoensin et al., 2016). However, in view of the gaps in the literature described herein, researchers must continue to investigate new ways to frame the discussion and messaging around STI prevention in a way that is meaningful to the individual, in order to reduce the social, physiological, psychological and financial burden of STIs.
SUMMARY BOX.
What does this Paper contribute to the wider global clinical community?
Our review found that PrEP provides an opportunity for MSM to access sexual health care, testing, treatment and counselling. We did not find any conclusive evidence that PrEP users are increasing their sexual risk behaviors.
Condom use and sexual risk counselling at every encounter appear to be effective in decreasing risk behavior. STI testing should include extra-genital testing in MSM regardless of PrEP use, in order to prevent health deficits and onward transmission.
Providing privacy for MSM to complete sexual health questionnaires improves accurate risk reporting in turn decreasing morbidity and mortality.
RELEVANCE TO CLINICAL PRACTICE.
In an era where HIV prevention methods are rapidly improving, changing the perceived threat of disability or death, strategies for STI prevention and reducing sexual risk behavior cannot remain stagnant. Nurses, community health workers, doctors, outreach workers, social workers are all well positioned to promote sexual health through the dissemination of accurate information to the communities they serve, including marginalized MSM throughout the world.
Footnotes
Contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Contributor Information
Kellie Freeborn, Nurse Practitioner, Voluntary Assistant Clinical Professor and PhD candidate at School of Nursing, Department of Community Health Systems, University of California, San Francisco Recipient of NINR/NIH F31 Grant 1NR016179-01A1.
Carmen J. Portillo, School of Nursing, University of California, San Francisco.
References
- Bissessor M, Whiley DM, Fairley CK, Bradshaw CS, Lee DM, Snow AS, … Chen MY. Persistence of Neisseria gonorrhoeae DNA Following Treatment for Pharyngeal and Rectal Gonorrhea Is Influenced by Antibiotic Susceptibility and Reinfection. Clin Infect Dis. 2015;60(4):557–563. doi: 10.1093/cid/ciu873. [DOI] [PubMed] [Google Scholar]
- Bolan RK, Beymer MR, Weiss RE, Flynn RP, Leibowitz AA, Klausner JD. Doxycycline Prophylaxis to Reduce Incident Syphilis among HIV-Infected Men Who Have Sex With Men Who Continue to Engage in High-Risk Sex: A Randomized, Controlled Pilot Study. Sex Transm Dis. 2015;42(2):98–103. doi: 10.1097/olq.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks RA, Landovitz RJ, Kaplan RL, Lieber E, Lee SJ, Barkley TW. Sexual risk behaviors and acceptability of HIV pre-exposure prophylaxis among HIV-negative gay and bisexual men in serodiscordant relationships: a mixed methods study. AIDS Patient Care STDS. 2012;26(2):87–94. doi: 10.1089/apc.2011.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2011. 2013 (n/a). Retrieved from Atlanta: http://www.cdc.gov/hiv/surveillance/resources/reports/2011report/index.htm.
- Chakrapani V, Newman PA, Shunmugam M, Mengle S, Varghese J, Nelson R, Bharat S. Acceptability of HIV Pre-Exposure Prophylaxis (PrEP) and Implementation Challenges Among Men Who Have Sex with Men in India: A Qualitative Investigation. AIDS Patient Care STDS. 2015;29(10):569–577. doi: 10.1089/apc.2015.0143. [DOI] [PubMed] [Google Scholar]
- Collumbein M, Busza Joanna, Cleland John, Campbell Oona. Social Science Methods for research on Sexual and Reproductive Health. Malta: WHO Press; 2012. [Google Scholar]
- Eaddy MT, Cook CL, O’Day K, Burch SP, Cantrell CR. How Patient Cost-Sharing Trends Affect Adherence and Outcomes: A Literature Review. Pharmacy and Therapeutics. 2012;37(1):45–55. [PMC free article] [PubMed] [Google Scholar]
- Gersh JK, Fiorillo SP, Burghardt L, Nichol AC, Thrun M, et al. Attitudes and Barriers towards Pre-Exposure Prophylaxis (Prep) among High-Risk HIV-Seronegative Men who have Sex with Men. Journal of AIDS & Clinical Research. 2014;05(08) doi: 10.4172/2155-6113.1000335. [DOI] [Google Scholar]
- Golub, Kowalczyk, Weinberger, Parsons Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. J Acquir Immune Defic Syndr. 2010;54(5):548–555. doi: 10.1097/QAI.0b013e3181e19a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RM, Lama JR, Anderson PL, Anderson Pl, McMahan VM, Liu AY, … Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. 2010 doi: 10.1056/NEJMoa1011205. (1533-4406 (Electronic)). doi:D - NLM: NIHMS264954,D - NLM: PMC3079639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm P. Wiley International Encyclopedia of Marketing. John Wiley & Sons, Ltd; 2010. Social Desirability Bias. [Google Scholar]
- Hewett PC, Mensch BS, Ribeiro MC, Jones HE, Lippman SA, Montgomery MR, van de Wijgert JH. Using sexually transmitted infection biomarkers to validate reporting of sexual behavior within a randomized, experimental evaluation of interviewing methods. Am J Epidemiol. 2008;168(2):202–211. doi: 10.1093/aje/kwn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD … Cochrane Statistical Methods, G. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff CC, Chakravarty D, Bircher AE, Campbell CK, Grisham K, Neilands TB, … Dworkin S. Attitudes Towards PrEP and Anticipated Condom Use Among Concordant HIV-Negative and HIV-Discordant Male Couples. AIDS Patient Care STDS. 2015;29(7):408–417. doi: 10.1089/apc.2014.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojilla CJ, Koester KA, Cohen SE, Buchbinder S, Ladzekpo D, Matheson T, Liu AY. Sexual Behavior, Risk Compensation, and HIV Prevention Strategies Among Participants in the San Francisco PrEP Demonstration Project: A Qualitative Analysis of Counseling Notes. AIDS Behav. 2015 doi: 10.1007/s10461-015-1055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtgraves T. Social desirability and self-reports: testing models of socially desirable responding. Pers Soc Psychol Bull. 2004;30(2):161–172. doi: 10.1177/0146167203259930. [DOI] [PubMed] [Google Scholar]
- Hotton AL, Gratzer B, Mehta SD. Association between serosorting and bacterial sexually transmitted infection among HIV-negative men who have sex with men at an urban lesbian, gay, bisexual, and transgender health center. Sex Transm Dis. 2012;39(12):959–964. doi: 10.1097/OLQ.0b013e31826e870d. [DOI] [PubMed] [Google Scholar]
- Kauth MR, St Lawrence JS, Kelly JA. Reliability of retrospective assessments of sexual HIV risk behavior: a comparison of biweekly, three-month, and twelve-month self-reports. 1991 (0899-9546 (Print)) [PubMed] [Google Scholar]
- Kennedy CaFV. A systematic review PrEP for MSM - systematic review – to inform the WHO Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. World Health Organization; 2014. pp. 1–83. (WHO/HIV/2014.9) [PubMed] [Google Scholar]
- Knight R, Small W, Carson A, Shoveller J. Complex and Conflicting Social Norms: Implications for Implementation of Future HIV Pre-Exposure Prophylaxis (PrEP) Interventions in Vancouver, Canada. PLoS One. 2016;11(1):e0146513. doi: 10.1371/journal.pone.0146513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek K, Arauz-Cuadra C, Kipke MD. Attitudes and perceptions of biomedical HIV prevention methods: voices from young men who have sex with men. Arch Sex Behav. 2015;44(2):487–497. doi: 10.1007/s10508-014-0398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth AE, Martin DP, Golden MR, Weiss NS, Heagerty PJ, Spielberg F, … Holmes KK. A Comparison Between Audio Computer-Assisted Self-Interviews and Clinician Interviews for Obtaining the Sexual History. Sex Transm Dis. 2004;31(12):719–726. doi: 10.1097/01.olq.0000145855.36181.13. [DOI] [PubMed] [Google Scholar]
- Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, … Kolber MA. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JL, Glidden DV, Mayer KH, Liu AY, Buchbinder SP, Amico KR, … Grant RM. No Evidence of Sexual Risk Compensation in the iPrEx Trial of Daily Oral HIV Preexposure Prophylaxis. PLoS One. 2013;8(12):e81997. doi: 10.1371/journal.pone.0081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe TL, DiFranceisco W, Reed BR. Effects of question format and collection mode on the accuracy of retrospective surveys of health risk behavior: a comparison with daily sexual activity diaries. Health Psychol. 2007;26(1):60–67. doi: 10.1037/0278-6133.26.1.60. [DOI] [PubMed] [Google Scholar]
- McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, … Gill ON. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. The Lancet. 2015 doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, … Delfraissy JF. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015;373(23):2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- O’Neal JD, Berteau LC. Revitalizing condom-centered HIV prevention strategies. Curr HIV/AIDS Rep. 2015;12(1):139–144. doi: 10.1007/s11904-014-0250-8. [DOI] [PubMed] [Google Scholar]
- Pluye P, Robert E, Cargo M, Bartlett G, O’Cathain A, Griffiths F, Boardman F, Gagnon MP, Rousseau MC. Mixed Methods Appraisal Tool (MMAT) 2011 Retrieved from http://mixedmethodsappraisaltoolpublic.pbworks.com. Archived by WebCite® at http://www.webcitation.org/5tTRTc9yJ.
- Punyacharoensin N, Edmunds WJ, De Angelis D, Delpech V, Hart G, Elford J, … White RG. Effect of pre-exposure prophylaxis and combination HIV prevention for men who have sex with men in the UK: a mathematical modelling study. The Lancet HIV. 2016 doi: 10.1016/S2352-3018(15)00056-9. [DOI] [PubMed]
- Underhill K, Morrow KM, Colleran C, Holcomb R, Calabrese SK, Operario D, … Mayer KH. A Qualitative Study of Medical Mistrust, Perceived Discrimination, and Risk Behavior Disclosure to Clinicians by U.S. Male Sex Workers and Other Men Who Have Sex with Men: Implications for Biomedical HIV Prevention. J Urban Health. 2015 doi: 10.1007/s11524-015-9961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Population Fun. Global Forum on MSM & HIV, United Nations Development Programme. World Health Organization, United States Agency for International Development, World Bank; 2015. Implementing comprehensive HIV and STI programmes with men who have sex with men: practical guidance for collaborative interventions. [Google Scholar]
- Venter F, Allais L, Richter M. Exposure Ethics: Does Hiv Pre-Exposure Prophylaxis Raise Ethical Problems for the Health Care Provider and Policy Maker? Bioethics. 2014;28(6):269–274. doi: 10.1111/bioe.12021. [DOI] [PubMed] [Google Scholar]
- Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, Hare CB. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis. 2015;61(10):1601–1603. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhardt LS, Forsyth AD, Carey MP, Jaworski BC, Durant LE. Reliability and Validity of Self-Report Measures of HIV-Related Sexual Behavior: Progress Since 1990 and Recommendations for Research and Practice. Arch Sex Behav. 1998;27(2):155–180. doi: 10.1023/a:1018682530519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs. 2005;52(5):546–553. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- Wilton J, Senn H, Sharma M, Tan DH. Pre-exposure prophylaxis for sexually-acquired HIV risk management: a review. HIV AIDS (Auckl) 2015;7:125–136. doi: 10.2147/HIV.S50025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Strategy for the Prevention and Control and Sexually Transmitted Infections: 2006–2015. Geneva: World Health Organization; 2007. [Google Scholar]
- World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections. Geneva: World health Organization; 2012a. [Google Scholar]
- World Health Organization. Guidance on pre-exposure oral prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. Geneva: World Health Organization; 2012b. [PubMed] [Google Scholar]
- Zenilman JM, Weisman CS, Rompalo AM, Ellish N, Upchurch DM, Hook EWI, Celentano D. Condom Use to Prevent Incident STDs: The Validity of Self-Reported Condom Use. Sex Transm Dis. 1995;22(1):15–21. doi: 10.1097/00007435-199501000-00003. [DOI] [PubMed] [Google Scholar]