Abstract
Stem rust of wheat caused by the fungal pathogen Puccinia graminis f. sp. tritici historically caused major yield losses of wheat worldwide. To understand the genetic basis of stem rust resistance in contemporary North American spring wheat, genome-wide association analysis (GWAS) was conducted on an association mapping panel comprised of 250 elite lines. The lines were evaluated in separate nurseries each inoculated with a different P. graminis f. sp. tritici race for 3 years (2013, 2015, and 2016) at Rosemount, Minnesota allowing the evaluation of race-specificity separate from the effect of environment. The lines were also challenged with the same four races at the seedling stage in a greenhouse facility at the USDA-ARS Cereal Disease Laboratory. A total of 22,310 high-quality SNPs obtained from the Infinium 90,000 SNPs chip were used to perform association analysis. We observed often negative and sometimes weak correlations between responses to different races that highlighted the abundance of race-specific resistance and the inability to predict the response of the lines across races. Markers strongly associated with resistance to the four races at seedling and field environments were identified. At the seedling stage, the most significant marker-trait associations were detected in the regions of known major genes (Sr6, Sr7a, and Sr9b) except for race QFCSC where a strong association was detected on chromosome arm 1AL. We postulated the presence of Sr2, Sr6, Sr7a, Sr8a, Sr9b, Sr11, Sr12, Sr24, Sr25, Sr31, and Sr57 (Lr34) in this germplasm based on phenotypic and marker data. We found over half of the panel possessed three or more Sr genes, and most commonly included various combinations of Sr6, Sr7a, Sr8a, Sr9b, Sr11, Sr12, and Sr57. Most of these genes confer resistance to specific P. graminis f. sp. tritici races accounting for the prevalent stem rust resistance in North American spring wheat.
Keywords: genome-wide association, stem rust, Triticum aestivum, major genes, multi-locus model
Introduction
Stem rust of wheat (Triticum aestivum L.), caused by Puccinia graminis f. sp. tritici (Pgt) Erikss. & Henning, is potentially a destructive disease of wheat at the global scale (Singh et al., 2011). In North America, the use of resistant varieties coupled with efforts to eradicate the alternate host of Pgt, barberry (Berberis vulgaris L.) led to successful control of wheat stem rust (Jin and Singh, 2006). Unlike in the decades 1900–1960, major losses due to wheat stem rust have not recently occurred in the United States (Roelfs et al., 1993; Kolmer et al., 2007). Pgt isolates have been classified as races depending on their reactions to a total of 20 differential wheat lines with single stem rust resistance genes (Jin et al., 2008). Pgt races have been denoted by a five-letter code, such as TTKSK, that represents the reaction of a given Pgt isolate to the 20 wheat stem rust resistance genes in the differential set. Although there was a large decline in the number of Pgt races since 1960, race TPMKC was the most prevalent race in the United States until the late 1980s. After this time, the frequency of race TPMKC was low because of the use of resistance cultivars of both hard red winter and spring wheats that carried the stem rust resistance (Sr) gene Sr6 (Kolmer et al., 2007). The most common Pgt race in the US since 2003 has been race QFCSC that is more avirulent compared to race TPMKC (Jin, 2005; Kolmer et al., 2007). Races QTHJC and RCRSC have also been observed at various frequencies in the US Pgt population (McVey et al., 2002). McVey et al. (2002) also noticed the decline of race TPMKC from 1997 to 1998 on wheat and the increase of race RCRSC in 1998. Based on the reaction of the four races to the wheat stem rust resistance genes in the differential set, race TPMKC is virulent to Sr8a, Sr9e, and SrTmp but avirulent to Sr9a and Sr9b, whereas RCRSC is virulent to Sr9a and Sr9b but avirulent to Sr8a, Sr9e, and SrTmp (Supplemental Table 1). QTHJC is the only race virulent to Sr6 out of the four races. TPMKC is also the only race virulent to both Sr9e and SrTmp among the four races. In a population structure analysis of North American Pgt races conducted by Stoxen (2012), a Neighbor-joining analysis of 62 North American Pgt isolates (the four races TPMKC, RCRSC, QTHJC, and QFCSC were included) with 20 SSR markers assigned the isolates into 9 well-supported clades. The four races were placed in different clades. The pairwise genetic differentiation values (Rst values) among the nine groups were also significant at P < 0.05 (Stoxen, 2012). These values ranged from 0.188 (QFCSC-QTHJC groups) to 0.607 (TPMKC-RCRSC). In summary, both phenotypic and genotypic analyses indicated that these races are distinct from each other.
Spring wheat breeders in the North Central United States and the Prairie Provinces of Canada have aggressively selected for stem rust resistance resulting in the deployment of wheat cultivars with resistance to the prevalent races of Pgt. Widely grown cultivars that were selected specifically for their resistance to stem rust include “Thatcher” released in 1935 (Thatcher Wheat, 1936), “Chris” released in 1966 by the Minnesota Agricultural Experiment Station (Kolmer et al., 2007), and “Selkirk” released in 1953 (Martens and Dyck, 1989).
These cultivars served as important sources of stem rust resistance for the deployment of stem rust resistant wheat cultivars worldwide through the Green Revolution (Singh et al., 2008). The genetics basis of resistance to stem rust in these cultivars was characterized in multiple studies. Thatcher was found to possess Sr genes Sr5, Sr9g, Sr12, and Sr16 (Luig, 1983; Knott, 2000). Selkirk had Sr6, Sr7b, Sr9d, Sr17, and Sr23 (Roelfs and McVey, 1979). Chris possessed genes including Sr5, Sr7a, Sr8a, Sr9g, and Sr12 (Singh and McIntosh, 1987). The genetic basis of stem rust resistance in more recently developed wheat cultivars in North America was postulated to be conferred by combinations of race-specific Sr genes (Kolmer et al., 1991) including Sr6, Sr7, Sr9, and Sr17. Though conventional spring wheat cultivars in North America have continued to be selected for resistance to stem rust, the genetic basis of resistance in these cultivars has not been elucidated.
The emergence of a new Pgt race virulent to several deployed Sr genes, commonly known as Ug99, presented global concerns which has resulted in thorough phenotypic characterization of available genetic resources for resistance (Jin et al., 2007; Rouse et al., 2011; Rahmatov et al., 2016), characterization of races in the Ug99 race group including TTKSK (Pretorius et al., 2000; Jin et al., 2008, 2009; Newcomb et al., 2016), identification of stem rust resistance loci (Hiebert et al., 2010; Yu et al., 2011, 2012, 2014; Haile et al., 2012; Letta et al., 2013, 2014; Njau et al., 2013; Singh et al., 2013; Periyannan et al., 2014; Zurn et al., 2014; Babiker et al., 2015, 2016; Basnet et al., 2015; Chen et al., 2015; Dunckel et al., 2015; Kumssa et al., 2015; Nirmala et al., 2016), Sr gene cloning (Periyannan et al., 2013; Saintenac et al., 2013; Mago et al., 2015; Steuernagel et al., 2016), and the development of Ug99-resistant wheat cultivars (Njau et al., 2010; Singh et al., 2015).
A total of 250 contemporary spring wheat cultivars and advanced breeding lines from North America were evaluated for response to Ug99 stem rust in Africa and assessed with molecular markers to facilitate GWAS (Bajgain et al., 2015; Singh et al., 2015). The response of these 250 lines to North American Pgt races was not characterized and thus no information was available on correlation of resistance to Ug99 and resistance to North American Pgt races.
The goal of the current study was to determine the genetic basis of resistance in North American spring wheat to multiple races of Pgt in seedling and field tests. Through this goal, we wanted to (1) test if resistance to North American races of Pgt is correlated to resistance to Ug99, (2) identify markers linked to stem rust resistance genes effective to North American Pgt races, and to (3) postulate the presence of Sr genes effective to North American Pgt races in North American spring wheat.
Materials and methods
Study materials and phenotyping
The Triticeae Coordinated Agricultural Project (TCAP) spring wheat association mapping panel consisted of 250 wheat lines from breeding programs in the U.S (69.6%), Agricultural and Agri-Food Canada (20.4%), and the International Maize and Wheat Improvement Center (CIMMYT, 10%; Table 1). The panel was assessed for response to four Pgt races: QFCSC (isolate 95MN1080), RCRSC (isolate 00MN99C), QTHJC (isolate 0069MN399), and TPMKC (isolate 74MN1409) for 3 years (2013, 2015, and 2016) in the field and in a greenhouse for seedling responses. Each isolate was originally collected in Minnesota, and the reaction of the four Pgt races is given in Supplemental Table 1. The field evaluations were conducted at the Rosemount Research and Outreach Center of the University of Minnesota in Rosemount, MN. Each year, four separate field nurseries were maintained at Rosemount. In each nursery, spreaders were inoculated with a different Pgt isolate. The location of each nursery was no closer than 500 m to another nursery in order to maintain adequate isolation of Pgt races at Rosemount (Roelfs, 1972). Each year, the four Pgt isolates were randomly matched to specific nursery locations at Rosemount. The wheat lines were planted in 1 m long rows and replicated twice, in separate blocks, in each year, and infection response (IR) (Roelfs et al., 1992) and disease severity (SEV) on the modified Cobb scale (Peterson et al., 1948) were recorded twice between milky-ripe and soft-dough growth stages. The average of the two records within each year and race were used for subsequent data analyses. The 1 m long rows were planted perpendicular to single rows of a mixture of stem rust susceptible wheat varieties “Morocco” and “Baart” used as stem rust spreaders. The spreaders were planted 1–2 weeks prior to the experimental plots. Stem rust epidemics in each nursery were initiated by inoculating a light mineral oil suspension of Pgt urediniospores onto the spreader rows using an Ulva+ sprayer (Micron Sprayers Ltd., Bromyard, UK). A unique sprayer was used for each Pgt isolate. Two inoculations of each nursery were conducted in between booting and heading growth stages of the spreader rows.
Table 1.
Sources of wheat lines in the TCAP spring wheat association mapping panel.
| Origin/source | No. of lines |
|---|---|
| Ag-Canada Alberta | 10 |
| CIMMYT | 25 |
| Ag-Canada Manitoba | 13 |
| Montana State University (MSU) | 25 |
| Ag-Canada Saskatchewan | 28 |
| South Dakota State University (SDSU) | 30 |
| University of California-Davis (UCD) | 34 |
| University of Idaho (UI) | 32 |
| University of Minnesota (UMN) | 27 |
| Washington State University (WSU) | 26 |
Field IR, SEV, and seedling infection types (IT) were subjected to analysis of variance. Since the year effect was not significant, best linear unbiased predictors (BLUPs) were calculated in SAS v. 9.3 software (SAS Institute, 2011), and combined data across years for IR and SEV were used for GWAS analysis. Categorical IR classes were converted to a linear 0–9 scale (Gao et al., 2016). The seedling stage IT evaluation for the same isolates of the four races used in the field was performed according to conditions described in Rouse et al. (2011) using the 0 to 4 Stakman IT scale (Stakman et al., 1962) and converted to a 0–9 linear scale (Zhang et al., 2014; Gao et al., 2016) for subsequent analyses. Two replications of each isolate were evaluated on the 250 lines. Pearson correlation coefficients among races were calculated both for seedling and field responses.
SNP genotyping and data filtering
All of the 250 lines were previously genotyped with 90,000 gene-based SNPs using a custom Illumina Infinium iSelect bead chip assay (Illumina Inc., Hayward, CA; Wang et al., 2014). The genotypic data were downloaded from the Triticeae Toolbox (https://triticeaetoolbox.org/wheat). All SNPs with missing values above 20%, heterozygosity level > 2%, and minor allele frequency (MAF) < 5% were removed. Lines with 10% missing values across the SNPs were also removed (nine accessions were eliminated). The final filtered data set consisted of 22,310 SNPs for 241 lines. The remaining missing data points were imputed with the LD-KNNI imputation algorithm (Money et al., 2015) in TASSEL 5 (Bradbury et al., 2007).
Linkage disequilibrium (LD), population structure, and kinship analyses
Intra-chromosomal LD between all possible pairwise comparisons of SNPs was calculated as squared allele frequency correlation (r2) in Synbreed R package (http://synbreed.r-forge.r-project.org/). The pattern of LD decay over genetic distance was previously reported for this population (Bajgain et al., 2015). We calculated the pairwise LD values between SNPs to check whether QTL/gene-associated markers were in LD and to select neutral markers for assessing population structure.
As the presence of population structure is one of the limiting factors in association mapping, we employed a Bayesian clustering approach using a model-based quantitative population structure assessment method implemented in STRUCTURE v.2.3.4 (Pritchard et al., 2000). A total of 1,575 SNPs that had LD values (r2) < 0.2 compared to all other SNPs were selected as neutral markers for population structure analysis. An admixture model with correlated allele frequency and a burn-in of 50,000 iterations followed by 100,000 Monte Carlo Markov Chain (MCMC) iterations was performed to test k values from k = 1 to 10. For each k, five independent runs were conducted to test the sampling variance of population structure inference. To determine the most likely number of clusters (k), the output from STRUCTURE was submitted to the web-based STRUCTURE HARVESTER (Earl and vonHoldt, 2012). The optimum number of clusters was determined based on the Δk statistics from the 2nd order rate of change in the log probability of the likelihood function between successive k values (Evanno et al., 2005). Genetic distance-based cluster analysis was also conducted using the “hclust” script for the Ward cluster method in R (R Core Team, 2016). A population kinship matrix based on the complete SNP data was calculated in GAPIT R package (Lipka et al., 2012).
Association analysis
Marker-trait associations for the identification of chromosome regions associated with stem rust resistance were computed with the single-locus mixed linear model (SLMM) (Yu et al., 2006) that was implemented in GAPIT R package (Lipka et al., 2012) using a compressed mixed linear model (Li et al., 2014; Tang et al., 2016), and by a multi-locus mixed model (MLMM; Segura et al., 2012). For the two model selection criteria for MLMM, the extended Bayesian information criterion (EBIC) and multiple-Bonferroni criterion (mBonf), we used EBIC across the traits and races. The genome-wide p-values of the selected models were adjusted for false discovery rate (FDR) for the purpose of comparing results with that of the single-locus mixed model. In both models, population structure using the 1st 10 principal components (PCA) and genetic relatedness among individuals (K) were fitted as covariates in the respective mixed models. To declare a significant QTL in the single locus model, initially a more relaxed marker-wise p-value of ≤ 0.001 to identify candidate QTL was used. For multiple comparison adjustment, a false discovery rate (FDR) of 10%, was applied for both single and multi-locus models as a threshold for identifying robust QTL. Common QTL between races or traits were also considered as evidence of true QTL.
Finally, genes/QTL that each line possessed were postulated based on the phenotype and haplotype of markers associated with disease response. Several of the identified QTL were coincident with previously characterized stem rust resistance genes. Gene postulations were made for wheat stem rust resistance genes Sr2, Sr6, Sr7a, Sr9b, Sr11, Sr12, Sr24, Sr25, Sr31, and Sr57. Wheat lines were postulated to possess Sr7a based on (1) the presence of the resistance allele of Sr7a-associated (Bajgain et al., 2015; this study) marker IAAV3545 (synonym IWB34733) and (2) the presence of a resistant IT in response to Sr7a-avirulent race TKTTF (Bajgain et al., 2015). Wheat lines were postulated to possess Sr8a based on (1) the presence of the resistance allele of Sr8a-associated (Bajgain et al., 2015) and linked (Hiebert et al., 2017) marker kwh54 (synonym Excalibur_c12085_276; IWB22036), and (2) the presence of a resistant IT in response to Sr8a-avirulent race TRTTF (Bajgain et al., 2015). Wheat lines were postulated to possess Sr11 based on (1) the presence of the resistance allele of Sr11-associated (Bajgain et al., 2015) and linked (Nirmala et al., 2016) markers KASP_6BL_IWB73072 (synonym Tdurum_contig67619_532) and KASP_6BL_IWB46893 (synonym Kukri_c60966_205) and (2) the presence of a resistant IT in response to Sr11-avirulent race TKTTF (Bajgain et al., 2015). Genes Sr6 and Sr9b were postulated based on the results in the current study (see Results). For Sr12, we screened the population with the marker NB-LRR3 from Hiebert et al. (2016). For the remaining genes (Sr2, Sr24, Sr25, Sr31, and Sr57), our gene postulations were based on previously described molecular marker data for the same population (Bajgain et al., 2015).
Results
Seedling stem rust response
The distribution of seedling IT scores based on the linearized 0–9 scale revealed that the majority of the tested lines were resistant (< 6, corresponding to less than IT “2+” on the 0–4 Stakman scale) to the four tested races (Supplemental Figure 1). However, the proportion of resistant entries was different among the races. For instance, only 1.2% of the lines were susceptible (>6) to race QFCSC and about 14% of the lines were susceptible to race QTHJC. The proportions for the remaining two races (TPMKC and RCRSC) fell within these two values. A total of 85% (206 out 241) of the lines showed resistance (IT < 6) to all four races whereas the proportion of resistant lines for combinations of three races varied from 85.5 to 91.3%. Similarly, the proportion of lines that showed resistance to two races ranged from 85.5 to 95.8%. Although the majority of the tested lines showed resistant ITs, analysis of variance (ANOVA) indicated that there were highly significant (p < 0.01) differences among the entries for all races (Supplemental Table 2). The phenotypic correlation coefficients among the four races were in the range of moderate (r = 0.47 between QTHJC and QFCSC) to high (r = 0.7 between TPMKC and RCRSC; Supplemental Figure 2).
Field stem rust response
For field responses of the association panel, combined analysis of variance was conducted across 3 years (2013, 2015, and 2016) for IR and SEV since the effect of year was not significant (Table 2). Highly significant (P < 0.01) phenotypic variation was observed among the lines for all four races for both traits (Table 2). The frequency distribution of BLUP values for both IR and SEV were approximately normal with a slight shift toward resistance in some cases especially for IR (Supplemental Figures 3, 4). Since square root and logarithm transformation did not improve normality of the data, the original data was used for SEV and the linearized data was used for IR for subsequent analyses.
Table 2.
Variance components for random variables for infection response (IR) and severity (SEV) across three environments (2013, 2015, and 2016).
| Subject | IR@ | SEV@ |
|---|---|---|
| Genotype | 0.01071*** | 69.95*** |
| Genotype × Race | 0.006516*** | 32.91*** |
| Genotype × year | 0.001370** | 16.36*** |
| Year | 0.000045ns | 25.23ns |
| Year × Race | 0.001062ns | 5.63ns |
| Bloc | 0.000350ns | 1.84* |
| Genotype(Year × Race) | 0.002282ns | 17.86* |
| Residual | 0.006136ns | 23.37** |
@, Race significant at P < 0.05;
significant at P < 0.05;
significant at P < 0.01;
significant at P < 0.0001.
There was strong correlation between field IR and SEV recorded for each race. However, the correlation coefficients between races were either low or in most cases negative both for IR and SEV (Supplemental Figure 5). The correlation coefficients of seedling IT with field IR and SEV were moderate (0.37–0.45) for all races except for TPMKC where seedling IT was weakly correlated with field SEV. No significant correlations were identified between our data on these North American Pgt races compared to the field data of the response of this panel to Ug99 in Ethiopia and Kenya (Bajgain et al., 2015; Supplemental Figure 6).
Population structure analysis
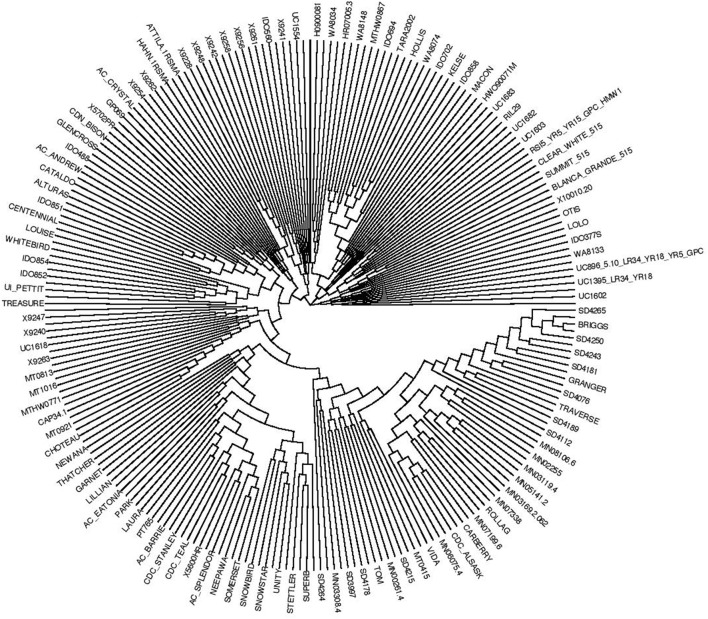
The rate of change in the log probability (Δk) from the model-based population structure analysis indicated that the most likely number of subpopulations was two (Supplemental Figure 7). Among the five replicated runs at k = 2, the run with the highest posterior probability of the data (run seven for this analysis) was used as representative of the replications for downstream analysis. Although each line shared some amount of similarity to lines in both sub-populations, the two groups consisted of a similar number of lines with 106 (44%) lines in the 1st cluster (C1) and 135 (56%) lines in the 2nd cluster (C2) (Supplemental Figure 8). Majority of the lines in the C1 group were obtained from the North Central United States and Canada. Similarly, most of the lines in the C2 group were from the Western United States and CIMMYT. Few accessions from Canada (three from Alberta and two from Manitoba) and also eight lines from MSU were classified into the Western cluster (C2) (Supplemental Table 3). In distance-based cluster analysis using the whole marker data set (22,310 SNPs), the majority of the lines from the North Central United States and Canada grouped in the nearby cluster branches whereas lines from the Western United States and CIMMYT were also in another group of closest branches (Figure 1), largely supporting the results from the Bayesian-based clustering method.
Figure 1.
Genetic relationship among TCAP spring wheat association mapping germplasm.
Association analyses for stem rust resistance
Association analysis was conducted for seedling infection type (IT), field infection response (IR), and field disease severity (SEV) separately for each race. The single-locus model output was validated by MLMM. The QTL detected with the single-locus model and confirmed by MLMM for four races at the seedling stage are presented in Table 3.
Table 3.
Marker-trait associations that were identified for four races of Puccinia graminis f. sp. tritici in seedling assays.
| Race | Marker | Chromosome | Position (cM) | R2 (%) | p-value | Gene postulated |
|---|---|---|---|---|---|---|
| QFCSC | Excalibur_c1066_303 | 1A | 102.32 | 9.14 | 3.32 × 10−5 | New QTL |
| QTHJC | IWB17135 | 2D | 40.05 | 9.61 | 7.17 × 10−7 | Sr6 |
| TPMKC | IWA2415 | 2D | 40.05 | 7.89 | 1.102 × 10−5 | Sr6 |
| IWB1190 | 2B | 119.07 | 9.05 | 3.47 × 10−6 | Unknown | |
| RCRSC | IWA1505 | 4A | 145.19 | 12.34 | 1.84 × 10−7 | Sr7a |
Association analysis for seedling response
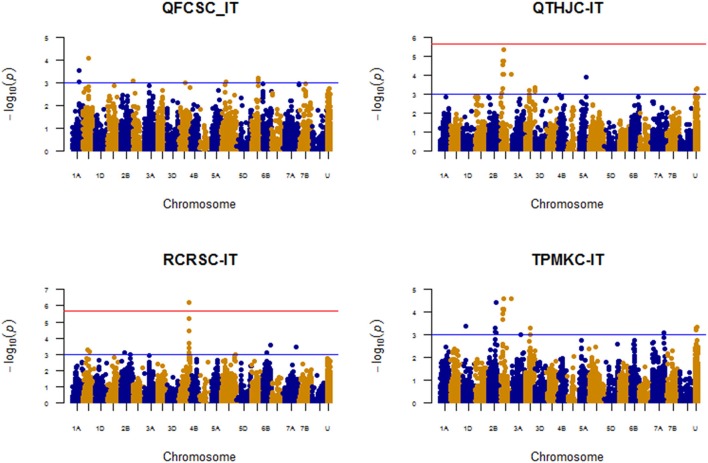
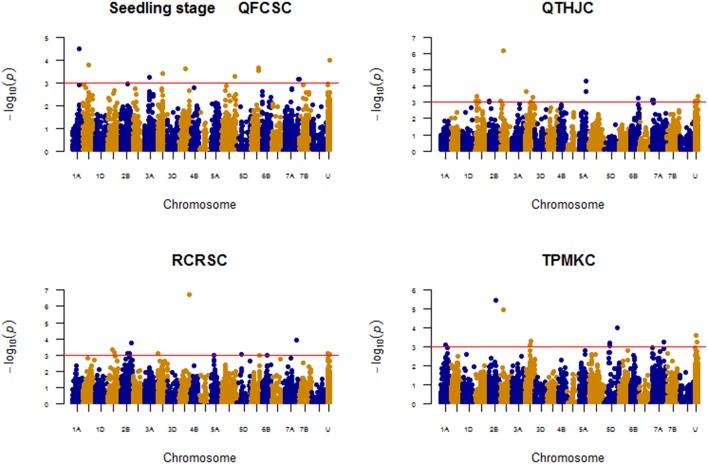
The reactions of the TCAP association mapping panel to the four Pgt races (QFCSC, QTHJC, RCRSC, and TPMKC) were used to identify chromosome regions associated with resistance. The compressed single-locus model that included kinship and the 1st 10 principal components accounted for the population structure well (Supplemental Figure 9). The number of marker-trait associations (MTAs) ranged from 11 for QFCSC to 28 for QTHJC (Supplemental Table 4, Figure 2) with a total of 79 candidate MTAs at P < 0.001 for all four races at the seedling stage (69 unique SNPs on 14 chromosomes). The highest number of MTAs were located on chromosome 2D (38%) followed by chromosomes 3B (11.4%) and 4A (8.9%). A total of seven (8.9%) MTAs were from markers of unknown chromosome location. By categorizing MTAs within 5 cM as one QTL, we identified a total of 25 QTL regions for markers with known chromosome locations. After applying the more stringent threshold (FDR of 10%), a QTL detected for race QTHJC on chromosome arm 2DS (36.54–40.05 cM), and another QTL detected on chromosome arm 4AL for race RCRSC (144.38–145.19 cM) showed significant association signals. The MLMM model was applied to identify the most significant markers in the QTL regions for these two races (Figure 3). Marker D_contig73920_552 (IWB17135) on 2DS (at 40.05 cM) was significantly associated with resistance against race QTHJC (FDR P < 0.1). This marker is in LD (average r2 = 0.74) with markers in the range of 34.15–40.05 cM. Similarly, marker IWA1505 (4AL at 145.19 cM) showed strong association (FDR P < 0.1) with race RCRSC and explained 12.31% of the phenotypic variation.
Figure 2.
Manhattan plots for response to four races based on seedling infection type genome-wide association analysis.
Figure 3.
Manhattan plots for response to four races based on seedling infection type with MLMM model.
None of QTL regions for races QFCSC and TPMKC passed the stringent multiple comparison threshold. However, the most significant QTL detected for QFCSC was on chromosome 1B [at position 90.60 cM with marker Ku_c9909_1766 (IWB40197)], and for race TPMKC was detected on 2DS [at position 40.05 cM with markers RAC875_c57_1178 (IWB59246) and IWA2415, and at position 152.84 cM with marker IWA2414]. With MLMM model, a QTL conferring resistance against QFCSC was detected on 1A (102.32 cM) with marker Excalibur_c1066_303 (IWB21700) (p-value = 3.32E-05) instead of 1B (90.60 cM). To resolve these conflicting results, we re-ran the analysis using another multi-locus model that was implemented in FarmCPU R package (Liu et al., 2016) and found that SNP marker on chromosome 1A (position 102.32 cM) was actually the most significantly (p-value = 3.63E-05) associated marker with QFCSC resistance at the seedling stage. For race TPMKC, a model with two markers Bobwhite_c18540_97 (IWB1190; 2B; 119.07 cM; p-value = 3.47E-06) and IWA2415 (2D, 40.05 cM; p-value = 1.10E-05) was selected as optimum in the MLMM model. However, only the former was significant at FDR p-value of 10%. We used marker Bobwhite_c18540_97 (IWB1190) in addition to haplotypes of three other markers associated with seedling resistance to race TPMKC on chromosome arm 2BL to postulate the presence of Sr9b that is likely the wheat stem rust resistance gene conferring the observed QTL (see section Discussion). We used marker IWA2415 in addition to three other markers associated with seedling resistance to race QTHJC on chromosome 2D to postulate the presence of Sr6. Both markers jointly explained 16.94% of the phenotypic variation for race TPMKC.
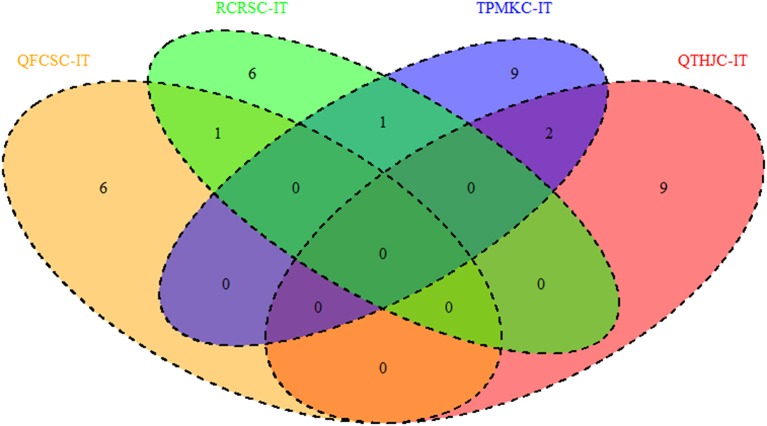
In addition, MTAs detected across two or more races were also used as criteria for identifying reliable MTAs. Although there were no QTL shared among the four races, a total of four QTL regions were common between two races at the seedling stage. As shown in Figure 4, races QFCSC and RCRSC shared a common QTL region on chromosome 1B (90.60–94.40 cM); races QTHJC and TPMKC had two QTL in common on chromosome 2D (34.12–40.05 cM; 152.84 cM with marker IWA2414). Similarly, races RCRSC and TPMKC shared QTL on chromosome arm 7AL (map position 201.46–201.78 cM).
Figure 4.
QTL regions shared among response to four races based on seedling infection type genome-wide association analysis.
Association analysis for field response
The field association analysis was conducted using combined data across 3 years (2013, 2015, and 2016) for infection response (IR) and disease severity (SEV) for each of the four Pgt races (RCRSC, QTHJC, TPMKC, and QFCSC) using both single-locus mixed model and MLMM.
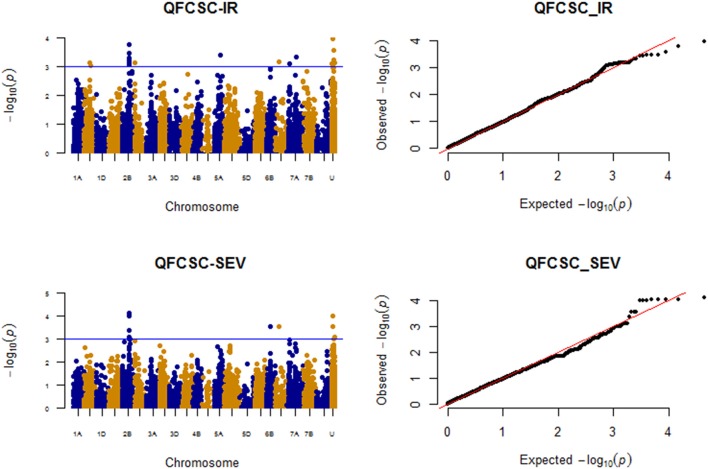
Race: QFCSC
The markers associated with resistance against race QFCSC are displayed on Figure 5.
Figure 5.
Manhattan plots for response to race QFCSC field data (IR and SEV).
Infection response (IR)
A total of eight significant (P < 0.001) QTL regions were identified on six chromosomes (1B, 2B, 2D, 5A, 6D, and 7A) after MTAs within 5 cM were merged together. Another eight unmapped markers also showed significant associations (Supplemental Table 5). None of the QTL regions were significant at FDR of 10%, but unmapped marker IAAV2323 showed relatively strong association (p-value < 0.0001) followed by markers GENE-0966_173 (IWB32165) and RAC875_c15396_90 on chromosome arm 2BL. Applying MLMM model, these three markers showed strong associations (FDR p < 0.1) for race QFCSC IR.
Disease severity (SEV)
Four chromosome regions on chromosomes 2B (108.45–110.87 cM; 119.07 cM), 6B (61.78 cM), and 6D (63.58 cM) were significantly associated with QFCS-SEV at P < 0.001; four unmapped markers were also significantly associated at this threshold level. With respect to QTL regions/MTAs common across IR and SEV for QFCSC, the QTL on chromosomes 2B (108.45–110.87 cM), 6D (63.58 cM with marker CAP11_c4727_205), and unmapped SNPs Ex_c47157_508, Tdurum_contig12459_405and IAAV2323 were significantly associated with both IR and SEV for QFCSC (Figure 5; Supplemental Table 5). With MLMM, a model with markers Excalibur_c76665_98 (IWB28807; 2B; 109.25 cM) and IAAV2323 was found optimum, and both markers were significant at FDR p-value of 10% similar to associations detected with IR for QFCSC. These two markers jointly explained 15.94% of the phenotypic variation. The unmapped marker IAAV2323 is in linkage equilibrium (r2 < 0.1) with other mapped markers detected in marker-trait associations in this study.
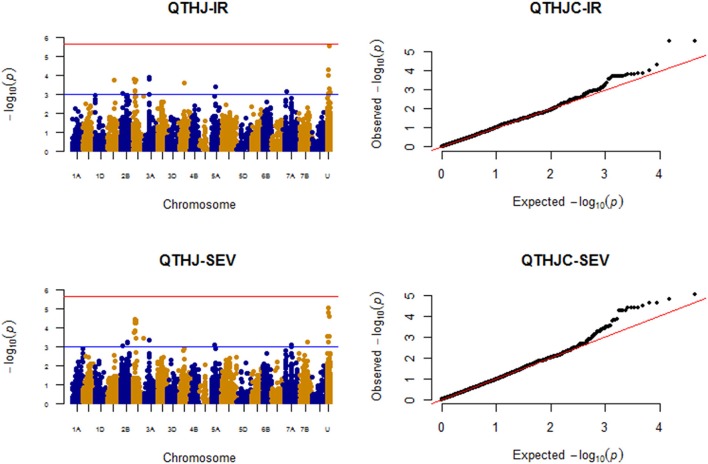
Race: QTHJC
Infection response (IR)
As shown on Figure 6, a total of 15 QTL were identified and eight of them corresponded to markers with known chromosome positions on chromosomes 2A (101.97 cM), 2B (48.54 cM), 2D (22.46 cM; 34.15–40.05 cM), 3A (85.39–86.66 cM), 4A (74.51 cM), 5A (62.72 cM), and 7A (65.41 cM). Two unmapped markers IWA760 and RAC875_c7319_195 were significant at the FDR adjusted p-value of 10%, and each of these two markers explained about 8% of the phenotypic variation. In the MLMM, the selected optimum model consisted of two unmapped markers IWA760 (P = 5.64E-07) and Excalibur_c3574_607 (P = 2.07E-05) that were strongly associated with resistance against QTHJC based on IR with the former SNP marker passed multiple test FDR P < 0.1. Both markers together explained 11.46% of the phenotypic variation. Marker IWA760 was also identified as the most significantly associated with QTHJC resistance with single-locus mixed model.
Figure 6.
Manhattan plots for response to race QTHJC field data (IR and SEV).
Disease severity (SEV)
A total of 15 significant QTL were detected for QTHJC SEV and eight of these were distributed on six chromosomes: 2B, 2D, 3A, 5A, 7A, and 7B. Four of the seven unmapped markers Excalibur_rep_c69347_697, Excalibur_c3574_607, IWA760, and RAC875_c7319_195 showed significant association at FDR p-value of 10%. Similarly, a QTL region on chromosome arm 2DS (34.15–40.05 cM with nine MTAs) was also significant at FDR (P < 0.1). The QTL on chromosomes 2B (48.54 cM), 2D (22.46 cM), 2D (34.15–40.05 cM), and 3A (85.39–86.66 c) were common QTL between IR and SEV for QTHJC. Unmapped markers such as Excalibur_c3574_607, Excalibur_rep_c69347_697, IWA760, RAC875_c7319_195, and RAC875_rep_c72596_466 were also shared between IR and SEV (Figure 6). From MLMM, three unmapped markers IWA760 (p-value = 4.50E-07), Excalibur_c3574_607 (p-value = 1.45E-07), and RAC875_rep_c72596_466 (p-value = 4.68E-06) were strongly associated with QTHJC based on SEV data with all three together explaining 22.33% of the phenotypic variation. All three of them showed significant associations at FDR p-value of 10%. The LD among these three markers is very negligible (r2 < 0.01) indicating that they are in linkage equilibrium and likely represent different QTL.
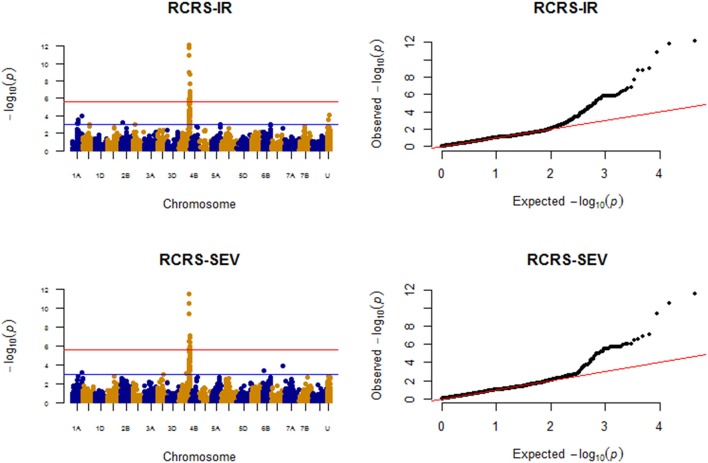
Race RCRSC
MTAs detected for race RCRSC are shown in Figure 7.
Figure 7.
Manhattan plots for response to race RCRSC field data (IR and SEV).
Infection response (IR)
Twelve QTL were significantly associated with IR to RCRSC. Out of these, QTL on chromosomes 1A (at position 94.46 cM; 142.31 cM), 4A (142.29–147.53 cM; 149.53–153.00 cM; 163.83–164.13 cM) and two unmapped SNPs, IWA2771 and Kukri_c10641_584 were significant at FDR p-value < 0.1 (Figure 7; Supplemental Table 6). A maximum phenotypic variation of 17.45% was explained by SNP IAAV3545 (4A; 144.37 cM). A model with marker IAAV3545 (4A, 144.38 cM; P = 3.17E-20) and Excalibur_c15048_488 (IWB22544) on chromosome 2D (37.62 cM; P = 2.97E-06) was selected as the optimum model in MLMM. The two SNPs together explained 26.40% of the phenotypic variation. Marker IAAV3545 showed an exceptionally low p-value that was significant at the Bonferroni threshold level (P = 2.24E-06) and explained 21.53% of the phenotypic variation by itself.
Disease severity (SEV)
A total of seven QTL regions were significant (P < 0.001) for RCRSC-SEV. However, only QTL regions on 4A (142.29–147.54 cM; 151.33–152.99 cM; 163.83–164.13 cM) and 7A (6.38 cM) were significant at FDR p-value < 0.1. Similar to IR, the SNP IAAV3545 (on 4A at position 144.37 cM) explained the most phenotypic variation (16.23%). QTL regions on chromosomes 1A with marker Kukri_c44201_497 (IWB45411) (142.31 cM) and 4A (142.29–147.54 cM; 149.53–153.00 cM; 163.83–164.13 cM) were shared between RCRSC IR and SEV (Figure 7). With MLMM, a model with marker IAAV3545 (4A, 144.38 cM; P = 2.95E-16) was found optimum, and this marker showed strong association (significant with Bonferroni P = 2.24E-06) with resistance against race RCRSC explaining 20.14% of the phenotypic variation. It was also mapped in the QTL region detected for RCRSC resistance with the single-locus mixed model.
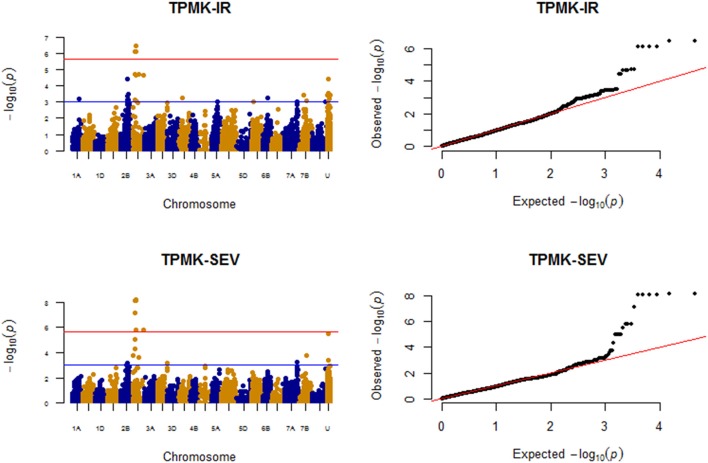
Race TPMKC
The MTAs detected for resistance against race TPMKC based on both IR and SEV are indicated in Figure 8.
Figure 8.
Manhattan plots for response to race TPMKC field data (IR and SEV).
Infection response (IR)
A total of 13 QTL regions (excluding unmapped marker MTAs) were significantly associated with TPMKC IR at P < 0.001. However, only QTL on chromosomes 2D (80.41 cM), 2D (36.54–40.05 cM), 2B (115.01 cM) and unmapped SNP Excalibur_c60964_203 were significantly associated with resistance against TPMKC (Figure 8; Supplemental Table 7) based on FDR of 10%. Applying MLMM, a model with four markers was found optimum for resistance against race TPMKC. Out of these markers, D_contig73920_552 (IWB17135) on 2D (40.05 cM; P = 1.41E-09) and Excalibur_c60964_203 (unmapped, P = 5.20-07) were significant with the Bonferroni test. Both markers jointly explained 18.46% of the phenotypic variation.
Disease severity (SEV)
Chromosomes 2B, 2D, 3B, 7A, and 7B harbored QTL regions that were significantly associated with resistance against TPMKC based on disease severity. Two QTL regions on chromosome 2D (34.15–40.05 cM; 152.84 cM) and unmapped SNP IWA2414 passed the multiple comparison test (FDR p-value < 0.1). The former was strongly associated with resistance against TPMKC both with IR and SEV. However, with relaxed threshold level (P < 0.001), there were a total of 10 QTL regions that were shared by IR and SEV for race TPMKC (Figure 8). With MLMM analysis, a model with two SNPs, D_contig73920_552 (IWB17135; 2D, 40.05 cM) and IWA8141 (2B, 109.53 cM) were found optimum for SEV. Marker D_contig73920_552 (IWB17135; P = 1.12E-10) was significantly associated at Bonferroni threshold level P < 2.24E-06 explaining 13.28% of the phenotypic variation.
Generally, none of the QTL regions were common across the four races with IR, but there was a QTL region (34.15–40.45 cM) on chromosome arm 2DS that was shared among races QTHJC, RCRSC, and TPMKC (Figure 9). Similarly, one QTL region on chromosome 2B (108.45–110.87 cM) was common among QTHJC, QFCSC, and TPMKC (Figure 10).
Figure 9.
QTL regions shared among response to four races based on field IR.
Figure 10.
QTL regions shared among response to four races based on field SEV.
Gene postulation
We postulated genes/QTL that each line possessed by manually assessing the relationship of its disease response and marker haplotype for each line. We used both seedling IT and field disease responses for Pgt races QFCSC, RCRSC, TPMKC, and QTHJC to predict the presence of resistance genes for each line. The postulations were made for 11 previously known genes. A total of 12 lines (5%) lacked any of these genes, and 27 (11%) lines possessed only a single postulated gene (Supplemental Table 8). The remaining 202 (84%) of the lines carried two or more genes (gene combination lines). Over half of the lines (54%) possessed three or more genes. Gene combination lines tended to be composed of various combinations of the most frequent Sr genes including Sr6, Sr7a, Sr8a, Sr9b, S11, Sr12, and Sr57 (Lr34). However, only line 9,263 from the International Maize and Wheat Improvement Center (CIMMYT) carried the maximum observed nine postulated genes (Sr6, Sr8a, Sr9b, Sr11, Sr12, Sr24, Sr31, and Sr57). The average number of Sr genes per line was 2.8 and varied across breeding programs. Lines from UC Davis possessed an average 1.9 Sr genes whereas those from Manitoba possessed an average of 4.4 Sr genes. Resistance gene Sr8a is known to confer resistance to the virulent race TRTTF (Olivera et al., 2012). Resistance genes Sr7a and Sr11 confer resistance to race TKTTF, a non-Ug99 virulent race that caused the 2013–2014 stem rust epidemic in Ethiopia (Olivera et al., 2015). We also assessed the distribution of each gene in this panel, and found that genes such as Sr7a (present in 122 lines), Sr9b, and Sr12 are common in this population and as close to 50% of the population carried each of these genes (Figure 11). Genes Sr6, Sr8a, and Sr57 were present in more than 30% of the population. A total of 21% of the lines in this study were postulated to have Sr11. On the contrary, alleles of Sr2, Sr24, Sr25, and Sr31 were rare in this panel (not more than 10% of the lines). The Sr28-linked marker IWB1208 (Babiker et al., 2017) was not available in the dataset from Triticeae Toolbox.
Figure 11.
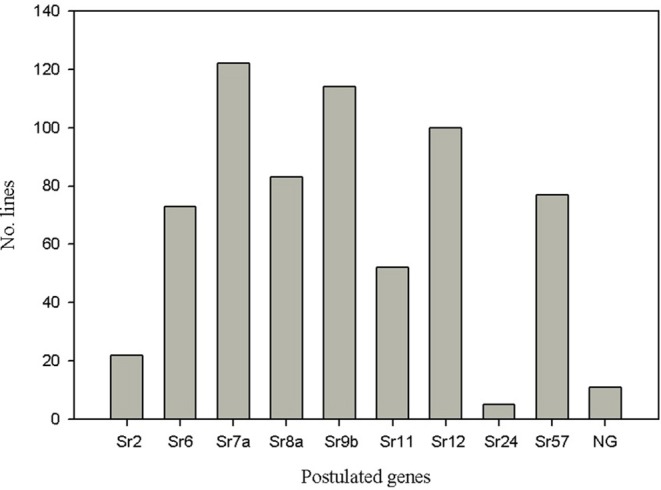
Postulated stem rust resistance genes in the TCAP spring wheat association mapping panel.
Discussion
Though several studies have evaluated seedling and/or field response of wheat association mapping panels to Pgt isolates (Yu et al., 2011, 2012; Letta et al., 2014; Zhang et al., 2014; Gao et al., 2017; Mihalyov et al., 2017; Muleta et al., 2017), only one has evaluated field response of the wheat population studied to more than one Pgt race (Chao et al., 2017). In that study, field response of a bulk of six Pgt isolates in Minnesota was compared to response to a bulk of Pgt isolates in Ethiopia, and differences between these datasets could be due to environment and/or race. This study is the first to use GWAS to evaluate race-specificity, separate from the effect of environment, of stem rust field response. Our finding often negative and sometimes weak correlations between response to different Pgt races highlights the abundance of race-specific resistance and the inability to apply results derived from one Pgt race to others. Our finding of usually moderate correlations of seedling and field stem rust responses within each race combined with our finding that usually only the most significant QTL detected between seedling and field tests were consistent also has an important implication: weakly-associated QTL detected in seedling assays by GWAS likely do not translate to effective QTL in the field. As resistance in the field is most desired, caution should be employed when interpreting results from GWAS using seedling assays.
The negative correlations between field resistance to various Pgt races, especially between races QFCSC and QTHJC, is a striking finding of our study. This suggests that selecting for increased resistance to one race will actually result in selecting for increased susceptibility to another race. In order to test if QTL were significantly associated with each other, we used Chi-square independence tests. The results indicated that QTL associated with QFCSC and QTHJC resistance were not independent of each other (data not shown). The lack of correlation between Ug99 field data and response to any of the races used in this study suggests that stem rust evaluations to North American Pgt isolates cannot be used as a proxy for predicting stem rust response in Africa and may not be applicable for predicting response to future emerging virulent Pgt strains.
Phenotypic variability for resistance to stem rust
The purpose of evaluating the TCAP AM panel for response to Pgt races QTHJC, RCRSC, QFCSC, and TPMKC was to facilitate GWAS in order to characterize the genetics of resistance. Also, MTAs identified through this effort can be used for combining multiple genes/QTLs in breeding programs. Analysis of variance showed significant differences among the genotypes in phenotypic resistance against all four Pgt races both in seedling evaluations and in the field.
Population structure and genetic relatedness of the TCAP AM panel
For the current AM panel, all analysis methods considered here (model-based, principal component, and cluster analyses) revealed that there were two major groups that broadly agree with geographic origins of the genotypes. A similar result was also reported previously for the same panel (Bajgain et al., 2015).
Seedling marker-trait associations (MTA)
With the single-locus mixed model, initially the candidate QTL were identified using a relaxed threshold level with p-value of 0.001 without adjusting for multiple comparisons. Although a large number of MTAs (11–28) were detected for each race, there were a total of 25 QTL regions after grouping of MTAs within 5 cM as one QTL region. Out of these, QTL regions detected on chromosome arm 2DS (36–40 cM with four markers) for race QTHJC, on chromosome arm 4AL (144.38–145.19 cM with markers IAA3545 and IWA1505) for race RCRSC were significant at FDR value of 10%. However, with the MLMM, marker D_contig73920_552 (IWB17135) (2DS, 40.05 cM) was strongly associated with QTHJC resistance. Similarly, IWA1505 (4AL at position 145.19 cM) was the most significantly associated marker with resistance against race RCRSC.
Although none of the candidate QTL regions passed multiple comparisons tests for race QFCSC and TPMKC, with the single-locus model, marker Excalibur_c1066_303 (1AL at position 102.32 cM) showed strong association with resistance against QFCSC with the MLMM. Similarly, markers Bobwhite_c18540_97 (IWB1190) on chromosome arm 2BL (119.07 cM) and IWA2415 (2DS, 40.05 cM) were strongly associated with TPMKC resistance with joint-explained phenotypic variation of 16.94%.
The integrated map developed by Maccaferri et al. (2015) was used a reference for comparing our results with previously reported QTL. On chromosome 1A, QTL for QFCSC (at position 102.32 cM) that was detected with marker Excalibur_c1066_303 (integrated map position 110.01 cM) may be similar to a previously reported stem rust resistance QTL in the region. DArt maker wpt-0128 (125 cM integrated map position) that flanked stem rust resistance in the PBW343/Kingbird population (Bhavani et al., 2011) is about 15 cM away from the map location of marker Excalibur_c1066_303 that was associated with QFCSC resistance in the current study. However, other stem rust resistance-associated DArt markers on 1AL such as wpt-734078 in Bhavani et al. (2011) and wpt-6869 in Rouse et al. (2014) were mapped on proximal end of chromosome 1AS on the integrated map (Maccaferri et al., 2015). Another study indicated that diagnostic markers for Sr1RSAmigo on a rye-wheat translocation (T1AL.1RS) were associated with QFCSC, RCRSC, and TPMKC resistance at the seedling stage (Zhang et al., 2014). We also detected a moderate QTL conferring resistance against QFCSC on chromosome arm 1BL (integrated map position 114.13 cM) with marker IWB40197. The SNP marker IWA435 (1B, integrated map position 60.13 cM) identified as a reliable marker to discriminate between Sr31 (1BL.1RS) and Sr1RSAmigo (1AL.1RS) by Mihalyov et al. (2017). We used the data from this marker to postulate the presence of Sr31 (Supplemental Table 8). However, there appears to be one false-positive as line Selkirk clearly does not possess Sr31 based on susceptible seedling responses to races TRTTF and TKTTF (Bajgain et al., 2015). Association of marker IWA435 with the four races was also tested and we found that it did not pass the significance threshold level (P < 0.001). The association was only significant at p-value < 0.05 for RCRSC at the seedling stage. We do not expect either Sr31 or Sr1RSAmigo on wheat-rye translocations to be more frequent than 5% in this panel. Therefore, we expect the QTL on 1A effective to QFCSC to be similar to the QTL reported in the Kingbird population or possibly novel.
Marker BobWhite_c18540_97 (IWB1190) on chromosome 2B (119.07 cM and integrated map position 242.14 cM) that was significantly associated with TPMKC resistance mapped at a similar position as the SSR marker wmc332 (integrated position 242.14 cM) that was linked with major gene Sr28 on 2BL (Rouse et al., 2012). However, Sr28 does not confer resistance to TPMKC (Rouse et al., 2012). The haplotype of IWB1190 in combination with Sr6 gene-linked marker IWA2415 also explained only 16.94% of phenotypic variation for race TPMKC seedling response. Moreover, the SNP marker IWA840 (integrated map position 222.71 cM) recently reported to be linked with Sr28 (by Mihalyov et al., 2017) is about 20 cM proximal to the QTL-linked marker IWB1190, and its associations with all four races are not significant in the current work.
Although the MLMM resolved the association signals detected within a 6 cM segment on chromosome arm 2DS for QTHJC and TPMKC, the single model analysis enabled the comparison of detected QTL across different studies. The MLMM-identified markers, IWB17135 for QTHJC and IWA2415 for TPMKC resistance both at the same position (2DS, 40.05 cM) were mapped 16 and 6 cM away, respectively, from the SSR marker wmc453 (78.5 cM) which was 1.1 cM distal to Sr6 (Tsilo et al., 2009). With the single model, among the markers within a 6 cM segment on 2DS, markers Kukri_c20972_618 (IWB42406, integrated position 78.7 cM) and Excalibur_c39215_100 (IWB26013, integrated position 74.7 cM) were strongly associated (FDR P < 0.1) with QTHJC resistance. These markers were 0.2 cM distal to wmc453 and 3.8 cM proximal to wmc453, respectively. Given that the LD among all markers on chromosome 2DS within the range 34–40 cM is strong (average r2 = 0.74), Sr6 may be flanked by these two SNP markers. Gene Sr6 confers resistance against the predominant Pgt races in Unites States such as TPMKC, QFCSC, MCCFC, and QCCJB (McVey et al., 2002; Jin, 2005). Zhang et al. (2014) also found that Sr6 confers resistance against race QFCSC and TPMKC, but not for race QTHJC contrary to our seedling QTHJC result. Variable levels of virulence or avirulence to Sr6 have been observed in Pgt isolates (Watson and Luig, 1968), so it is possible that Sr6, though not qualitatively effective to race QTHJC, does confer a significant quantitative resistance effect to race QTHJC under specific environmental conditions (Forsyth, 1956).
Marker IWA1505 (4AL at position 145.19 cM) consistently showed strong association with race RCRSC resistance. Documented resistance genes on 4AL are Sr7 (McIntosh et al., 1995) and SrND643, which may be an allele of Sr7 (Basnet et al., 2015). Comparing the map position of IWA1505 (integrated map position 180.86 cM) with the positions of SSR markers (Xgwm350 and Xwmc219) that flanked SrND643 indicated that IWA1505 was 2.26 cM proximal to Xwmc219 and 0.64 cM distal to the 2nd closest SSR marker proximal to SrND643. This marker was also mapped 12.47 distal and 8.94 cM proximal to SSR marker barc78 (168.39 cM) and wmc313 (199.8 cM) that flanked the Sr7 locus (Turner et al., 2016), respectively. Marker barc78 was also associated race JRCQC resistance in a durum wheat association mapping panel (Letta et al., 2014). We expect that the RCRSC resistance locus corresponds to Sr7a in our study based on the overlap of markers postulated to be associated with Sr7a in Bajgain et al. (2015) and proximity to markers linked to Sr7a in Turner et al. (2016).
Field resistance QTL
Markers such as GENE-0966_173 and Excalibur_c76665_98 (IWB28807) on chromosome arm 2BL (consensus map position 109.25 cM; integrated position 201.209 cM) and unmapped marker IAAV2323 consistently associated with response to race QFCSC. Marker IAAV2323 is in linkage equilibrium (r2 < 0.1) with all other markers involved in MTAs and it is most likely a different locus. Those two markers on chromosome 2B are very close (1.84 cM) to the DArt marker wPt-8460 (integrated position 199.37 cM), a marker linked to Sr9h gene both in biparental mapping (Rouse et al., 2014) and association mapping (Yu et al., 2014). Other major genes such as Sr47 and Sr28 are also nearby on chromosome arm 2BL (Singh et al., 2011; Yu et al., 2014), but are unlikely candidates as they are either derived from alien species and not present in this panel or they are not effective to QFCSC (Sr28). We expect the QTL on 2BL that confers resistance to QFCSC to likely be an allele at the Sr9 locus based on map position. Of the described Sr9 alleles, Sr9b is likely the allele conferring resistance to race QFCSC that is associated with markers GENE-0966_173 and Excalibur_c76665_98. The similar detection of this locus on 2BL in response to TPMKC fits the Sr9b hypothesis based on race-specificity of this QTL compared to Sr9b.
On chromosome 4A, the marker IAAV3545 (4A at 144.38 cM) explained 16.23–21.53% of the phenotypic variation and consistently showed the strongest association with resistance against RCRSC across models, traits, and growth stages (seedling and adult plant). Since the Sr7b allele is not effective to RCRSC, the resistance at this locus most likely conferred by the Sr7a allele. A previous association study included all the four races (QFCSC, QTHJC, RCRSC, and TPMKC) also showed that Sr7a was associated with race RCRSC resistance but not with the resistance against the remaining three races (Zhang et al., 2014).
The QTL region that was detected on 2DS (34–40 cM) with several markers including D_contig73920_552 (IWB17135) showed strong associations with QTHJC and TPMKC resistance both at seedling and adult plant stages. Comparing the map positions markers from different platforms and LD analysis, the QTL detected on chromosome arm 2DS is in the region of the previously known major gene Sr6. This indicates the possibility of tracking Sr6 in breeding programs by converting those chip-based SNPs that showed strong associations (e.g., D_contig73920_552) into PCR-based SNP markers. Unmapped markers IWA760, RAC875_c7319_195 and Excalibur_c3574_607 showed strong associations with race QTHJC resistance across traits and models for field resistance in this study. The 1st two markers in perfect LD (r2 = 1) and had moderate LD (r2 = 0.3) with a marker mapped at 22.46 cM on chromosome 2DS. However, both are in linkage equilibrium (r2 = 0.01) with the 3rd marker. Interestingly marker Excalibur_c3574_607 showed strong LD that ranged from 0.5 to 0.94 (average r2 = 0.76) with markers within the range 34–40 cM on chromosome 2DS indicating this marker is linked with Sr6.
Although numerous moderate MTAs were detected for each race in the current study, the consistently-detected QTL across traits, plant growth, or QTL detection methods with strong association signals were mainly in the regions of known major stem rust resistance genes Sr6, Sr7a, and Sr9b. In addition, the majority of the detected MTAs are race-specific indicating the absence of broadly effective genes in this population (Bajgain et al., 2015). However, based on our gene postulation, there are lines that carry combinations of genes that confer resistance to both North American and virulent African and West Asian Pgt races (e.g., TRTTF and TKTTF). Our data do not allow postulation of all Sr genes present in this panel. For example, we postulated the presence of zero Sr genes in wheat line Marquis. Previous studies demonstrated that Marquis possesses at least four Sr genes (Sr7b, Sr18, Sr19, and Sr20; Knott, 1965). These and other Sr genes were not detected in our study because of any of the following reasons: ineffectiveness to the Pgt races assayed, low frequency in the panel, and lack of data from additional Pgt isolates that would allow accurate postulation. The Sr genes that we postulated are effective to one or more of the modern North American Pgt races. Our gene postulation data could be used to guide breeders seeking donor lines for particular Sr genes. For example, Sr genes Sr7a, Sr9b, and Sr12 were frequently present in the Minnesota and Manitoba lines, but other potentially valuable Sr genes including Sr2 and Sr25 were absent. Overall our data suggest the presence of Sr gene combinations in the majority of North American spring wheat breeding lines. Though many of these Sr genes are race-specific, combination of them results in resistance to multiple North American Pgt isolates.
Author contributions
EE: conducted data analyses and prepared the first draft; MP: provided seeds of the mapping panel and reviewed the manuscript; MR: conceived the study, designed, and conducted both field and greenhouse experiments, and reviewed the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00052/full#supplementary-material
Seedling infection type phenotypic mean (BLUPs) distribution for four the four stem rust races (QTHJC, TPMKC, QFCSC, and RCRSC).
Phenotypic correlation coefficients among four races based on the infection type.
Phenotypic distribution of field infection response mean (BLUPs).
Phenotypic distribution of field disease severity mean (BLUPS).
Phenotypic correlation coefficients both seedling data and field data sets.
Phenotypic correlation between disease response of North American stem rust races evaluated in Rosemont, MN, and Ug99 evaluated in Kenya and Ethiopia.
Graph showing optimum number of population structure of TCAP Spring wheat association mapping panel.
Population structure plot for the TCAP Spring wheat Association mapping panel.
Q-Q plot for four races based on seedling infection type genome-wide association analysis.
Isolates of Puccinia graminis f. sp. tritici used in field and seedling assays and their reaction to differential wheat stem rust resistance genes.
Variance components for four stem rust races for infection type at seedling stage for 250 entries evaluated in Greenhouse.
Cluster groups of spring wheat association mapping panel based on model-based population structure analysis.
Marker-trait associations identified for four stem rust races at seedling stage.
Marker-trait associations for race QFCSC-IR.
Marker-trait associations for race RCRSC-SEV.
Marker-trait associations for race TPMC-IR.
Gene postulation for spring wheat association mapping panel based on phenotypic and marker data.
References
- Babiker E. M., Gordon T. C., Chao S., Newcomb M., Rouse M. N., Jin Y., et al. (2015). Mapping resistance to the Ug99 race group of the stem rust pathogen in a spring wheat landrace. Theor. Appl. Genet. 128, 605–612. 10.1007/s00122-015-2456-6 [DOI] [PubMed] [Google Scholar]
- Babiker E. M., Gordon T. C., Chao S., Rouse M. N., Wanyera R., Acevedo M., et al. (2017). Molecular mapping of stem rust resistance loci effective against the Ug99 race group of the stem rust pathogen and validation of a single nucleotide polymorphism marker linked to stem rust resistance gene Sr28 Phytopathology 107, 208–215. 10.1094/PHYTO-08-16-0294-R [DOI] [PubMed] [Google Scholar]
- Babiker E. M., Gordon T. C., Chao S., Rouse M. N., Wanyera R., Newcomb M., et al. (2016). Genetic mapping of resistance to the Ug99 race group of Puccinia graminis f. sp. Tritici in a spring wheat landrace CItr 4311. Theor. Appl. Genet. 129, 2161–2170. 10.1007/s00122-016-2764-5 [DOI] [PubMed] [Google Scholar]
- Bajgain P., Rouse M. N., Bulli P., Bhavani S., Gordon T., Wanyera R., et al. (2015). Association mapping of North American spring wheat breeding germplasm reveals loci conferring resistance to Ug99 and other African stem rust races. BMC Plant Biol. 15:249. 10.1186/s12870-015-0628-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basnet B. R., Singh S., Lopez-Vera E. E., Huerta-Espino J., Bhavani S., Jin Y., et al. (2015). Molecular mapping and validation of SrND643: a new wheat gene for resistance to the stem rust pathogen Ug99 race group. Phytopathology 105, 470–476. 10.1094/PHYTO-01-14-0016-R [DOI] [PubMed] [Google Scholar]
- Bhavani S., Singh R. P., Argillier O., Huerta-Espino J., Singh S., Njau P., et al. (eds.). (2011). Mapping durable adult plant stem rust resistance to the race Ug99 group in six CIMMYT wheats, in BGRI 2011 Technical Workshop (St Paul, MN: Borlaug Global Rust Initiative; ). [Google Scholar]
- Bradbury P. J., Zhang Z., Kroon D. E., Casstevens T. M., Ramdoss Y., Buckler E. S. (2007). TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635. 10.1093/bioinformatics/btm308 [DOI] [PubMed] [Google Scholar]
- Chao S., Rouse M. N., Acevedo M., Szabo-Hever A., Bockelman H., Bonman J. M., et al. (2017). Evaluation of genetic diversity and host resistance to stem rust in USDA NSGC durum wheat accessions. Plant Genome 10. 10.3835/plantgenome2016.07.0071 [DOI] [PubMed] [Google Scholar]
- Chen S., Rouse M. N., Zhang W., Jin Y., Akhunov E., Wei Y., et al. (2015). Fine mapping and characterization of Sr21, a temperature-sensitive diploid wheat resistance gene effective against the Puccinia graminis f. sp. tritici Ug99 race group. Theor. Appl. Genet. 128, 645–656. 10.1007/s00122-015-2460-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckel S. M., Olson E. L., Rouse M. N., Bowden R. L., Poland J. P. (2015). Genetic mapping of race-specific stem rust resistance in the synthetic hexaploid W7984 X Opata M85 mapping population. Crop Sci. 55, 2580–2588. 10.2135/cropsci2014.11.0755 [DOI] [Google Scholar]
- Earl D. A., vonHoldt B. M. (2012). STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361. 10.1007/s12686-011-9548-7 [DOI] [Google Scholar]
- Evanno G., Regnaut S., Goudet J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14, 2611–2620. 10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- Forsyth F. R. (1956). Interaction of temperature and light on the seedling reaction of McMurachy wheat to race 15B of stem rust. Can. J. Bot. 34, 745–749. 10.1139/b56-054 [DOI] [Google Scholar]
- Gao L., Rouse M. N., Mihalyov P. D., Bulli P., Pumphrey M. O., Anderson J. A. (2017). Genetic characterization of stem rust resistance in a global spring wheat germplasm collection. Crop Sci. 57, 2575–2589. 10.2135/cropsci2017.03.0159 [DOI] [Google Scholar]
- Gao L., Turner M. K., Chao S., Kolmer J., Anderson J. A. (2016). Genome wide association study of seedling and adult plant leaf rust resistance in elite spring wheat breeding lines. PLoS ONE 11:e0148671. 10.1371/journal.pone.0148671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile J. K., Nachit M. M., Hammer K., Badebo A., Roder M. S. (2012). QTL mapping of resistance to race Ug99 of Puccinia graminis f. sp. Tritici in durum wheat (Triticum durum Desf.). Mol. Breed. 30, 1479–1493. 10.1007/s11032-012-9734-7 [DOI] [Google Scholar]
- Hiebert C. W., Kolmer J. A., McCartney C. A., Briggs J., Fetch T., Bariana H., et al. (2016). Major gene for field stem rust resistance co-locates with resistance gene Sr12 in 'Thatcher' wheat. PLoS ONE 11:e0157029 10.1371/journal.pone.0157029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert C. W., Rouse M. N., Nirmala J., Fetch T. (2017). Genetic mapping of stem rust resistance to Puccinia graminis f. sp. Triticici race TRTTF in the Canadian wheat cultivar Harvest. Phytopathology 107, 192–197. 10.1094/PHYTO-05-16-0186-R [DOI] [PubMed] [Google Scholar]
- Hiebert C. W., Fetch T. G., Zegeye T. (2010). Genetics and mapping of stem rust resistance to Ug99 in the wheat cultivar Webster. Theor. Appl. Genet. 121, 65–69. 10.1007/s00122-010-1291-z [DOI] [PubMed] [Google Scholar]
- Jin Y. (2005). Races of Puccinia graminis identified in the United States during 2003. Plant Dis. 89, 1125–1127. 10.1094/PD-89-1125 [DOI] [PubMed] [Google Scholar]
- Jin Y., Singh R. P. (2006). Resistance in U.S. wheat to recent Eastern African isolates of Puccinia graminis f. sp. Tritici with virulence to resistance gene Sr31. Plant Dis. 90, 476–480. 10.1094/PD-90-0476 [DOI] [PubMed] [Google Scholar]
- Jin Y., Singh R. P., Ward R. W., Wanyera R., Kinyua M., Njau P., et al. (2007). Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f. sp. Trtici. Plant Dis. 91, 1096–1099. 10.1094/PDIS-91-9-1096 [DOI] [PubMed] [Google Scholar]
- Jin Y., Szabo L. J., Pretorius Z. A., Singh R. P., Ward R., Fetch T., Jr. (2008). Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis 92, 923–926. 10.1094/PDIS-92-6-0923 [DOI] [PubMed] [Google Scholar]
- Jin Y., Szabo L. J., Rouse M. N., Fetch T., Jr., Pretorius Z. A., Wanyera R., et al. (2009). Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f. sp. tritici. Plant Dis. 93, 367–370. 10.1094/PDIS-93-4-0367 [DOI] [PubMed] [Google Scholar]
- Knott D. R. (1965). A comparison of the reaction to stem rust of wheat lines backcrossed five and nine times to Marquis that carry the same resistance genes. Can. J. Plant Sci. 45, 106–107. 10.4141/cjps65-015 [DOI] [Google Scholar]
- Knott D. R. (2000). The inheritance of stem rust resistance in Thatcher wheat. Can. J. Plant Sci. 80, 53–63. 10.4141/P99-016 [DOI] [Google Scholar]
- Kolmer J. A., Dyck P. L., Roelfs A. P. (1991). An appraisal of stem and leaf rust resistance in North American hard red spring wheats and the probability of multiple mutations to virulence in populations of cereal rust fungi. Am. Phytopathol. Soc. 81, 237–239. [Google Scholar]
- Kolmer J. A., Jin Y., Long D. L. (2007). Wheat leaf and stem rust in the United States. Aust. J. Agric. Res. 58, 631–638. 10.1071/AR07057 [DOI] [Google Scholar]
- Kumssa T. T., Baenziger P. S., Rouse M. N., Guttieri M., Dweikat I., Brown-Guedira G., et al. (2015). Characterization of stem rust resistance in wheat cultivar Gage. Crop Sci. 55, 229–239. 10.2135/cropsci2014.05.0348 [DOI] [Google Scholar]
- Letta T., Maccaferri M., Badebo A., Ammar K., Ricci A., Crossa J., et al. (2013). Searching for novel sources of field resistance to Ug99 and Ethiopian stem rust races in durum wheat via association mapping. Theor. Appl. Genet. 126, 1237–1256. 10.1007/s00122-013-2050-8 [DOI] [PubMed] [Google Scholar]
- Letta T., Olivera P., Maccaferri M., Jin Y., Ammar K., Badebo A., et al. (2014). Association mapping reveals novel stem rust resistance loci in durum wheat at the seedling stage. Plant Genome 7. 10.3835/plantgenome2013.08.0026 [DOI] [Google Scholar]
- Li M., Liu X., Bradbury P., Yu J., Zhang Y. M., Todhunter R. J., et al. (2014). Enrichment of statistical power for genome-wide association studies. BMC Biol. 12:73. 10.1186/s12915-014-0073-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka A. E., Tian F., Wang Q., Peiffer J., Li M., Bradbury P. J., et al. (2012). GAPIT: genome association and prediction integrated tool. Bioinformatics 28, 2397–2399. 10.1093/bioinformatics/bts444 [DOI] [PubMed] [Google Scholar]
- Liu X., Huang M., Fan B., Buckler B. S., Zhang Z. (2016). Iterative Usage of fixed and random effect models for powerful and efficient genome-wide association studies. PLoS Genet. 12:e1005767. 10.1371/journal.pgen.1005767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luig N. H. (1983). A Survey of Virulence Genes in Wheat Stem Rust, Puccinia graminis f. sp. tritici. Advances in Plant Breeding, Supplement 11 to Journal of Plant Breeding, Berlin: Paul Parey. [Google Scholar]
- Maccaferri M., Ricci A., Salvi S., Milner S. G., Noli E., Martelli P. L., et al. (2015). A high-density, SNP-based consensus map of tetraploid wheat as a bridge to integrate durum and bread wheat genomics and breeding. Plant Biotechnol. 13, 648–663. 10.1111/pbi.12288 [DOI] [PubMed] [Google Scholar]
- Mago R., Zhang P., Vautrin S., Šimková H., Bansal U., Luo M. C., et al. (2015). The wheat Sr50 gene reveals rich diversity at a cereal disease resistance locus. Nat. Plants 1:15186. 10.1038/nplants.2015.186 [DOI] [PubMed] [Google Scholar]
- Martens J. W., Dyck P. L. (1989). Genetics of resistance to rust in cereals from a Canadian perspective. Can. J. Plant Pathol. 11, 78–85. 10.1080/07060668909501152 [DOI] [Google Scholar]
- McIntosh R. A., Wellings C. R., Park R. F. (1995). Wheat Rusts. An Atlas of Resistance Genes. CSIRO Australia; Dordrecht: Kluwer Academic Publication. [Google Scholar]
- McVey D. V., Long D. L., Roberts R. V. (2002). Races of Puccinia graminis in the United States during 1997 and 1998. Plant Dis. 86, 568–572. 10.1094/PDIS.2002.86.6.568 [DOI] [PubMed] [Google Scholar]
- Mihalyov P. D., Nichols V. A., Bulli P., Rouse M. N., Pumphery M. O.. (2017). Multi-locus mixed model analysis of stem rust resistance in winter wheat. Plant Genome 10. 10.3835/plantgenome2017.01.0001 [DOI] [PubMed] [Google Scholar]
- Money D., Gardner K., Migicovsky Z., Schwaninger H., Zhong G. Y., Myles S. (2015). LinkImpute: fast and accurate genotype imputation for nonmodel organisms. G3 5, 2383–2390. 10.1534/g3.115.021667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muleta K. T., Rouse M. N., Rynearson S., Chen X., Buta B. G., Pumphrey M. O. (2017). Characterization of molecular diversity and genome-wide mapping of loci associated with resistance to stripe rust and stem rust in Ethiopian bread wheat accessions. BMC Plant Biol. 17:134. 10.1186/s12870-017-1082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb M., Olivera P. D., Rouse M. N., Szabo L. J., Johnson J., Gale S., et al. (2016). Kenyan isolates of Puccinia graminis f. sp. tritici from 2008 to 2014: Virulence to SrTmp in the Ug99 race group and implications for breeding programs. Phytopathology 106, 729–736. 10.1094/PHYTO-12-15-0337-R [DOI] [PubMed] [Google Scholar]
- Nirmala J., Chao S., Olivera P., Babiker E. M., Abeyo B., Tadesse Z., et al. (2016). Markers linked to wheat stem rust resistance gene sr11 effective to Puccinia graminis f. sp. tritici race TKTTF. Phytopathology 106, 1352–1358. 10.1094/PHYTO-04-16-0165-R [DOI] [PubMed] [Google Scholar]
- Njau P. N., Bhavani S., Huerta-Espino J., Keller B., Singh R. P. (2013). Identification of QTL associated with durable adult plant resistance to stem rust race Ug99 in wheat cultivar ‘Pavon 76.’ Euphytica 190, 33–44. 10.1007/s10681-012-0763-4 [DOI] [Google Scholar]
- Njau P. N., Jin Y., Huerta-Espino J., Keller B., Singh R. P. (2010). Identification and evaluation of sources of resistance to stem rust race Ug99 in wheat. Plant Dis. 94, 413–419. 10.1094/PDIS-94-4-0413 [DOI] [PubMed] [Google Scholar]
- Olivera P. D., Jin Y., Rouse M. N., Badebo A., Fetch T., Singh R. P., et al. (2012). Races of Puccinia graminis f. sp. Tritici with combined virulence to Sr13 and Sr9e in a field stem rust screening nursery in Ethiopia. Plant Dis. 96, 623–628. 10.1094/PDIS-09-11-0793 [DOI] [PubMed] [Google Scholar]
- Olivera P. D., Newcomb M., Szabo L. J., Rouse M., Johnson J., Gale S., et al. (2015). Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis f. sp. Tritici that caused a wheat stem rust epidemic in Southern Ethiopia in 2013-14. Phytopathology 105, 917–928. 10.1094/PHYTO-11-14-0302-FI [DOI] [PubMed] [Google Scholar]
- Periyannan S., Bansal U., Bariana H., Deal K., Luo M. C., Dvorak J., et al. (2014). Identification of a robust molecular marker for the detection of the stem rust resistance gene Sr45 in common wheat. Theor. Appl. Genet. 127, 947–955. 10.1007/s00122-014-2270-6 [DOI] [PubMed] [Google Scholar]
- Periyannan S., Moore J., Ayliffe M., Bansal U., Wang X., Huang L., et al. (2013). The gene Sr33, an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99. Science 341, 786–788. 10.1126/science.1239028 [DOI] [PubMed] [Google Scholar]
- Peterson R. F., Campbell A. B., Hannah A. E. (1948). A diagrammatic scale for estimating rust intensity of leaves and stem of cereals. Can. J. Res. Sect. C 26, 496–500. 10.1139/cjr48c-033 [DOI] [Google Scholar]
- Pretorius Z. A., Singh R. P., Wagoire W. W., Payne T. S. (2000). Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis. 94, 413–419. 10.1094/PDIS.2000.84.2.203B [DOI] [PubMed] [Google Scholar]
- Pritchard J. K., Stephens M., Donnelly P. (2000). Inference of population structure using multilocus genotype data. Genetics 155, 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmatov M., Rouse N. M., Nirmala J., Danilova T., Friebe B., Steffenson B. J., et al. (2016). A new 2DS•2RL Robertsonian translocation transfers stem rust resistance gene Sr59 into wheat. Theor. Appl. Genet. 129, 1383–1392. 10.1007/s00122-016-2710-6 [DOI] [PubMed] [Google Scholar]
- R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; Available online at: http://www.R-project.org/ [Google Scholar]
- Roelfs A. P. (1972). Gradients in horizontal dispersal of cereal rust uredospores. Phytopathology 62, 70–76. 10.1094/Phyto-62-70 [DOI] [Google Scholar]
- Roelfs A. P., Long D. L., Roberts J. J. (1993). Races Puccinia graminis in the United States during 1990. Plant Dis. 77, 125–128. 10.1094/PD-77-0125 [DOI] [Google Scholar]
- Roelfs A. P., McVey D. V. (1979). Low infection types produced by Puccinia graminis f. sp. Tritici and wheat lines with designated genes for resistance. Phytopathology 69, 722–730. 10.1094/Phyto-69-722 [DOI] [Google Scholar]
- Roelfs A. P., Singh R. P., Saari E. E. (1992). Rust Disease of Wheat: Concepts and Methods of Disease Management. Mexico: CIMMYT. [Google Scholar]
- Rouse M. N., Nava I. C., Chao S., Anderson J. A., Jin Y. (2012). Identification of markers linked to the race Ug99 effective stem rust resistance Sr28 in wheat (Triticum aestivum L.). Theor. Appl. Genet. 125, 877–885. 10.1007/s00122-012-1879-6 [DOI] [PubMed] [Google Scholar]
- Rouse M. N., Talbert L. E., Singh D., Sherman J. D. (2014). Complementary epistasis involving Sr12 explains adult plant resistance to stem rust in thatcher wheat (Triticum aestivum L.). Theor. Appl. Genet. 127, 1549–1559. 10.1007/s00122-014-2319-6 [DOI] [PubMed] [Google Scholar]
- Rouse M. N., Wanyera R., Njau P. N., Jin Y. (2011). Sources of resistance to stem rust race Ug99 in spring wheat germplasm. Plant Dis. 95, 762–766. 10.1094/PDIS-12-10-0940 [DOI] [PubMed] [Google Scholar]
- Saintenac C., Zhang W., Salcedo A., Rouse M. N., Trick N. H., Akhunov E., et al. (2013). Identification of wheat gene Sr35 that confers resistance to Ug99 stem rust race group. Science 341, 783–786. 10.1126/science,.1239022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute (2011). The SAS System for Windows. Version 9.3. Cary, NC: SAS Institute. [Google Scholar]
- Segura V., Vilhjálmsson B. J., Platt A., Korte A., Seren Ü, Long, Q., et al. (2012). An efficient multi-locus mixed model approach for genome-wide association studies in structured populations. Nat. Genet. 44, 825–830. 10.1038/ng.2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Hodson D. P., Huerta-Espino J., Jin Y., Bhavani S., Njau P. N., et al. (2011). The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu. Rev. Phytopathol. 49, 465–481. 10.1146/annurev-phyto-072910-095423 [DOI] [PubMed] [Google Scholar]
- Singh R. P., Hodson D. P., Huerta-Espino J., Jin Y., Njau P. N., Wanyera R., et al. (2008). Will stem rust destroy the World's wheat crop? Adv. Agron. 98, 271–309. 10.1016/S0065-2113(08)00205-8 [DOI] [Google Scholar]
- Singh R. P., Hodson D. P., Jin Y., Lagudah E. S., Ayliffe M. A., Bhavani S., et al. (2015). Emergence and spread of new races of wheat stem rust fungus: continued threat to food security and prospects of genetic control. Phytopathology 105, 872–884. 10.1094/PHYTO-01-15-0030-FI [DOI] [PubMed] [Google Scholar]
- Singh R. P., McIntosh R. A. (1987). Genetics of resistance to Puccinia graminis tritici in ‘Chris’ and ‘W3746’ wheats. Theor. Appl. Genet. 73, 846–855. 10.1007/BF00289389 [DOI] [PubMed] [Google Scholar]
- Singh S., Singh R. P., Bhavani S., Huerta-Espino J., Eugenio L. V. E. (2013). QTL mapping of slow-rusting, adult plant resistance to race Ug99 of stem rust fungus in PB343/Muu RIL population. Mol. Breed. 126, 1367–1375. 10.1007/s11032-015-0362-x [DOI] [PubMed] [Google Scholar]
- Stakman E. C., Stewart D. M., Loegering W. Q. (1962). Identification of Physiologic Races of Puccinia graminis var. tritici. USDA-ARS E617. Washington, DC:USDA. [Google Scholar]
- Steuernagel B., Periyannan S. K., Hernández-Pinzón I., Witek K., Rouse M. N., Yu G., et al. (2016). Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 34, 652–655. 10.1038/nbt.3543 [DOI] [PubMed] [Google Scholar]
- Stoxen S. (2012). Population Structure of Puccinia graminis f. sp. tritici in the Unites States. University of Minnesota Digital Conservancy. Available online at: https://conservancy.umn.edu/handle/11299/131092
- Tang Y., Liu X., Wang J., Li M., Wang Q., Tian F., et al. (2016). GAPIT Version 2: an enhanced integrated tool for genomic association and prediction. Plant Genome 9. 10.3835/plantgenome2015.11.0120 [DOI] [PubMed] [Google Scholar]
- Thatcher Wheat (1936). University of Minnesota, Agricultural Research Station. St. Paul, MN, Bulletin: 325. [Google Scholar]
- Tsilo T. J., Chao S., Jin Y., Anderson J. A. (2009). Identification and validation of SSR markers linked to the stem rust resistance gene Sr6 on the short arm of chromosome 2D in Wheat. Theor. Appl. Genet. 118, 515–524. 10.1007/s00122-008-0917-x [DOI] [PubMed] [Google Scholar]
- Turner M. K., Jin Y., Rouse M. N., Anderson J. M. (2016). Stem rust resistance in “Jagger” winter wheat. Crop Sci. 56, 1–7. 10.2135/cropsci2015.11.0683 [DOI] [Google Scholar]
- Wang S., Wong D., Forrest K., Allen A., Chao S., Huang B. E., et al. (2014). Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol. J. 12, 787–796. 10.1111/pbi.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson I. A., Luig N. H. (1968). Progressive increase in virulence in Puccinia graminis f. sp. trtici. Phytopathology 58, 70–73. [Google Scholar]
- Yu J. M., Pressoir G., Briggs W. H., Bi I. V., Yamasaki M., Doebley J. F., et al. (2006). A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 38, 203–208. 10.1038/ng1702 [DOI] [PubMed] [Google Scholar]
- Yu L. X., Barbier H., Rouse M. N., Singh S., Singh R. P., Bhavani S., et al. (2014). A consensus map for Ug99 stem rust resistance loci in wheat. Theor. Appl. Genet. 127, 156–1581. 10.1007/s00122-014-2326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. X., Lorenz A., Rutkoski J., Singh R. P., Bhavani S., Huerta-Espino J., et al. (2011). Association mapping and gene-gene interaction for stem rust resistance in CIMMYT spring wheat germplasm. Theor. Appl. Genet. 123, 1257–1268. 10.1007/s00122-011-1664-y [DOI] [PubMed] [Google Scholar]
- Yu L. X., Morgounov A., Wanyera R., Keser M., Singh S. K., Sorrells M. (2012). Identification of Ug99 stem rust resistance loci in winter wheat germplasm using genome-wide association analysis. Theor. Appl. Genet. 125, 749–758. 10.1007/s00122-012-1867-x [DOI] [PubMed] [Google Scholar]
- Zhang D., Bowden R. L., Yu J., Carver B. F., Bai G. (2014). Association analysis of stem rust resistance in U.S. winter wheat. PLoS ONE 9:e103747. 10.1371/journal.pone.0103747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurn J. D., Newcomb M., Rouse M. N., Jin Y., Chao S., Sthapit J., et al. (2014). High-density mapping of a resistance gene to Ug99 from the Iranian landrace PI 626573. Mol. Breed. 34, 871–881. 10.1007/s11032-014-0081-8 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seedling infection type phenotypic mean (BLUPs) distribution for four the four stem rust races (QTHJC, TPMKC, QFCSC, and RCRSC).
Phenotypic correlation coefficients among four races based on the infection type.
Phenotypic distribution of field infection response mean (BLUPs).
Phenotypic distribution of field disease severity mean (BLUPS).
Phenotypic correlation coefficients both seedling data and field data sets.
Phenotypic correlation between disease response of North American stem rust races evaluated in Rosemont, MN, and Ug99 evaluated in Kenya and Ethiopia.
Graph showing optimum number of population structure of TCAP Spring wheat association mapping panel.
Population structure plot for the TCAP Spring wheat Association mapping panel.
Q-Q plot for four races based on seedling infection type genome-wide association analysis.
Isolates of Puccinia graminis f. sp. tritici used in field and seedling assays and their reaction to differential wheat stem rust resistance genes.
Variance components for four stem rust races for infection type at seedling stage for 250 entries evaluated in Greenhouse.
Cluster groups of spring wheat association mapping panel based on model-based population structure analysis.
Marker-trait associations identified for four stem rust races at seedling stage.
Marker-trait associations for race QFCSC-IR.
Marker-trait associations for race RCRSC-SEV.
Marker-trait associations for race TPMC-IR.
Gene postulation for spring wheat association mapping panel based on phenotypic and marker data.