Abstract
Accumulating evidence has suggested that the dysregulation of miRNA is an important factor in the pathogenesis of lung cancer. Here, we demonstrate that miR‐335 expression is reduced in non‐small cell lung cancer (NSCLC) tumors relative to non‐cancerous adjacent tissues, while the expression of Tra2β is increased. In addition, clinical data revealed that the increased Tra2β and decreased miR‐335 expression observed in NSCLC cells was associated with poor patient survival rates. In vitro experimentation showed that the overexpression of miR‐335 inhibited the growth, invasion and migration capabilities of A459 lung cancer cells, by targeting Tra2β. In contrast, inhibition of miR‐335 or overexpression of the Tra2β target gene stimulated the growth, invasion and migratory capabilities of A459 lung cancer cells in vitro. Furthermore, overexpression of miR‐335 or inhibition of Tra2β decreased the phosphorylation of Rb‐S780 and Rb‐AKT. Overall, these findings suggest that the downregulation of miR‐335 in A459 lung cancer cells promoted cell proliferation through upregulation of Tra2β, mediated via activation of the AKT/mTOR signaling pathway, and suggest that miR‐335 may have potential as a novel therapeutic target for NSCLC.
Keywords: Cell proliferation, miR‐335, non‐small cell lung cancer, prognosis, Tra2β
1. INTRODUCTION
Lung cancer is a leading cause of cancer‐related deaths worldwide.1 Among the different types of the disease that include adenocarcinoma, squamous carcinoma, adenosquamous carcinoma, large cell carcinoma and non‐small cell lung cancer (NSCLC), the latter accounts for more than 80% of cases.2 Despite recent advances in treatments for lung cancer and in understanding lung tumor molecular biology, prognosis of the disease remains poor; a comprehensive analysis incorporating all subtypes and stages of the disease revealed that overall 5‐year survival rates for NSCLC remain below 15%.3, 4
The transformer 2 beta homolog (Tra2β), also known as RA301 and SFRS10, is a gene that encodes a serine/arginine‐rich (SR)‐like nuclear protein. The Tra2β protein belongs to the SR‐like protein family and has an RNA recognition motif (RRM) and two RS domains.5 It has been shown to play an important role in RNA processing and normal tissue development, and is essential for the normal development of the embryo and brain in mice, where Tra2β deficiency results in early embryonic lethality.6 Furthermore, Tra2β has been reported to be associated with several pathologies, including stroke, tumorigenesis, silicosis, nerve injury and atherosclerosis.5, 7, 8 The Tra2β protein is now considered to be one of the most important splicing factors that are involved in the progression of several diseases, including cancer.9 A recent study has shown that Tra2β was upregulated in NSCLC and that this upregulation was correlated with a poor prognosis.10 Conversely, downregulation of Tra2β inhibited cell proliferation and induced apoptosis of A549 lung carcinoma cells.10 Based on these findings, the authors of the study speculated that Tra2β might serve as a novel molecular target for the diagnosis and treatment of NSCLC.10
MicroRNA (miRNA) are a class of small, single‐stranded endogenous non‐coding RNA comprising 18‐22 nucleotides.11 They regulate gene expression post‐transcriptionally through binding to the 3′ untranslated region (3′UTR region) of their target mRNA, inhibiting production of the encoded protein.12 The differential expression of miRNA in the cells of tumor tissues and normal tissues has been described for many different tumor types.13, 14 Accumulating evidence has shown that miRNA play an important role in many biological processes, including cell growth, metabolism, differentiation and apoptosis.15 and further research has demonstrated that they play a vital role in the development of cancers.11, 15 On this basis, some miRNA may become treatment targets for cancers. Indeed, recent studies in a range of different cancers have shown that dysregulated miRNA can cause tumor initiation, development and metastasis, by regulating the expression of multiple target genes.16 In particular, studies have shown that the expression of miRNA in NSCLC tissue compared with normal tissues is dysregulated.17, 18 However, no studies have previously reported a specific correlation between miR‐335 expression and NSCLC, and the effects and molecular mechanisms of miR‐335 in the pathogenesis of NSCLC are currently unclear. Against this background, we sought to investigate the relationship between the expression of miR‐335 and Tra2β in NSCLC tissues and adjacent non‐cancerous tissues, and to explore experimentally the roles of miR‐335 and Tra2β in the pathogenesis of NSCLC.
2. MATERIALS AND METHODS
Ethical approval for the study was sought and granted by the Ethics Committee of the Affiliated Hospital of Nantong University, Jiangsu Province, China. Written informed consent was obtained from all patients who participated in the study.
2.1. Histology
Fresh, surgically resected samples of NSCLC tumor tissues and adjacent noncancerous tissues were obtained from 292 patients attending the Department of Thoracic Surgery at the Affiliated Hospital of Nantong University, Jiangsu Province, China. Samples were obtained between January 2014 and September 2016. None of the patients had undergone chemotherapy prior to their surgery.
2.2. Cell lines and cell culture
Human A549 lung cancer cells were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). Cells were cultured in DMEM medium supplemented with 10% FBS (Gibco, USA), 100 IU/mL penicillin and 100 mg/mL streptomycin (Life Technologies, USA) and maintained in a cell culture incubator under conditions of 37°C with 5% CO2.
2.3. Cell transfection and dual luciferase reporter assay
The oligonucleotides of miR‐335 mimics, miR‐335 antagomir and scrambled oligonucleotides (miR‐control) were purchased from Gene Pharma (Shanghai, China). Single‐stranded DNA fragments containing wild type or mutated miR‐335 seed sequences as well as their complementary fragments were synthesized by Sangon Biotech (Shanghai, China). After annealing the respective single‐stranded DNA, the resultant double‐stranded DNA was inserted into the dual‐luciferase reporter gene vector psicheck‐2 (Promega, Madison, WI, USA) to obtain the recombinant wild type reporter gene vector Tra2β‐3′ UTR‐WT and the mutant reporter gene vector Tra2β‐3′ UTR‐Mut. For the dual luciferase reporter gene experiments, A549 cells were seeded in 96‐well plates at a density of 5000 cells per well, and co‐transfected with 25 ng pischeck‐2 reporter gene vector and 50 ng of either the miR‐335 mimics or miRNA control, using Lipofectamine2000 (Invitrogen, USA) according to the manufacturer's instructions. Cells transfected with pischeck‐2 vector only were used as a blank control. Forty‐eight hours after transfection, the luciferase activity was conducted using the Dual‐Luciferase Reporter Assay System (Promega, USA). All experiments were performed in triplicate.
2.4. Protein extraction and western blotting
The total protein content of the transfected and control cells was extracted by mixing with RIPA lysis buffer for 30 minutes on ice. Protein concentration was determined using a BCA Quantification Kit (Beyotime, Beijing, China). Protein lysates (50 μg) were separated by 12% SDS‐PAGE and blotted onto a 0.45‐μm PVDF membrane (Millipore, Hercules, CA, USA). The membrane was then incubated with HRP‐conjugated secondary antibody (Jackson, USA) at room temperature for 2 hours. Immuno‐reactivity of the separated proteins was visualized using the ECL Western Blotting kit (Invitrogen, USA) according to the manufacturer's instructions, using GAPDH as a loading control. Rabbit anti‐AKT, anti‐pAKT‐S473, anti‐S6K and anti‐pS6K‐T389 were purchased from Cell Signaling Technology (Boston, MA, USA). Anti‐rabbit IgG‐HRP and GAPDH mRB antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All experiments were performed in triplicate.
2.5. Cell viability
To determine the effects of miR‐335 on cell viability, A549 cells were seeded in 96‐well plates at a density of 6000 cells per well, in 100 μL. After transfection of exogenous miR‐335 mimics, miR‐335 siRNA or scrambled miRNA for 48 hours, cell viability was assessed using the MTT colorimetric assay. A 10‐μL aliquot of 5 mg/mL MTT (Sigma‐Aldrich, St. Louis, MO, USA) was added to each well and incubated for 4 hours, after which the MTT solution was removed and dimethyl sulfoxide was added to each well to dissolve the metabolic product. Absorbance was measured at 490 nm using a microplate reader (Thermo Fisher, USA).
2.6. Cell apoptosis
To determine the effects of miR‐335 on apoptosis of NSCLC cells, A549 cells were seeded in 6‐well plates with a density of 2 × 105 per well. When the cells had reached 70% to 80% confluence, they were transfected with miR‐335 mimics, miR‐335 antagomir or scrambled miRNA. Forty‐eight hours after transfection, cells were collected for processing using a Cell Apoptosis Kit (BD Biosciences, San Jose, CA, USA) and sorted to separate out apoptotic cells using a flow cytometer (Beckman Coulter, Brea, CA, USA). Data were processed and analyzed using a Beckman Coulter Epics XL Flow Cytometer.
2.7. Real‐time quantitative PCR analysis
The total RNA was isolated from transfected A549 cells using TRIzol Reagent (Invitrogen, USA) according to the manufacturer's instructions, and used for quantitative RT‐PCR analysis. For miR‐335 and the RNA gene RNU6B, reverse transcription and real‐time PCR were performed using the TakMan MicroRNA Reverse Transcription Kit and TaqMan Universal PCR Master Mix, respectively, and using Ambion miRNA primers. The expression levels of the miRNA were normalized to RNU6B. For quantifying the expression levels of other genes, reverse transcription and PCR were performed using the Quant One Step RT‐PCR Kit (TIANGEN, China) and TransStart Green qPCR SuperMix (TRANSGEN, China), respectively. The primer sequences for the target genes were as follows: Tra2β forward 5′–CATAGACGATCACGTAGCAGGT‐3′, reverse 5′‐GAGAGCTGCCATAGGTAGGTC‐3′; β‐actin forward 5′‐GATGAGATTGGCATGGCTTT‐3′, reverse 5′‐GTCACCTTCACCGTTCCAGT‐3′.
2.8. Cell invasion and migration assay
Transwells comprising 24‐wells coated with Matrigel (8‐μm poresize; BD Biosciences, San Jose, CA, USA) were used for the cell invasion assays. Equal numbers (1 × 105) of untransfected cells and cells transfected with miR‐335 mimics, miR‐335 siRNA, sh‐Trra2β or Tra2β vector were plated onto separate wells. Cells were cultured overnight in serum‐free medium before being trypsinized and resuspended at a density of 2 × 105 cells/mL in culture medium containing 2% FBS. The cells were then added to the upper chamber of the transwell, with medium containing 20% FBS as a chemoattractant in the lower chamber. Medium containing 2% FBS was added to the lower chamber as a control. The Matrigel and cells remaining in the upper chamber were removed after 24‐h incubation using cotton swabs. The number of cells in at least five random microscopic fields of view (×200 magnification) were counted and photographed. For the cell migration assay, transfected cells were plated. The cells were incubated until the formation of an approximately 80%‐90% confluent monolayer. Micropipette tips (AP‐20) were then used to mimic a scratch wound. The ratio of cell migratory capacity was determined by measuring the width of the scratch at 0 hour and 24 hours under bright‐field microscopy.
2.9. Detection of bromodeoxyuridine incorporation
Untreated cells or cells transfected with plasmids were washed thoroughly with medium and cultured in fresh medium containing 10 μM bromodeoxyuridine (BrdU; Sigma‐Aldrich) for 1 hour. Cells were then either harvested directly or left to grow in BrdU‐free medium for varying lengths of time prior to being harvested.
The resulting cell pellets were washed with PBS. Cells were then hybridized with a mouse monoclonal anti‐BrdU antibody (Abcam, USA), diluted at a ratio 1:100 in PBST and incubated overnight at 40°C. After rinsing with PBST, cells were incubated with FITC‐conjugated rabbit anti‐mouse immunoglobulin antibody (Immuno Jackson, USA) diluted at a ratio1:400 in PBST. After 2 hour incubation at room temperature in the dark, cells were washed with PBS and stained with DAPI solution for 10 minutes before being photographed.
2.10. Statistical analysis
The survival rate of NSCLC patients in the study sample was calculated using Kaplan–Meier survival analysis. Differences between groups were determined by one‐way ANOVA using SPSS Statistics v17.0 (IBM, Armonk, NY, USA). All experiments were performed in triplicate as a minimum and data are expressed as means ± SD. P < .05 was considered statistically significant.
3. RESULTS
3.1. Expression of miR‐335 and Tra2β in lung cancer tissues
Expression of miR‐335 was significantly decreased in NSCLC tissues compared with adjacent non‐tumorous tissue samples (Figure 1A), while the expression of Tra2β was significantly increased (Figure 1B).
Figure 1.
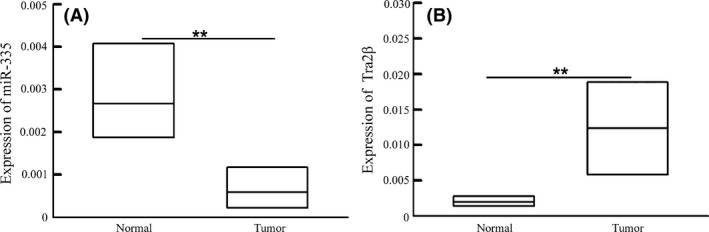
miR‐335 and Tra2β expression in tissue. A, miR‐335 expression significantly decreased in lung cancer patients (n = 292). B, Tra2β expression increased in lung cancer patients compared with non‐cancerous adjacent tissues (n = 292). The data are presented as the mean ± SD. **P < .01, vs normal group
3.2. Effects of miR‐335 on cell growth, cell migration and invasion
Effects of miR‐335 on A549 cell growth were investigated by overexpression or inhibition of miR‐335. We first assessed the levels of expression of miR‐335 in A549 cells following transfection of miR‐335 mimics or miR‐335 antagomir. The results showed that transfection of miR‐335 mimics increased the expression of miR‐335 by these cells, while transfection of miR‐335 antagomir decreased miR‐335 expression (Figure 2A). The overexpression of miR‐335 was found to significantly inhibit A549 cell growth, as indicated by the ratio of BrdU positive cells (Figure 2B). In contrast, inhibition of miR‐335 stimulated A549 cell growth, as indicated by an increase in the ratio of BrdU‐positive cells (Figure 2C,D). These findings were confirmed through an apoptosis assay in which apoptotic cells were sorted and quantified by flow cytometry. The results showed that the overexpression of miR‐335 induced cell apoptosis, whereas inhibition of miR‐335 significantly reduced the number of apoptotic cells (Figure 2E). We further investigated the effects of miR‐335 on the migration of A549 cells through an in vitro transwell migration assay using Matrigel, because the migration of cancer cells is usually identified as a key factor in cancer metastasis. By counting the number of cells that migrated through the Matrigel into the lower compartment of the transwell, we estimated the extent of migration of the cells. The results showed that miR‐335 significantly reduced the invasion capability of A549 cells (Figure 2F,G). A wound‐healing assay similarly showed that miR‐335 significantly reduced the migration capability of A549 cells (Figure 2H,I).
Figure 2.
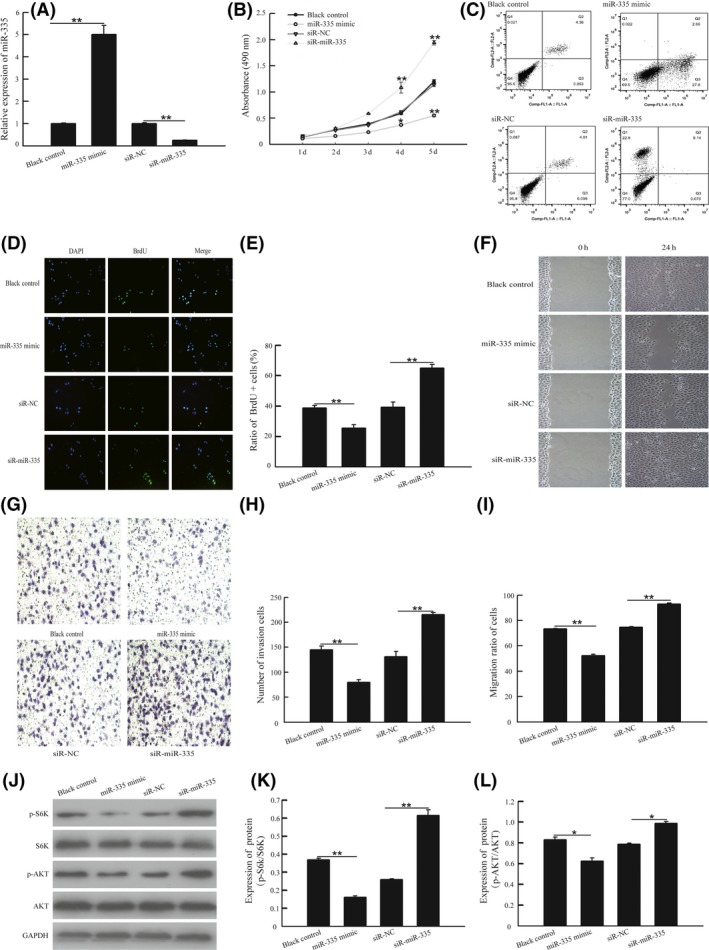
miR‐335 inhibited cell growth, cell invasion and cell migration in vitro through the activation of the AKT/mTOR signaling pathway. A, A549 cells was transfected with exogenous miR‐335, miR‐335 antagomir or scrambled; the expression of miR‐335 was detected by quantitative RT‐PCR methods. B, Cell viability was assessed by MTT assay after transfection with different plasmids. C,D, A549 cells were transfected with miR‐335 siRNA, pre‐miR‐335 or negative controls for 24 h; then the cells were cultured with medium containing 10 μM BrdU for 1 h. Cells were fixed and stained for BrdU incorporation, immunofluorescence images of BrdU and DAPI were analyzed with Image J software and the ratio of BrdU‐positive cells was calculated. E, Cell apoptosis was detected by flow cytometric assay. F,G, Cell invasion was detected by transwell Matrigel assay, and number of invasion was measured with Image J software. H,I, Cell migration was detected by wound‐healing assay, and ratio of migration was measured with Photoshop CS5. J‐L, A549 cells were transfected with exogenous miR‐335, miR‐335 antagomir or scrambled for 48 h. Total proteins were extracted for immunoblotting of AKT, S6K, phosphorylation of AKT(S473) and S6K1(T389) and GAPDH. *P < .05 or **P < .01, vs pcDNA3.1 group. *P < .05 or **P < .01, vs ASO‐NC group
To test whether miR‐335 suppresses apoptosis of NSCLC cells, and to investigate the implicated signaling pathways, we measured changes in the AKT‐mTOR signaling pathway after transfection of A549 cells with miR‐335 mimics or miR‐335 antagomir. The antibodies used assessed the phosphorylated state of AKT at Ser473 and S6K at Thr389. The results showed that miR‐335 antagomir increased pAKT at Ser473 and pS6K1 at Thr389, while miR‐335 mimics had the opposite effects (Figure 2J‐L).
3.3. Identification of the functional target of miR‐335 in non‐small cell lung cancer cells
MicroRNA have been functionally classified as proto‐oncogenes or tumor suppressors, and are known to be aberrantly expressed in different cancers.19 Previous studies have demonstrated that the miR‐335 protein is a molecule involved in the pathogenesis and progression of lung cancer.10 Using three miRNA databases (Targetscan, Pictar and Miranda), we identified a putative miR‐335‐binding site located in the 3′‐UTR of Tra2β mRNA. To confirm whether miR‐335 directly binds to this location in Tra2β, wild type or mutant Tra2β 3′‐UTR were cloned and inserted into a luciferase reporter vector. The results of the luciferase reporter assay showed that enhanced miR‐335 expression remarkably reduced the luciferase activity of the wild‐type 3′‐UTR, but not the mutant reporter constructs (Figure 3B). Moreover, our data showed that both the protein and gene levels of Tra2β were reduced in the miR‐335 transfected cell lines compared to the negative controls, suggesting that miR‐335 can directly bind to Tra2β (Figure 3A,C,D).
Figure 3.
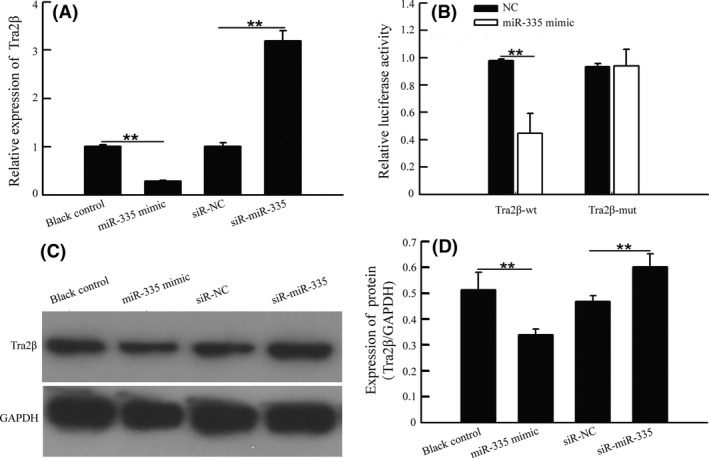
Tra2β is a direct functional target of miR‐335 in non‐small cell lung cancer cells. A, A549 cells were transfected with exogenous miR‐335, miR‐335 antagomir or scrambled control for 48 h. Expression of Tra2β were detected by quantitative RT‐PCR method. B, Dual luciferase reporter assay showed that miR‐335 mimics significantly reduced the luciferase activity of Tra2β 3′‐UTR wt but not Tra2β 3′‐UTR mut in A549 cells. C,D, A549 cells was transfected with exogenous miR‐335, miR‐335 antagomir or scrambled control for 48 h. Proteins were immunoblotted with Tra2β and GAPDH antibody separately, and the density of protein was measured with Image J software. *P < .05 or **P < .01, vs pcDNA3.1 group. **P < .01, vs ASO‐NC group
3.4. Effects of Tra2β on cell growth, cell invasion and cell migration
To investigate the effects of Tra2β on A459 cell growth, invasion and migration capabilities, we first assessed the level of expression of Tra2β in A549 cells following transfection with Tra2β vector or shTra2β vector. We found that transfection with shTra2β decreased the expression of Tra2β while transfection with Tra2β vector increased expression, at both the gene and protein levels (Figure 4A‐C).10 As shown in Figure 4D, inhibition of Tra2β significantly inhibited A549 cell growth, a finding that is consistent with results of our previous research. In contrast, overexpression of Tra2β stimulated A549 cell growth, as evidenced by an increase in the ratio of BrdU‐positive cells increased (Figure 4E,F). We further confirmed these results through an apoptosis assay using cytometric analysis to identify apoptotic cells. The results showed that the overexpression of Tra2β reduced cell apoptosis in A549 cells, whereas inhibition of Tra2β significantly increased the number of apoptotic cells (Figure 4G). Furthermore, we observed that Tra2β overexpression significantly increased the migration of A549 cells compared with controls, and also enhanced their invasion capability (Figure 4H,I). A wound‐healing assay further showed that the inhibition of Tra2β significantly reduced this migration capability (Figure 4J,K).
Figure 4.
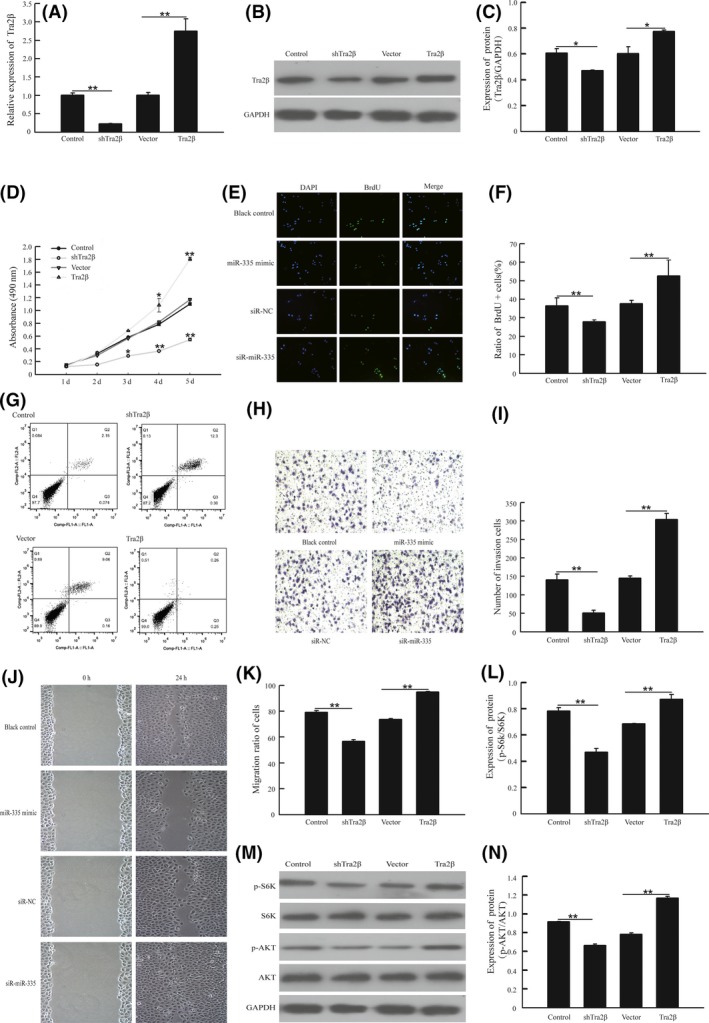
Tra2β promoted cell growth, cell invasion and cell migration in vitro by activation of the AKT/mTOR signaling pathway. A,B,C, A549 cells was transfected with shTra2β, Tra2β or negative control vector, and the expression of Tra2β was detected by quantitative RT‐PCR methods and western blot methods D, Cell viability was assessed by MTT assay after transfection with different plasmids. E,F, A549 cells transfected with shTra2β and Tra2βor negative vector separately for 24 h, then the cells were cultured with medium containing 10 μM BrdU for 1 h. Cells were fixed and stained for BrdU incorporation, immunofluorescence images of BrdU and DAPI were analyzed with Image J software, and the ratio of BrdU‐positive cells was calculated. G, Cell apoptosis was detected by flow cytometric assay. H,I, Cell invasion was detected by transwell Matrigel assay, and number of invasion was measured with Image software. J,K, Cell migration was detected by wound‐healing assay, and ratio of migration was measured with Photoshop CS5. L‐N, A549 cells was transfected with shTra2β, Tra2β or vector for 48 h. Total proteins were extracted for immunoblotting of AKT, S6K, phosphorylation of AKT(S473) and S6K1(T389). **P < .01, vs control group. **P < .01, vs vector group
To test whether Tra2β suppresses apoptosis in NSCLC cells, and to investigate the signaling pathway by which such effects may be mediated, we identified the changes in the AKT‐mTOR signaling pathway after transfection of A459 cells with Tra2β vector or shTra2β vector. These changes were assessed by measuring the phosphorylation of AKT at Ser473 and S6K at Thr389. The results showed that transfection with the Tra2β vector increased phosphorylation at these sites, while shTra2β vector decreased their phosphorylation in NSCLC cells (Figure 4L‐N).
4. DISCUSSION
The initiation and progression of NSCLC is an extensive pathological process that involves complex alterations in the expression of various oncogenes and tumor suppressor genes that play key roles in cell proliferation and cell apoptosis. In spite of the development of therapeutic approaches for the treatment of NSCLC, such as surgical resection, chemotherapy and radiation therapy, the disease is rarely curable and has a poor prognosis.20 A deeper understanding of the genes and associated mechanisms involved in the progression of NSCLC is urgently needed to improve current treatments and prognoses. In the present study, we investigated the role of Tra2β and miR‐335 in the development of NSCLC.
There is an accumulating body of evidence suggesting that miRNA play an important role in tumorigenesis as well as in tumor progression, diagnosis and treatment.21 Despite the identification of a number of miRNA targets involved in human tumors, in the majority of cases their mechanisms of effect remain unclear. The expression of miR‐335 has been shown to be aberrant in tumor tissue from several types of cancer, and is associated with cell proliferation and inflammation.22, 23, 24 The present study found that miR‐335 was downregulated in NSCLC tumor tissues compared with adjacent non‐tumor tissues. In addition, miR‐335 expression was found to be associated with both histological differentiation and clinical stage of disease in NSCLC. Calculation of a survival curve revealed that low expression of miR‐335 in tumor samples was associated with a poor prognosis of NSCLC. We also observed that overexpression of miR‐335 inhibited proliferation of A549 cells and induced cell apoptosis, while high expression of miR‐335 by these cells also significantly reduced their migration and invasion capabilities. Furthermore, overexpression of miR‐335 increased the phosphorylation of AKT at S473 and S6K at T389 in NSCLC cells.
The Tra2β gene encodes a nuclear protein that is an important splicing factor that results in multiple transcript variants. Splicing activation by the Tra2β protein is concentration‐dependent; thus it is important that Tra2β levels are tightly regulated to maintain homeostasis.25 Dysregulation of Tra2β has been closely linked to various human diseases, including cancer,26, 27 but the role Tra2β in NSCLC has remained unclear. Previous studies have indicated that Tra2β participates in various cellular processes, such as cell proliferation, diversification and apoptosis.25, 28 The findings of the present study have added to and extended these findings by showing that knockdown of Tra2β inhibited proliferation of A549 lung cancer cells and induced cell apoptosis, as well as reducing their migratory and invasion capabilities. Furthermore, it was observed that the overexpression of Tra2β increased phosphorylation of AKT at S473 and S6K at T389 in NSCLC cells and that miR‐335 appeared to regulate the expression of Tra2β through binding the 3′UTR.
Taken together, the findings of the present study have demonstrated that Tra2β is upregulated in NSCLC and is associated with a poor prognosis. Downregulation of Tra2β inhibited proliferation and induced apoptosis of A549 lung cancer cells. Furthermore, Tra2β was identified as a direct target of miR‐335. Our results indicate that the interaction between miR‐335 and Tra2β may play a vital role in the pathogenesis of NSCLC, that may be mediated via the AKT/mTOR signaling pathway. This suggests that miR‐335 may be a novel therapeutic target for the treatment of NSCLC.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Liu J, Bian T, Feng J, et al. miR‐335 inhibited cell proliferation of lung cancer cells by target Tra2β. Cancer Sci. 2018;109:289–296. https://doi.org/10.1111/cas.13452
Funding information
Six talent peaks project in Jiangsu Province, China (No. WSN‐059); the Science Foundation of Nantong City, Jiangsu, China (No. MS12015007); Scientific research topic of Jiangsu provincial health and Family Planning Commission, China (No. H201626); Key talents of Medical Science in Jiangsu Province, China (No.QNRC2016682).
Contributor Information
Yifei Liu, Email: ntdxliuyifei@sina.com.
Jiahai Shi, Email: ntshijiahai0513@163.com.
REFERENCES
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11‐30. [DOI] [PubMed] [Google Scholar]
- 2. Zhang T, Zhang DM, Zhao D, et al. The prognostic value of osteopontin expression in non‐small cell lung cancer: a meta‐analysis. J Mol Histol. 2014;45:533‐540. [DOI] [PubMed] [Google Scholar]
- 3. Cai J, Fang L, Huang Y, et al. miR‐205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non‐small cell lung cancer. Cancer Res. 2013;73:5402‐5415. [DOI] [PubMed] [Google Scholar]
- 4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 5. Best A, Dagliesh C, Ehrmann I, et al. Expression of Tra2 beta in Cancer Cells as a Potential Contributory Factor to Neoplasia and Metastasis. Int J Cell Biol. 2013;2013:843781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grellscheid S, Dalgliesh C, Storbeck M, et al. Identification of evolutionarily conserved exons as regulated targets for the splicing activator tra2beta in development. PLoS Genet. 2011;7:e1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daoud R, Mies G, Smialowska A, et al. Ischemia induces a translocation of the splicing factor tra2‐beta 1 and changes alternative splicing patterns in the brain. J Neurosci. 2002;22:5889‐5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsukamoto Y, Matsuo N, Ozawa K, et al. Expression of a novel RNA‐splicing factor, RA301/Tra2beta, in vascular lesions and its role in smooth muscle cell proliferation. Am J Pathol. 2001;158:1685‐1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeo K, Kawai T, Nishida K, et al. Oxidative stress‐induced alternative splicing of transformer 2beta (SFRS10) and CD44 pre‐mRNAs in gastric epithelial cells. Am J Physiol. 2009;297:C330‐C338. [DOI] [PubMed] [Google Scholar]
- 10. Ji L, Ni T, Shen Y, et al. Transformer 2beta (Tra2beta/SFRS10) positively regulates the progression of NSCLC via promoting cell proliferation. J Mol Histol. 2014;45:573‐582. [DOI] [PubMed] [Google Scholar]
- 11. Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281‐297. [DOI] [PubMed] [Google Scholar]
- 13. Thayanithy V, Sarver AL, Kartha RV, et al. Perturbation of 14q32 miRNAs‐cMYC gene network in osteosarcoma. Bone. 2012;50:171‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends Mol Med. 2006;12:580‐587. [DOI] [PubMed] [Google Scholar]
- 15. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen KH, Hung JH, Chang CW, Weng YT, Chen PS. Solasodine inhibits invasion of human lung cancer cell through downregulation of miR‐21 and MMPs expression. Chem Biol Interact. 2017;268:129‐135. [DOI] [PubMed] [Google Scholar]
- 18. Huang YK, Fan XG, Qiu F. TM4SF1 promotes proliferation, invasion, and metastasis in human liver cancer cells. Int J Mol Sci. 2016;17:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3‐L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372‐31378. [DOI] [PubMed] [Google Scholar]
- 20. Sun S, Schiller JH, Spinola M, Minna JD. New molecularly targeted therapies for lung cancer. J Clin Invest. 2007;117:2740‐2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834‐838. [DOI] [PubMed] [Google Scholar]
- 22. Li H, Zhu H, Zhou Y, et al. Long non‐coding RNA MSTO2P promotes the proliferation and colony formation in gastric cancer by indirectly regulating miR‐335 expression. Tumour Biol. 2017;39:1010428317705506. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Wang N, Zeng X, et al. MicroRNA‐335 and its target Rock1 synergistically influence tumor progression and prognosis in osteosarcoma. Oncol Lett. 2017;13:3057‐3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Chen L, Bian ZL, Diao HY, Shao JG. [Expression of miR‐335 in serum in patients with hepatocellular carcinoma and its significance]. Zhonghua Gan Zang Bing Za Zhi. 2017;25:47‐49. [DOI] [PubMed] [Google Scholar]
- 25. Kajita K, Kuwano Y, Kitamura N, et al. Ets1 and heat shock factor 1 regulate transcription of the Transformer 2beta gene in human colon cancer cells. J Gastroenterol. 2013;48:1222‐1233. [DOI] [PubMed] [Google Scholar]
- 26. Diao Y, Wu D, Dai Z, Kang H, Wang Z, Wang X. Prognostic value of transformer 2beta expression in prostate cancer. Int J Clin Exp Pathol. 2015;8:6967‐6973. [PMC free article] [PubMed] [Google Scholar]
- 27. Yang L, Tao T, Wang Y, Bao Z, He X, Cui G. Knocking down the expression of TRA2beta inhibits the proliferation and migration of human glioma cells. Pathol Res Pract. 2015;211:731‐739. [DOI] [PubMed] [Google Scholar]
- 28. Roberts JM, Ennajdaoui H, Edmondson C, Wirth B, Sanford JR, Chen B. Splicing factor TRA2B is required for neural progenitor survival. J Comp Neurol. 2014;522:372‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]


