Sir
Clioquinol (5-chloro-7-iodo-8-quinolinol) (Fig. 1A), an ionophore and metal chelator, is being explored as treatment of cancer and the neurodegenerative conditions Alzheimer’s, Parkinson’s, and Huntington’s diseases [1]. Zinc ions in complex with another ionophore, pyrithione, were recently shown to inhibit acetylation of aminoglycosides by aminoglycoside modifying enzymes (AMEs) effectively reversing resistance in Gram-negatives [2]. The aminoglycoside 6′-N-acetyltransferase type Ib [AAC(6′)-Ib] is among the most prevalent AMEs [3]. It confers resistance to a large group of aminoglycosides including amikacin, one of the most refractory to enzymatic inactivation [3]. This antibiotic is used in life threatening infections and it has been instrumental in the treatment of neonatal outbreaks [4]. Identification of compounds that interfere with the action of resistance enzymes will permit the continuous use of existing aminoglycosides. Here we show that Zn+2 in complex with clioquinol reduces resistance to amikacin in Acinetobacter baumannii and Escherichia coli harboring aac(6′)-Ib. A. baumannii A155 is a multidrug-resistant urine isolate that carries the aac(6′)-Ib gene [2]. E. coli TOP10(pNW1) includes aac(6′)-Ib in the plasmid pNW1 [2]. Clioquinol, kanamycin A, and amikacin were purchased from Sigma-Aldrich. Purification and acetylating activity of AAC(6′)-Ib procedures were described before [2]. Bacterial cultures were carried out in Lennox Luria (L) or Mueller-Hinton medium. In cellulo activity of zinc complexed to clioquinol was assessed culturing the cells in 100-μl Mueller-Hinton broth containing 0.1% dimethyl sulfoxide (DMSO) with the specified additions in microtiter plates using the BioTek Synergy 5 microplate reader. Cultures were carried out for 20 h at 37°C with shaking. The OD600 of the cultures was measured every 20 min [2].
Fig. 1.
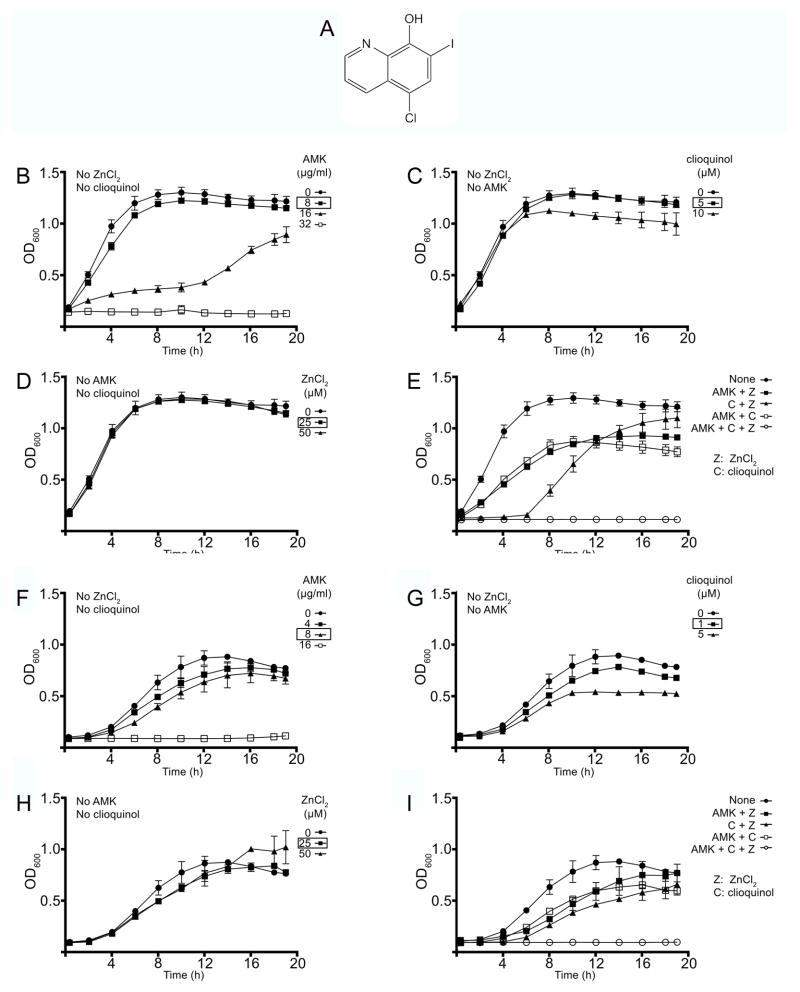
Chemical structure and activity of clioquinol. A, chemical structure of clioquinol. B–I, comparison of the effects of ZnCl2 and clioquinol on A. baumannii (B–E) and E. coli (F–I) resistance to amikacin. A. baumannii A155 or E. coli TOP10(pNW1) cells were cultured in 100 μl Mueller-Hinton broth in microtiter plates at 37°C with the indicated additions. Panels show the effect of addition of different concentrations of amikacin (B, F), clioquinol (C, G), ZnCl2 (D, H), or combinations of two or the three reagents (E, I). Boxed concentrations are those used in panels E and I.
Since the ionophore clioquinol is already being investigated for treatment of human diseases, it is an attractive candidate for testing its efficacy, in combination with zinc, as an inhibitor of the resistance to amikacin mediated by AAC(6′)-Ib. An initial assessment of AAC(6′)-Ib-mediated acetylation of kanamycin A in vitro in the presence of clioquinol showed that, as expected, it does not have any inhibitory activity in the absence of Zn+2. These results suggest that if the addition of clioquinol to Zn+2 ions significantly reduces the concentration of metal needed to inhibit amikacin resistance in bacterial cells harboring aac(6′)-Ib, the effect could be attributed to the stimulation of Zn+2 uptake by the ionophore properties of clioquinol. A. baumannii A155 exhibits robust growth in up to 8 μg/ml amikacin (Fig. 1B), and growth was not significantly disturbed by the addition of clioquinol or ZnCl2 at the concentrations tested (Fig. 1C and D). Growth curves of A. baumannii A155 cultured in the presence of 8 μg/ml amikacin with the addition of either 5 μM clioquinol or 25 μM ZnCl2 showed a slight reduction in doubling time and after 20 h incubation at 37°C the cultures reached an optical density indicative of healthy growth (Fig. 1E). When the cultures occurred in the presence of 5 μM clioquinol and 25 μM ZnCl2 there was an extended lag phase, but then the doubling time and final optical density were comparable to the values observed in the culture carried out without additions (Fig. 1E). Conversely, when these two compounds were added to the medium in combination with amikacin, growth was completely inhibited (Fig. 1E). Nearly identical results were observed when the bacterium tested was E. coli harboring aac(6′)-Ib. No significant effect on growth was seen in the presence of 8 μg/ml amikacin, 1 μM clioquinol, or 25 μM ZnCl2 (Fig. 1F, G, and H). Clioquinol was slightly toxic to this bacterium at 5 μM and therefore it was used at 1 μM, a concentration at which toxicity was minimal (Fig. 1G). There was a slight reduction in doubling time in cultures containing combinations of two of the testing components, but the optical density reached after 20 h incubation at 37°C was identical to that when only amikacin was present (Fig. 1I). As it was the case for A. baumannii, the presence of all three components resulted in complete growth inhibition (Fig. 1I).
Our results strongly suggest that combinations of Zn+2 and ionophores are firm candidates to be developed as adjuvants to aminoglycosides to overcome resistance mediated by AMEs. Although the mechanism by which Zn+2 interferes with enzymatic acetylation of aminoglycosides mediated by AAC(6′)-Ib remains to be elucidated, an attractive possibility is that formation of a coordination complex [5] protects the substrate aminoglycoside from modification.
The components of the combination used in this work to achieve phenotypic conversion to susceptibility are both already being used, or tested, for numerous diseases. Zinc is used to treat common cold, children diarrhea, and a long list of chronic diseases. Clioquinol is being tested as treatment for cancer and major neurodegenerative conditions [1]. These ongoing studies and uses suggest that toxicity might not be a serious impediment in their use to overcome resistance to amikacin or other aminoglycosides.
Acknowledgments
Funding: This work was supported by Public Health Service grant 2R15AI047115-04 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (to MET) and a grant from the California State University Program for Education and Research in Biotechnology.
Footnotes
Competing Interests: None declared.
Ethical Approval: Not required
References
- 1.Bareggi SR, Cornelli U. Clioquinol: review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci Ther. 2012;18:41–6. doi: 10.1111/j.1755-5949.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin DL, Tran T, Alam JY, Herron SR, Ramirez MS, Tolmasky ME. Inhibition of aminoglycoside 6′-N-acetyltransferase type Ib by zinc: reversal of amikacin resistance in Acinetobacter baumannii and Escherichia coli by a zinc ionophore. Antimicrob Agents Chemother. 2014;58:4238–41. doi: 10.1128/AAC.00129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat. 2010;13:151–71. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labaune JM, Bleyzac N, Maire P, Jelliffe RW, Boutroy MJ, Aulagner G, et al. Once-a-day individualized amikacin dosing for suspected infection at birth based on population pharmacokinetic models. Biol Neonate. 2001;80:142–7. doi: 10.1159/000047133. [DOI] [PubMed] [Google Scholar]
- 5.Kozlowski H, Kowalik-Jankowska T, Jezowska-Bojczuk M. Chemical and biological aspects of Cu2+ interactions with peptides and aminoglycosides. Coord Chem Rev. 2005;249:2323–34. [Google Scholar]