Abstract
Malignant mesothelioma (MM) is a rare but socially important neoplasm due to its association with asbestos exposure. Malignant mesothelioma is difficult to diagnose at an early stage, yet there are no particularly effective treatments available at the advanced stage, thus necessitating efficient strategies to prevent MM in individuals already exposed to asbestos. We previously showed that persistent oxidative damage caused by foreign body reaction and affinity of asbestos both to hemoglobin and histones is one of the major pathogeneses. Accordingly, as an effective strategy to prevent asbestos‐induced MM, we undertook the use of an iron chelator, deferasirox, which decreased the epithelial–mesenchymal transition in a crocidolite‐induced rat MM model. However, this agent may show adverse effects. Here, we studied the effects of iron removal by phlebotomy as a realistic measure on the same rat model. We injected a total of 5 mg crocidolite i.p. to F1 hybrid rats between the Fischer‐344 and Brown‐Norway strains at the age of 6 weeks. We repeated weekly or biweekly phlebotomy of 6‐8 mL/kg/time from 10 to 60 weeks of age. The animals were observed until 120 weeks. In male rats, phlebotomy significantly decreased the weight and nuclear grade of MM, and modestly reduced the associated ascites and the fraction of more malignant sarcomatoid subtype. Weekly phlebotomy prolonged long‐term survival. Our results indicate that appropriate phlebotomy may be a practical preventive measure to attenuate the initiation and promotion capacity of asbestos towards MM by reducing iron in individuals exposed to asbestos.
Keywords: asbestos, malignant mesothelioma, oxidative stress, phlebotomy, prevention
Abbreviations
- 8‐OHdG
8‐hydroxy‐2′‐deoxyguanosine
- HPF
high‐power field
- MDA
malondialdehyde
- MM
malignant mesothelioma
- NT
non‐therapeutic
- Phleb‐2
phlebotomy twice per month
- Phleb‐4
phlebotomy four times per month
- RBC
red blood cell
- ROS
reactive oxygen species
1. INTRODUCTION
Malignant mesothelioma (MM) is a neoplasm caused primarily by asbestos exposure.1, 2 In the 1960s, epidemiological studies firmly linked MM with asbestos exposure. Asbestos is one of the natural fibrous silicate minerals, consisting of several types including crocidolite, amosite, and chrysotile. The former two contain iron as ~30% of their components.1 Asbestos, as a mineral, presents characteristics such as acid‐, alkali‐, heat‐, and friction‐resistance with versatility and low cost for industrial use. Thus, it was used worldwide in the 20th century. Asbestos is now recognized as a human carcinogen; its use is legally prohibited in most developed countries, but it is not yet banned in developing countries.3 Because it takes 30‐40 years to develop MM after asbestos inhalation, the incidence of MM is expected to rise in the coming decades.4, 5 In Japan, the number of the patients will peak in 2025, and 100 000 new patients are expected in the coming 40 years.4 MM is one of the most aggressive tumors when diagnosed, and the median survival is expected to be 4‐18 months for pleural forms.2 Therefore, it is important to develop preventive intervention in high‐risk people exposed to asbestos. There are three histopathological subtypes (epithelioid, biphasic, and sarcomatoid) and various variants.2 Clinically, patients with the sarcomatoid subtype have the poorest prognosis with a remarkably short survival.6
The molecular mechanisms of asbestos‐induced mesothelial carcinogenesis have been clarified recently. Inhaled asbestos fibers are not digested by macrophages and, after passing through visceral pleura, eventually reach parietal pleura, where they initiate and promote mesothelial carcinogenesis. There are several distinct mechanisms in asbestos‐induced carcinogenesis, including oxidative stress (free radical generation), molecular adsorption (hemoglobin and histones), chromosome tangling, and chronic inflammation, which work in combination.7, 8, 9, 10 Excess iron both from adsorption (heme from hemoglobin) on an asbestos surface and from asbestos components catalyzes the Fenton reaction, generating hydroxyl radicals, which plays an important role in mesothelial carcinogenesis.11, 12, 13
As much as 60% of iron exists as heme in hemoglobin, which transports oxygen in mammals. Although iron plays an essential role in our body, excess iron is a risk for cancer, as exemplified by hepatocellular carcinoma in viral hepatitis B/C and ovarian cancer in ovarian endometriosis.12, 14, 15 In this context, iron removal indeed can reduce the risk of hepatic cancer,16, 17 and presumably of other cancers in humans.18 We previously reported that i.p. injection of iron saccharate, an in situ depositable form of iron, induced MM, in which the homozygous deletion of Cdkn2a/2b was observed in the sarcomatoid subtype.19 The same genetic alteration is observed in most (92.6%) of the asbestos‐induced peritoneal MM in rats.20 Homozygous deletion of CDKN2A (p16 INK4A) is one of the most common genetic alterations in human MM.21, 22 Moreover, we observed that repeated administration of nitrilotriacetate, an iron chelator to increase the catalytic activity of iron, significantly shortened the period of asbestos‐induced MM carcinogenesis, confirming the involvement of iron in this carcinogenesis.20 Therefore, we hypothesized that iron removal may reduce free radical generation even after exposure to asbestos fibers.
Iron chelators, such as deferasirox, deferoxamine, and deferiprone, are used to improve iron overload conditions in patients who received repeated blood transfusion.23, 24, 25 Recently, we reported that deferasirox and deferoxamine revealed preventive effects on asbestos‐induced MM carcinogenesis in rats.26, 27 However, these agents after long‐term use may present some adverse effects, including renal and hepatic dysfunction, as well as susceptibility to infectious diseases. Here, we evaluated the effects of iron removal by phlebotomy on crocidolite‐induced MM carcinogenesis in rats. Phlebotomy has been applied in Japan to patients with chronic active hepatitis C16, 17, 28, 29 and polycythemia vera.30 We found that phlebotomy significantly decreased the tumor weight, modestly prolonged survival, and reduced the fraction of the sarcomatoid subtype.
2. MATERIALS AND METHODS
2.1. Materials
Crocidolite was obtained from Union for International Control of Cancer (Geneva, Switzerland). All rats were Fischer‐344/Brown‐Norway F1 hybrids, which do not generate mesothelioma spontaneously.20 Fischer‐344 female rats and Brown‐Norway male rats were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan). The animal experiment committee of Nagoya University Graduate School of Medicine (Nagoya, Japan) approved these experiments.
2.2. Carcinogenesis experiments
Six‐week‐old male and female rats were injected twice with 1 mL of 2.5 mg/mL crocidolite suspension in saline (a total of 5 mg; male, n = 29; female, n = 16) or saline alone with 1‐week interval as untreated controls (male, n = 7; female, n = 10). One month after the initial injection of crocidolite (10 weeks old), we initiated the iron‐reduction procedures by performing phlebotomy. We divided the rats that received crocidolite into three groups: NT (non‐therapeutic; male, n = 10; female, n = 6); Phleb‐2 (biweekly treatment; male; n = 11; female, n = 5); and Phleb‐4 (weekly treatment; male, n = 8; female, n = 5). The rat group of saline alone with phlebotomy was omitted because the effects of phlebotomy on this group were already analyzed in our previous study.26 We calculated the appropriate volumes of phlebotomy as follows. For comparison, in human therapeutics: RBC turnover, 120 days; hemoglobin, 12‐14 g/dL; total blood volume (mL) = body weight (kg) × 80 (mL/kg);16, 31, 32 and phlebotomy as a second‐line therapy for chronic active hepatitis C as 400 mL/month comes to 400 mL/(60 × 80 mL) = 1/12 (with 60 kg as the average human body weight). Regarding adult rats:33, 34, 35 RBC turnover, 40‐60 days; hemoglobin, 14‐18 g/dL; total blood volume (mL) = body weight (kg) × 60 (mL/kg). The following calculation was performed using 12 g/dL human hemoglobin and 18 g/dL rat hemoglobin. For adult rats, (body weight × 60) × (1/12) × (18/12) × (120/40) = 22.5 × body weight mL/month ≈ 6 × body weight mL/time every week (calculated as 40 days of turnover), or (body weight × 60) × (1/12) × (18/12) × (120/60) = 15 × body weight mL/month ≈ 8 × body weight mL/time every other week (calculated as 60 days of turnover). Furthermore, we finely adjusted the dose of phlebotomy, considering the hematocrit and general condition of the rats. The male Phleb‐2 group was scheduled as follows: 6 mL/kg/time for weeks 10‐17 after birth, and 8 mL/kg/time for weeks 18‐60. The male Phleb‐4 group was scheduled as follows: 6 mL/kg/time for weeks 10‐17 after birth, and 7 mL/kg/time for weeks 18‐60. The female Phleb‐2 group was scheduled as follows: 6 mL/kg/time for weeks 10‐17 after birth, 7 mL/kg/time for weeks 18‐33, and 8 mL/kg/time for weeks 34‐60, based on lower body weight and hematocrit. The female Phleb‐4 group was scheduled as follows: 6 mL/kg/time for weeks 10‐33 after birth, and 7 mL/kg/time for weeks 34‐60. The hematocrit was used as an indicator, controlled appropriately, and there was no death attributable to phlebotomy. We collected a small amount of blood from all rats at weeks 6, 10, 20, 30, 40, 50, 60, 70, 80, and 90 to measure the hematocrit and serum iron. The rats were euthanized when ascites or more than 10% weight loss per week was observed. Ascites could be noticed by body weight gain and was ascertained by test puncture using a 21‐gauge syringe. The remaining rats were euthanized at week 120, and a complete autopsy of each rat was performed.
The hematocrit was measured by centrifuging a hematocrit tube whenever phlebotomy or blood collection was carried out. A blood examination using plasma was undertaken at week 50 with VetScan HM5 (Abaxis, Union City, CA, USA). Each phlebotomy and blood collection was carried out at approximately 2:00 pm. The rats were anesthetized with isoflurane during phlebotomy. The serum iron concentration was measured using the Fe C‐test Wako (Wako Pure Chemical Industries, Osaka, Japan).
2.3. Macroscopic and histopathological analysis of MM
Concerning macroscopic analysis, we observed the tumor mass/spread and recorded images of abdominal organs at autopsy. We collected all the excisable tumors and measured the total weight and size of the tumor and volume of ascites. Primary tumors (>10 mm) were observed on greater omentum, mesentery, and epididymal adipose tissues. Disseminated tumors (<1 mm) were scattered on peritoneal surface, and were collected whichever possible. In the analysis of the tumor weight, one male NT rat, one male Phleb‐4 rat, and one female Phleb‐2 rat were excluded because they were outliers. Nuclear grade was calculated as “nuclear atypia score plus mitotic counts score.” Nuclear atypia score was obtained as follows: 0, no malignancy; 1, low; 2, moderate; and 3, high. Mitotic counts in 10 HPF were scored as follows: 0, no malignancy; 1, 0‐4/10 HPF; 2, 5‐9/10 HPF; 3, 10‐19/10 HPF; and 4, ≥20/10 HPF. Hot spots (areas with the highest nuclear grade) in tumors were chosen for analysis.
Tissues were fixed in neutral‐buffered 10% formalin solution for 24 hours then washed with 70% ethanol and embedded in paraffin, from which 4‐μm sections were cut and stained with H&E. Three registered pathologists (K.Y., Ya.O., and S.T.) and one resident pathologist (Yu.O.) diagnosed each mesothelioma subtype as either epithelioid, biphasic, or sarcomatoid. We made a diagnosis of biphasic when either the epithelioid or sarcomatoid subtype occupied >10% of the tumor area with H&E staining. There are several variants in the epithelioid or sarcomatoid subtypes of MM.2 In our model, MMs were present with one or more variants, and we regarded them as a variant when they occupied >10% of the tumor area with H&E staining. Using these fractions, we analyzed the appearance of variants in the three groups (NT, Phleb‐2, and Phleb‐4). All the images were obtained using an Olympus microscope (BX53; Tokyo, Japan; objective lens; UPLSAPO) and a DP22/U‐TV0.5XC‐3 camera (Olympus).
2.4. Analysis of serum oxygenated metabolites
Serum 8‐OHdG was measured using a highly sensitive ELISA kit for 8‐OHdG (Japan Institute for the Control of Aging, NIKKEN SEIL, Fukuroi, Japan). In the analysis of the 8‐OHdG, one male Phleb‐2 rat and one female NT rat were excluded as outliers. Serum MDA was measured using a TBARS (TCA Method) assay kit (Cayman Chemical, Ann Arbor, MI, USA).
2.5. Statistical analysis
The data were analyzed using spss Statistics 24.0 (IBM, Armonk, NY, USA) and GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). The cumulative survival rate (disease‐specific survival rate) was calculated for each group according to the Kaplan–Meier method and compared using the log‐rank test. Statistical significance between the two groups of interest was analyzed using the unpaired Student's t‐test, if not indicated otherwise. Mann–Whitney non‐parametric U‐test were used if indicated. If the data did not show normal distribution, correlation analysis was performed using non‐parametric method (Spearman's rank correlation coefficient). The results were shown as mean ± SEM except for where noted. A P‐value <.05 was considered significant. Using Grubbs’ test with GraphPad software QuickCalcs (https://www.graphpad.com/quickcalcs/), outliers were detected and excluded from the analysis.
3. RESULTS
3.1. Appropriate phlebotomy decreases iron stores without apparent adverse events
We started phlebotomy at the age of 10 weeks, 4 weeks after the asbestos injection. The phlebotomy was finely designed during the experiment to reduce body iron sufficiently but not to kill the rats (Section 2), based on our experience in previous experiments.26 The body weight of each rat was recorded every week. No significant weight loss was observed in any group (Figure 1A,B). We modified the phlebotomy, if necessary, and the hematocrit was maintained at a constant level but significantly lower than that of the NT control group (Figure 1C,D). In male rats, the hematocrit of Phleb‐2 was lower at 30‐50 weeks and that of Phleb‐4 was lower at 20‐60 weeks compared with NT (P < .05; Figure 1C). In female rats, the hematocrit of Phleb‐4 was lower than that of NT at week 20 and 30 (P < .05; Figure 1D), but the maximal phlebotomy of the safety dose did not reduce the hematocrit of Phleb‐2 (Figure 1D). We did not perform phlebotomy after 60 weeks because a decrease in hematocrit was observed, thought to be caused by crocidolite‐induced chronic inflammation or carcinogenesis. The serum iron level was measured at 20, 30, 40, 50, and 60 weeks, showing lower levels in the Phleb‐4 group than in the NT group during the whole period (P < .05; Figure 1E,F). We undertook blood tests with VetScan HM5 at week 50 (Table S1). The reduction of RBCs and hemoglobin by phlebotomy was constant and significant, whereas there was no significant difference in the hematocrit by this method at 50 weeks. Other common alterations were an increase in the mean corpuscular volume and red cell distribution width, which may be explained by the increase in reticulocytes, which are larger in volume than mature RBCs. No rat died from repeated phlebotomy, distress, or infectious diseases.
Figure 1.
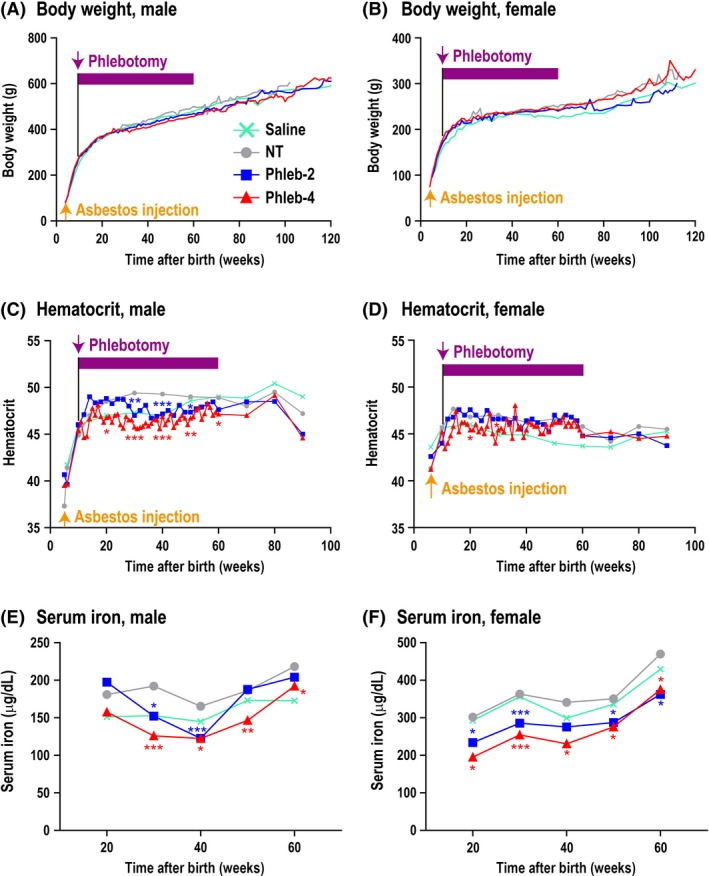
Effects of repeated long‐term phlebotomy in rats. A,B, Body weight. There was no significant weight loss in any group. C,D, Hematocrit. The hematocrit was maintained as low as possible. In female rats that underwent phlebotomy twice per month (biweekly; Phleb‐2), we could not reduce the hematocrit at the safe maximal phlebotomy dose. E,F, Serum iron. Rats that underwent phlebotomy four times per month (weekly; Phleb‐4) showed a low level. Serum was collected at ~2:00 pm. A‐D, Treatment groups: Saline (male, n = 7; female, n = 10); non‐therapeutic (NT; male, n = 10; female, n = 6); Phleb‐2 (male, n = 11; female, n = 5); and Phleb‐4 (male, n = 8; female, n = 5). E,F, Treatment groups: Saline (male, n = 5; female, n = 5), NT (male, n = 9; female, n = 5), Phleb‐2 (male, n = 11; female, n = 5); and Phleb‐4 (male, n = 8; female, n = 5). Means, *P < .05, **P < .01, ***P < .005. All groups were compared with the NT group by Student's t‐test. The saline group was not injected with asbestos, and the other groups were injected with 5 mg crocidolite
3.2. Sufficient phlebotomy prolongs disease‐specific survival by reducing tumor weight and ascites
Phlebotomy of the Phleb‐4 group revealed a tendency to prolong the disease‐specific survival rate after crocidolite injection only in male rats (P = .0539; Figure 2A), although this tendency was not clear in the other groups (Figure 2A,B). Alternatively, the survival fraction at week 103 of male Phleb‐2 + Phleb‐4 rats (7 alive and 12 dead) was significantly higher (P = .032; Fisher's exact test) than that of male NT rats (0 alive and 10 dead). Because there was no gender difference in rats’ lifespan, mixed survival rates of male and female rats was also calculated. Phleb‐2 treatment moderately (P = .0833) and Phleb‐4 treatment significantly (P = .0271) prolonged survival (Figure 2C). At autopsy, more than half of the rats that received crocidolite injections eventually developed ascites, which were generally bloody. We defined ≥5 g ascites as the presence of ascites, and judged that the rats with ascites caused by tumors died of hypovolemic shock, and the others died of cachexia or old age. In addition to the survival rate classified by gender (Figure 2A,B), the survival rate based on ascites was also analyzed (Figure 3). Interestingly, Phleb‐4 rats revealing ascites at autopsy showed significantly longer survival in comparison to NT rats (P = .0218).
Figure 2.
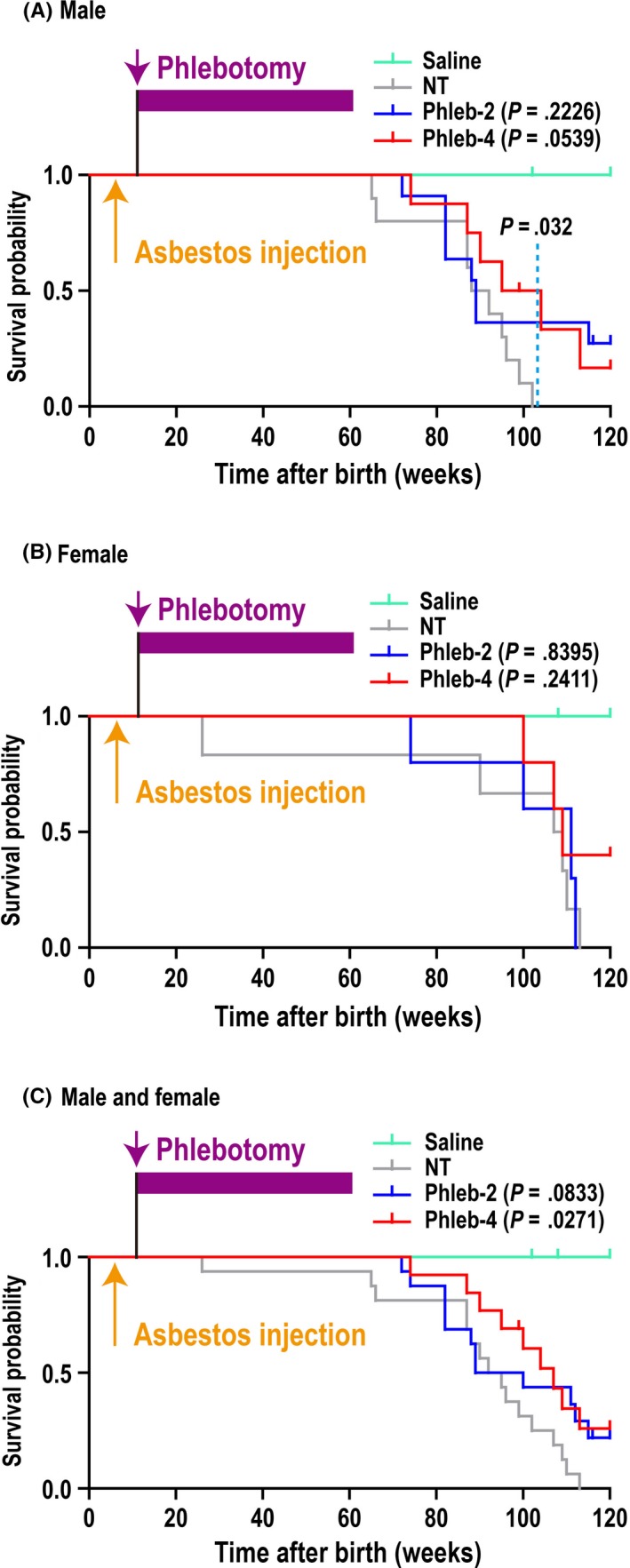
Effects of phlebotomy on the survival of crocidolite‐induced malignant mesothelioma (MM) in rats. A, Disease‐specific survival rate of male rats. Male rats that underwent phlebotomy four times per month (weekly; Phleb‐4) tended to survive longer than male rats injected with 5 mg crocidolite that did not receive phlebotomy (NT; P = .0539). The survival rate at week 103 of male rats that underwent phlebotomy twice per month (biweekly; Phleb‐2) + Phleb‐4 rats (7 alive and 12 dead) was higher (P = .032, Fisher's exact test) than that of male NT rats (0 alive and 10 dead). Phleb‐2: hazard ratio (HR), 0.539; 95% confidence interval (CI), 0.199‐1.460; Phleb‐4: HR, 0.342; 95% CI, 0.115‐1.020. B, Disease‐specific survival rate of female rats. Phleb‐2: HR, 0.872; 95% CI, 0.231‐3.290; Phleb‐4: HR, 0.441; 95% CI, 0.112‐1.73. C, Disease‐specific survival rate of male and female rats. Phleb‐2 rats tended to survive longer and Phleb‐4 rats survived longer than NT rats. Phleb‐2: HR, 0.498; 95% CI, 0.226‐1.100; Phleb‐4: HR, 0.393; 95% CI, 0.172‐0.900. Treatment groups: Saline (male, n = 7; female, n = 10); NT (male, n = 10; female, n = 6); Phleb‐2 (male, n = 11; female, n = 5); and Phleb‐4 (male, n = 8, female, n = 5). P‐values were determined using the log–rank test and HR and 95% CI were calculated, compared with the NT group. One male saline rat (102 wk), one male Phleb‐2 rat (116 wk), one male Phleb‐4 rat (99 wk), and one female Phleb‐2 rat (100 wk) died of senescence, and one female saline rat died of sarcoma (>100 mm); these were regarded as censored cases. Two male Phleb‐2 rats, one male Phleb‐4 rat, and two female Phleb‐4 rats survived until 120 wk; these were also regarded as censored cases, in which one male Phleb‐2 rat and one female Phleb‐4 rat showed MM with ascites at autopsy. These were included for macroscopic and histopathological analysis as with the other MM cases. All other rats died of MM
Figure 3.
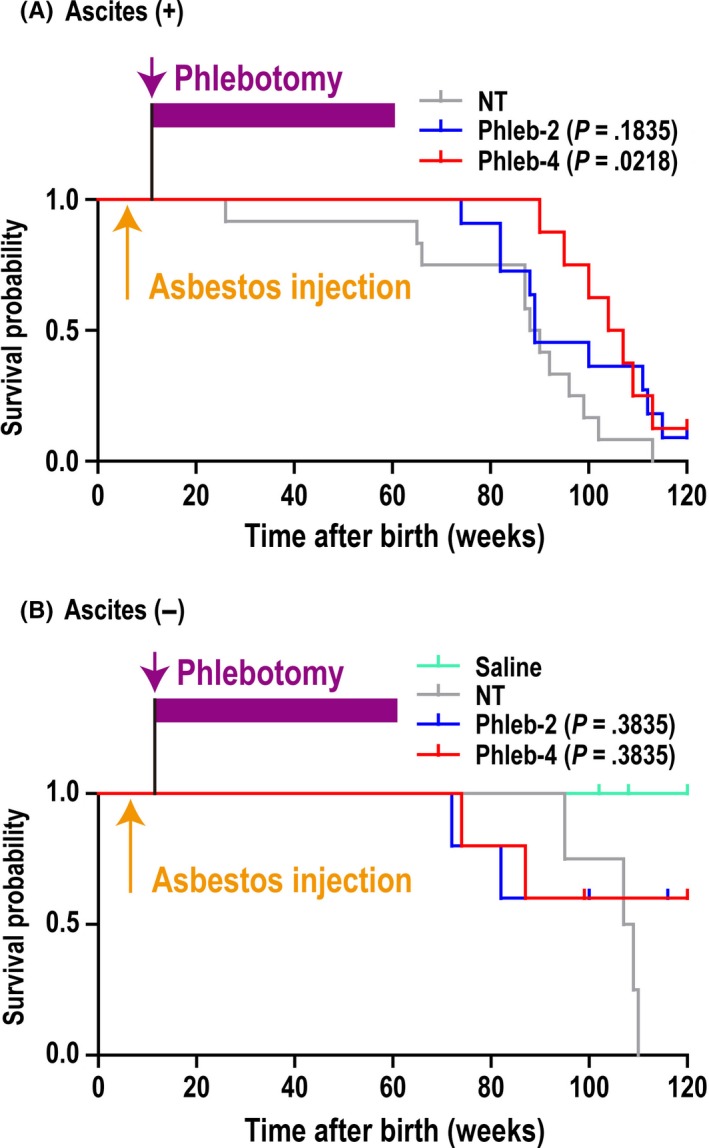
Disease‐specific survival rate in rats with crocidolite‐induced malignant mesothelioma, classified by the existence of ascites. Survival rate was analyzed based on the absence or presence of ascites, defined as ≥5 g ascites. A, Presence of ascites. Rats that underwent phlebotomy four times per month (weekly; Phleb‐4) survived significantly longer than rats that did not undergo phlebotomy (NT; P = .0218). Rats that underwent phlebotomy twice per month (biweekly; Phleb‐2): hazard ratio (HR), 0.546; 95% confidence interval (CI), 0.224‐1.330; Phleb‐4: HR, 0.315; 95% CI, 0.117‐0.845. B, Absence of ascites. There was no significant difference among the groups. Phleb‐2: HR, 0.486; 95% CI, 0.0956‐2.47; Phleb‐4: HR, 0.486; 95% CI, 0.0956‐2.47. Treatment groups: A, NT (male, n = 9; female, n = 3); Phleb‐2 (male, n = 7; female, n = 4); and Phleb‐4 (male, n = 4; female, n = 4). B, Saline (male, n = 7; female, n = 10); NT (male, n = 1; female, n = 3); Phleb‐2 (male, n = 4; female, n = 1); and Phleb‐4 (male, n = 4; female, n = 1)
At autopsy, MM were observed as distinct large nodules at various locations in the peritoneal cavity, including the hepatic surface, greater omentum, mesentery, and epididymal adipose tissue (Figure 4A). We collected and weighed the tumors and ascites. The tumor weight was significantly lower in male Phleb‐4 rats (P = .039; Figure 4B) than in male NT rats. In this analysis, one male NT rat (tumor weight, 59 g), one male Phleb‐4 rat (tumor weight, 49.8 g), and one female Phleb‐2 rat (tumor weight, 54.7 g) were excluded as outliers. Although the tumors were small in female Phleb‐4 rats, there was no significant difference between treatments (Figure 4B). The amounts of ascites tended to be lower in male Phleb‐4 rats than in male NT rats (Figure 4C). However, there was no significant difference in the other groups. Fisher's exact test on NT vs (Phleb‐2 + Phleb‐4) rats was carried out based on the presence or absence of ascites, which indicated that phlebotomy induced the tendency for the absence of ascites in male rats (Figure 4C).
Figure 4.
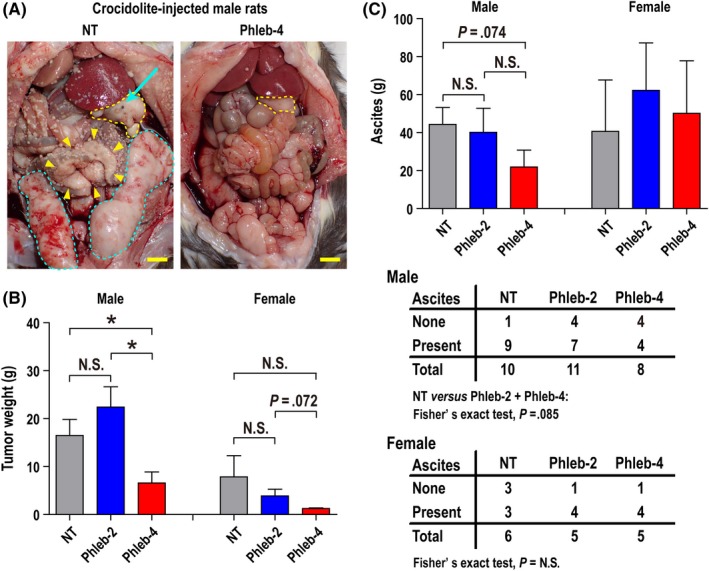
Phlebotomy reduces the tumor weight and ascites of crocidolite‐induced malignant mesothelioma (MM) in rats. A, Macroscopic findings at autopsy. Greater omentum (yellow dotted line), mesentery (yellow arrowheads), and epididymal adipose tissues (blue dotted line) were replaced by the primary tumor (MM) of non‐therapeutic (NT) rats (left). Countless disseminated minute tumors of MM were observed in the whole peritoneal cavity (left). Blue arrow indicates residual crocidolite on MM of the greater omentum (left). Only primary tumor was observed on the hepatic surface and greater omentum (right, yellow dotted line) and dissemination was not observed in rats that underwent phlebotomy four times per month (weekly; Phleb‐4; right). Scale bar = 10 mm. B, Tumor weight of male and female rats. Tumor weight was significantly lower in male Phleb‐4 rats than in male NT rats. C, Ascites of male and female rats. Male Phleb‐4 rats showed fewer ascites, resulting from phlebotomy. B, Treatment groups: NT (male, n = 9; female, n = 6); rats that underwent phlebotomy twice per month (biweekly; Phleb‐2; male, n = 11; female, n = 4); and Phleb‐4 (male, n = 7; female, n = 5). One male NT, one male Phleb‐4, and one female Phleb‐2 rat were excluded as outliers; mean ± SEM, *P < .05. All the groups were compared with the NT group by Student's t‐test. C, Treatment groups: NT (male, n = 10; female, n = 6); Phleb‐2 (male, n = 11; female, n = 5); and Phleb‐4 (male, n = 8, female, n = 5). Mean ± SEM, all groups were compared with the NT group by Student's t‐test except male NT vs Phleb‐2 or Phleb‐4 rats (Mann–Whitney U‐test). Refer to text for details
We observed three subtypes of MM with several variant histologies (Figure S1). In the phlebotomy groups, the number of rats with the biphasic subtype or with no tumor was increased (Figure 5A). The χ2‐test revealed that phlebotomy modestly reduced the fraction of the sarcomatoid subtype when we compared the non‐sarcomatoid vs sarcomatoid subtype in NT vs Phleb rats (P = .098; Figure 5B). Regarding histological variants, we observed more cases in the phlebotomy group with variant histology, especially the osteosarcomatoid variant (Figure S1i) belonging to the sarcomatoid subtype (Table S2). However, the solid variant (Figure S1d) in the epithelioid subtype, which may be a poor prognostic factor in humans,36 was reduced in the phlebotomy group. In addition, the well‐differentiated papillary variant (Figure S1b), a far better variant in prognosis than others,37 and no malignancy case (Figure S1a) were increased in the phlebotomy group. Phlebotomy significantly decreased the nuclear grade (P = .0456; Figure 6A,B). Nuclear grade was higher in solid and lower in well‐differentiated papillary variants (Table S3). There was a strong positive correlation between this grade and tumor weight (P < .0001, r s = .702; Figure 6C).
Figure 5.
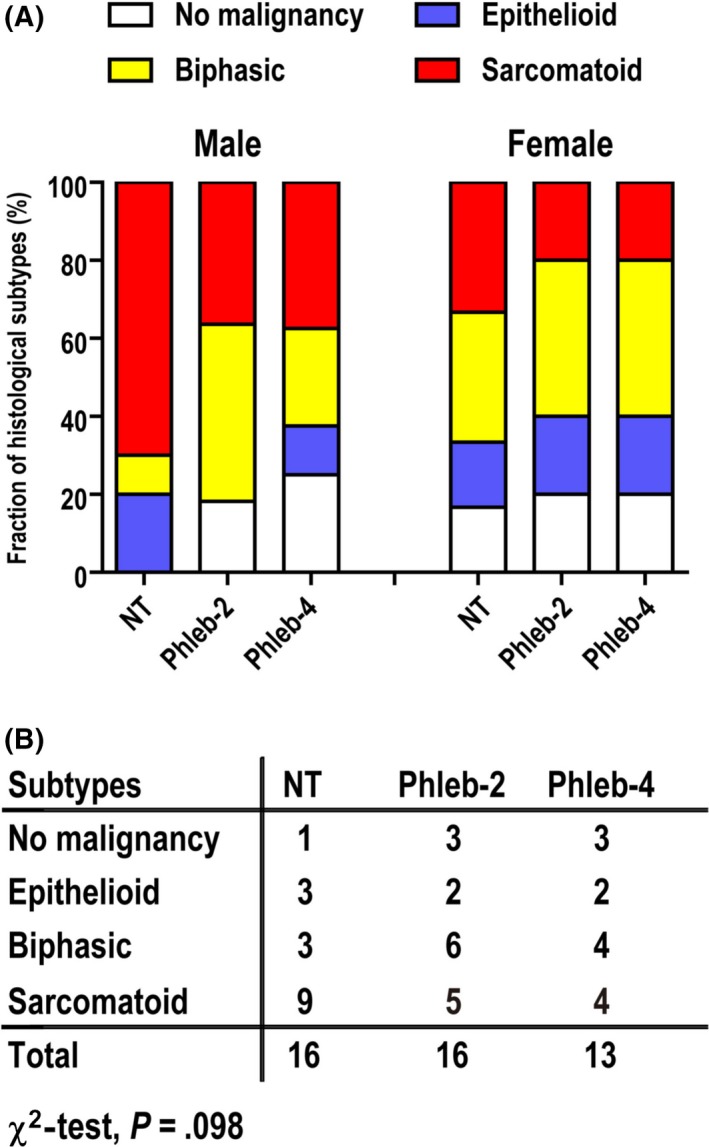
Phlebotomy influences the histopathology of crocidolite‐induced malignant mesothelioma (MM) in rats. A, Difference in the fraction of MM subtypes. The number of rats with the biphasic subtype and those without a tumor were increased among those that underwent phlebotomy four times per month (weekly; Phleb‐4) or twice per month (biweekly; Phleb‐2). B, Summary table of the fraction of MM subtypes. Phlebotomy modestly reduced the sarcomatoid subtype, comparing the non‐therapeutic (NT) vs Phleb groups for non‐sarcomatoid vs sarcomatoid groups. Analysis by the χ2‐test
Figure 6.
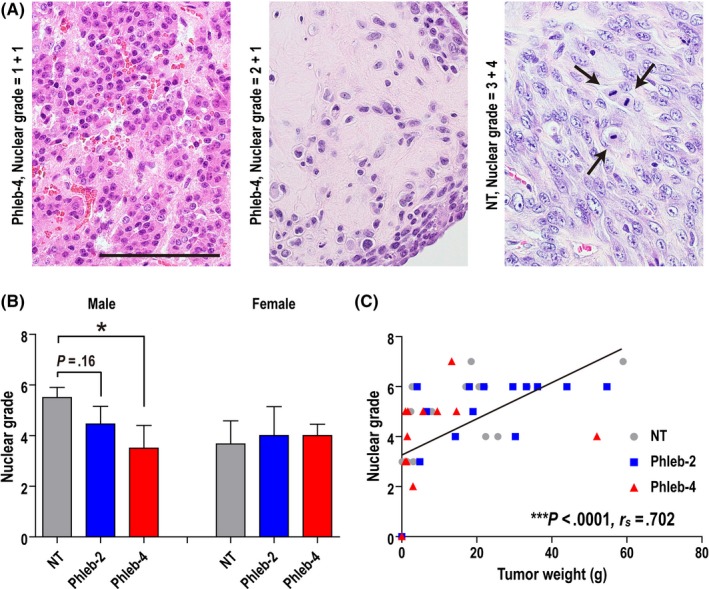
Phlebotomy reduces the nuclear grade of crocidolite‐induced malignant mesothelioma in rats. A, Assessment of nuclear grade using H&E staining. Nuclear grade = nuclear atypia score (0, no malignancy; 1, low; 2, moderate; 3, high) + mitotic counts score (0, no malignancy; 1, 0‐4/10 high‐power fields [HPF]; 2, 5‐9/10 HPF; 3, 10‐19/10 HPF; 4, ≥20/10 HPF). Left, nuclei are small and uniform in size. Center, nuclei are non‐uniform. Right, larger/non‐uniform nuclei with distinct nucleoli and frequent mitoses (arrows) are observed. Scale bar = 100 μm. (b) Nuclear grade was significantly reduced in male rats that underwent phlebotomy four times per month (weekly; Phleb‐4). Treatment groups: non‐therapeutic (NT; male, n = 10; female, n = 6); rats that underwent phlebotomy twice per month (biweekly; Phleb‐2; male, n = 11; female, n = 5); and Phleb‐4 (male, n = 8; female, n = 5). Mean ± SEM, *P < .05. All the groups were compared with the NT group by Student's t‐test. C, Correlation between tumor weight and nuclear grade. There was a strong positive correlation (***P < .0001; Spearman's rank correlation coefficient = .702)
We assumed that judging the phlebotomy effect would be difficult after mesothelial carcinogenesis. Because animal death from MM is apparently increased at 65‐70 weeks, 8‐OHdG and MDA in serum at week 50 were measured. In Phleb‐2, 8‐OHdG was significantly reduced, but the influence in Phleb‐4 was not clear (Figure S2a). Malondialdehyde was modestly reduced in male Phleb‐2 and Phleb‐4 rats (Figure S2b).
4. DISCUSSION
Asbestos‐induced MM is a social problem in various countries.1, 4 Because its carcinogenesis takes 30‐40 years based on epidemiological data, we have even more preventive room to intervene for those who were exposed to asbestos than those with other cancers. We previously showed that iron removal using an iron chelator, deferasirox, prevented the epithelial–mesenchymal transition and delayed the progression of tumors in rat MM.26 In the present study, we used crocidolite‐induced rat MM and intervention by finely tuned phlebotomy. We found that repeated phlebotomy decreased the total tumor weight (Figure 4B), ascites (Figure 4C), and the fraction of the sarcomatoid subtype (Figure 5). The survival rate was higher in the male phlebotomy group than the male NT group. These results are consistent with previous results of deferasirox26 but novel in that we used the low‐risk intervention of phlebotomy. Dissemination coexistent with the appearance of ascites is a condition in which MM invades the peritoneal cavity. Phlebotomy reduced the risk of progression to this condition. Because ascites and pleural effusion cause malaise and pulmonary dysfunction, it may improve the quality of life of patients.
However, we also obtained a contradictory result. Regarding tumor weight, Phleb‐2 revealed higher levels than NT in male rats, despite a lack of statistical significance (Figure 4B). Several rats in the Phleb‐2 group indeed showed prolonged survival (Figure 2A). In these rats, biweekly phlebotomy was probably not sufficient to attenuate the tumor progression because a low level of oxidative stress might activate cellular proliferation, angiogenesis, and metastasis in tumors.38, 39 Notably, there was a dose‐dependence of phlebotomy in the MM cases with ascites, which may suggest that iron removal is useful to suppress peritoneal dissemination at the progression stage.
We showed that phlebotomy reduced the solid variant in the epithelioid subtype and induced the osteosarcomatoid variant in the sarcomatoid subtype (Table S2). It was reported that high levels of ROS induce adipogenesis through Forkhead box O (FOXO), peroxisome proliferator‐activated receptor γ (PPARγ), and CCAAT‐enhancer‐binding protein signaling, whereas the absence of ROS induces osteogenesis through Wnt/β‐catenin, mitogen‐activated protein kinase (MAPK), and Hedgehog signaling in mesenchymal stem cells.40 We speculate that iron removal by phlebotomy reduces ROS produced by the Fenton reaction. However, local evaluation of oxidative stress in situ in the peritoneal cavity was difficult in the present study due to the mixed pathology of chronic peritoneal inflammation and mesothelial carcinogenesis with the present experimental design. Phlebotomy reduced serum 8‐OHdG in the Phleb‐2 group but not in the Phleb‐4 group, which might be affected by the acute inflammatory changes associated with repeated phlebotomy. However, we do not believe that phlebotomy‐induced distant local acute inflammation altered MM carcinogenesis in our study because the peritoneum is an anatomical location of relatively poor blood flow.
In a previous study, it was difficult to achieve volume control of phlebotomy, and many rats died from progressive anemia, allowing us to undertake phlebotomy for less than 20 weeks compared to the intervention using an oral iron chelator, deferasirox, for 50 weeks.26 Here, we determined the upper limit of phlebotomy as 8 mL/kg/time, aimed the lower hematocrit value in the Phleb group than the NT group, and controlled the volume. Male Phleb‐2 rats showed a tendency of an increase in hematocrit, especially at 50‐60 weeks old, which was thought to be a shortage of phlebotomy. However, increasing the amount of each phlebotomy was difficult because carcinogenesis was thought to start at ~60 weeks, when rats became sensitive to this intervention. In contrast, we could accomplish the target hematocrit value in male Phleb‐4 rats. Regarding female rats, we could not achieve the target hematocrit value, due to lower body weight and hematocrit than males. Notwithstanding, the levels of serum iron in female phlebotomized rats were lower than those in the NT group during the whole period. The levels of serum iron in females were conversely higher than males, confirming our previous study.26 The effects of phlebotomy on tumor weight and survival were more significant in male than in female rats. These results suggest that the hematocrit value might reflect the production of ROS accompanied with hemoglobin adsorption11, 12, 13 more precisely than the serum iron levels. Therefore, hematocrit or hemoglobin levels of the non‐therapeutic status could be clinical markers when preventive phlebotomy is applied to humans.
No rat died from the overbalance of phlebotomy, distress, or infectious diseases in the present experiment, indicating that appropriate phlebotomy was carried out. There are some reports that a high neutrophil‐to‐lymphocyte ratio is associated with poor survival in patients with some tumors41, 42 or chronic distress in rats.43 Although there were no significant differences, the average neutrophil‐to‐lymphocyte ratio of Phleb‐2 was higher and that of Phleb‐4 was lower in rats of both sexes than that of the NT groups (Table S1). The result of Phleb‐2 may indicate distress from phlebotomy, whereas that of Phleb‐4 may indicate the effect of phlebotomy. Practically, in human patients, changing the puncture sites and finer procedures would be possible. Serum ferritin is a good marker for iron stores in humans, but we did not obtain a significant reduction of serum ferritin (data not shown), presumably because rats are more sensitive to repeated phlebotomy.
A limitation in this study is that the appropriate volume of phlebotomy in rats was still unclear, especially in female rats. We could not decrease the hematocrit of female rats by phlebotomy as expected; this was thought to be the reason why the tumor weight and survival of the female phlebotomized groups were not significantly affected. A relatively low number of animals in each group is a limitation of this study, although these numbers have not been uncommon in phlebotomy studies.26, 28, 44
In conclusion, the present preclinical study suggests that appropriate phlebotomy is effective as a preventive measure for asbestos‐induced MM carcinogenesis, regarding tumor size, ascites, histology, and disease‐specific survival. At the very least, it delays tumor progression. Phlebotomy is a relatively safe intervention in humans and has been shown to decrease visceral cancer incidence and mortality in humans.18 Although the preventive effects in this study were observed only in male rats, we believe that this does not prevent using this procedure in female humans, because iron metabolism in female rats that lack bleeding menstruation appears different from that in humans. If the high‐risk individuals are not iron‐deficient, we recommend considering regular phlebotomy. Clinical trials are necessary for confirmation of the present results.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Mr. Nobuaki Misawa, Mr. Daiki Somiya, Ms. Tomomi Aoyama, and Ms. Naomi Tagami (Nagoya University Graduate School of Medicine) for technical assistance. This work was supported, in part, by the Japan Society for the Promotion of Science (Kakenhi grant nos. JP17H04064, JP221S0001‐04, and JP24108008) and a Private University Research Branding Project to Shinya Toyokuni. Yuuki Ohara was a recipient of a Takeda Science Foundation Fellowship (April 2014‐March 2018).
Ohara Y, Chew S‐H, Shibata T, Okazaki Y, Yamashita K, Toyokuni S. Phlebotomy as a preventive measure for crocidolite‐induced mesothelioma in male rats. Cancer Sci. 2018;109:330–339. https://doi.org/10.1111/cas.13460
REFERENCES
- 1. IARC, WHO . Asbestos (Chrysotile, Amosite, Crocidolite, Tremolite, Actinolite, and Anthophyllite). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens; Part C: Arsenic, Metals, Fibres, and Dusts. Lyon, France: IARC, WHO; 2012:219‐309. [Google Scholar]
- 2. Oury TD, Roggli VL, Sporn TA, eds. Pathology of Asbestos‐Associated Diseases, 3rd edn New York, NY: Springer Verlag; 2014. [Google Scholar]
- 3. Le GV, Takahashi K, Karjalainen A, et al. National use of asbestos in relation to economic development. Environ Health Perspect. 2010;118:116‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591‐1603. [DOI] [PubMed] [Google Scholar]
- 5. Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer. 2005;92:587‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol. 2012;7:1631‐1639. [DOI] [PubMed] [Google Scholar]
- 7. Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagai H, Toyokuni S. Biopersistent fiber‐induced inflammation and carcinogenesis: lessons learned from asbestos toward safety of fibrous nanomaterials. Arch Biochem Biophys. 2010;502:1‐7. [DOI] [PubMed] [Google Scholar]
- 9. Chew SH, Toyokuni S. Malignant mesothelioma as an oxidative stress‐induced cancer: An update. Free Radic Biol Med. 2015;86:166‐178. [DOI] [PubMed] [Google Scholar]
- 10. Nagai H, Toyokuni S. Differences and similarities between carbon nanotubes and asbestos fibers during mesothelial carcinogenesis: shedding light on fiber entry mechanism. Cancer Sci. 2012;103:1378‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagai H, Ishihara T, Lee WH, et al. Asbestos surface provides a niche for oxidative modification. Cancer Sci. 2011;102:2118‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toyokuni S, Ito F, Yamashita K, Okazaki Y, Akatsuka S. Iron and thiol redox signaling in cancer: an exquisite balance to escape ferroptosis. Free Radic Biol Med. 2017;108:610‐626. [DOI] [PubMed] [Google Scholar]
- 14. Stevens RG, Jones DY, Micozzi MS, Taylor PR. Body iron stores and the risk of cancer. N Engl J Med. 1988;319:1047‐1052. [DOI] [PubMed] [Google Scholar]
- 15. Kato J, Kobune M, Kohgo Y, et al. Hepatic iron deprivation prevents spontaneous development of fulminant hepatitis and liver cancer in Long‐Evans Cinnamon rats. J Clin Invest. 1996;98:923‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kato J, Kobune M, Nakamura T, et al. Normalization of elevated hepatic 8‐hydroxy‐2′‐deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697‐8702. [PubMed] [Google Scholar]
- 17. Kato J, Miyanishi K, Kobune M, et al. Long‐term phlebotomy with low‐iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830‐836. [DOI] [PubMed] [Google Scholar]
- 18. Zacharski LR, Chow BK, Howes PS, et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996‐1002. [DOI] [PubMed] [Google Scholar]
- 19. Hu Q, Akatsuka S, Yamashita Y, et al. Homozygous deletion of CDKN2A/2B is a hallmark of iron‐induced high‐grade rat mesothelioma. Lab Invest. 2010;90:360‐373. [DOI] [PubMed] [Google Scholar]
- 20. Jiang L, Akatsuka S, Nagai H, et al. Iron overload signature in chrysotile‐induced malignant mesothelioma. J Pathol. 2012;228:366‐377. [DOI] [PubMed] [Google Scholar]
- 21. Xio S, Li D, Vijg J, Sugarbaker DJ, Corson JM, Fletcher JA. Codeletion of p15 and p16 in primary malignant mesothelioma. Oncogene. 1995;11:511‐515. [PubMed] [Google Scholar]
- 22. Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108‐2113. [PubMed] [Google Scholar]
- 23. Takatoku M, Uchiyama T, Okamoto S, et al. Retrospective nationwide survey of Japanese patients with transfusion‐dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol. 2007;78:487‐494. [DOI] [PubMed] [Google Scholar]
- 24. Lindsey WT, Olin BR. Deferasirox for transfusion‐related iron overload: a clinical review. Clin Ther. 2007;29:2154‐2166. [DOI] [PubMed] [Google Scholar]
- 25. Guariglia R, Martorelli MC, Villani O, et al. Positive effects on hematopoiesis in patients with myelodysplastic syndrome receiving deferasirox as oral iron chelation therapy: a brief review. Leuk Res. 2011;35:566‐570. [DOI] [PubMed] [Google Scholar]
- 26. Nagai H, Okazaki Y, Chew SH, Misawa N, Yasui H, Toyokuni S. Deferasirox induces mesenchymal‐epithelial transition in crocidolite‐induced mesothelial carcinogenesis in rats. Cancer Prev Res. 2013;6:1222‐1230. [DOI] [PubMed] [Google Scholar]
- 27. Jiang L, Chew SH, Nakamura K, Ohara Y, Akatsuka S, Toyokuni S. Dual preventive benefits of iron elimination by desferal in asbestos‐induced mesothelial carcinogenesis. Cancer Sci. 2016;107:908‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hayashi H, Takikawa T, Nishimura N, Yano M, Isomura T, Sakamoto N. Improvement of serum aminotransferase levels after phlebotomy in patients with chronic active hepatitis C and excess hepatic iron. Am J Gastroenterol. 1994;89:986‐988. [PubMed] [Google Scholar]
- 29. Yano M, Hayashi H, Wakusawa S, et al. Long term effects of phlebotomy on biochemical and histological parameters of chronic hepatitis C. Am J Gastroenterol. 2002;97:133‐137. [DOI] [PubMed] [Google Scholar]
- 30. Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk‐stratification, and management. Am J Hematol. 2017;92:94‐108. [DOI] [PubMed] [Google Scholar]
- 31. Greer JP, Arber DA, Glader B, et al. Wintrobe's Clinical Hematology, 12th edn Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 32. Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J. Harrison's Principles of Internal Medicine, 19th edn New York, NY: McGraw‐Hill Professional; 2015. [Google Scholar]
- 33. Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72‐76. [PubMed] [Google Scholar]
- 34. Matsuzawa T, Nomura M, Unno T. Clinical pathology reference ranges of laboratory animals. Working Group II, Nonclinical Safety Evaluation Subcommittee of the Japan Pharmaceutical Manufacturers Association. J Vet Med Sci. 1993;55:351‐362. [DOI] [PubMed] [Google Scholar]
- 35. Burns KF, De Lannoy CW Jr. Compendium of normal blood values of laboratory animals with indication of variations. I. Random‐sexed populations of small animals. Toxicol Appl Pharmacol. 1966;8:429‐437. [DOI] [PubMed] [Google Scholar]
- 36. Cerruto CA, Brun EA, Chang D, Sugarbaker PH. Prognostic significance of histomorphologic parameters in diffuse malignant peritoneal mesothelioma. Arch Pathol Lab Med. 2006;130:1654‐1661. [DOI] [PubMed] [Google Scholar]
- 37. Butnor KJ, Sporn TA, Hammar SP, Roggli VL. Well‐differentiated papillary mesothelioma. Am J Surg Pathol. 2001;25:1304‐1309. [DOI] [PubMed] [Google Scholar]
- 38. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. North S, Moenner M, Bikfalvi A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 2005;218:1‐14. [DOI] [PubMed] [Google Scholar]
- 40. Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 2015;24:1150‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil‐lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181‐184. [DOI] [PubMed] [Google Scholar]
- 42. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst. 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- 43. Swan MP, Hickman DL. Evaluation of the neutrophil‐lymphocyte ratio as a measure of distress in rats. Lab Anim. 2014;43:276‐282. [DOI] [PubMed] [Google Scholar]
- 44. Mizote A, Hida AI, Hosako M, Fujisawa M, Kamekawa M, Okada S. Effects of phlebotomy on the growth of ferric nitrilotriacetate‐induced renal cell carcinoma. Acta Med Okayama. 2002;56:199‐204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


