Abstract
Heart failure in older adults is frequently accompanied by sleep disordered breathing (SDB). Treatment of SDB in persons with heart failure with preserved ejection fraction (HFpEF) is unclear because most data is on heart failure with reduced ejection fraction (HFrEF). The purpose of this paper was to evaluate studies that report on the effects of positive airway pressure on patient outcomes in older adults with HFpEF and comorbid SDB. A search of the literature found six data-based studies (N = 36 to 126). Treatment with positive airway pressure reduced nighttime SDB symptoms and improved daytime functional status in persons with HFPEF and SDB (New York Heart Association Functional Class: effect sizes= −.67 to −1.60). Limitations (i.e. only two studies were randomized controlled trials, small sample sizes, and women were under-represented) suggest that additional evidence is needed to guide treatment of SDB in older adults with HFpEF.
Keywords: Heart failure, heart failure with preserved ejection fraction, sleep-disordered breathing, sleep apnea syndrome, adaptive servo-ventilation, positive airway pressure
Introduction
According to the American Heart Association, in 2014 over 6.5 million adults in the U.S. had heart failure. The prevalence of heart failure is expected to increase by 46% by 2030 to over 8 million.1 Heart failure is associated with significant decreases to health-related quality of life (HRQoL), daytime dysfunction, fatigue, dyspnea, and increased mortality.2 The total cost of heart failure in 2012 was estimated as almost 31 billion dollars; this cost is projected to increase to 70 billion dollars by 2030.3 Although cardiac disease is not part of healthy aging, older adults have a disproportionate risk. The prevalence of heart failure is less than 2% of adults under 60 years old, it increases to 6% in the 60 to 79 year-old age group and is greater than 13% in persons over 80 years old.1
Heart failure is characterized as either heart failure with reduced ejection fraction (HFrEF; left ventricular ejection fraction [LVEF] ≤ 40%) or as heart failure with preserved ejection fraction (HFpEF; LVEF ≥ 50%).4 Half of all patients with heart failure present with a preserved LVEF;1 these patients have a unique phenotype from HFrEF. Patients with HFpEF are frequently older (often in the 8th decade of life), more likely to be female, without a myocardial infarction, and have a history of hypertension, coronary artery disease, obesity compared to patients with HFrEF.1,5–7 The prevalence of HFpEF relative to HFrEF is increasing at a rate of 1% per year and may become the predominant form of heart failure in the developed world.2 Unlike HFrEF, there are no established therapies to improve mortality and morbidity in patients with HFpEF.8 There are differences in the etiology of morbidity and mortality between HFrEF and HFpEF, with morbidity in HFpEF influenced more by non-heart failure related conditions.2,9 Thus, therapy in HFpEF is aimed at treating the more prevalent comorbidities that coexist in persons with HFpEF in order to reduce symptom burden and to improve HRQoL.2
Sleep disordered breathing (SDB) is a highly prevalent comorbidity in adults with HFpEF, estimated to range from 50% to over 80% when compared to persons who have no history of heart failure.10–14 According to the American Academy of Sleep,15 SDB refers to a number of breathing disruptions that occur during sleep that include:
obstructive apneas (cessation of airflow for 10 seconds or longer because of an obstruction in airflow in the upper pharynx with continued respiratory effort),
hypopneas (a decrease in airflow by 50% from baseline accompanied by a decrease of 3% in oxygen saturation),
central apneas (absence of a central nervous system signal to breath with a cessation in breathing for at least 10 seconds),
mixed apneas (an apnea that starts as an obstructive event but converts to a central apnea, or vice versa), and
Cheyne-Stokes respirations (a cyclic pattern of waxing and waning airflow with respirations).
Data from previous research suggests that SDB results in sympathetic activation, sleep fragmentation, and intermittent hypoxia that is associated with a variety of adverse consequences in older adults including excessive daytime sleepiness,16 depression,16 cognitive decline,17,18 reduced functional status,19 cardiovascular disease,20,21 and increased mortality.21
In patients with heart failure, the choice of treatment for SDB depends on the severity and type of breathing disturbance, symptom presentation (i.e. presence of snoring or excessive daytime sleepiness), and the type of cardiovascular disease present.22–24 Positive airway pressure (e.g., continuous positive airway pressure [CPAP], bi-level positive airway pressure [bi-PAP], or adaptive servo-ventilation [ASV]) is the mainstay treatment of SDB.22 CPAP involves wearing a mask that delivers a steady stream of air to prevent airway collapse and obstructive apneas. Unlike CPAP, Bi-PAP machines have two pressure settings with a higher pressure for inhalation and a lower pressure for exhalation. Bi-PAP is useful for patients who find it difficult to exhale against positive pressure, require a high positive pressure setting to prevent airway obstruction, require oxygen, have a neuro-muscular disorder, or who have HFrEF. ASV is a further refinement as a type of positive airway pressure that uses an algorithm based on the detection of apneas to the level of pressure required to maintain respiration with inspiratory support to treat central apneas.25,26 ASV is used for patients with more complex SDB consisting with mixed obstructive and central apneas and Cheyne-Stokes respirations.
While the prevalence of SDB is similar between the populations of HFrEF and HFpEF, whether the predominant type of sleep apnea (e.g. obstructive versus central sleep apnea) is similar remains unclear.14,27 Central sleep apnea is more common in persons with heart failure compared to the general population.12 The prevalence of central sleep apneas has been shown to increase significantly (p<.05) with increasing heart failure severity.27,28 In general, patients with HFpEF tend to have more obstructive apneas in comparison with patients with HFrEF.14 In a prospective study of both HFpEF and HFrEF, OSA was the predominant type of SDB in the HFpEF patients (62%) but not in HFrEF patients.14 A larger study of 244 persons with only HFpEF also found more OSA (40%) versus central sleep apnea (30%) in those identified with SDB.27 However, several studies suggest that the pattern of central apneas, Cheyne-Stokes respirations, obstructive apneas, and hypopneas changes on a night-to-night basis further complicating the delineation of SDB between persons with HFrEF and HFpEF as well as the management of SDB in each population.26,29
A growing body of evidence over the past decade supports positive airway pressure (e.g., CPAP, bi-PAP, or ASV) as effective methods to treat SDB with demonstrated improvements to nocturnal respiratory function and daytime sleepiness.30–32 The impact of positive airway pressure on cardiac function and mortality is less clear.30–33 For example, the Sleep Apnea Cardiovascular Endpoints (SAVE) study33 was a randomized control trial to evaluate the effectiveness of CPAP in reducing adverse cardiovascular events among patients with cardiovascular disease and comorbid moderate-to-severe OSA (apnea + hypopnea index [AHI] ≥ 15).33 Those patients randomized to CPAP (n = 1346) had significant improvements in daytime sleepiness, respiratory function, and quality of life measures but not in mortality or the recurrence of myocardial infarction when compared to the usual-care group (n = 1341).
Additionally, adverse results occurred in a second study the Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo-Ventilation in Patients with Heart Failure (SERVE-HF).25 The SERVE-HF study, a large randomized controlled trial (N= 1,325) of subjects with HFrEF (LVEF ≤ 45%) and comorbid moderate-to-severe central sleep apnea (AHI ≥ 15), prompted healthcare professionals to question the safety of using ASV in persons with heart failure. The SERVE-HF trial was prematurely stopped because of a statistically significant increase in the primary end-point of all-cause mortality and cardiovascular deaths in those participants randomized to ASV (p <.01). Although the results from the SERVE-HF study cannot be generalized to persons with HFpEF, both the SAVE and SERVE-HF trials have rendered the treatment of SDB among heart failure patients a controversial topic regardless of treatment type.
While the negative prognostic impact of HFpEF is similar to that of HFrEF,34 each type of heart failure represents a different clinical syndrome that should be studied and treated separately.35 Important differences exist between HFpEF and HFrEF with respect to the extent of myocardial dysfunction, patterns of cardiac remodeling in heart chambers, and in the response to therapeutic interventions.35 Additionally, OSA in older adults greater than 65 years has a distinctly different physiological phenotype in old versus young individuals.36 As a result of these differences, outcomes during positive airway pressure therapy in patients with HFpEF, typically persons greater than 65 years with comorbid SDB, would be expected to be different from the samples in both the SERVE-HF and SAVE trials.
Previous studies on the treatment of SDB in adults with heart failure have historically focused on middle aged adults, featured samples for which the type of heart failure was not identified, or where the majority of the participants had symptoms suggestive of HFrEF. Therefore, the effect of positive airway therapy on patient-centered and clinical outcomes in older adults with HFpEF is poorly understood. The purpose of this paper is to evaluate the studies that report on the effects of positive airway pressure on patient-centered and clinical outcomes in older adults with HFpEF and comorbid SDB.
Methods
Search and data abstraction
In June 2017, a systematic all-field and no-date-limit literature search using PubMed, OVID, and Web of Science was conducted. Two investigators, working independently, used the following combination of search terms: sleep apnea, or sleep apnea—obstructive, or sleep apnea—central, or sleep apnea—mixed; AND continuous positive airway pressure, or CPAP, or adaptive servo ventilation, or ASV, or bi-level positive airway pressure, or BiPAP; AND heart failure, or heart failure—diastolic, or heart failure—preserved ejection fraction, or HFpEF. The identified articles were analyzed using the protocol below.
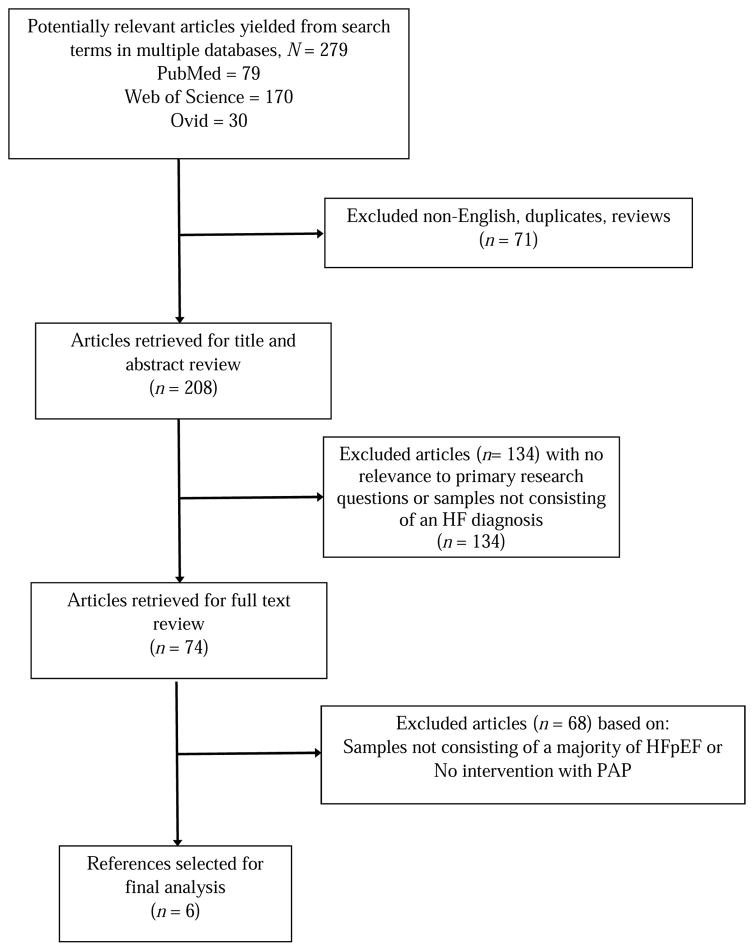
The search identified 279 publications (i.e., articles in peer-reviewed journals); 71 were excluded from further analysis because they were written in languages other than English or were not data-based research articles. The titles and abstracts of the remaining 208 articles were screened to determine whether or not the associated full-text articles would be analyzed (Figure 1). One-hundred thirty four publications were excluded for further analysis because the abstracts either did not mention heart failure participants, or lacked content relevant to our topic. This yielded 74 publications that qualified for our full-text review. Following this full-text review, 68 articles were excluded because (1) no delineation was made between the HFrEF and the HFpEF participants or the sample comprised a majority of adults with HFrEF, and/or (2) the study reported did not feature a positive airway pressure intervention for the treatment of sleep apnea in the participants with co-existing HFpEF and SDB.
Figure 1.
Results of systematic review process.
As a result of this search protocol, six articles were evaluated. A common outcome in four of the studies, the New York Heart Association Functional Class, was used to determine the effect of SDB treatment. A standardized effect size for each study was calculated by subtracting the average pre-treatment functional class from the average post-intervention functional class and then dividing the difference by the standard deviation of the pretreatment functional class.37 The relative magnitude of change was based on Cohen’s definitions of a small (0.2), moderate (0.5), and large (> 0.8) effect size.38
Results
Design and samples
The six studies that examined the effect of treatment of sleep apnea in adults with HFpEF with positive airway pressure are described in Table 1.39–43 Four of these studies39,40,42,43 had a prospective observational design39,40,42 and two were randomized controlled trials with a control group that received usual care (i.e. cardiac medications).41,44 Four studies included a homogenous sample of participants with HFpEF39,41–43 while two studies included a mixed sample of participants with either HFrEF or HFpEF.41,44 The majority (56%) of the sample in the Oldenburg study41 were diagnosed with HFpEF while in O’Connor study44 only 19% (n=24) of the sample had HFpEF, although subgroup analyses were completed within this population. The studies were conducted by four independent groups of investigators: two groups in Japan,41–43 one in Germany,39,40 and one multicenter study that included investigators from both Germany and United States.44 The six studies recruited convenience samples from clinical populations that were small-to-modest in size; the smallest study included 36 participants and the largest study included 126.
Table 1.
Key characteristics of studies on treatment of sleep disordered breathing in adults with heart failure with preserved ejection fraction.
| 1st author, county, year | Sampl e size | Study Design | Sample | Mean age (years) | % Women, % HFpEF | PAP Device/ Comparison Group | Study Duration | Therapeutic Benefits |
|---|---|---|---|---|---|---|---|---|
| Bitter, Germany 2010 | N= 60 | Observational Prospective | Type of HF: HFpEF class II – III, LVEF ≥ 50% Type of SDB: Moderate to Severe, AHI > 15/h with CSR |
69 ± 8 | 15, 100% | ASV (n = 39)/Comparison (rejected treatment, stopped treatment, or noncompliant; n = 21) | 11.6 ± 3 months | Improved respiratory function (AHI longest apnea and hypopnea length, maximum and mean oxygen desaturation, percentage of study time with an oxygen saturation of < 90%), functional capacity (cardiopulmonary exercise testing), cardiac function (echocardiography measure), and NYHA functional class (ES = −0.67); all p- values < 0.05. |
| Oldenburg, Germany, 2013 | N= 45 | Observational Prospective | Type of HF: Both HFpEF and HFrEF, LVEF ≥ 50% Type of SDB: Moderate to Severe, AHI ≥ 15/h with Central and Mixed apneas |
71 ± 10 | 9, 56% | ASV (N=38)/No comparison | 3.6 ± 1.2 months | Improved NYHA functional class (ES = −0.80), exercise tolerance and oxygen uptake via cardiopulmonary exercise test, and AHI. |
| Yoshihisa, Japan, 2013 | N= 36 | Randomized control trial | Type of HF: HFpEF (LVEF > 50%) Type of SDB: Moderate to Severe, AHI > 15/h with OSA and CSR-CSA |
64 ± 14 | 19, 100% | ASV (n = 18)/Comparison (Usual care; n = 18) | 6 months, long term follow up | Improved cardiac diastolic function, arterial stiffness, NYHA Functional class (ES = −1.60), and decrease in cardiac death and hospitalizations due to cardiac disease, all p-values < 0.05. |
| Yoshihisa, Japan, 2015 | N= 109 | Observational Prospective | Type of HF: HFpEF (LVEF > 50%) Type of SDB: AHI ≥ 15/h with OSA, CSA, or Mixed |
68 ± 13 | 37, 100% | CPAP & ASV (n = 31)/Comparison (Usual care; n = 78) | 6 months, long term follow up | Improved, systolic and diastolic blood pressure, % vital capacity, peak oxygen uptake, NYHA Functional class (ES = −1.60), and a reduced cardiac and all-cause mortality, all p- values < 0.05. |
| Arikawa, Japan, 2016 | N = 58 | Observational Prospective | Type of HF: HFpEF (LVEF ≥ 50%) Type of SDB: Mild to severe, AHI ≥ 5, OSA |
65 ± 15 | 41%, 100% | CPAP (n=39)/ Comparison (non-OSA; n = 19) | 36 months | BNP levels were higher in those with OSA + CPAP vs comparison group at: 6 months [221 (137–324) vs 76 (38–96) pg/ml, p<0.05], 12 months [123 (98–197) vs 52 (38–76) pg/ml, p<0.05] and 36 months [115 (64–174) vs 56 (25–74) pg/ml, p<0.05] |
| O’Connor, Germany and United States 2017 | N = 126 | Randomized, control trial, prospective, multicenter | Type of HF: Both HFrEF (LVEF ≤ 45%) and HFpEF (LVEF > 45%) Type of SDB: Moderate to Severe, AHI ≥ 15, CSA, OSA, and mixed |
62 ± 14 | 32, 19% | ASV (n = 65)/Comparison (usual care; n = 61) | 6 months | Enrollment stopped early (126 of 215 planned) following the release of the SERVE-HF results, limiting statistical power. Improvements to AHI only. Subgroup analysis with HFpEF suggested significant positive effect on a composite global rank score (hierarchy of death, cardiovascular hospitalizations, and percent changes in 6-min walk distance) at 6 months (p = 0.036). |
Note: AHI = apnea/hypopnea index, ASV = adaptive servo-ventilation, BNP = Plasma Brain Natriuretic Peptide, CPAP = continuous positive airway pressure, CSA = central sleep apnea, CSR = Cheyne-Stokes Respirations, ES = Effect Size, HF = Heart Failure, HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection fraction, LVEF = left ventricular ejection fraction, OSA = obstructive sleep apnea, PAP = positive airway pressure, SDB = sleep disordered breathing.
Effect of positive airway pressure on outcomes in persons with HFpEF and comorbid SDB
Bitter et al. examined persons with stable HFpEF (NYHA classes II–III) and primarily central sleep apnea and Cheyne-Stokes respiration (i.e., a ratio of central to obstructive apneas > 50%).39 All participants (N=60) were offered ASV. Assessments were made between the treatment group (n = 39) and a non-equivalent comparison group (n = 21) of participants who either refused or could not tolerate treatment. Significant improvements were observed in cardiopulmonary exercise testing, NYHA class, and cardiac function among the participants of the ASV treatment group (mean follow up 11.6 ± 3 months; all p-values < .01) compared to the untreated comparison group.
A study by Oldenburg et al.40 screened 45 consecutive patients with heart failure (HFrEF or HFpEF) and moderate-to-severe SDB (either central or mixed sleep apneas) to reveal the long-term (i.e., 3.6 ± 1.2 months) effects of a tri-level ASV device on cardiac function.40 Only 38 patients were successfully titrated to tri-level ASV. On follow-up (3.6 ± 1.2 months) those patients (n=23) adherent to ASV (mean ASV use of ≥ 4 hours per night for ≥ 5 days a week) exhibited significant improvements in AHI, NYHA class functional class, and in cardiopulmonary exercise testing (all p-values < .01).
Yoshihisa et al. (2013) conducted a small (N = 36) experimental study of adults with HFpEF and SDB (AHI > 15) randomly assigned to either an ASV group or a medication only group.41 Compared to the medication only group, the ASV group had significant improvements (all p values < .05) to AHI, NYHA functional class, and cardiac function after 6 months.41 A Kaplan-Meier analysis found that persons in the ASV group had a lower event-free rate for cardiac events (i.e., cardiac deaths or hospitalizations; p < .05) after one year. The Cox proportional hazard analysis revealed that only ASV was an independent predictor of cardiac events in HFpEF and SDB (HF = 0.576, 95% CI 0.183, 0.799; p = .0164).
Yoshihisa et al. (2015) reported a larger observational study (N = 109) of persons with HFpEF and moderate to severe SDB.42 Treatment devices were distributed according to the dominant type of sleep apnea: CPAP for OSA- patients and ASV to central sleep apnea-Cheyne Stokes Respiration each representing approximately 50% of the sample. While all participants were offered treatment, comparisons were made between those who accepted the positive airway pressure treatment (n=31) and those participants who refused or were intolerant of the device (n=78). The intervention group exhibited statistically significant improvements (i.e., all p values ≤ 0.05) in NYHA functional class, cardiac function, and cardiopulmonary exercise tests after 6 months, compared to the control group.42 Long-term cardiac and all-cause mortality were lower in the positive airway pressure group than in the control group, as demonstrated by Kaplan-Meier analysis (p < 0.05).
Arikawa et al. (2016) was a small observational study (N = 58) of patients with new-onset HFpEF (66 ± 15 years) to examine the effect of treatment of SDB on plasma brain natriuretic peptide (BNP) levels, a marker for heart failure severity, during a 36 month follow-up.43 Presence of SDB was determined after enrollment into the study and all patients with OSA (n = 39) were treated with CPAP, as well as with lifestyle modifications. Comparisons were made between those HFpEF patients without OSA (n = 19) and those HFpEF patients with OSA. Both groups received usual care for heart failure management. BNP levels reduced in both groups over time, however, the reductions were less evident in the OSA group at each time point. The OSA group had higher BNP levels at six months (221 vs 76 pg/ml, 12 months (123 vs 52 pg/ml, and at 36 months (115 vs 56 pg/ml); all p-values < 0.05.43
Most recently, O’Connor et al. (2017) described results from the Cardiovascular Improvements with Minute Ventilation-targeted ASV Therapy in Heart Failure (CAT-HF) study,44 a randomized controlled trial (RCT) designed to evaluate the effect of ASV in hospitalized patients with heart failure. The primary endpoint was a composite global rank endpoint of survival, CV hospitalization, and functional capacity measured by 6-minute walk distance. The study proposed randomized 215 subjects with heart failure; however only 126 participants were randomized in this study before it was the trial was terminated because of release of data suggesting higher mortality in the SERVE-HF study. Preliminary data from the CAT-HF study suggested that SDB was effectively and safely treated by ASV (AHI decreased from 35.7/h to 2.1/h) in a HF population that included both HFrEF and HFpEF. Data collected prior to study termination did not find that use of ASV resulted in an improvement in cardiac status. A limitation of this study was that average adherence to ASV was very low (2.7h/night), limiting the ability to determine the effect of ASV on cardiac function. Despite this, a subgroup analysis of participants with HFpEF (n = 24) showed a positive effect of ASV on the composite global rank score (hierarchy of death, cardiovascular hospitalizations, and percent changes in 6-min walk distance) at 6 months (p = 0.036).44
Effect of positive airway pressure treatment pre-and post-intervention on NYHA functional class
Although the type of intervention device varied, the NYHA functional class was a common metric used in four of the studies.39–42 Overall, NYHA functional class improved from pre-intervention to post-intervention with the use of positive airway pressure treatment (see Table 2). More specifically, treatment of SDB with positive airway pressure was associated with moderate to large improvements in the NYHA functional class with effect sizes that ranged from −.67 to −1.6.
Table 2.
Effect of positive airway pressure on the New York Heart Association functional class.
| M | SD | Mean Difference | Effect Size | 95% CI | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Group | Lower | Upper | |||||
| Bitter | Pre | 2.4 | 0.6 | −0.40 | −0.67 | −1.12 | −0.21 |
| Post | 2.0 | 0.8 | |||||
| Oldenburg | Pre | 2.4 | 0.5 | −0.40 | −0.80 | −1.40 | −0.20 |
| Post | 2.0 | 0.4 | |||||
| Yoshihisa, 2013 | Pre | 2.3 | 0.5 | −0.80 | −1.60 | −2.35 | −0.85 |
| Post | 1.5 | 0.5 | |||||
| Yoshihisa, 2015 | Pre | 2.4 | 0.5 | −0.80 | −1.60 | −2.07 | −1.13 |
| Post | 1.6 | 0.5 | |||||
Note. Standardized effect size of treatment on the New York Health Association (NYHA) functional class for each study was based on the change score between pre-intervention and post-intervention divided by the standard deviation at pre-intervention (Becker, 1988). M = mean; SD = standard deviation; CI = confidence interval.
Discussion
This review of studies suggests that treatment of SDB in patients with HFpEF may result in improved nighttime sleep and daytime function that included statistically significant improvements in cardiac function, especially among those participants who were adherent to positive airway pressure treatment (e.g. CPAP or ASV) for more than 4 hours each night.39,41,42 In addition, treatment of SDB with positive airway pressure resulted in improved exercise tolerance39,40,42 and reduced cardiac complications resulting in hospitalizations and/or mortality.41,42,44 The significant improvement in NYHA class, an outcome in four of the studies, suggests treatment of SDB results in better overall functional status in persons with HFpEF. However, these results should be interpreted with caution because of the conflicting evidence between previous studies in patients with HFrEF and because of the quality of the studies reviewed.
Quality Appraisal of Each Study
All of the studies had risks of bias that threatened their validity including non-experimental designs,39,40,42,43 small sample sizes,41 use of non-equivalent comparison groups,39,42,43 and attrition (40%).40 The use of a one group pretest/posttest analysis in the study by Oldenburg et. al (2013) raises the possibility of rival hypotheses that account for the improvement in functional and cardiac status. None of the studies qualified as a superiority RCT that featured a comparative analysis between different types of positive airway pressure devices. Moreover, none of the studies reported use of a power analysis to determine sample size except for the study by O’Conner44 however, this study was terminated when only half the needed sample was recruited.
Five studies had similar eligibility criteria: a diagnosis of HFpEF and moderate-to-severe SDB (mean apnea + hypopnea per hour sleep [AHI] ≥ 15/hour)39–42,44 while one study43 included patients with HFpEF without SDB as an part of the inclusion criteria. A standard HFpEF diagnosis according to European Society of Cardiology guidelines was used in the two studies that were conducted in Germany39,40, while the three studies from Japan41–43 utilized the Framingham Criteria for Congestive Heart Failure with a LVEF ≥ 50%. The study by O’Connor used an LVEF > 45% to identify HFpEF in hospitalized patients admitted for acute decompensated heart failure. In all studies, SDB was diagnosed by an objective sleep study device using modified American Academy of Sleep Medicine guidelines. In-laboratory polysomnography was used in all but two studies; the study by O’Connor and colleagues44 utilized a four-channel type III home sleep study device while the study by Arikawa and colleagues used a two stage screening process with first with evaluation of nocturnal desaturations with pulse oximetry and then use of portable polysomnography with EEG monitoring to assess for OSA while the patient was hospitalized with new-onset heart failure.
Additional threats to validity include the low number of female participants and the lack of data on positive airway pressure device adherence. Positive airway pressure device adherence is especially relevant in Arikawa et al. who concluded that “appropriately” treated OSA may worsen long-term cardiac function in persons with HFpEF, although CPAP adherence was not reported.43 There was no one consistent intervention (CPAP, ASV and tri-level ASV) for all of the studies precluding direct comparison of the impact of treatment on outcomes. Even though all of the studies used cardiac measures as their main outcomes, none had patient-oriented outcomes as endpoints other than the New York Heart Association (NYHA) functional class in four of the studies.39–42 The NYHA functional class is a useful clinical tool for determining the eligibility of patients for certain medical services and informing therapeutic management. However, the NYHA classification does not fully explain patient-oriented outcomes or health-related quality of life.
Limitations of the review
While there are numerous studies that examine treatment of SDB in unspecified heart failure or in samples with HFrEF, a limitation of this review was the limited number of databased studies on the effect of treatment of SDB in persons with HFpEF and that only two studies had an experimental design. In addition, studies that were non-English, in the grey-literature (i.e. abstracts to conferences), or not published because of publication bias to non-statistically significant findings were not accessed.
Conclusions and Recommendations
In conclusion, the five out of six studies reviewed suggest that treatment of SDB in older adults with HFpEF may have positive effects on cardiac function that may result in decreased cardiac events and hospitalizations. However, minimal evidence exists from randomized clinical trials with HFpEF samples to guide practice regarding appropriate treatment of SDB within this population. Effective management of SDB is especially important because HFpEF is rapidly becoming the predominant form of heart failure among older adults, and complex SDB is highly prevalent in this population.26
Effective treatment of SDB is difficult concerning the choice of which type of positive airway pressure device to use. The different type of device appears to have different effectiveness and consequences depending on type of heart failure and type of SDB. According to the American Academy of Sleep Medicine (AASM), CPAP is indicated for treatment of obstructive apneas in patients with either HFrEF or HFpEF. Treatment of central apneas or Cheyne-Stokes respirations requires use of Bi-PAP or ASV. The updated 2016 AASM guidelines do not recommend ASV therapy in persons with HFrEF (LVEF ≤ 45%) and moderate to severe SDB with predominantly central sleep apneas. However, the guidelines state that ASV can be used in those persons with HFpEF, mild sleep-disordered breathing, or those with OSA predominant SDB (including both HFrEF and HFpEF).24
Until the mechanism behind the increased mortality observed in the SERVE-HF trial is determined, treatment with ASV in patients with HFpEF and predominant central sleep apnea needs to be closely monitored. Additional well designed clinical trials are needed with samples that accurately reflect this demographic group and that have adequate sample sizes to determine the effectiveness of treatment of SDB in persons with HFpEF. Particularly, this can help determine whether or not treatment decisions need to be made according to the type of heart failure (i.e., HFrEF vs. HFpEF) or on the mix of obstructive to central events in the individual patient. Finally, further study is needed among older adults with HFpEF to examine more holistically the impact of treatment of SDB on patient-oriented outcomes such as self-management of this chronic disease and quality of life.
Nursing Implications
Sleep disordered breathing is commonly observed in older patients with HFpEF but is not part of the routine clinical evaluation and management of heart failure.45 Therefore, SDB remains untreated in many of these patients.45 The adverse physiological and functional effects from SDB on health-related quality of life illustrates the importance of evaluation not only for the presence of but also the risk of SDB in the heart failure population. To date, there is no approved therapy to reduce hospitalization for HFpEF46 and evidence based interventions with proven efficacy failed to demonstrate mortality benefits.2,47 If SDB in patients with HFpEF is an important “hidden variable” for impaired symptom management, it may be a crucial therapeutic target and may improve treatment regimens and health-related quality of life. In addressing SDB in this older adult population with HFpEF, nurses can play a vital role in cardiovascular risk reduction and symptom management.
Acknowledgments
This research was supported by the T32, Technology: Research in Chronic and Critical Illness (2T32 NR008857) at the University of Pittsburgh, School of Nursing (L. Baniak).
This research was supported by the NINR: K24 NR016685 (E. Chasens).
Note
- AHI
apnea/hypopnea index
- ASV
adaptive servo-ventilation
- CPAP
continuous positive airway pressure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HRQoL
health related quality of life
- LVEF
left ventricular ejection fraction
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- SDB
sleep disordered breathing
Footnotes
Conflict of Interest
‘Conflicts of interest: none’.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics--2017 update: A report from the American Heart Association. Circulation. 2017;131(135):e146–e603. doi: 10.1161/CIR.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma K, Kass DA. Heart failure with preserved ejection fraction: Mechanisms, clinical features, and therapies. Circ Res. 2014;115(1):79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States a policy statement from the American Heart Association. Circ Hear Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: A report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation. 2012;126(1):65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 6.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. http://www.nejm.org/doi/full/10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 7.Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119(24):3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128(16):240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 9.Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59(11):998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Circulation. 1998;97:2154–2159. doi: 10.1161/01.CIR.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 11.Jilek C, Krenn M, Sebah D, et al. Prognostic impact of sleep disordered breathing and its treatment in heart failure: An observational study. Eur J Heart Fail. 2011;13(1):68–75. doi: 10.1093/eurjhf/hfq183. [DOI] [PubMed] [Google Scholar]
- 12.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association council for high blood pressure research professional education committee, council on. Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 13.Tremel F, Pépin JL, Veale D, et al. High prevalence and persistance of sleep apnoea in patients referred for acute left ventricular failure and medically treated over 2 months. Eur Hear J. 1999;20:1201–1209. doi: 10.1053/euhj.1999.1546. [DOI] [PubMed] [Google Scholar]
- 14.Herrscher TE, Akre H, Overland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: Even in patients with preserved systolic function. J Card Fail. 2011;17(5):420–425. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Sleep Medicine. International Classification of Sleep Disorders. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 16.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathieu A, Mazza S, Décary A, et al. Effects of obstructive sleep apnea on cognitive function: A comparison between younger and older OSAS patients. Sleep Med. 2008;9(2):112–120. doi: 10.1016/j.sleep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia and risk of mild cognitive impairment and dementia in older women. J Am Med Assoc. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redeker NS, Muench U, Zucker MJ, et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 2010;33(4):551–560. doi: 10.1093/sleep/33.4.551. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2849795&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb D, Yenokyan G, Newman A, et al. A prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The Sleep Heart Health Study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg H, Lakticova V, Scharf SM. Obstructive sleep apnea: Clinical features, evaluation, and principles of management. In: Kryger M, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. 6. Missouri: Elsevier Saunders; 2017. pp. 1110–1124. [Google Scholar]
- 23.Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: An update for 2015. J Clin Sleep Med. 2015;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aurora RN, Chowdhuri S, Ramar K, et al. Updated adaptive servo-ventilation recommendations for the 2012 AASM guideline: “The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses”. J Clin Sleep Med. 2016;12(5):757–761. doi: 10.5664/jcsm.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javaheri S. Heart failure. In: Kryger M, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. Vol. 6. Philadelphia: Elsevier; 2017. pp. 1271–1285. [Google Scholar]
- 27.Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602–608. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 28.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep-disordered breathing in patients with symptomatic heart failure: A contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9(3):251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Vazir A, Hastings PC, Papaioannou I, et al. Variation in severity and type of sleep-disordered breathing throughout 4 nights in patients with heart failure. Respir Med. 2008;102(6):831–839. doi: 10.1016/j.rmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal S, Nadeem R, Loomba RS, Nida M, Vieira D. The effects of continuous positive airways pressure therapy on cardiovascular end points in patients with sleep-disordered breathing and heart failure: A meta-analysis of randomized controlled trials. Clin Cardiol. 2014;37(1):57–65. doi: 10.1002/clc.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko Y, Floras JS, Phil D, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 32.Naughton MT, Liu PP, Bernard DC, Goldstein RS, Bradley DT. Treatment of congestive heart failure and Cheyne-Stokes respiration during sleep by continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151(1):92–97. doi: 10.1164/ajrccm.151.1.7812579. [DOI] [PubMed] [Google Scholar]
- 33.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia SR, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. http://www.nejm.org/doi/full/10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 35.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes of the heart failure syndrome. Circulation. 2011;123:2006–13. doi: 10.1161/CIRCULATIONAHA.110.954388. discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards BA, Wellman A, Sands SA, et al. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37(7):1227–1236. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker BJ. Synthesizing standardized mean change measures. Br J Math Stat Psychol. 1988;41:257–278. [Google Scholar]
- 38.Cohen J. A power primer. Quant Methods Psychol. 1992;112(1):155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 39.Bitter T, Westerheide N, Faber L, et al. Adaptive servoventilation in diastolic heart failure and Cheyne-Stokes respiration. Eur Respir J. 2010;36(2):385–392. doi: 10.1183/09031936.00045609. [DOI] [PubMed] [Google Scholar]
- 40.Oldenburg O, Bitter T, Wellmann B, et al. Trilevel adaptive servoventilation for the treatment of central and mixed sleep apnea in chronic heart failure patients. Sleep Med. 2013;14(5):422–427. doi: 10.1016/j.sleep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Yoshihisa A, Suzuki S, Yamaki T, et al. Impact of adaptive servo-ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep-disordered breathing. Eur J Heart Fail. 2013;15(5):543–550. doi: 10.1093/eurjhf/hfs197. [DOI] [PubMed] [Google Scholar]
- 42.Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;421:413–421. doi: 10.1002/clc.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arikawa T, Toyoda S, Haruyama A, et al. Impact of obstructive sleep apnoea on heart hailure with preserved ejection fraction. Hear Lung Circ. 2016;25(5):435–441. doi: 10.1016/j.hlc.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 44.O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation–targeted adaptive servo-ventilation therapy in heart failure: The CAT-HF trial. J Am Coll Cardiol. 2017;69(12):1577–1587. doi: 10.1016/j.jacc.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 45.Khayat R, Small R, Rathman L, et al. Sleep disordered breathing in heart failure: Identifying and treating an important but often unrecognized comorbidity in heart failure patients. J Card Fail. 2014;19(6):431–444. doi: 10.1016/j.cardfail.2013.04.005.Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butler J, Fonarow G, Zile M, et al. Developing therapies for heart failure with preserved ejection fraction: Current state and future directions. JACC Heart Fail. 2014;2(2):97–112. doi: 10.1016/j.jchf.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senni M, Paulus W, Gavazzi A, et al. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35(40):2797–2811d. doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]