Abstract
Podoplanin (PDPN) is expressed on many tumors and is involved in tumor metastasis. The objective of the present study was to develop an ELISA for determining soluble PDPN (sPDPN) levels as a potential novel tumor marker in plasma of patients with cancers for detection of tumor occurrence and metastasis. Mouse monoclonal antibodies (mAb) against human PDPN were developed and characterized. Two anti‐PDPN mAb, SZ‐163 and SZ‐168, were used in a sandwich ELISA to detect plasma sPDPN in patients with cancers and in normal individuals. The levels of sPDPN were detected in patients with adenocarcinoma (87 cases, 31.09 ± 5.48 ng/ml), squamous cell carcinoma (86 cases, 6.91 ± 0.59 ng/ml), lung cancer (45 cases, 26.10 ± 7.62 ng/ml), gastric cancer (38 cases, 23.71 ± 6.90 ng/ml) and rectal cancer (27 cases, 32.98 ± 9.88 ng/ml), which were significantly higher than those in normal individuals (99 cases, 1.31 ± 0.13 ng/ml) (P < .0001). Moreover, the sPDPN levels in patients with metastatic cancers were higher (192 cases, 30.35 ± 3.63 ng/ml) than those in non‐metastatic cancer patients (92 cases, 6.28 ± 0.77 ng/ml) (P < .0001). The post‐treatment sPDPN levels of cancer patients (n = 156) (4.47 ± 0.35 ng/ml) were significantly lower compared with those seen pre‐treatment (n = 128) (43.74 ± 4.97 ng/ml) (P < .0001). These results showed that an ELISA method was successfully established for quantitation of plasma sPDPN and plasma sPDPN levels correlate significantly with tumor occurrence and metastasis.
Keywords: ELISA, monoclonal antibody, soluble podoplanin, tumor metastasis, tumor occurrence
1. INTRODUCTION
Podoplanin (PDPN), also known as aggrus, is a type‐I transmembrane sialoglycoprotein that is highly expressed in lymphatic endothelial cells, but not vascular endothelial cells.1 PDPN is also expressed in many tumor cells, including squamous cell carcinoma,2, 3, 4 malignant mesothelioma,5, 6 Kaposi's sarcoma,7 angiosarcoma,1 hemangioblastoma,8 testicular seminoma,9 dysgerminoma2 and brain tumors.10 PDPN is composed of 3 structural domains: a highly O‐glycosylated extracellular domain, a hydrophobic transmembrane domain, and a short cytoplasmic domain.11, 12 PDPN induces platelet aggregation through direct binding of its extracellular domain to C‐type lectin‐like receptor 2 (CLEC‐2) expressed on the platelet surface.13 Thus, PDPN plays an important role in lymphatic vascular development and in cancer metastasis through interaction with CLEC‐2.14, 15 PDPN is also expressed in cancer‐associated fibroblasts (CAF) where it facilitates lung adenocarcinoma cell invasion by activating the Rho‐ROCK pathway via its cytoplasmic domain.16
In addition to the form of PDPN expressed on the cell surface, a soluble form of PDPN (sPDPN) has recently been detected and investigated. Carrasco‐Ramirez et al report that cells ectopically or endogenously expressing PDPN release membrane‐shed micro‐vesicles (MV) and endosomal‐derived exosomes (EXO) that contain PDPN mRNA and protein. In vitro study suggested a crucial role of PDPN in EXO and MV production and/or release and for PDPN‐EXO in tumorigenesis.17 Those PDPN‐EXO may account for PDPN in circulating fluids in vivo because PDPN+ microparticles were identified in pleural effusions of both cancer and benign origin in humans.18 However, given the expression of PDPN in tumors, sPDPN is a promising biomarker because of the potential ease of its measurement in serum. To this end, Sankiewicza et al used surface plasmon resonance to quantitate sPDPN in the serum and urine of bladder cancer patients and healthy volunteers and found that the PDPN levels in the patient samples were higher than that of the healthy controls.19
To date, several anti‐PDPN monoclonal antibodies with high sensitivity and specificity have been developed. Among these, NZ‐1 reacts with the platelet aggregation‐inducing (PLAG) domain of human PDPN, thereby inhibiting PDPN‐induced platelet aggregation in vitro and suppressing cancer metastasis in vivo.20, 21 Immunohistochemistry conducted using another PDPN antibody, D2‐40, has been used to detect tumor metastasis and significantly increased the detection frequency of lymphatic invasion compared to the use of conventional HE staining in squamous cell carcinoma.22 However, few antibodies targeting sPDPN have been generated, and both immunohistochemistry and surface plasmon resonance are relatively difficult and expensive analyses.
Therefore, we recently developed 2 novel mouse mAb against human PDPN (SZ163 and SZ168) and established a double‐antibody sandwich ELISA, which we then used for quantitation of sPDPN in the plasma of 283 cancer patients and 99 normal controls. The results show that plasma sPDPN could be a novel tumor marker for detection and diagnosis of both tumor occurrence and metastasis. Moreover, our double‐antibody sandwich ELISA is a highly desirable screening method for biomarkers because of its relatively low cost and minimal invasiveness.
2. MATERIALS AND METHODS
2.1. Plasma collection from cancer patients or normal individuals
The study conformed to the ethical guidelines of the 2004 Declaration of Helsinki and was approved by the Institutional Ethics Committee at the First Affiliated Hospital of Soochow University, China and Luoyang Central Hospital Affiliated to Zhengzhou University, China. Informed consent was obtained from all normal individuals and patients. 2 ml samples of blood were collected by vein puncture into tubes containing 3.6 mg ethylene diaminetetraacetic acid. Plasma was prepared by centrifugation at 1500 g for 5 minute before storage at −80°C. As a control, plasma was collected from 100 normal individuals with various ABO blood groups. Blood samples from patients with adenocarcinoma (87 cases), squamous cell carcinoma (86 cases), lung cancer (45 cases), gastric cancer (38 cases) or rectal cancer (27 cases) were collected from the First Affiliated Hospital of Soochow University and Luoyang Central Hospital Affiliated to Zhengzhou University. Specifically, the 87 cases of adenocarcinomas consisted of 3 origins: gastric (32 cases), lung (31 cases) and rectal (24 cases). The 86 cases of squamous cell carcinomas consisted of 7 origins: esophageal (36 cases), lung (25 cases), cervical (15 cases), gastric (4 cases), nasopharyngeal (3 cases), rectal (2 cases) and neck (1 case). Criteria for diagnosis of cancer were derived from the recommendations of The Union for International Cancer Control.
2.2. Animals and cell lines
Female BALB/c mice (8 weeks old) were purchased from Shanghai SLRC Experimental Animal (Shanghai, China). The Chinese hamster ovary (CHO) cell line, CHO cells ectopically expressing PDPN (CHO‐PDPN),23 and the U87 astroglioma cell line were stored in our laboratory. The NCI‐H226 lung squamous cell line was purchased from Jiangsu KeyGEN BioTECH (Nanjing, China).
2.3. Cell culture
Chinese hamster ovary, CHO‐PDPN and U87 cells were cultured in DMEM (Hyclone, Logan, Utah, America), supplemented with 10% FBS (Gibco, Carlsbad, CA, USA). NCI‐H226 cells were cultured in RMPI‐1640 medium (Gibco), supplemented with 10% FBS (Gibco).
2.4. Polypeptide synthesis and coupling
A 22‐amino‐acid polypeptide, DTETTGLEGGVAMPGAEDDVVC, was synthesized and coupled with keyhole limpet hemocyanin (KLH) by Shanghai Ziyu Biotechnology. (Shanghai, China). The following 21 amino acids, DTETTGLEGGVAMPGAEDDVV, correspond to amino acids 31‐51 of human PDPN. The interaction with CLEC‐2 was primarily observed at Glu47 and Asp48 in the platelet aggregation‐stimulating (PLAG) domain (amino acids 29‐54) and the α2‐6 linked sialic acid at Thr52 of hPDPN.24 The sequence of our ploypeptide was chosen within the PLAG domain, for the purpose of developing mAb targeting the PDPN‐CLEC‐2 interaction with potential therapeutic application against cancer metastasis.
2.5. Hybridoma production and antibody purification
Female BALB/c mice were immunized by subcutaneous injection of DTETTGLEGGVAMPGAEDDVVC‐KLH (100 μg/100 μl/mouse), with 100 μl/mouse of Freund's complete adjuvant. A secondary subcutaneous immunization was performed 4 weeks later, followed by a final injection given by tail vein an additional 4 weeks later. The animal experiments were approved by the Animal Ethics Committee of Soochow University, Suzhou, China. Murine anti‐human PDPN mAb were developed using standard hybridoma technology. The supernatants from positive clones were screened by ELISA for binding to the synthetic peptide and to CHO‐PDPN cells immobilized on 96‐well plates. The isotype of the mAb was determined using immune double diffusion. The IgG was purified from ascites using a protein G‐Sepharose 4B column (Amersham Biosciences, Buckinghamshire, UK).
2.6. ELISA screening of positive monoclonal antibody clones
The 22‐amino‐acid polypeptide or CHO‐PDPN cells were immobilized on 96‐well plates at 1 μg/ml or 4 × 104 cells/ml, respectively, using 50 μl of solution per well, at 4°C overnight. The wells coated with the peptide were washed with PBS containing 0.05% Tween‐20 (PBST) to remove non‐adsorbed antibodies and then blocked with 2% (w/v) BSA in PBS for 2 hours at 37°C. The wells coated with the cells were fixed with 4% paraformaldehyde for 30 minute at room temperature, washed with PBST, blocked with 2% BSA, and incubated for 2 hours at 37°C. After washing, the plates were incubated with hybridoma supernatant or SZ163 and SZ168, which are two anti‐human PDPN mAb we generated by synthetic peptide immunization and hybridoma screening as described in the methods, at various concentrations (1000, 500, 250, 125, 62.5 and 31.25 ng/ml) for 2 hours at 37°C. After washing, the bound mAb were then incubated with peroxidase‐conjugated goat anti‐mouse IgG for 1 hour at 37°C. After further washing, the enzymatic reaction was conducted with a substrate solution containing tetramethylbenzidine (TMB) (Thermo Fisher, Waltham, MA, USA). The reaction was stopped with 50 μl/well of 2 mol/L H2SO4, and the optical density was measured at 450 nm using a microplate reader (Thermo Fisher).
2.7. Analysis of human podoplanin expression by flow cytometry
The specificity of the anti‐PDPN mAb were characterized by flow cytometry. Cultured U87, NCI‐H226 or CHO‐PDPN cells were collected by trypsin‐EDTA treatment and incubated with 18H5 as a positive control (Abcam, Cambridge, UK), mouse IgG as a negative control, or anti‐PDPN antibodies (SZ163 and SZ168, 2 μg/tube) for 1 hour at room temperature. The cells were then incubated with PE‐goat anti‐mouse antibody (Invitrogen, Carlsbad, CA, USA) at room temperature for 30 minute. Flow cytometry was performed using FACSCalibur (BD Biosciences, San Jose, CA, USA).
2.8. Western blot analysis
Cells were solubilized using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) and the lysates were separated in reduced SDS‐PAGE followed by transfer to a PVDF membrane (Millipore, Darmstadt, Germany). After blocking with 8% skim milk in 0.1% PBST, the membrane was probed with either SZ‐163 or SZ‐168 (3 μg/ml in PBS + 0.1% Tween 20) for 2 hours at room temperature. Specifically bound primary antibodies were detected with HRP‐conjugated goat anti‐mouse IgG (Immunotech, Marseille, France) and ECL substrate (Sigma‐Aldrich, St. Louis, MO, USA) according to the manufacturers’ instructions.
2.9. Analysis of plasma soluble podoplanin by ELISA
One hundred microliter of 10 μg/ml of SZ‐163 mAb IgG was coated into a 96‐well microtiter plate overnight at 4°C. The wells were washed with PBST to remove non‐adsorbed antibody and then blocked with 2% (w/v) BSA in PBS for 2 hours at 37°C. After washing, the plates were incubated with 100 μl of the recombinant human PDPN‐Fc (R&D Systems, Minneapolis, MN, USA) (125, 62.5, 31.25, 15.63, 7.82, 3.91 and 1.95 ng/ml) as the standard and plasma samples for 2 hours at 37°C. After washing, bound sPDPN was incubated with HRP‐conjugated SZ‐168 IgG (HRP‐SZ‐168) for 1 hour at 37°C and the binding of HRP‐SZ‐168 was detected with TMB.
2.10. Statistical analyses
Statistical analysis was performed using SPSS 19.0 statistical software (SPSS, Chicago, IL, USA). Data were expressed as the mean ± SD. The nonparametric test was used to compare the results obtained from the cancer patients and normal individuals, and between the metastatic and non‐metastatic groups of cancer patients. Differences among groups were considered as statistically significant if the P‐value was ≤.05.
3. RESULTS
3.1. Production and characterization of anti‐podoplanin monoclonal antibodies
A total of 8 anti‐PDPN mAb (named SZ‐161 to SZ‐168) were obtained. Both SZ‐163 and SZ‐168 were identified as IgG1 subtypes and both antibodies bound specifically with the synthetic PDPN peptide (Figure 1A). Furthermore, immunoblotting indicated that both SZ‐163 and SZ‐168 bound to the human recombinant protein PDPN‐Fc (67 kDa), and to endogenous PDPN (46 or 36 kDa depending on glycosylation status) in U87 astroglioma cells, NCL‐H226 lung squamous cell and CHO‐PDPN cells (Figure 1B). In addition, flow cytometric analyses indicated that SZ163 and SZ168 recognized PDPN expressed on the tumor cell surface, although SZ168 appeared to have a higher affinity (Figure 1A,C).
Figure 1.

SZ163 and SZ168 monoclonal antibodies (mAb) specifically recognized human podoplanin (PDPN). A, ELISA analysis of SZ163 and SZ168 binding to recombinant human PDPN‐Fc. SZ‐163 and SZ‐168 were incubated with coated, recombinant human PDPN‐Fc (2 μg/ml). Specific binding was detected with HRP‐conjugated goat anti‐mouse IgG antibody. B, Immunoblotting assay using SZ‐163 and SZ‐168. U87, NCI‐H226 or CHO‐PDPN cell lysates, and recombinant human PDPN‐Fc were separated on 10% reduced SDS‐PAGE and then immunoblotted with different PDPN antibodies including SZ‐163 or SZ‐168. C, Flow cytometric analyses of SZ163 and SZ168 binding to PDPN expressed on the cell surface. U87, NCI‐H226 and CHO‐PDPN or CHO cells were incubated with SZ163, SZ168, 18H5 (positive control) or mouse IgG (negative control), then phycoerythrin (PE)‐conjugated goat anti‐mouse IgG for detecting PDPN. The rPDPN‐Fc = recombinant human PDPN/Fc fusion protein
3.2. Plasma soluble podoplanin levels in normal individuals and cancer patients
A dilution series of PDPN‐Fc from 125 to 1.95 ng/ml was created to assess the function of the SZ163 and SZ168 antibodies in ELISA. Plotting the OD450 nm versus the PDPN‐Fc levels resulted in a binding curve of PDPN‐Fc to SZ163 and HRP‐SZ168. A curve in a semi‐log scale was obtained by plotting optical density versus PDPN‐Fc (Figure 2A). The linear regression gave a correlation of 0.99. According to the ELISA results, the sPDPN levels in the plasma of normal individuals (n = 99) ranged from 0.52 to 8.67 ng/ml, while that in the plasma of cancer patients (n = 284) ranged from 0.52 to 185.40 ng/ml. The average sPDPN level in the cancer patients (21.95 ± 2.49 ng/ml) was significantly higher than that in the normal individuals (1.33 ± 0.14 ng/ml) as compared by the Mann‐Whitney test analyses (P < .0001) (Figure 2B).
Figure 2.
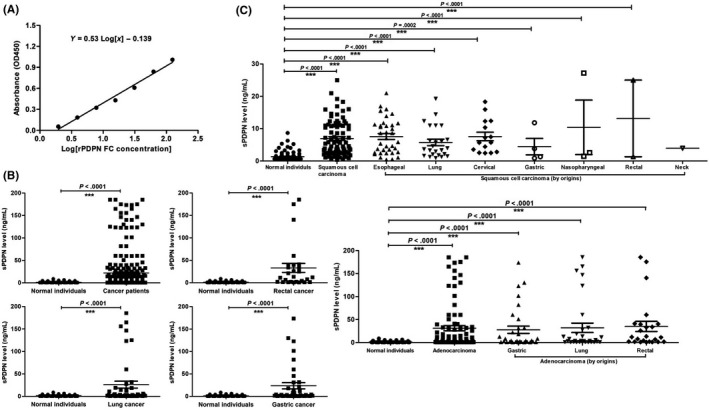
Establishment of a double‐antibody sandwich ELISA and detection of plasma soluble podoplanin (sPDPN) levels in normal individuals and cancer patients. A, Representative binding curve of PDPN to anti‐PDPN antibody generated by plotting absorbance versus concentration of rPDPN‐Fc. Linear regression analysis resulted in a correlation of 0.99. B and C, Plasma sPDPN levels in normal individuals and inpatients of different cancer types
3.3. Soluble podoplanin levels in patients with various cancer types before and after treatment
Plasma samples from tumor patients with adenocarcinoma (87 cases), squamous cell carcinoma (86 cases), rectal cancer (27 cases), lung cancer (45 cases) or gastric cancer (38 cases) were collected before and after treatment. As assessed by ELISA, there were significant increases in the plasma levels of sPDPN in squamous cell carcinoma (6.91 ± 0.59 ng/ml), adenocarcinoma (31.09 ± 5.48 ng/ml), rectal cancer (20.98 ± 6.30 ng/ml), lung cancer (19.50 ± 5.59 ng/ml) and gastric cancer (23.71 ± 6.90 ng/ml) compared with that of normal individuals (1.31 ± 0.13 ng/ml) (P < .0001) (Figure 2B,C). Moreover, the post‐treatment plasma sPDPN levels of cancer patients (n = 156) (4.47 ± 0.35 ng/ml) were significantly lower compared with those seen pre‐treatment (n = 128) (43.74 ± 4.97 ng/ml) (Figure 3). For each cancer type, the plasma sPDPN level of the post‐treatment group was significantly lower than that of the pre‐treatment group, especially in squamous cell carcinoma, adenocarcinoma and lung cancer (Figure 3). The pre‐treatment group consisted of patients that did not receive any surgery, chemotherapy or radiotherapy, while the post‐treatment group consisted of patients 3‐7 days after surgery or those that received at least one course of radiation/chemotherapy.
Figure 3.
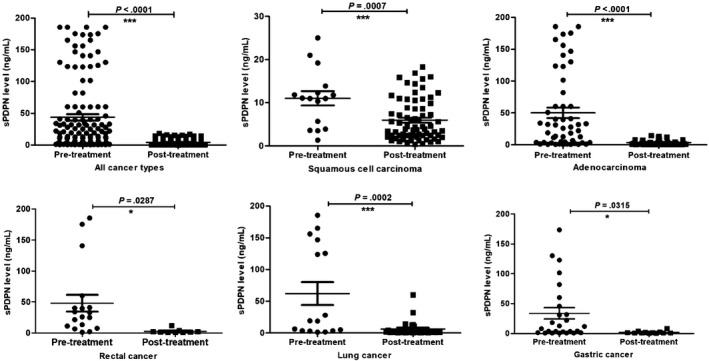
Plasma levels of soluble podoplanin (sPDPN) in patients with squamous cell carcinoma, adenocarcinoma, rectal, lung and gastric cancer before or after treatment
3.4. Analysis of the soluble podoplanin plasma levels in metastatic and non‐metastatic cancer patients at pre‐treatment and post‐treatment
The cancer patients were divided into 2 groups according to whether their tumor was metastatic or not, and sPDPN levels in the 2 groups were analyzed using a Mann‐Whitney test. The sPDPN level was 30.35 ± 3.63 ng/ml in the plasma of the metastatic group (n = 192) and 6.28 ± 0.77 ng/ml in that of the non‐metastatic group (n = 92), and this difference was statistically significant (P < .0001) (Figure 4A).
Figure 4.
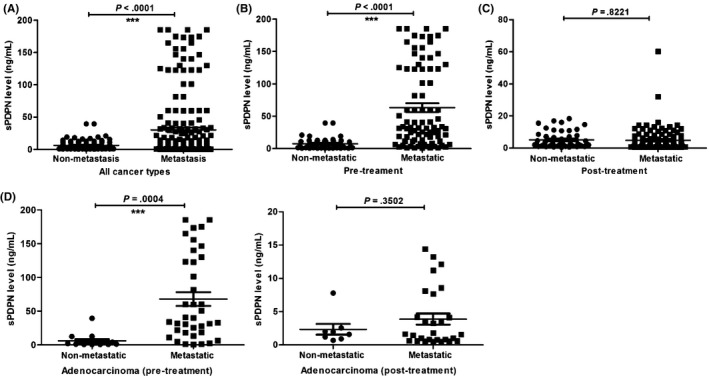
Analysis of the plasma soluble podoplanin (sPDPN) levels in metastatic and non‐metastatic cancer patients. A, Plasma sPDPN in the metastatic and non‐metastatic groups before treatment. B, Plasma sPDPN in non‐metastatic and metastatic groups before treatment. C, Plasma sPDPN levels in non‐metastatic and metastatic groups post‐treatment. D, Plasma sPDPN levels in non‐metastatic and metastatic adenocarcinoma groups pre‐ and post‐treatment
For pre‐treatment cancer patients (n = 129), the sPDPN values ranged from 0.90 to 185.40 ng/ml (63.16 ± 6.74 ng/ml) in the metastatic group (n = 84), compared with 0.64 to 39.60 ng/ml (7.43 ± 1.35 ng/ml) in the non‐metastatic group (n = 45). The plasma sPDPN level in the non‐metastatic group was significantly lower than that in the metastatic group of the pre‐treatment patients (P < .0001) (Figure 4B).
For post‐treatment cancer patients (n = 155), the sPDPN values were equivalent in the metastatic and non‐metastatic groups (0.50 to 60.20 ng/ml [4.86 ± 0.70 ng/ml] in the metastatic group [n = 108] and 0.42 to 18.28 ng/ml [5.12 ± 0.75 ng/ml] in the non‐metastatic group [n = 47] [P = .8221]) (Figure 4C).
For specific cancer types, the plasma sPDPN level in the non‐metastatic group with adenocarcinoma pretreatment was significantly lower than that in the metastatic group (P = .0004) (Figure 4D). However, the difference between the non‐metastatic and metastatic groups diminished after treatment (P = .3502) (Figure 4D). The comparison of plasma sPDPN levels between the non‐metastatic and metastatic groups pre‐treatment and post‐treatment was more obvious in adenocarcinoma than other cancer types, for example, lung cancer (P = .1566 vs P = .9113) and rectal cancer (P = .2803 vs P = .2007) (Fig. S1).
3.5. Soluble podoplanin was as sensitive as carcinoembryonic antigen for use as an indicator of cancer
Carcinoembryonic antigen is a heavily glycosylated protein primarily found in the apical membrane of enterocytes. CEA levels are established markers in clinical practice for prognostic purposes of colorectal adenocarcinoma, lung cancer and breast cancer.25, 26 Thus, the diagnostic efficiency of sPDPN for predicting cancer was compared to that of CEA. The CEA level ranged from 0.42 to 5.35 ng/ml (average, 1.48 ng/ml) in the plasma of normal individuals (n = 99), compared with 0.5 to 1330.0 ng/ml (average, 31.09 ng/ml) in the plasma of cancer patients (n = 110). The area under the curve (AUC) was analyzed and the specificity and sensitivity of the 2 indexes (sPDPN and CEA) were compared using receiver operating characteristic curve (ROC) analysis (Figure 5). The results suggested that as a biomarker, sPDPN accurately reflected tumor occurrence and development.
Figure 5.
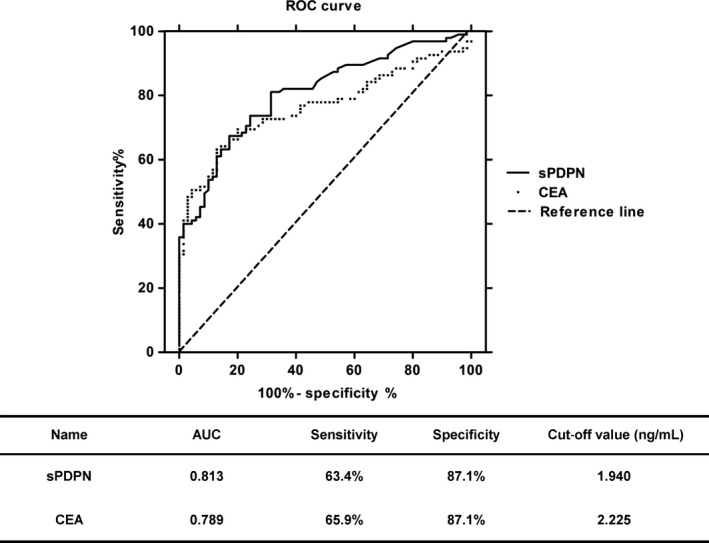
Comparison of the diagnostic efficiency of carcinoembryonic antigen (CEA) and soluble podoplanin (sPDPN) for cancer
4. DISCUSSION
Thus far, PDPN has been considered a promising therapeutic target for treatment of tumors, especially metastatic tumors. In previous reports, anti‐PDPN monoclonal antibodies have been examined for their treatment and diagnostic potential. NZ‐1 and MS‐1 were shown to inhibit PDPN‐induced platelet aggregation in vitro and suppress cancer metastasis in mice,14, 27 although it is unknown whether these antibodies suppress human cancer metastasis. Another anti‐PDPN antibody, D2‐40, was proposed as a potential diagnostic marker for many different types of human tumors.6, 8, 22, 27, 28 However, quantitation of the sPDPN level in plasma by ELISA has not been previously reported. Moreover, the relationships between sPDPN and tumor invasion, metastasis and malignant progression have remained largely unclear.
To detect sPDPN in the plasma and explore its biological function, we generated 2 new anti‐human PDPN mAb (SZ163 and SZ168) and developed a double‐antibody sandwich ELISA to quantitate plasma sPDPN, using SZ‐163 as the coating antibody and HRP‐SZ‐168 as the detecting antibody. The ELISA results showed that the levels of sPDPN in tumor patients with adenocarcinoma, squamous cell carcinoma, and rectal, lung and gastric cancers were significantly higher than those of normal individuals (P < .0001) (Figure 2). Furthermore, sPDPN levels in metastatic cancer patients were significantly higher than those of non‐metastatic cancer patients (P < .0001) (Figure 4). Therefore, our ELISA provided an easier, faster and more reliable way to detect sPDPN in plasma compared with the previously reported surface plasmon resonance method. The ELISA could be applied on a large scale at a reasonable cost to diagnose patients with tumors or to differentiate patients with metastasis. In addition, our results implied that PDPN on the surface of tumor cells can be hydrolyzed, spontaneously resulting in the release of the extracellular domain into the plasma. PDPN is known to possess multiple cleavage sites for different types of proteolytic enzymes, such as serine protease, trypsin, elastase, cysteine protease calpain‐2, MMP2/9 and presenilin‐1/gamma‐secretase in the extracellular and cytoplasmic domains.12, 29 Therefore, the extracellular domain of PDPN may be shed through the action of these proteolytic enzymes, especially in the tumor environment where O‐glycosylation of PDPN is known to be misregulated. This speculation is supported by recent progress in production of a series of PDPN cancer‐specific monoclonal antibodies (CasMab) by Kato group.20, 30, 31, 32, 33, 34, 35, 36, 37, 38 For example, the CasMabLpMab‐2 recognizes cancer‐type aberrant glycosylation (O‐glycosylation or sialylation, not keratan sulfate) of Thr55 and/or Ser56; therefore, it reacts with hPDPN‐expressing cancer cells but not with normal cells.34 Another CasMab LpMab‐21, the epitope of which contains sialyated Thr76, detects PDPN in glioblastomas, oral cancers and seminomas as well as in normal cells such as lymphatic endothelial cells, but does not recognize PDPN in the renal glomerulus or type I alveolar cells of lung.30 LpMab‐7, an anti‐non‐PLAG hPDPN mAb, can be used for detecting different glycan profiles of hPDPN, including the poly LacNAc structure.35 Evidence from the PDPN CasMab suggest the diversity of PDPN glycosylation among different cancer cells.
In our study, the plasma sPDPN level in cancer patients post‐treatment was significantly lower compared with pre‐treatment cancer patients. The difference was more significant in cancer types with high expression of PDPN, such as squamous cell carcinoma, adenocarcinoma or lung cancer than other cancers (Figure 3), suggesting the specificity of sPDPN as a cancer biomarker. In addition, in squamous cell carcinoma, the plasma sPDPN from patients who underwent chemotherapy or surgery and chemotherapy were significantly lower than those of pre‐treatment patients, while this was not true of patients who underwent surgery alone (Fig. S2). These results suggested that cancer treatment decreased the formation or increased the clearance of plasma sPDPN, and that the sPDPN levels in plasma may reflect the efficiency of cancer treatment. Furthermore, the comparison of the diagnostic efficiency of sPDPN and CEA suggested that sPDPN might be a better cancer marker, with an AUC value closer to 1 and a lower cut‐off value.
Additional studies that include expanded numbers of cancer patients and cancer types will be required for further testing of the sPDPN‐specific mAb and ELISA system. However, the results of this study strongly suggest that optimization of sPDPN as a reliable marker for detection and diagnosis of tumor occurrence and metastasis under more complicated pathological circumstances is warranted.
DISCLOSURE STATEMENT
The authors have no conflict of interest to declare.
Supporting information
Zhao X, Pan Y, Ren W, et al. Plasma soluble podoplanin is a novel marker for the diagnosis of tumor occurrence and metastasis. Cancer Sci. 2018;109:403–411. https://doi.org/10.1111/cas.13475
Xingpeng Zhao, Yanfang Pan and Weihua Ren contributed equally to this study.
Funding information
National Scientific Foundation of China (Grant Nos. 81270593, 31400692, and 81520108005); National Key Technology R&D Program of China (2012BA118B02); National Institutes of Health (HL128390).
Contributor Information
Lijun Xia, Email: lijun-xia@omrf.org.
Yiming Zhao, Email: zhaoyimingbox@163.com.
REFERENCES
- 1. Breiteneder‐Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: Podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up‐regulation of the lymphatic marker podoplanin, a mucin‐type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial‐mesenchymal transition: podoplanin‐mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261‐272. [DOI] [PubMed] [Google Scholar]
- 4. Yuan P, Temam S, El‐Naggar A, et al. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107:563‐569. [DOI] [PubMed] [Google Scholar]
- 5. Kimura N, Kimura I. Podoplanin as a marker for mesothelioma. Pathol Int. 2005;55:83‐86. [DOI] [PubMed] [Google Scholar]
- 6. Ordonez NG. D2‐40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372‐380. [DOI] [PubMed] [Google Scholar]
- 7. Weninger W, Partanen TA, Breiteneder‐Geleff S, et al. Expression of vascular endothelial growth factor receptor‐3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi's sarcoma tumor cells. Lab Invest. 1999;79:243‐251. [PubMed] [Google Scholar]
- 8. Roy S, Chu A, Trojanowski JQ, Zhang PJ. D2‐40, a novel monoclonal antibody against the M2A antigen as a marker to distinguish hemangioblastomas from renal cell carcinomas. Acta Neuropathol. 2005;109:497‐502. [DOI] [PubMed] [Google Scholar]
- 9. Kato Y, Sasagawa I, Kaneko M, Osawa M, Fujita N, Tsuruo T. Aggrus: a diagnostic marker that distinguishes seminoma from embryonal carcinoma in testicular germ cell tumors. Oncogene. 2004;23:8552‐8556. [DOI] [PubMed] [Google Scholar]
- 10. Shibahara J, Kashima T, Kikuchi Y, Kunita A, Fukayama M. Podoplanin is expressed in subsets of tumors of the central nervous system. Virchows Arch. 2006;448:493‐499. [DOI] [PubMed] [Google Scholar]
- 11. Fernández‐Muñoz B, Yurrita MM, Martín‐Villar E, et al. The transmembrane domain of podoplanin is required for its association with lipid rafts and the induction of epithelial‐mesenchymal transition. Int J Biochem Cell Biol. 2011;43:886‐896. [DOI] [PubMed] [Google Scholar]
- 12. Yurrita MM, Fernández‐Muñoz B, del Castillo G, Martín‐Villar E, Renart J, Quintanilla M. Podoplanin is a substrate of presenilin‐1/γ‐secretase. Int J Biochem Cell Biol. 2014;46:68‐75. [DOI] [PubMed] [Google Scholar]
- 13. Navarro‐Núñez L, Pollitt AY, Lowe K, Latif A, Nash GB, Watson SP. Platelet adhesion to podoplanin under flow is mediated by the receptor CLEC‐2 and stabilised by Src/Syk‐dependent platelet signalling. Thromb Haemost. 2015;113:1109‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bertozzi CC, Schmaier AA, Mericko P, et al. Platelets regulate lymphatic vascular development through CLEC‐2‐SLP‐76 signaling. Blood. 2010;116:661‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takagi S, Sato S, Oh‐hara T, et al. Platelets Promote tumor growth and metastasis via Direct Interaction between Aggrus/podoplanin and CLEC‐2. PLoS ONE. 2013;8:e73609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neri S, Ishii G, Hashimoto H, et al. Podoplanin‐expressing cancer‐associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int J Cancer. 2015;137:784‐786. [DOI] [PubMed] [Google Scholar]
- 17. Carrasco‐Ramirez P, Greening DW, Andres G, et al. Podoplanin is a component of extracellular vesicles that reprograms cell‐derived exosomal proteins and modulates lymphatic vessel formation. Oncotarge. 2016;7:16070‐16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roca E, Lacroix R, Judicone C, et al. Detection of EpCAM‐positive microparticles in pleural fluid: a new approach to mini‐invasively identify patients with malignant pleural effusions. Oncotarget. 2016;7:3357‐3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sankiewicz A, Guszcz T, Mena‐Hortelano R, Zukowski K, Gorodkiewicz E. Podoplanin serum and urine concentration in transitional bladder cancer. Cancer Biomarker. 2016;16:343‐350. [DOI] [PubMed] [Google Scholar]
- 20. Kaneko MK, Kunita A, Abe S, et al. Chimeric anti‐podoplanin antibody suppresses tumor metastasis through neutralization and antibody‐dependent cellular cytotoxicity. Cancer Sci. 2012;103:1913‐1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chandramohan V, Bao X, Kato Kaneko M, et al. Recombinant anti‐podoplanin (NZ‐1) immunotoxin for the treatment of malignant brain tumors. Int J Cancer. 2013;132:2339‐2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bai B, Ma W, Wang K, et al. Detection of D2‐40 monoclonal antibody‐labeled lymphatic vessel invasion in esophageal squamous cell carcinoma and its clinicopathologic significance. Cancer Biol Med. 2013;10:81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qu L, Zhao X, Fu J, et al. Stable expression of recombinant human podoplanin in Chinese hamster ovary (CHO) cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:25‐28. [PubMed] [Google Scholar]
- 24. Nagae M, Morita‐Matsumoto K, Kato M, Kaneko MK, Kato Y, Yamaguchi Y. A platform of C‐type lectin‐like receptor CLEC‐2 for binding O‐glycosylated podoplanin and nonglycosylated rhodocytin. Structure. 2014;22:1711‐1721. [DOI] [PubMed] [Google Scholar]
- 25. Bersinger NA, Rageth JC. A comparison between pregnancy‐associated alpha 2‐glycoprotein (alpha 2‐PAG), carcino‐embryonic antigen (CEA), CA‐125, and CA‐15‐3 as tumor markers in breast cancer. Eur J Gynaecol Oncol. 1990;11:135‐139. [PubMed] [Google Scholar]
- 26. Loeser A, Neumann M, Kocot A, Vergho DC, Spahn M, Riedmiller H. Serum carcino‐embryonic antigen (CEA) and its possible use as tumor marker for secondary tumors in urinary intestinal reservoirs. Urol Oncol. 2013;31:644‐648. [DOI] [PubMed] [Google Scholar]
- 27. Tajima S, Fukayama M. Possibility of D2‐40 as a diagnostic and tumor differentiation‐suggestive marker for some of phosphaturic mesenchymal tumors. Int J Clin Exp Pathol. 2015;8:9390‐9396. [PMC free article] [PubMed] [Google Scholar]
- 28. Braun M, Flucke U, Debald M, et al. Detection of lymphovascular invasion in early breast cancer by D2‐40 (podoplanin): a clinically useful predictor for axillary lymph node metastases. Breast Cancer Res Treat. 2008;112:503‐511. [DOI] [PubMed] [Google Scholar]
- 29. Pan Y, Yago T, Fu J, et al. Podoplanin requires sialylated O‐glycans for stable expression on lymphatic endothelial cells and for interaction with platelets. Blood. 2014;124:3656‐3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaneko MK, Nakamura T, Honma R, et al. Development and characterization of anti‐glycopeptide monoclonal antibodies against human podoplanin, using glycan‐deficient cell lines generated by CRISPR/Cas9 and TALEN. Cancer Med. 2017;6:382‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaneko MK, Oki H, Hozumi Y, et al. Monoclonal antibody LpMab‐9 recognizes O‐glycosylated N‐terminus of human podoplanin. Monoclon Antib Immunodiagn Immunother. 2015;34:310‐317. [DOI] [PubMed] [Google Scholar]
- 32. Kaneko MK, Oki H, Ogasawara S, Takagi M, Kato Y. Anti‐podoplanin monoclonal antibody LpMab‐7 detects metastatic lesions of osteosarcoma. Monoclon Antib Immunodiagn Immunother. 2015;34:154‐161. [DOI] [PubMed] [Google Scholar]
- 33. Kaneko MK, Yamada S, Nakamura T, et al. Antitumor activity of chLpMab‐2, a human‐mouse chimeric cancer‐specific antihuman podoplanin antibody, via antibody‐dependent cellular cytotoxicity. Cancer Med. 2017;6:768‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kato Y, Kaneko MK. A cancer‐specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci Rep. 2014;4:5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kato Y, Kunita A, Abe S, et al. The chimeric antibody chLpMab‐7 targeting human podoplanin suppresses pulmonary metastasis via ADCC and CDC rather than via its neutralizing activity. Oncotarget. 2015;6:36003‐36018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kato Y, Ogasawara S, Oki H, et al. LpMab‐12 established by CasMab technology specifically detects sialylated O‐Glycan on Thr52 of platelet aggregation‐stimulating domain of human podoplanin. PLoS ONE. 2016;11:e0152912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kato Y, Ogasawara S, Oki H, et al. Novel monoclonal antibody LpMab‐17 developed by CasMab technology distinguishes human podoplanin from monkey podoplanin. Monoclon Antib Immunodiagn Immunother. 2016;35:109‐116. [DOI] [PubMed] [Google Scholar]
- 38. Kato Y, Kaneko MK, Kuno A, et al. Inhibition of tumor cell‐induced platelet aggregation using a novel anti‐podoplanin antibody reacting with its platelet‐aggregation‐stimulating domain. Biochem Biophys Res Commun. 2006;349:1301‐1307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


