Abstract
Inhibitory luminopsins (iLMO2) integrate opto- and chemo-genetic approaches and allow for cell-type specific inhibition of neuronal activity. When exposed to a Renilla luciferase substrate, Coelenterazine (CTZ), iLMO2 generates bioluminescence-mediated activation of its amino-terminal halorhodopsin, resulting in neuronal inhibition. Moderate daily exercise in the form of interval treadmill-training (IT) applied following a peripheral nerve injury results in enhanced motor axon regeneration and muscle fiber reinnervation in female mice. We hypothesized that iLMO2 mediated inhibition of motoneuron activity during IT would block this enhancement. Unilateral intramuscular injections of Cre-dependent AAV2/9-EF1a-DIO-iLMO2 (~8.5×1013 vg/ml) were made into the gastrocnemius and tibialis anterior muscles of young female ChAT-IRES-Cre mice, thereby limiting iLMO2 expression specifically to their motoneurons. Four to six weeks were allowed for retrograde viral transduction after which a unilateral sciatic nerve transection (Tx) and repair was performed. Animals were randomized into four groups: IT only, IT+CTZ, CTZ only, and untreated (UT). Three weeks post Tx-repair, the maximal amplitude direct muscle responses (M-max) in both muscles in the IT only group were significantly greater than in UT mice, consistent with the enhancing effects of this exercise regimen. Inhibiting motoneuron activity during exercise by a single injection of CTZ, administered 30 minutes prior to exercise, completely blocked the enhancing effect of exercise. Similar treatments with CTZ in mice without iLMO2 had no effect on regeneration. Neuronal activity is required for successful enhancement of motor axon regeneration by exercise.
Keywords: Motoneuron activity, Treadmill training, Inhibitory luminopsin, Sciatic nerve injury, M-response, RRID:MGI:3689725, RRID:CVCL_0045
Graphical abstract
INTRODUCTION
Approximately 200,000 individuals are affected by traumatic peripheral nerve injuries every year, in the United States alone (Bekelis et al. 2015; Immerman et al. 2014; Noble et al. 1998; Taylor et al. 2008). The regeneration of injured axons and their nerves is possible, however, recovery of function after a peripheral nerve injury is slow and inadequate (English et al. 2014; Gordon 2016; Gordon et al. 2008). Several experimental therapies have been employed in attempts to speed up the process of axon regeneration (Gordon et al. 2003) and improve functional outcomes (Gordon et al. 2008) after a peripheral nerve injury. These include brief electrical stimulation (Gordon and English 2015), repeated electrical stimulation (Al-Majed et al. 2000), and exercise (English et al. 2009; Gordon and English 2015; Sabatier et al. 2008). More recently, chemogenetic (Jaiswal and English 2017) and optogenetic (Ward et al. 2016) approaches have been utilized to target regeneration of axons and evaluate its impact on recovery of function. These studies have demonstrated that increasing the activity of injured axons and their neuronal cell bodies is sufficient to promote accelerated regeneration of injured axons.
The type of exercise utilized determines the outcomes of the experimental therapy. Rodents voluntarily utilize patterned locomotion where brief intervals of high-intensity running are interspersed with periods of rest (De Bono et al. 2006). Our laboratory has previously used this paradigm of exercise, called interval training (IT), in mice (Sabatier et al. 2008; Wood et al. 2012). Mice that are exercise trained starting three days after a sciatic nerve transection for two weeks, have significantly improved axon regeneration (Sabatier et al. 2008) and increases in functionally appropriate target reinnervation (English et al. 2009) after a sciatic nerve injury. A significantly increased number of retrogradely labeled motoneurons are also found in exercise trained animals compared to brief electrical stimulation four weeks after sciatic nerve injury (English et al. 2009). Exercise-induced enhancements of axon regeneration occur in a sex dependent manner; high intensity interval training benefits only female mice (English et al. 2011; Wood et al. 2012). These results form the rationale for utilizing only female mice and are the basis of the exercise protocol used in the present study. Although exercise is known to produce significant improvements in regeneration of injured axons (Boeltz et al. 2013; Cannoy et al. 2016; English et al. 2009; Gordon and English 2015; Lerman et al. 2002; Udina et al. 2011) the specific requirement for neuronal activation in the observed enhancements is not known. To address this question, we employed the use of inhibitory luminopsins (iLMO2) to inhibit motoneuron activity during exercise.
Inhibitory optogenetic techniques are valued for the precise and reversible control of neuronal activity that they provide (Liske et al. 2013). These primarily involve the use of a halorhodopsin pump, which, when illuminated by yellow light, hyperpolarizes the neuronal cell membrane by pumping chloride ions into cells, thus effectively blocking action potential generation and its propagation (Guru et al. 2015; Liske et al. 2013). Difficulties in the long-term implantation of the required light delivery hardware impede the utility of this approach in chronic experimental studies and hinder its clinical translation potential (Birkner et al. 2014; Liske et al. 2013). Using iLMO2 enables us to produce a non-invasive and repeatable control of neuronal activity in specific cell types in vivo (Berglund et al. 2016; Birkner et al. 2014; Tung et al. 2015). iLMO2 are fusion proteins (Figure 1c) with a light-emitting Renilla luciferase (RLuc), light-sensing inhibitory opsin, Natromonas halorhodopsin (NpHR), and a fluorescent protein tag, Venus. In addition to being a fluorescent tag, Venus also serves as bioluminescent resonance energy transfer (BRET). Its action is critical for achieving amplification of emitted light and to drive the iLMO2. A small molecule substrate, h-Coelenterazine (CTZ) is oxidized by RLuc (Saito et al. 2012) and converted into photons that activate the NpHR pump (Schobert and Lanyi 1982) to hyperpolarize cells expressing the iLMOs (Berglund et al. 2016; Tung et al. 2015). We employed the use of iLMO2 to investigate the importance of motoneuron activity in enhancement of functional recovery with exercise after a sciatic nerve injury. We hypothesized that, inhibition of motoneuron activity during IT, using iLMO2, will attenuate the exercise induced enhancement of functional recovery in female mice after a sciatic nerve injury. A preliminary report of some of these findings has been made (Jaiswal et al. 2016).
Figure 1.
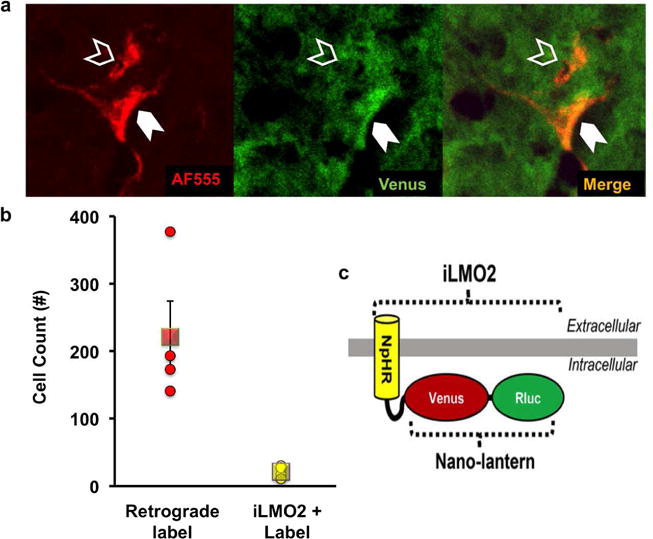
Detection of AAV-iLMO2 expression in the spinal cord. (a) Representative retrogradely labeled sciatic motoneurons with (solid arrowhead) and without (hollow arrowhead) Venus fluorescence, indicative of iLMO2 expression. (b) Quantification of detected iLMO2 in 10.7±3% of all retrogradely labeled sciatic motoneurons, (○) individual animal cell counts and (□) group mean ± SEM. (c) Schematic representation of iLMO2 (Tung et al. 2015).
MATERIALS & METHODS
Animals
Animals were housed in a 12-hour light/12-hour dark facility with access to water and standard chow ad libitum. The Institutional Animal Care and Use Committee of Emory University approved all procedures. Female ChAT-IRES-Cre mice (P10 age n=4, The Jackson Laboratory, stock# 018957, obtained from parental line #06410) were used for experiments characterizing the expression of iLMO2 in motoneurons. ChAT-IRES-Cre (4 weeks age, male n=3 and female n= 3, The Jackson Laboratory, stock# 006410, parental line, MGI Cat# 3689725, RRID:MGI:3689725) mice were used for experiments characterizing the inhibition of motor responses and its time-course after h-CTZ administration. Since no differences were observed in the results obtained from male and female mice, these data were pooled. ChAT-IRES-Cre (4–5 weeks age, female n=12) and sex and age matched mice untreated control mice (4–5 weeks age, female n=8) were used to assess the effects of motoneuron inhibition during treadmill walking on exercise-induced benefits in functional recovery after a sciatic nerve Tx-repair. See Table 1 for detailed itemization of animal numbers used for every experiment presented herein.
Table 1.
Detailed itemization of animal numbers used for each experiments presented herein.
| Experiment/Treatment | Animal number (n) | Sex | Age at Start | Virus injection | Sciatic nerve injury | Exercise | Comment |
|---|---|---|---|---|---|---|---|
| Histological analysis | 4 | F | P10 | Intramuscular | Tx + retrograde labeling | None | iLMO2 detection |
| Epidural stimulation | 3 | F | 4–5 weeks | In sciatic nerve | None | None | Inhibition of spinally evoked motor potentials and time course of CTZ administration |
| 3 | M | ||||||
| Exercise study | 12 | F | 4–5 weeks | Intramuscular | Tx-repair | IT (n=2) IT+CTZ (n=5) CTZ (n=5) |
Effects of motoneuron inhibition during treadmill walking on exercise-induced benefits in functional recovery after a sciatic nerve Tx-repair |
| 8 | None | IT (n=3*) UT (n=5*) |
Data from one animal each in the IT and UT groups could not be collected, as they died unexpectedly.
Virus Injections
The development of the iLMO2 (Ef1a-DIO-iLMO2) construct is described in detail in supplemental methods. This construct was packaged into a viral particles (AAV2/9) by the Viral Vector Core of Emory University. Mice (female ChAT-IRES-Cre, age P10, n=4; female ChAT-IRES-Cre age 4–5 weeks, n=12) were anesthetized with Ketamine (80mg/kg) and Xylazine (10mg/kg) administered intraperitoneally. Additional Ketamine was administered as required during the surgical procedure. The right lateral gastrocnemius (LG) and tibialis anterior (TA) muscles were exposed and injected with 1 μl of the AAV2/9 vector in sterile 1X PBS (8.5×1013 viral genomes/μl). Using a Hamilton syringe (26G, 2.5 μl; Hamilton, Reno, NV), four injection sites through each of the whole LG and TA muscles were injected with 0.25 μl of the viral vector, each. Each injection was performed over 30 seconds and the needle was held in place for approximately 30 seconds after the injection to avoid retrograde leakage of viral vector. The LG and TA muscles in the remainder of animals (untreated control mice n=8) were not exposed or injected. Animals recovered for four to six weeks after intramuscular injections to allow for sufficient time for retrograde transduction of targeted LG and TA neurons. These animals were used in experiments involving iLMO2 expression and effects of motoneuronal inhibition during treadmill walking on exercise-induced benefits in functional recovery after a sciatic nerve Tx-repair.
In a separate group of animals (ChAT-IRES-Cre, age 4 weeks, n=6) the right sciatic nerve was exposed in the mid-thigh area, approximately 5mm proximal to the trifurcation of the sciatic nerve into tibial, common fibular, and sural branches and blunt dissected away from surrounding tissue. Using a pair of angled forceps slight tension was applied to the exposed sciatic nerve. A small rectangle piece of parafilm (Parafilm M™ wrapping film, Fisher Scientific Co LLC, Pittsburgh PA) was placed under and around the nerve so as to prevent leakage and spread of virus injection to surrounding tissue. Using a Hamilton syringe (30G, 2.5μl; Hamilton, Reno, NV) approximately 1.5μl of the AAV2/9 vector in sterile hypertonic salt solution (AAV2/9-Ef1a-DIO-iLMO2, 5M NaCl, 1x PBS, 2.12×1011 viral genomes/μl) was injected under the epineurium at multiple places. Some fluid collected in the parafilm boat and was taken up by the nerve by letting it sit for 5 minutes. After ensuring no additional viral vector fluid was remaining, the parafilm boat was removed and the incision was sutured. These animals recovered for four weeks to allow for sufficient time for retrograde transduction of targeted sciatic neurons. They were then used for epidural electrical stimulation experiments.
Peripheral nerve injury
We used the sciatic nerve transection-repair (Tx-repair) as a model of peripheral nerve injury in our study. Previously virus injected and untreated control mice (ChAT-IRES-Cre, n=12; and untreated control mice n=8, age 8 weeks) were subjected to a sciatic nerve Tx-repair. These animals were injected with Ketamine (80mg/kg) and Xylazine (10mg/kg) cocktail anesthesia. Additional ketamine was administered as required during the surgical procedure. The sciatic nerves were exposed unilaterally in the mid-thigh area, approximately 5mm proximal to the trifurcation of the sciatic nerve into tibial, common fibular, and sural branches. It was then secured on a small rectangle of SILASTIC film (Dow Corning 501-1) using 5–7μl of fibrin glue: a mixture of fibrinogen, fibronectin, and thrombin (Sigma-Aldrich, St. Louis, MO). This mixture was formulated immediately prior to use. The components of this mixture formed a fibrin-glue ‘clot’ that provided mechanical stabilization of the repaired nerve for approximately 72 hours, until normal tissue fibrosis provided support (MacGillivray 2003; Ward et al. 2016). Once secured to the film, the nerve was cut with sharp scissors near the center of the film. Securing the nerve to the film prevents the withdrawal of the two stumps of the nerve after transection. A second application of 5–7μl of fibrin glue was then applied to secure the ends of the cut nerve together. Post-surgical analgesia was not administered. Animals were allowed to recover for three days before experimental procedures were performed.
Exercise protocol - Interval training (IT)
Animals subjected to a peripheral nerve injury were randomly divided into four groups; IT only (n=4), IT with CTZ treatment (n=5), CTZ treatment only (n=5), and untreated, UT (n=4). Data from one animal each in the IT and UT groups could not be collected, as they died unexpectedly. Animals in the exercise groups, IT only and IT+CTZ, walked on a level treadmill at 20m/min for 2 minutes followed by 5 minutes of rest period (English et al. 2009). This was repeated a total of four times each day. IT began three days post sciatic nerve Tx-repair and was performed one time/day, five days/week for a total of two weeks. For animals in the IT+CTZ group, IT began 30 minutes after systemic administration of h-CTZ administration to allow maximal motoneuron inhibition during training.
Coelenterazine injections
Coelenterazine (Inject-A-Lume, h-CTZ, NanoLight Technology, Pinetop, AZ) powder was fresh solubilized in the vehicle solution provided, immediately prior to administration. 250μg of dissolved h-CTZ solution was used for intra-peritoneal (i.p.) injection in all animals.
Electrically Evoked Responses
a) Epidural Stimulation
Animals (ChAT-IRES-Cre female n=3, male n=3) that previously received virus injections in the sciatic nerve were anesthetized using Ketamine (80mg/kg) and Xylazine (10mg/kg). Additional ketamine was administered as required during the surgical procedure. A midline skin incision of approximately 2–3 cm was made on the back. A dorsal lumbar (L2) laminectomy was performed to expose the dura mater covering the spinal cord. A bipolar tungsten wire needle electrode made from 100μm tungsten stock, designed and made in our laboratory, were threaded caudally into the epidural space beneath the L3 laminae and gently positioned on either side of the midline (~2mm apart). In one animal, two commercially available needle electrodes (Neuroline monopolar, 28G, Ambu/AS, Copenhagen, Denmark) were used similarly. Short (0.1 ms) constant voltage stimulus pulses were applied to the needle electrode to produce a spinally evoked motor potential (SEMP) (Gerasimenko et al. 2015) recorded from the LG and TA muscles. Stimulus intensity was gradually increased until a maximal amplitude response was recorded. The inter-stimulation interval was set at three seconds in an effort to minimize any damage to the spinal cord. Bipolar EMG electrodes (Basmajian and Stecko 1963), for acute use, were made using fine wire (California Fine Wire Company, Grover Beach, CA; Stablohm 800 A, material number cfw-100189) and were placed into the LG and TA muscles using 25G hypodermic needle. SEMPs were recorded before (Pre CTZ) and at 10, 30, 60 and 90 minutes after treatment (Post CTZ).
b) Sciatic Nerve Stimulation
Animals were anesthetized using Ketamine (80mg/kg) and Xylazine (10mg/kg) cocktail anesthesia. Additional ketamine was administered as required during the surgical procedure. Bipolar stimulating electrical cuffs were assembled using a short length of Silastic tubing and stranded stainless steel micro wire, AWG size 40 (Cooner Wire, Chatsworth, CA; part# AS631) (Sabatier et al. 2011). These cuffs were placed around the sciatic nerve just proximal to the branching into the common fibular and tibial nerves to evoke responses in intact animals (n=20). Three weeks after sciatic nerve Tx-repair recordings, stimulating needle electrodes (Neuroline monopolar, 28G, Ambu/AS, Copenhagen, Denmark), positioned 1–2mm apart proximal to the injury site under the exposed sciatic nerve, were used to evoke responses (Redondo-Castro and Navarro 2013). Needle electrodes, instead of electrical cuffs, were used after sciatic nerve Tx-repair in an effort to minimize the influence of invasive recordings on regenerating axons. Bipolar EMG electrodes (Basmajian and Stecko 1963), for acute use, were made using fine wire (California Fine Wire Company, Grover Beach, CA; Stablohm 800 A, material number cfw-100189) and were placed into the LG and TA muscles using 25G hypodermic needle. Electrically evoked EMG activity was recorded from these electrodes in response to sciatic nerve stimulation (0.3 ms pulses). Stimulus intensity was gradually incremented until a maximal M response amplitude was recorded. The inter-stimulation interval was set at three seconds to minimize any muscle fatigue. All animals were euthanized at ten weeks after sciatic nerve Tx-repair data with an overdose of sodium pentobarbital anesthesia (150mg/kg).
Histological Analysis
The sciatic nerves of mice (female ChAT-IRES-Cre, n=4, previously injected with AAV-iLMO2 intramuscularly, Table 1) were completely transected and the proximal stump was soaked with Alexa Fluor 555 (10,000 MW, fixable; Invitrogen, Carlsbad, CA) crystals for one hour to retrogradely label all sciatic neurons (English 2005; English et al. 2009). Following one hour of this soak, the surgical site was washed with saline three times, surgical wounds were sutured closed and the animals were returned to their home cage for three days to allow for retrograde uptake of tracer. These animals were then euthanized (sodium pentobarbital anesthesia, 150mg/kg), perfused transcardially with 4% periodatelysate-paraformaldehyde (PLP), (English 2005; English et al. 2009) and lumbar spinal cords and L4-L5 dorsal root ganglia were harvested and stored in 20% sucrose solution at 4°C for cryoprotection. Frozen cross sections of the lumbar spinal cord (20μm) and L4 DRG (12μm) tissue were placed on slides, cover slipped with VectaShield antifade mounting medium (Vector Laboratories, Burlingame, CA). High-resolution RGB images (20×) of the spinal cord ventral horn and L4 DRG were captured using an upright fluorescent microscope (Leica DM6000), a low light camera and Simple PCI software (Hamamatsu, Sewickley, PA). The total number of retrogradely labeled sciatic neurons (AF555 positive) and cells expressing iLMO2 (Venus positive) were counted from all cryosections obtained from the spinal cord and DRG. No stereological corrections were used.
Data & Statistical Analyses
The amplitudes of SEMPs evoked via epidural electrical stimulation prior to and at different times after CTZ injections were compared using two-tailed, paired t-tests. Data are presented as mean ± standard deviation. The extent of functional recovery three weeks after sciatic nerve Tx-repair was compared between the different treatment groups using one-way ANOVA and Fisher’s least significant differences (LSD) post-hoc analysis, where relevant. Data are presented as mean ± standard error of mean. According to our a priori power analysis, a sample size of four to five animals per experimental group was adequate for power of 0.8 and statistically significant differences of p<0.05.
RESULTS
Detection of iLMO2 in retrogradely labeled sciatic motoneurons
iLMO2 was selectively expressed in motoneurons by injecting a Cre-dependent adeno-associated viral vector (Supplemental methods and data Figure S1) into the spinal cord of ChAT-IRES-Cre female mice (n=4). From 20× high-resolution images of the spinal cord (Figure 1a), retrogradely labeled sciatic motoneurons (AF555 positive; 221±53.1 cells) and cells also expressing iLMO2 (Venus positive; 21.25±4.4 cells) were counted. Average numbers of retrogradely labeled cells with and without iLMO2 expression are presented in Figure 1b. iLMO2 expression could be detected in 10.7±3% of the total number of retrogradely labeled sciatic motoneurons (Figure 1b). No iLMO2 expression was observed in retrogradely labeled L4 DRG neurons (data not shown), as was expected. AAV2/9 has been used successfully as a vector for retrograde transport and delivery of transgenes to the central nervous system (Castle et al. 2014; Choi et al. 2005; Hollis et al. 2008; Jaiswal and English 2017; Smith et al. 2017). No attempt was made to amplify the fluorescent signal from Venus positive cells using antibody staining (Kim et al. 2007). Detection of cells expressing transgenes from formaldehyde fixed tissues may not always be reflective of the true number of cells expressing the transgene and its associated fluorescent reporter (Kim et al. 2007; Zhu et al. 2016). Results from electrophysiological studies presented herein indicate the likelihood that a significantly greater number of cells express iLMO2 than those that could be detected in this histological assay.
Blocking motoneuron activity significantly decreased the amplitude of spinally evoked motor potentials
As the intensity of epidural electrical stimulation was gradually increased, the amplitude of the spinally evoked motor potentials (SEMPs) from the LG and TA muscles also increased. The amplitude of these evoked responses eventually reached a maximum response for each muscle studied. The maximal amplitude of this SEMP was measured for Pre CTZ and 10, 30, 60 and 90 mins Post CTZ administration. All data were scaled to Pre CTZ maximal amplitude values and percentages were calculated (Figure 2; %Pre CTZ). The SEMP response amplitude thus scaled was significantly decreased (two-tailed, paired t-test) in the LG (31±19% Pre CTZ, Figure 2a; p=0.0003) and TA (52±17% Pre CTZ, Figure 2b; p=0.0009) at 10–30mins Post CTZ and in the TA (71 ±21% Pre CTZ, Figure 2b; p=0.04) at 60–90mins Post CTZ. Thus, CTZ mediated activation of iLMO2 produced a rapid, robust and short duration inhibition of motoneuron activity. The inhibition of motoneuron activity significantly decreased the amplitude of muscle motor output in both LG and TA resulting from epidural stimulation.
Figure 2.
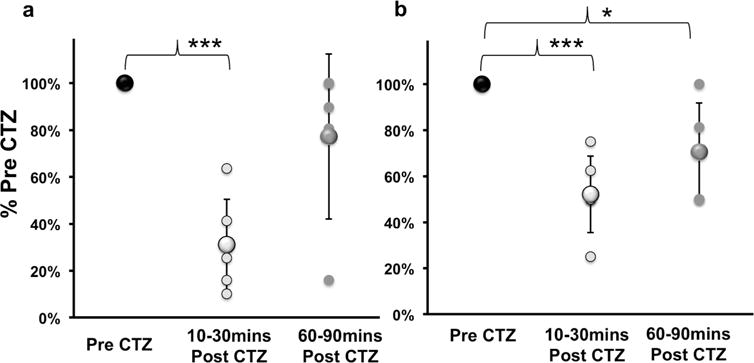
Spinally evoked motor responses in intact animals before (Pre CTZ), 10–30mins and 60–90mins after (Post CTZ) in the (a) lateral gastrocnemius (LG) and (b) tibialis anterior (TA) muscles. Data are presented as percentage mean ± SD of Pre CTZ values. Systemic administration of h-CTZ caused significant reductions in evoked responses in the LG (a; ***p=0.0003) and TA (b; ***p=0.0009) at 10–30mins Post CTZ and in the TA (b; *p=0.04) at 60–90mins Post CTZ.
Blocking motoneuron activity during exercise blocks exercise-induced enhancements in functional recovery
Electrical stimulation of the sciatic nerve produced a direct muscle response (M response) in the LG and TA muscles. The amplitude of this evoked potential increased gradually with increasing stimulus intensity until a maximal M-response was achieved. Maximal M-responses were electrically evoked in the LG (Figure 3a) and TA muscles at three weeks post sciatic nerve TX-repair. The amplitudes of these maximal responses were measured as the average rectified voltage in a user-defined latency window, and compared between the four groups: IT only, IT+CTZ, CTZ only and UT (Figure 3 b&c). Analyzed data are presented in bar- and-whisker plot format in Figure 3. The size of the bars indicate the mean ± SEM, the horizontal line in the center of the bar represents the mean, and whiskers are used to indicate the range of values in each data set. The one-way ANOVA omnibus test was significant for both LG (F= 3.81, 14, n=18, p=0.03) and TA (F= 11.56, 14, n=18, p=0.0004). Based on use of Fisher’s Least Significant Difference for post hoc paired testing between group comparisons, the amplitude of M-max responses in the IT group was significantly greater than that of the IT+CTZ group (Figure 3b: LG, p=0.026; Figure 3c: TA, p=0.0005), the CTZ only group (Figure 3b: LG, p=0.008; Figure 3c: TA, p=0.0001), and the UT group (Figure 3b: LG, p=0.017; Figure 3c: TA, p=0.0003). Post-hoc comparisons between results from the IT+CTZ, CTZ and UT groups were not significant. Therefore, blocking motoneuron activity during exercise, by CTZ administration, completely blocked any exercise-induced enhancements in the recovery of LG and TA motor function.
Figure 3.
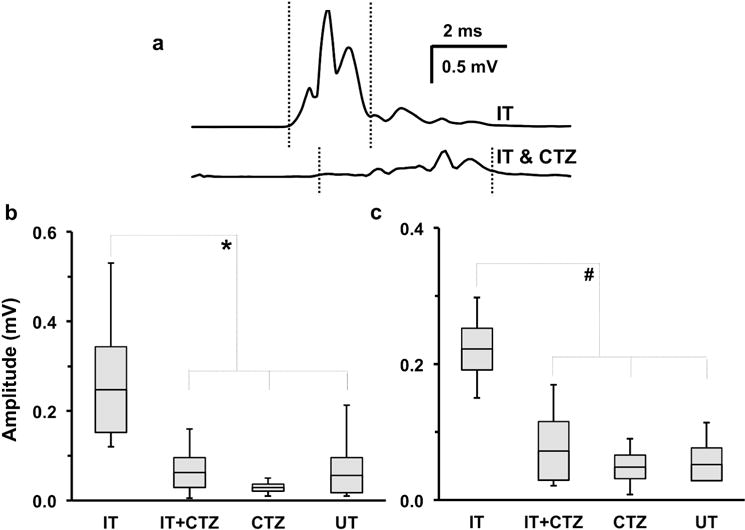
Recovery of electrically evoked maximum direct muscle M responses recorded three weeks after sciatic nerve transection and repair. (a) Representative traces recorded from lateral gastrocnemius (LG) in interval-trained mice that were either untreated (IT) or treated with h-CTZ prior to each exercise session (IT+CTZ). Vertical dotted lines indicate time window of the M response used for amplitude measurements. (b, c) In these bar-and-whisker plots, the size of the bars indicate the mean ± SEM, the horizontal line in the center of the bar represents the mean, and whiskers are used to indicate the range of values in each data set. The amplitude of M-max responses in the IT group was significantly greater than that of the IT+CTZ group (b: LG, *p=0.026; c: tibialis anterior (TA), #p=0.0005), as well as mice treated with h-CTZ (CTZ) (b: LG, *p=0.008; c: TA, #p=0.0001) and untreated mice (UT) (b: LG, *p=0.017; c: TA, #p=0.0003) groups.
DISCUSSION
The key findings in our study are as follows: a) Cre-dependent iLMO2 can be expressed in specific cell-types within the central nervous system using retrograde adenoviral vector transport from the periphery, in mice; b) Once expressed, iLMO2 activation by CTZ administration provides a rapid, robust and short duration inhibition of neuronal activity with cell-type specificity; c) iLMO2 mediated inhibition of motoneuron activity during exercise is sufficient to block the enhancements in motor axon regeneration and muscle fiber reinnervation produced by exercise after a sciatic nerve injury. Results from experiments presented herein employ the use of a combination of cutting-edge research tools including Cre-lox technology (Tsien 2016), iLMO2 (Tung et al. 2015) and retrograde viral vector transport from the periphery to the central nervous system (Castle et al. 2014; Jaiswal and English 2017) in mice. These tools together allowed us to reversibly and precisely inhibit motoneuron activity during exercise in conscious mice without the need of invasive hardware. We used these to address a fundamental biological question, whether neuronal activity during exercise is required for enhancement of axon regeneration and functional recovery. This is the first published report on the effect of iLMO2-mediated inhibition of intact motoneuron activity and its impact on recovery of function after a sciatic nerve injury.
Optogenetic techniques have had an unprecedented impact on investigations involving neuronal circuitry and behaviors in experimental models (Guru et al. 2015; Liske et al. 2013; Ward et al. 2016). However, many limiting features impede the use of optogenetics in chronic in vivo studies. These include; invasive nature of surgeries needed to implant light-delivery hardware, post surgical complications and ineffective light penetration required for activation of opsin. The use of inhibitory luminopsins bypasses these difficulties (Berglund et al. 2016; Birkner et al. 2014; Tung et al. 2015). These fusion proteins incorporate a light emitting Renilla luciferase (RLuc), a light sensing Natromonas halorhodopsin (NpHR) and a BRET fluorescent protein tag, Venus. RLuc oxidizes the small molecule substrate h-Coelenterazine (CTZ) thereby emitting photons of yellow light that activate the NpHR channel, effectively hyperpolarizing the cell membrane. The luminopsin construct used in our study (AAV2/9-EF1a-DIO-iLMO2) is designed to be under the control of a ubiquitous human elongation factor-1 alpha promoter (Ef-1a) in the double floxed inverse open reading frame (DIO) configuration. Therefore, in the presence of Cre the iLMO2 transgene will undergo Cre-mediated recombination and be effectively expressed. In the absence of Cre, iLMO2 will not be expressed. Thus, utilizing a robust construct design along with appropriate adenoviral vector, AAV2/9, (Castle et al. 2014; Jaiswal and English 2017; Jaiswal et al. 2016) was fundamental to the experimental results presented here. In mice in which this construct was expressed, robust inhibition of spinally evoked motor potentials (SEMPs) was found, consistent with robust expression of the viral vector in motoneurons. However, in histological analyses of spinal cord tissue we found that only ~10% of sciatic motor neurons could be detected that also expressed iLMO2 (Figure 1b). The poor detection of iLMO2 expression, relative to the amount of inhibition discerned using electrophysiological methods, could be due to the tissue fixation methods used. Fixation using paraformaldehyde is known to diminish fluorescent reporter signal strength (Zhu et al. 2016). In the future, amplification of the Venus fluorescence signal (Kim et al. 2007) with antibodies may enable detection of more cells expressing iLMO2 and help to explain the discrepancy in numbers of iLMO2 expressing cells that could be detected anatomically and the strong electrophysiological findings observed.
We used epidural electrical stimulation to activate the sciatic motor nucleus and produce SEMPs (Gerasimenko et al. 2015) in lateral gastrocnemius and tibialis anterior muscles of intact mice. Al Majed et al (2000) showed that the successful activation of cell bodies of injured axons is required for an increase in axon regeneration after a peripheral nerve injury (Al-Majed et al. 2000). We used iLMO2 to inhibit motoneurons specifically and reversibly. The inhibition of sciatic motoneuron cell bodies in our experiments is distinct from inhibition of action potential propagation along the injured axons described in other studies (Liske et al. 2013). The maximal inhibitory effect on epidural electrically evoked motor responses was observed between 10–30 minutes after systemic CTZ administration. This result was expected based on previous studies using a similar construct (Berglund et al. 2016; Tung et al. 2015). The relatively short duration of inhibition was desirable and effectively supplemented the design of our exercise study.
Exercise effectively promotes the regeneration of axons after a sciatic nerve injury (Boeltz et al. 2013; Cannoy et al. 2016; English et al. 2009; Gordon and English 2015). Treadmill exercise after a sciatic nerve injury is known to produce improvements in regeneration of injured axons (English et al. 2011; Sabatier et al. 2008). Specifically, interval treadmill training is known to improve axon regeneration in female mice after sciatic nerve injury (English et al. 2009; English et al. 2011; Sabatier et al. 2008; Wood et al. 2012). Activation of cell bodies of injured axons is required for accelerated axon regeneration (Al-Majed et al. 2000) and cell-type specific activation of motoneurons has been previously reported to be sufficient for functional motor axon regeneration (Ward et al. 2016) after a nerve injury. With the combination of this knowledge from previous reports and short duration of inhibition from results of our experiments, as detailed above, we focused our efforts on the effect of inhibiting motoneuron activity during exercise in female mice only. The results of our studies support our hypothesis. We demonstrate here that inhibition of motoneuron activity during IT, using iLMO2, completely blocked all exercise-induced enhancements of functional recovery in female mice after a sciatic nerve injury.
Male mice subjected to a sciatic nerve Tx-repair benefit from a continuous training paradigm lasting an hour but not the interval training protocol used here in female mice (Wood et al. 2012). The reasons for this pronounced sex difference are still emerging. We have proposed that the cellular mechanisms leading to enhanced axon regeneration with exercise may be fundamentally different in males and females (Gordon and English 2015), even though androgens and estrogens may be involved in both sexes (Acosta et al. 2017; Wood et al. 2012). Future studies involving male mice with longer lasting doses of CTZ, and therefore longer duration of inhibition, need to be conducted to investigate sex differences in the effect of blocking motoneuron activity in the cell bodies of injured axons during exercise. Coordinated activity between pools of motor neurons along with feedback from proprioceptive afferents is the basis of a locomotor pattern (Yakovenko et al. 2002) and inactivity as a result of neurological trauma can cause changes in central nervous system circuitry (Lundbye-Jensen and Nielsen 2008a; Lundbye-Jensen and Nielsen 2008b). Other future studies may include the use of iLMO2 to investigate the effect of blocking afferent activity during exercise and imposing task specific locomotor patterns to drive changes in central nervous system circuitry.
In summary, we conclude that motoneuron activity is required for exercise-associated enhancements in recovery of motor function as early as three weeks after sciatic nerve injury in female mice. We have demonstrated the Cre-dependent expression of iLMO2 in motoneurons specifically using retrograde adenoviral vector transport from the periphery, in female mice. iLMO2 activation via CTZ administration provides a rapid, robust and short duration inhibition of neuronal activity with cell-type specificity. Additionally we show that iLMO2 mediated inhibition of motoneuron activity during exercise is sufficient to block the enhancements in motor axon regeneration and muscle fiber reinnervation produced by exercise after a sciatic nerve injury.
Supplementary Material
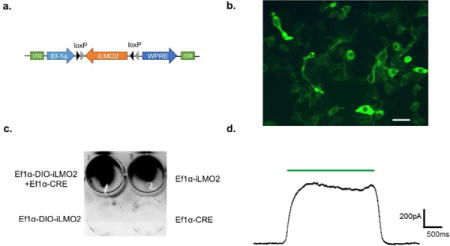
Supplemental Figure 1: Design and development of Ef1a-DIO-iLMO2 construct. (a) Schematic of the Ef1a-DIO-iLMO2 construct. (b) Fluorescence image showing HEK293 cells co-transfected with Ef1α-DIO-iLMO2 and Ef1α-Cre. iLMO2 expression (green) is localized to the cell membrane of transfected cells. Scale bar: 40um. (c) Bioluminescence imaging of HEK293 cells transfected with Ef1a-DIO-iLMO2, Ef1α-Cre, or both in a 12-well plate. Bioluminescence (black signal) was detected when DIO-iLMO2 was co-expressed with Cre and in positive control Ef1a-iLMO2. (d) Whole cell patch clamp recording of HEK293 cell co-transfected with Ef1a-DIO-iLMO2 and Ef1α-Cre showing hyperpolarizing outward current to green light (TRITC) illumination.
SIGNIFICANCE STATEMENT.
Peripheral nerve injuries are a major public health concern, with approximately 200,000 individuals affected every year in the USA alone. Exercise is often prescribed as a rehabilitation intervention in these individuals. However, the mechanism of action underlying exercise induced benefits in axon regeneration and recovery of function is not clear. The results of this study aim to address this gap in knowledge. By utilizing inhibitory luminopsins, we can probe cell-type specific contributions in a non-invasive fashion and optimize dosages for maximal exercise induced benefits.
Acknowledgments
The authors would like to thank the Emory Viral Vector Core, Emory University, Atlanta, GA for packaging the inhibitory Luminopsin into the adenovirus construct used in this study. We would also like to thank Dr. Francisco Alvarez, Department of Physiology, Emory University for generously providing the ChAT-IRES-Cre mice used for experiments characterizing the expression of iLMO2 in motoneurons. Also thanks to Olivia C. Mistretta for technical assistance.
GRANT SUPPORT: The authors would like to thank funding from NIH Grant NS057190, NS079757, NS086433, and NS079268 for supporting this research.
ABBREVIATIONS
- AAV2/9
Adeno associated virus serotype 2/9
- h-CTZ
h-Coelenterazine
- iLMO2
Inhibitory luminopsin
- LG
Lateral gastrocnemius
- TA
Tibialis anterior
- Tx
Transection
Footnotes
REQUESTED ASSOCIATE EDITOR: Ken Berglund, Ph.D.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHORS ROLES
Conceived and designed the experiments: PBJ AWE. Performed experiments: PBJ. Analyzed data: PBJ AWE. Supplemental data: JKT REG. Contributed materials/reagents/analysis tools: JKT REG AWE. Wrote the manuscript: PBJ AWE. Edited the manuscript: PBJ JKT REG AWE.
DATA ACCESSIBILITY STATEMENT
The authors have made available the data relevant to results presented in this study as a supplemental file.
References
- Acosta MC, Copley PA, Harrell JR, Wilhelm JC. Estrogen signaling is necessary for exercise-mediated enhancement of motoneuron participation in axon regeneration after peripheral nerve injury in mice. Developmental neurobiology. 2017 doi: 10.1002/dneu.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20(7):2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basmajian JV, Stecko G. The Role of Muscles in Arch Support of the Foot. J Bone Joint Surg Am. 1963;45:1184–1190. [PubMed] [Google Scholar]
- Bekelis K, Missios S, Spinner RJ. Falls and peripheral nerve injuries: an age-dependent relationship. Journal of neurosurgery. 2015:1–7. doi: 10.3171/2014.11.JNS142111. [DOI] [PubMed] [Google Scholar]
- Berglund K, Tung JK, Higashikubo B, Gross RE, Moore CI, Hochgeschwender U. Combined Optogenetic and Chemogenetic Control of Neurons. Methods Mol Biol. 2016;1408:207–225. doi: 10.1007/978-1-4939-3512-3_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkner E, Berglund K, Klein ME, Augustine GJ, Hochgeschwender U. Non-invasive activation of optogenetic actuators. Proc SPIE Int Soc Opt Eng. 2014;8928 doi: 10.1117/12.2044157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeltz T, Ireland M, Mathis K, Nicolini J, Poplavski K, Rose SJ, Wilson E, English AW. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J Neurophysiol. 2013;109(11):2645–2657. doi: 10.1152/jn.00946.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannoy J, Crowley S, Jarratt A, Werts KL, Osborne K, Park S, English AW. Upslope treadmill exercise enhances motor axon regeneration but not functional recovery following peripheral nerve injury. J Neurophysiol. 2016;116(3):1408–1417. doi: 10.1152/jn.00129.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle MJ, Gershenson ZT, Giles AR, Holzbaur EL, Wolfe JH. Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport. Hum Gene Ther. 2014;25(8):705–720. doi: 10.1089/hum.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi VW, McCarty DM, Samulski RJ. AAV hybrid serotypes: improved vectors for gene delivery. Curr Gene Ther. 2005;5(3):299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R926–934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol. 2005;490(4):427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- English AW, Cucoranu D, Mulligan A, Sabatier M. Treadmill training enhances axon regeneration in injured mouse peripheral nerves without increased loss of topographic specificity. J Comp Neurol. 2009;517(2):245–255. doi: 10.1002/cne.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Sabatier MJ. Enhancing recovery from peripheral nerve injury using treadmill training. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft. 2011;193(4):354–361. doi: 10.1016/j.aanat.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Ward PJ. Exercise, neurotrophins, and axon regeneration in the PNS. Physiology. 2014;29(6):437–445. doi: 10.1152/physiol.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, Reggie Edgerton V. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med. 2015;58(4):225–231. doi: 10.1016/j.rehab.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics. 2016;13(2):295–310. doi: 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Brushart TM, Chan KM. Augmenting nerve regeneration with electrical stimulation. Neurol Res. 2008;30(10):1012–1022. doi: 10.1179/174313208X362488. [DOI] [PubMed] [Google Scholar]
- Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci. 2015 doi: 10.1111/ejn.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8(4):236–250. doi: 10.1111/j.1085-9489.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- Guru A, Post RJ, Ho YY, Warden MR. Making Sense of Optogenetics. Int J Neuropsychopharmacol. 2015;18(11):pyv079. doi: 10.1093/ijnp/pyv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Kadoya K, Hirsch M, Samulski RJ, Tuszynski MH. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther. 2008;16(2):296–301. doi: 10.1038/sj.mt.6300367. [DOI] [PubMed] [Google Scholar]
- Immerman I, Price AE, Alfonso I, Grossman JA. Lower extremity nerve trauma. Bulletin of the Hospital for Joint Disease. 2014;72(1):43–52. [PubMed] [Google Scholar]
- Jaiswal PB, English AW. Chemogenetic enhancement of functional recovery after a sciatic nerve injury. Eur J Neurosci. 2017 doi: 10.1111/ejn.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal PB, Tung JK, Gross RE, English AW. Luminopsin mediated attenuation of exercise induced functional improvements after sciatic nerve injury. San Diego, CA: Society for Neuroscience; 2016. (Program No 675.06). Neuroscience 2016 Abstracts. 2016, Online. [Google Scholar]
- Kim IH, Nagel J, Otten S, Knerr B, Eils R, Rohr K, Dietzel S. Quantitative comparison of DNA detection by GFP-lac repressor tagging, fluorescence in situ hybridization and immunostaining. BMC Biotechnol. 2007;7:92. doi: 10.1186/1472-6750-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol (1985) 2002;92(6):2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- Liske H, Towne C, Anikeeva P, Zhao S, Feng G, Deisseroth K, Delp S. Optical inhibition of motor nerve and muscle activity in vivo. Muscle Nerve. 2013;47(6):916–921. doi: 10.1002/mus.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbye-Jensen J, Nielsen JB. Central nervous adaptations following 1 wk of wrist and hand immobilization. J Appl Physiol (1985) 2008a;105(1):139–151. doi: 10.1152/japplphysiol.00687.2007. [DOI] [PubMed] [Google Scholar]
- Lundbye-Jensen J, Nielsen JB. Immobilization induces changes in presynaptic control of group Ia afferents in healthy humans. J Physiol. 2008b;586(17):4121–4135. doi: 10.1113/jphysiol.2008.156547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray TE. Fibrin sealants and glues. J Card Surg. 2003;18(6):480–485. doi: 10.1046/j.0886-0440.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. The Journal of trauma. 1998;45(1):116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- Redondo-Castro E, Navarro X. Peripheral nerve alterations after spinal cord injury in the adult rat. Spinal Cord. 2013;51(8):630–633. doi: 10.1038/sc.2013.57. [DOI] [PubMed] [Google Scholar]
- Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211(2):489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier MJ, To BN, Nicolini J, English AW. Effect of slope and sciatic nerve injury on ankle muscle recruitment and hindlimb kinematics during walking in the rat. J Exp Biol. 2011;214(Pt 6):1007–1016. doi: 10.1242/jeb.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Chang YF, Horikawa K, Hatsugai N, Higuchi Y, Hashida M, Yoshida Y, Matsuda T, Arai Y, Nagai T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat Commun. 2012;3:1262. doi: 10.1038/ncomms2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B, Lanyi JK. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982;257(17):10306–10313. [PubMed] [Google Scholar]
- Smith BK, Martin AD, Lawson LA, Vernot V, Marcus J, Islam S, Shafi N, Corti M, Collins SW, Byrne BJ. Inspiratory muscle conditioning exercise and diaphragm gene therapy in Pompe disease: Clinical evidence of respiratory plasticity. Exp Neurol. 2017;287(Pt 2):216–224. doi: 10.1016/j.expneurol.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87(5):381–385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- Tsien JZ. Cre-Lox Neurogenetics: 20 Years of Versatile Applications in Brain Research and Counting. Front Genet. 2016;7:19. doi: 10.3389/fgene.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung JK, Gutekunst CA, Gross RE. Inhibitory luminopsins: genetically-encoded bioluminescent opsins for versatile, scalable, and hardwareindependent optogenetic inhibition. Scientific reports. 2015;5:14366. doi: 10.1038/srep14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina E, Puigdemasa A, Navarro X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve. 2011;43(4):500–509. doi: 10.1002/mus.21912. [DOI] [PubMed] [Google Scholar]
- Ward PJ, Jones LN, Mulligan A, Goolsby W, Wilhelm JC, English AW. Optically-Induced Neuronal Activity Is Sufficient to Promote Functional Motor Axon Regeneration In Vivo. PLoS One. 2016;11(5):e0154243. doi: 10.1371/journal.pone.0154243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K, Wilhelm JC, Sabatier MJ, Liu K, Gu J, English AW. Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Developmental neurobiology. 2012;72(5):688–698. doi: 10.1002/dneu.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovenko S, Mushahwar V, VanderHorst V, Holstege G, Prochazka A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol. 2002;87(3):1542–1553. doi: 10.1152/jn.00479.2001. [DOI] [PubMed] [Google Scholar]
- Zhu H, Aryal DK, Olsen RH, Urban DJ, Swearingen A, Forbes S, Roth BL, Hochgeschwender U. Cre dependent DREADD (designer receptors exclusively activated by designer drugs) mice. Genesis. 2016 doi: 10.1002/dvg.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Design and development of Ef1a-DIO-iLMO2 construct. (a) Schematic of the Ef1a-DIO-iLMO2 construct. (b) Fluorescence image showing HEK293 cells co-transfected with Ef1α-DIO-iLMO2 and Ef1α-Cre. iLMO2 expression (green) is localized to the cell membrane of transfected cells. Scale bar: 40um. (c) Bioluminescence imaging of HEK293 cells transfected with Ef1a-DIO-iLMO2, Ef1α-Cre, or both in a 12-well plate. Bioluminescence (black signal) was detected when DIO-iLMO2 was co-expressed with Cre and in positive control Ef1a-iLMO2. (d) Whole cell patch clamp recording of HEK293 cell co-transfected with Ef1a-DIO-iLMO2 and Ef1α-Cre showing hyperpolarizing outward current to green light (TRITC) illumination.


