Abstract
Niacin is effective in treating dyslipidemias but causes cutaneous vasodilation or flushing, a side effect that limits its clinical use. Blocking prostaglandins in humans reduces but does not consistently eliminate flushing, indicating additional mechanisms may contribute to flushing. The transient receptor potential vanilloid 1 (TRPV1) channel, when activated, causes cutaneous vasodilation and undergoes tachyphylaxis similar to that seen with niacin. Using a murine model, early phase niacin-induced flushing was examined and TRPV1 channel involvement demonstrated using pharmacologic blockade, desensitization, and genetic knockouts (TRPV1 KO). The TRPV1 antagonist AMG9810 reduced the magnitude of the initial and secondary peaks and the rapidity of the vasodilatory response (slope). TRPV1 desensitization by chronic capsaicin reduced the initial peak and slope. TRPV1 KO mice had a lower initial peak, secondary peak, and slope compared with wild-type mice. Chronic niacin reduced the initial peak, secondary peak, and slope in wild-type mice but had no effect in knockout mice. Furthermore, chronic niacin diminished the response to capsaicin in wild-type mice. Overall, these data demonstrate an important role for TRPV1 channels in niacin-induced flushing, both in the acute response and with chronic administration. That niacin-induced flushing is a complex cascade of events, which should inform pharmacological intervention against this side effect.
Keywords: niacin, cutaneous vasodilation, flushing, TRPV1 channels
INTRODUCTION
Niacin (nicotinic acid or vitamin B3) has been identified as an effective lipid-modifying agent and may be useful in reducing the risk of cardiovascular disease by lowering levels of total cholesterol, low-density lipoprotein cholesterol and triglycerides, and increasing high-density lipoprotein cholesterol.1,2 Despite these benefits, there is limited compliance with niacin because of a major side effect, cutaneous vasodilation, also commonly known as flushing. Flushing symptoms have been reported in more than 80% of patients, and approximately 30% of patients discontinue their use of niacin due to flushing in the first year of treatment.3,4 Thus, flushing is one cause of underutilization of niacin.
Niacin is known to interact with the hydroxyl-carboxylic acid (HCA2) receptor (also known as the GPR109A receptor). This receptor is expressed in adipocytes where it modulates free fatty acid release, contributing to niacin’s effect on lipids. HCA2 is also expressed in the Langerhans’ cells and keratinocytes of the skin where it is commonly believed to induce flushing because of prostaglandin-mediated D/E-prostanoid receptor activation through arachidonic acid release and metabolism.5–8 However, attempts to prevent niacin-induced flushing by inhibiting the prostaglandin signaling pathway has produced varying results. Although dual inhibition of cyclo-oxygenase pathways (COX-1 and COX-2) abolished niacin-induced vasodilation in mice,6 several studies in man who used pharmacological blockade of arachidonic acid metabolism or the downstream receptors were not able to fully ablate the flushing response.9–11 These findings indicate that mechanisms beyond arachidonic acid metabolism may contribute to the flushing response.
Recently, we demonstrated that niacin activates the transient receptor potential channel vanilloid subtype 1 (TRPV1) in vitro.12 Similar to niacin-induced flushing, acute TRPV1 activation causes cutaneous vasodilation in humans.13,14 Furthermore, both repetitive TRPV1 activation and repetitive administration of niacin result in tachyphylaxis.4,15 TRPV1 channel involvement in the vasodilatory response to niacin may explain, at least in part, both the acute response persisting despite blockade of the prostaglandin pathway and the observed tachyphylaxis. Given these similarities, we hypothesized that a portion of the vasodilatory response to acute niacin is mediated by activation of TRPV1 channels, and that tachyphylaxis to the vasodilatory effect of chronic niacin is due, in part, to desensitization of the TRPV1 channels. To test this hypothesis, we used a murine model of niacin-induced vasodilation and measured the acute and chronic response to niacin.
METHODS
All studies were approved by the UC Davis Institutional Animal Care and Use Committee. Animals were housed in a climate controlled room with ad libitum food and water and a 12:12 light:dark cycle.
Mouse Model
Wild-type C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA). TRPV1–/– (KO) mice were obtained from Jackson Laboratories (Sacramento, CA). All mice were adult males (3–6 months of age). For the experiments, mice were anesthetized using pentobarbital (Sigma-Aldrich, St. Louis, MO) at a dose of 60 mg/kg dissolved in 10% ethanol given by intraperitoneal (IP) injection. Niacin (Sigma-Aldrich) was administered by IP injection at a dose of 30 mg/kg dissolved in 0.9% saline (15 mg/mL). The TRPV1 antagonist AMG9810 [(E)-3-(4-t-Butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide] (Sigma-Aldrich) was administered by IP injection at doses of 20 and 40 mg/kg in PEG400 (20 mg/mL). The TRPV1 agonist capsaicin (Sigma-Aldrich) was administered topically at a dose of 0.2 mg in acetone (20 mg/mL).
Laser Doppler Flowmetry
Blood flow measurements were performed using a laser Doppler flow meter (BLF 21; Transonic Systems, Inc., Ithaca, NY) as previously described.10 Measurements were done on the ventral aspect of the ear with the anesthetized mouse supine on a warming pad (40°C). Blood flow was measured in relative tissue perfusion units (TPU) at a sampling rate of one measurement per second.
Blood Flow Profile Analysis
The blood flow response profile for acute niacin-induced vasodilation is classically described as a biphasic response, characterized by an early phase response that peaks within the first 2–3 minutes and a late phase response that peaks between 20 and 30 minutes after administration.6,7 For this study, the focus was on the early phase response. At the high sampling frequencies used in this study, the early phase exhibited 2 distinct peaks, termed the initial peak (occurring in the first minute) and a secondary peak (occurring within 5 minutes) (Fig. 1). After establishing baseline blood flow values for 3 minutes, niacin was administered by IP injection, and the magnitude of these peaks quantified. In addition, the rate of increase in blood flow of the first peak was termed the initial slope. Blood flow profiles for chronic niacin exposure showed a monophasic response without an initial peak. Peak values were analyzed as the percent change from baseline {[(TPUPeak − TPUBaseline)/TPUBaseline] ×100%}, and slope values were measured as the rate of change in blood flow [(TPUPeak − TPUBaseline)/Δtime].
FIGURE 1.
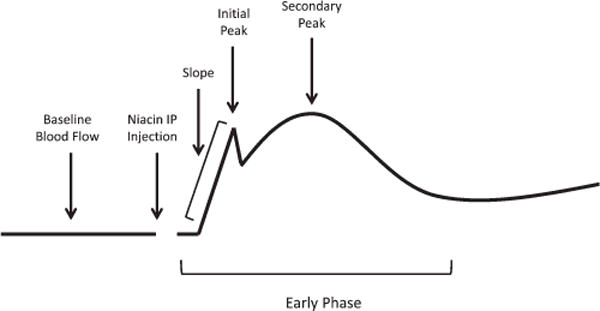
Schematic representation of blood flow profile analysis of the “early phase” of niacin-induced vasodilation. After establishing baseline blood flow, niacin was administered by IP injection. During early phase flushing, there was an initial rapid increase in blood flow (slope) that peaked briefly (initial peak), followed by another, more prolonged peak (secondary peak).
Acute Niacin Response
The role of TRPV1 channels in the acute response to niacin was assessed using 3 approaches (Fig. 2). First, TRPV1 channel activation was blocked using a known antagonist, AMG9810. The minimum effective dose to prevent capsaicin-induced hyperemia was previously found to be 20 mg/kg through pilot experiments (data not shown). Thus, mice were pretreated with AMG9810 (20 or 40 mg/kg) 10 minutes before niacin. The blood flow responses to niacin alone, niacin with AMG9810 pretreatment, and niacin with vehicle pretreatment were measured (Fig. 2A). Second, TRPV1 channel activity was reduced through repeated topical capsaicin application to the ear (3 times daily for 5 days) (Fig. 2B). Acute niacin-induced blood flow changes after desensitization were compared with the niacin response before desensitization. To verify desensitization, capsaicin-induced hyperemia in the ear was measured before and after repeated exposure. Finally, niacin-induced blood flow responses for TRPV1 KO mice were compared with that of wild-type mice (Fig. 2C).
FIGURE 2.
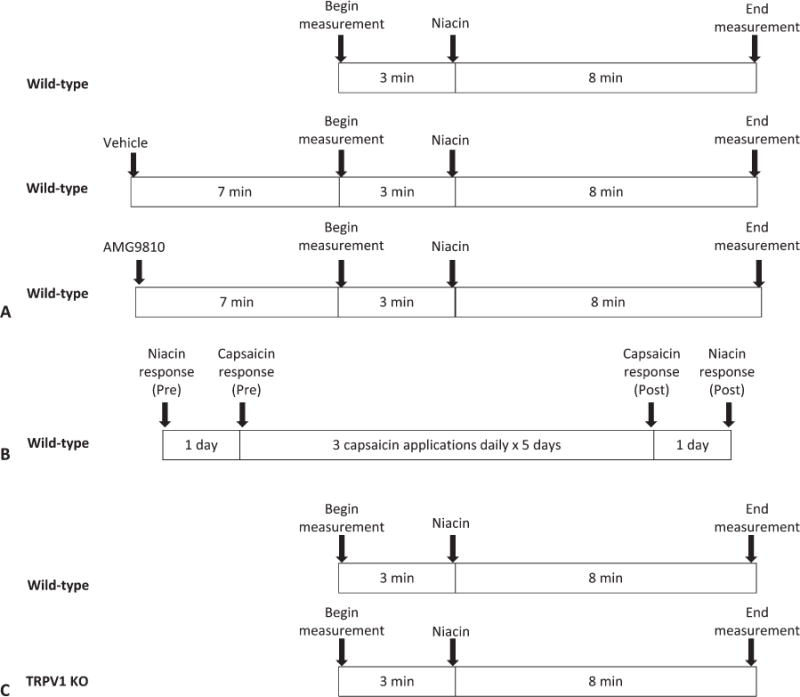
Schematic representation of the acute response niacin study. A, The role of TRPV1 channels in niacin-induced cutaneous vasodilation was assessed using the TRPV1 antagonist AMG9810 given 10 minutes before niacin. B, The role of TRPV1 channels in niacin-induced cutaneous vasodilation was assessed using capsaicin-induced desensitization of the TRPV1 channels. C, The role of TRPV1 channels in niacin-induced cutaneous vasodilation was assessed by comparing the response of TRPV1 KO mice to wild-type mice.
Chronic Niacin Response
Niacin-induced blood flow responses before and after chronic niacin exposure were measured in wild-type and TRPV1 KO mice (Fig. 3). Mice received daily niacin injections (30 mg/kg) IP for 2 weeks. The niacin response after 2 weeks was then compared with the initial response to verify tachyphylaxis. Niacin-induced changes in blood flow after repeated exposure were compared between wild-type and knockout mice. Capsaicin-induced hyperemia was used as a biologic measure before and after repeated niacin exposure to confirm reduced TRPV1 channel activity with niacin tachyphylaxis.
FIGURE 3.

Schematic representation of the chronic niacin study design. Blood flow responses to both capsaicin and niacin were measured in wild-type and TRPV1 KO mice before and after repeated niacin exposure.
Statistical Analysis
Statistical analysis was performed using SAS (Statistical Analysis System Institute, Inc., Cary, NC). Data were first tested for normality using the Shapiro–Wilk test. Multiple means comparisons in the pharmacologic blockade experiments were made using one-way ANOVA followed by Dunnett’s post hoc analysis with the niacin-only group set as the control. Capsaicin repeated exposure (pre and post) comparisons were made using paired Student’s t tests. Wild-type and knockout comparisons were made using unpaired Student’s t tests. Results were considered statistically significant at P < 0.05. Graphs show means ± SEM.
RESULTS
Acute Niacin-induced Flushing Is Mediated by TRPV1 Channel Activity
The effects of pretreatment with 20 mg/kg and 40 mg/kg AMG9810 were not different from each other (data not shown), and therefore data for both doses were combined for this condition (AMG9810). Pretreatment with vehicle (PEG400) did not affect the niacin blood flow response (Fig. 4A). Pretreatment with AMG9810 effectively eliminated the initial peak (Fig. 4A) and significantly reduced the magnitude of the blood flow response at that time point from 125 ± 13 to 22 ± 7 % change compared with niacin without pretreatment (P < 0.05, Fig. 4B). The magnitude of the secondary peak was also significantly reduced compared with niacin without pretreatment from 141 ± 22 to 72 ± 9 % change (P < 0.05, Fig. 4C). The slope of the initial niacin response was consequently significantly reduced after pretreatment with AMG9810 compared with niacin without pretreatment from 266 ± 25 to 84 ± 15 % change per minute (P < 0.05, Fig. 4D).
FIGURE 4.
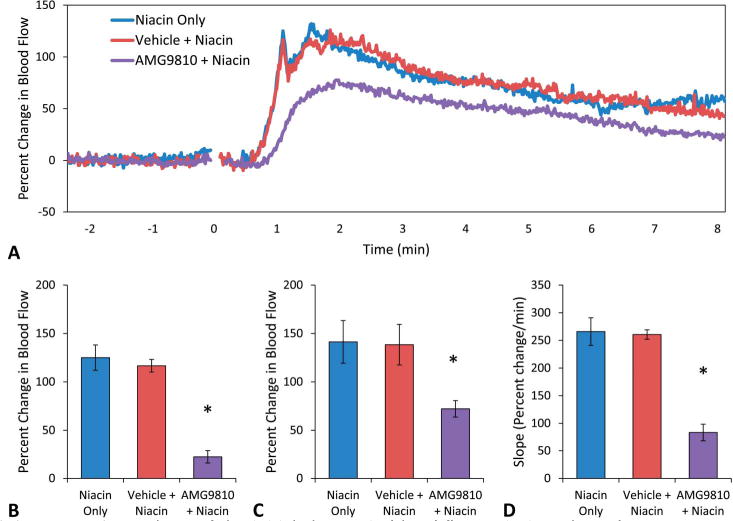
A, Mean blood flow responses to acute niacin. Niacin-only produced 2 distinct blood flow peaks (blue, n = 7). These were not changed by pretreatment with the vehicle (red, n = 6). Pretreatment with the TRPV1 antagonist AMG9810 (purple, n = 11) eliminated the initial peak, reduced the magnitude of the secondary peak, and reduced the slope. B, Blood flow at the initial peak. There was no statistical difference between niacin-only (125 ± 13 % change) and niacin after pretreatment with the vehicle (116 ± 16 % change). Pretreatment with AMG9810 significantly reduced the blood flow response at the time point of the initial peak to 22 ± 7 % change compared with niacin-only (*P < 0.05). C, Blood flow at the secondary peak. There was no statistical difference between niacin (141 ± 22 % change) and pretreatment with the vehicle (138 ± 21 % change). Pretreatment with AMG9810 reduced the secondary peak (72 ± 9 % change) (*P < 0.05). D, Slope of the initial change in blood flow. Niacin-only and pretreatment with vehicle were not significantly different (266 ± 25 and 261 ± 9 % change per minute). Pretreatment with AMG9810 reduced the slope of the response to 84 ± 15 % change per minute (*P < 0.05).
Repeated capsaicin exposure to desensitize the TRPV1 channels also diminished blood flow responses to acute niacin exposure (Fig. 5A). Postcapsaicin responses lacked the initial peak (Fig. 5A), and the change in blood flow at that time point was significantly reduced from 123 ± 24 to 53 ± 13 % change (P < 0.05, Fig. 5B). The secondary peak was also slightly lower compared with precapsaicin values, although this was not significant (103 ± 22 vs. 148 ± 24 % change) (Fig. 5C). The slope of the change in blood flow was significantly reduced compared with precapsaicin values (177 ± 27 vs. 276 ± 42 % change per minute) (P < 0.05, Fig. 5D). To verify reduced TRPV1 channel sensitivity, capsaicin-induced hyperemia was measured before and after repeated exposure. Capsaicin-induced cutaneous vasodilation was reduced from 222 ± 38 to 126 ± 19 % change after repeated exposure, demonstrating reduced TRPV1 channel activity (P < 0.05, Fig. 6).
FIGURE 5.
A, Mean blood flow responses to acute niacin. Before repeated capsaicin exposure (blue, n = 9), niacin produced 2 peaks in blood flow. After repeated capsaicin exposure (orange, n = 9), there was only a secondary peak, which was lower compared with precapsaicin. B, Blood flow at the initial peak. Post-capsaicin responses lacked the initial peak and the change in blood flow at that time point was significantly reduced from 123 ± 24 to 53 ± 13 % change (*P < 0.05). C, Blood flow at the secondary peak. The secondary peak postcapsaicin was not significantly different compared with precapsaicin values (148 ± 24 to 103 ± 22 % change, P = 0.098). D, Slope of the initial change in blood flow. The postcapsaicin rate of change in blood flow was significantly reduced to 177 ± 27 from 276 ± 42 % change per minute (*P < 0.05).
FIGURE 6.
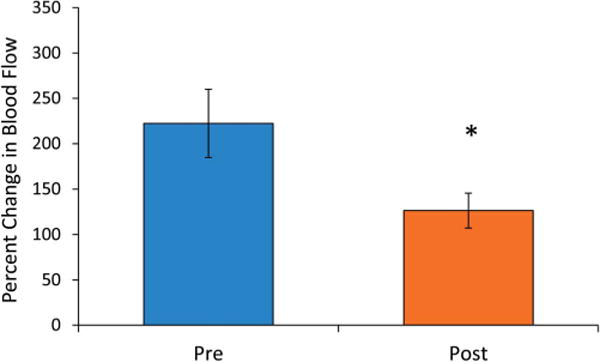
Peak blood flow responses to topical capsaicin before and after repeated capsaicin exposure. Percent change in blood flow decreased from 222 ± 38 % change precapsaicin to 126 ± 19 % change postcapsaicin (P < 0.05).
TRPV1 KO mice had a reduced blood flow response to acute niacin exposure compared with wild-type mice (Fig. 7A). There was no initial peak evident in the TRPV1 KO mice (Fig. 7A), and the blood flow at the time point corresponding to the initial peak was significantly lower compared with wild-type mice (43 ± 10 vs. 124 ± 15 % change) (P < 0.05, Fig. 7B). The secondary peak was also significantly reduced (80 ± 14 vs. 127 ± 12 % change) (P < 0.05, Fig. 7C), and the slope of the rate of change in blood flow was also significantly lower (103 ± 25 vs. 241 ± 28 % change per minute) (P < 0.05, Fig. 7D) in TRPV1 KO mice compared with wild-type mice.
FIGURE 7.
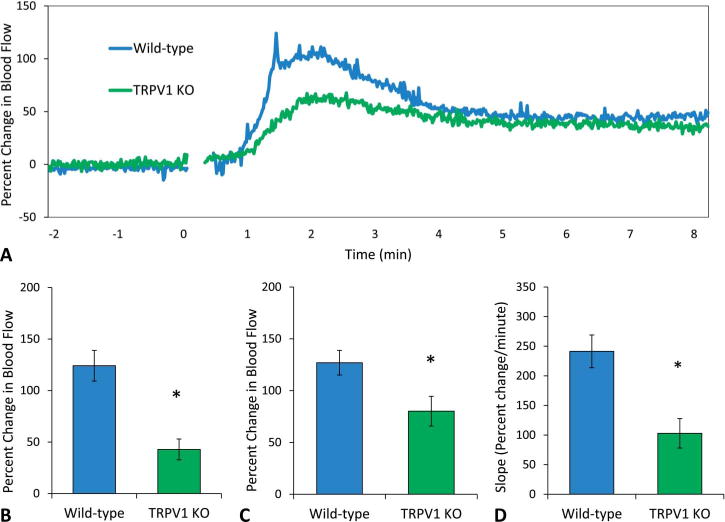
A, Mean blood flow responses to acute niacin. TRPV1 KO mice (green, n = 9) did not show an initial peak and overall had lower blood flow responses compared with wild-type mice (blue, n = 9). B, Blood flow at the initial peak. Compared with wild-type, TRPV1 KO mice had lower blood flow at the time point corresponding to the initial peak (42 ± 10 vs. 124 ± 15 % change) (P < 0.05). C, Blood flow at the secondary peak. TRPV1 KO mice had a secondary peak that was significantly less than wild-type mice (80 ± 14 vs. 127 ± 12 % change) (P < 0.05). D, Slope of the initial change in blood flow. TRPV1 KO mice had a significantly lower rate of change in blood flow compared with wild-type mice (103 ± 25 vs. 241 ± 28 % change per minute) (P < 0.05).
Chronic Niacin Response
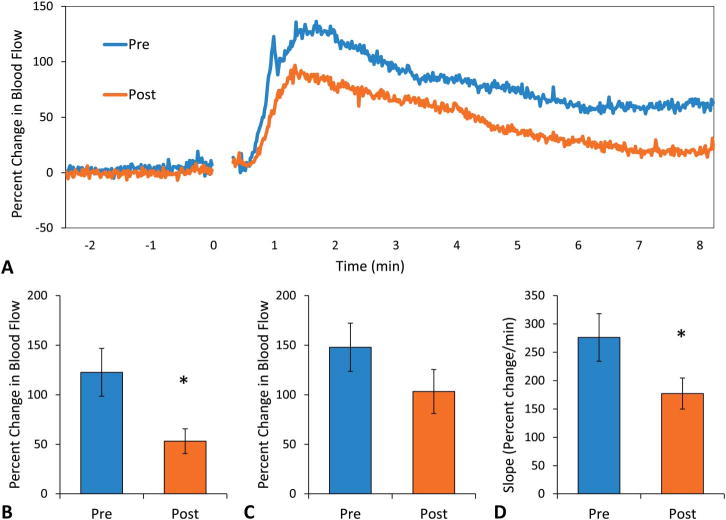
After 2 weeks of once daily niacin exposure, wild-type mice demonstrated a significant reduction in their blood flow response to niacin (ie, tachyphylaxis), whereas TRPV1 KO mice did not display any significant reduction in blood flow response with the same daily niacin exposure (Fig. 8A). In both wild-type and TRPV1 KO mice, the initial peak was absent after chronic niacin exposure (Fig. 8A), and the change in blood flow at this time point was significantly reduced in wild-type mice but not in the TRPV1 KO mice (20 ± 6 from 124 ± 15 vs. 23 ± 10 from 43 ± 10 % change, P < 0.05, Fig. 8B). The secondary peak was also significantly reduced in wild-type mice but not in TRPV1 KO mice (61 ± 12 from 127 ± 12 vs. 64 ± 17 from 80 ± 14 % change) (P < 0.05, Fig. 8C). The initial rate of change in blood flow was significantly lower after chronic niacin in wild-type mice but not in TRPV1 KO mice (34 ± 10 from 241 ± 28 vs. 43 ± 17 from 103 ± 25 % change per minute) (Fig. 8D). To assess if TRPV1 channel sensitivity was reduced after chronic niacin exposure in wild-type mice, capsaicin-induced hyperemia was measured before and after repeated exposure. Capsaicin-induced hyperemia was significantly lower after repeated niacin exposure (186 ± 31 to 106 ± 16 % change, P < 0.05), indicating reduced TRPV1 channel activity with niacin tachyphylaxis (Fig. 9).
FIGURE 8.
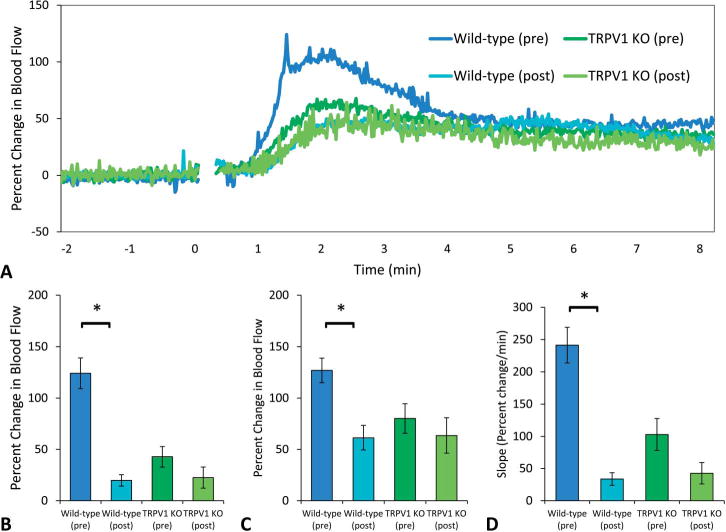
A, Mean blood flow responses before and after chronic niacin. After 2 weeks of repeated exposure to niacin, wild-type mice demonstrated significant tachyphylaxis (dark blue, n = 9 vs. light blue, n = 7), whereas TRPV1 KO mice showed only a slight reduction in blood flow responses (dark green, n = 9 vs. light green, n = 4). Both wild-type and TRPV1 KO mice lack the initial peak after chronic niacin exposure. B, Blood flow at the initial peak. At this time point, there was a reduction in blood flow from 124 ± 15 to 20 ± 6 % change in wild-type mice (P < 0.05) and a reduction from 43 ± 10 to 23 ± 10 % change in TRPV1 KO mice (P = 0.13). C, Blood flow at the secondary peak. Wild-type mice had a reduction from 127 ± 12 to 61 ± 12 % change in the secondary peak (P < 0.05), and TRPV1 KO mice had a reduction from 80 ± 14 to 64 ± 17 % change (P = 0.26). D, Slope of the initial change in blood flow. The rate of the initial rate of change in blood flow was lower postchronic niacin in wild types (34 ± 10 from 241 ± 28 % change per minute) (P < 0.05) and was lower in TRPV1 KO mice (43 ± 17 from 103 ± 25 % change per minute) (P = 0.08).
FIGURE 9.
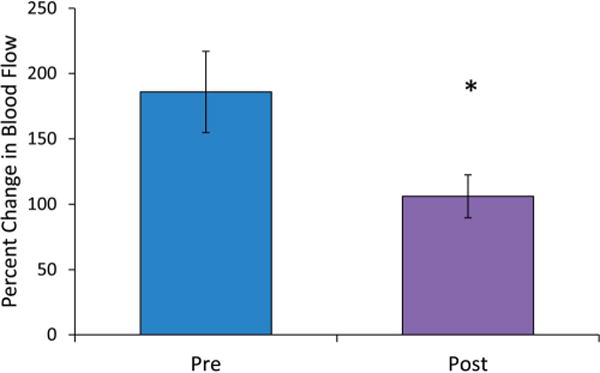
Peak blood flow responses to topical capsaicin before and after repeated niacin exposure. In wild-type mice, the peak change in blood flow was significantly lower after 2 weeks of chronic niacin (106 ± 16 % change) compared with preniacin (186 ± 31 % change per minute) (P < 0.05).
DISCUSSION
These experiments demonstrate that TRPV1 channels are likely involved in niacin-induced flushing and support the hypothesis that TRPV1 channels mediate the acute niacin response and tachyphylaxis with chronic use. Specifically, TRPV1 channels are responsible for the initial peak in the early phase flushing response (seen after the first minute) and contribute to the rapid rate of the onset of flushing. TRPV1 channels also mediate the magnitude of the secondary peak during early phase flushing (seen after the second minute). These effects were consistently observed in each of the 3 methods used to inhibit TRPV1 channel activity.
These data also support the hypothesis that reduced sensitivity of the TRPV1 channels is involved in niacin tachyphylaxis. Indeed, tachyphylaxis is observed for niacin-induced flushing but not for its antidyslipidemic effects, separating the 2 events as distinguishable pharmacologic effects. Chronic niacin exposure resulted in abolition of the initial peak and reductions in the slope and secondary peak, effects similar to those seen in the acute experiments when TRPV1 channels were inhibited. A reduction in sensitivity to capsaicin, a known TRPV1 channel agonist, was seen concurrently with a reduced blood flow response to niacin, further supporting a reduction in TRPV1 activity. Thus, TRPV1 channel activation contributes significantly to the early biphasic response to acute niacin administration and niacin tachyphylaxis is explained, at least in part, by TRPV1 channel desensitization.
Before these findings, niacin-induced flushing has largely been attributed to vasodilatory products of arachidonic acid metabolism, namely prostaglandins PGD2 and PGE2 through (COX) activity. Experiments by Hanson et al6,7 have demonstrated that the early phase flushing response is COX-1-dependent and the result of activation of Langerhans cell-based HCA2 receptors, whereas the late phase flushing response is COX-2–dependent and driven by activation of the HCA2 receptors expressed on keratinocytes. By using selective COX-1 and COX-2 inhibitors in a murine model, this group was able to block the early and late phase flushing responses, respectively, as well as abolish the entire response by coadministration of both inhibitors.6 Similarly, blocking the prostaglandin D2 receptor (DP1) using a selective antagonist, laropiprant, and using DP1 genetic knockout mice also reduced the flushing response to niacin,11,16 while complete abolition of the flushing response in mice required suprapharmacologic dose of aspirin (400 mg/kg).16 In contrast, other studies have found that reducing prostaglandin formation by blocking COX-1 activity with aspirin and COX-2 activity with celecoxib only partially reduced the incidence, severity, and duration of flushing symptoms.9,10
In addition to COX, arachidonic acid can also be metabolized by lipoxygenase and cytochrome P450 epoxygenase (CP450) to produce leukotrienes and epoxyeicosatrienoic acids, respectively. Our previous study demonstrated that pharmacologic inhibition of soluble epoxide hydrolase in the CP450 pathway inhibited niacin-induced vasodilation, potentially due to stabilization of epoxyeicosatrienoic acids levels, suppression of COX and lipoxygenase activity, and reduction of the production of prostaglandins such as PGE2.10,17 Although these findings support the role of prostaglandin production in niacin-induced flushing, blockade of this pathway, as with COX-1 blockade, was able to reduce but not eliminate flushing, indicating the possibility of additional mechanisms.
A promising candidate for this non-prostaglandin–based mechanism is the TRPV1 channel. TRPV1 is a member of the TRP cation channel superfamily. These nonselective cation channels respond to a wide range of stimuli. There are many known activators of TRPV1, including capsaicin, heat (>43°C), low pH, and many other endogenous agonists, including prostaglandins.18 Activation of this channel often results in pain and hyperemia or inflammation. TRPV1 channels are expressed in several different cell types, including sensory neurons, keratinocytes, and fibroblasts.18,19 The presence of this channel in the nociceptive neurons innervating the skin, as well as the keratinocytes themselves, make it ideally located for involvement in niacin-induced cutaneous vasodilation. In addition to its location, the similarity of the physiological response of activated TRPV1 channels (eg, hyperemia) to that of niacin-induced flushing also make it an appealing candidate. Furthermore, both repetitive TRPV1 activation and repetitive administration of niacin result in tachyphylaxis.4,20 These observations suggest potentially overlapping pathways in not only the acute but also the chronic response to niacin.
The potential for niacin to activate TRPV1 channels has been previously explored using an in vitro model and genetic knockouts.12 We demonstrated that niacin directly activated TRPV1 channels from the intracellular side, lowering the channel’s heat threshold, and increasing the open probability at physiological temperatures. This mechanism to activate TRPV1 channels at normal body temperature and cause pain and hyperemia has been demonstrated with other proinflammatory substances.18 Our earlier work in this area also demonstrated attenuation of the niacin-induced flushing response in TRPV1 KO mice for 1 hour after injection.12 These experiments provided strong evidence for the importance of TRPV1 channels in flushing. However, in contrast to this study, the sampling frequency was not high enough to discern the effects of this channel on the initial and secondary peaks during early phase flushing, and the administered dose far exceeded physiologic or pharmacologic concentrations. Nonetheless, the ability of niacin to lower threshold temperature and activate TRPV1 channels in vitro and the lack of flushing in TRPV1 KO mice in vivo indicated the need to further explore the role of this channel in niacin-induced flushing.
In contrast to previous studies using this murine model for niacin-induced cutaneous vasodilation,10,16 the higher laser Doppler sampling frequency (1 point per second) in this study allowed more precise characterization of the vascular response to niacin and revealed the presence of 2 distinct peaks within the early phase response.10,12,16 Although previous studies demonstrating 2 phases attributed the early phase to COX-1 and the late phase to COX-2,6,7 the greater resolution of this study showed a third response, namely an initial peak within the early phase, likely because of activation of TRPV1 channels.
Based on the current data, as well as prior in vitro work, we propose that a portion of the early flushing response, especially after ingestion of immediate release niacin, is due to opening of TRPV1 channels, followed by release of prostaglandins and activation of the DP1 receptor. This model may explain the reduced flushing response to slow release niacin, above and beyond the delay in absorption.
Limitations
Although these findings provide valuable insight into an additional mechanism that contributes to the niacin side effect of flushing, the applications of these findings in humans are unknown. Although the current murine model is an accepted animal model for studying niacin-induced cutaneous vasodilation and appears to reflect the response in humans,16 the transferability of these current findings to humans is yet to be examined. Thus, additional study is needed to determine if the role of TRPV1 channels in niacin-induced vasodilation is similar in humans or if TRPV1 inhibitors can be developed as a viable and tolerated therapy.18 Also, although these experiments have shown that TRPV1 channels are involved in the initial phase of flushing, these effects may be either parallel to, or a consequence of, prostaglandin production rather than a direct effect of TRPV1 activation by niacin. Specifically, previous studies have demonstrated that high doses of aspirin can completely block vasodilation in this model,16 as can dual inhibition of both COX-1 and COX-2 using selective inhibitors,6 and prostaglandins have been shown to lower the temperature threshold for the TRPV1 channel.18 Thus, the interplay between TRPV1 channels and prostaglandin-based signaling during the flushing response is unknown. Also, although in vitro data have demonstrated the effect of niacin on TRPV1 channels,12 and it has been previously established that TRPV1 channels are expressed in cell types that are commonly associated with the flushing response,6–8,18 the cell types and TRPV1 locations involved in this model of vasodilation were not explicitly examined in these experiments. Finally, the clinical value of niacin-based research has recently been called into question after publication of the findings of the HPS2-THRIVE and AIM-HIGH investigations.21,22 These large-scale clinical studies evaluated the addition of niacin to statin therapy alone. Although cholesterol and lipid profiles were improved with the inclusion of niacin, there was no significant difference in vascular event outcomes and a potential signal for harm in the HPS2-THRIVE study. Thus, although the future of niacin is uncertain due to these reports, it is currently FDA approved for prevention of recurrent myocardial infarction, slowing progression of atherosclerosis, and preventing pancreatitis. Given these indications and its likely use in patients not responsive to, or intolerant of, statin therapy, there is still a need for more complete understanding of its side effects, including flushing.
CONCLUSIONS
Using multiple methods of TRPV1 channel inhibition, these findings demonstrate that TRPV1 channels contribute, in part, to the acute flushing response to niacin and to tachyphylaxis after repeated exposure to niacin. Translation of these findings to humans may provide a method to ameliorate the often disabling side effect of flushing, which limits the clinical use of niacin.
Acknowledgments
Supported in part by resources from the VA Northern California Health Care System, Sacramento, CA.
Footnotes
The authors report no conflicts of interest.
The contents reported within do not represent the views of the Department of Veterans Affairs or the US Government.
References
- 1.Capuzzi DM, Morgan JM, Brusco OA, et al. Niacin dosing: relationship to benefits and adverse effects. Curr Atheroscler Rep. 2000;2:64–71. doi: 10.1007/s11883-000-0096-y. [DOI] [PubMed] [Google Scholar]
- 2.MacKay D, Hathcock J, Guarneri E. Niacin: chemical forms, bioavailability, and health effects. Nutr Rev. 2012;70:357–366. doi: 10.1111/j.1753-4887.2012.00479.x. [DOI] [PubMed] [Google Scholar]
- 3.Kamal-Bahl S, Watson DJ, Ambegaonkar BM. Patients’ experiences of niacin-induced flushing in clinical practice: a structured telephone interview. Clin Ther. 2009;31:130–140. doi: 10.1016/j.clinthera.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Paolini JF, Mitchel YB, Reyes R, et al. Measuring flushing symptoms with extended-release niacin using the flushing symptom questionnaire: results from a randomised placebo-controlled clinical trial. Int J Clin Pract. 2008;62:896–904. doi: 10.1111/j.1742-1241.2008.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blad CC, Ahmed K, Ijzerman AP, et al. Biological and pharmacological roles of HCA receptors. Adv Pharmacol. 2011;62:219–250. doi: 10.1016/B978-0-12-385952-5.00005-1. [DOI] [PubMed] [Google Scholar]
- 6.Hanson J, Gille A, Zwykiel S, et al. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest. 2010;120:2910–2919. doi: 10.1172/JCI42273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanson J, Gille A, Offermanns S. Role of HCA2 (GPR109A) in nicotinic acid and fumaric acid ester-induced effects on the skin. Pharmacol Ther. 2012;136:1–7. doi: 10.1016/j.pharmthera.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Lukasova M, Hanson J, Tunaru S, et al. Nicotinic acid (niacin): new lipid-independent mechanisms of action and therapeutic potentials. Trends Pharmacol Sci. 2011;32:700–707. doi: 10.1016/j.tips.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Cefali EA, Simmons PD, Stanek EJ, et al. Aspirin reduces cutaneous flushing after administration of an optimized extended-release niacin formulation. Int J Clin Pharmacol Ther. 2007;45:78–88. doi: 10.5414/cpp45078. [DOI] [PubMed] [Google Scholar]
- 10.Inceoglu AB, Clifton HL, Yang J, et al. Inhibition of soluble epoxide hydrolase limits niacin-induced vasodilation in mice. J Cardiovasc Pharmacol. 2012;60:70–75. doi: 10.1097/FJC.0b013e3182580a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai E, De Lepeleire I, Crumley TM, et al. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin Pharmacol Ther. 2007;81:849–857. doi: 10.1038/sj.clpt.6100180. [DOI] [PubMed] [Google Scholar]
- 12.Ma L, Lee BH, Mao R, et al. Nicotinic acid activates the capsaicin receptor TRPV1: potential mechanism for cutaneous flushing. Arterioscler Thromb Vasc Biol. 2014;34:1272–1280. doi: 10.1161/ATVBAHA.113.303346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrel WR, Wong BB, Lockhart JC, et al. Gender differences in regional cutaneous microcirculatory responses to capsaicin. Fundam Clin Pharmacol. 2004;18:195–200. doi: 10.1111/j.1472-8206.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 14.Munce TA, Kenney WL. Age-specific skin blood flow responses to acute capsaicin. J Gerontol A Biol Sci Med Sci. 2003;58:304–310. doi: 10.1093/gerona/58.4.b304. [DOI] [PubMed] [Google Scholar]
- 15.Roberts RG, Westerman RA, Widdop RE, et al. Effects of capsaicin on cutaneous vasodilation responses in humans. Agents Actions. 1992;37:53–59. doi: 10.1007/BF01987890. [DOI] [PubMed] [Google Scholar]
- 16.Cheng K, Wu TJ, Wu KK, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A. 2006;103:6682–6687. doi: 10.1073/pnas.0601574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lui JY, Yang J, Inceoglu B, et al. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010;79:880–887. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billeter AT, Hellmann JL, Bhatnagar A, et al. Transient receptor potential ion channels: powerful regulators of cell function. Ann Surg. 2014;259:229–235. doi: 10.1097/SLA.0b013e3182a6359c. [DOI] [PubMed] [Google Scholar]
- 19.Zheng J. Molecular mechanisms of TRP channels. Compr Physiol. 2013;3:221–242. doi: 10.1002/cphy.c120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novakova-Tousova K, Vyklicky L, Susankova K, et al. Functional changes in the vanilloid receptor subtype 1 channel during and after acute desensitization. Neuroscience. 2007;149:144–154. doi: 10.1016/j.neuroscience.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 21.The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 22.The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]


