Abstract
Purpose of Review
Posttraumatic stress disorder (PTSD) is associated with increased risk for dementia, yet mechanisms are poorly understood.
Recent Findings
Recent literature suggests several potential mechanisms by which sleep impairments might contribute to the increased risk of dementia observed in PTSD. First, molecular, animal, and imaging studies indicate that sleep problems lead to cellular damage in brain structures crucial to learning and memory. Second, recent studies have shown that lack of sleep might precipitate the accumulation of harmful amyloid proteins. Finally, sleep and PTSD are associated with elevated inflammation, which, in turn, is associated with dementia, possibly via cytokine-mediated neural toxicity and reduced neurogenesis.
Summary
A better understanding of these mechanisms may yield novel treatment approaches to reduce neurodegeneration in PTSD. The authors emphasize the importance of including sleep data in studies of PTSD and cognition and identify next steps.
Keywords: Stress, Alzheimer’s, Dementia, Veterans, Sleep, Inflammation, PTSD
Introduction
Convergent lines of evidence suggest multiple possible mechanisms by which inflammation and sleep impairments may mediate the increased risk of dementia in posttraumatic stress disorder (PTSD). In this review, we consider three broad categories of sleep-related mechanisms that may mediate or moderate the relationship between PTSD and increased risk of dementia. First, we present evidence that the sleep impairments associated with PTSD may lead to cellular damage. Second, we discuss research suggesting that sleep impairment associated with PTSD causes the accumulation of amyloid beta (Aβ) and tau protein deposits associated with neurodegeneration in multiple forms of dementia. Thirdly, we consider the possibility that inflammation stemming from PTSD-related sleep impairments precipitates cognitive impairment via reduced neurogenesis and increased neurodegeneration. We conclude by outlining a research agenda that could clarify the role of sleep deficits in the development of dementia in individuals with PTSD.
PTSD Is a Risk Factor for Dementia
In one large study of 181,093 male veterans enrolled at Veterans Affairs (VA) hospitals, 53,155 veterans diagnosed with PTSD had more than twice the risk for subsequent development of dementia compared to those without PTSD. Risk for incident dementia in the context of PTSD remained significantly elevated despite adjustment for sociodemographic variables, neuropsychiatric and medical comorbidities, and number of clinic visits [1]. In another VA study, receipt of the Purple Heart award was used as an indication that veterans had experienced psychologically traumatic events. Notably, dementia was more prevalent among veterans who had been diagnosed with PTSD compared to those without PTSD, regardless of Purple Heart status. These authors also controlled for a number of possible confounding variables, including number of clinic visits and psychiatric and medical comorbidities, including traumatic brain injury [2].
It remains unclear whether PTSD increases risk for some forms of dementia more than others. In the study by Yaffe et al., the dementia subtype associated with PTSD with the highest adjusted hazard ratio (HR) was frontotemporal dementia (FTD; a dementia associated with the deposition of intracellular tau protein aggregates) [3], with a HR of 2.19, after adjustment for demographic variables as well as medical and psychiatric comorbidity. Vascular dementia (VD) had the lowest adjusted HR at 1.69, but the effect was nonetheless statistically significant [1]. However, in a smaller study of 93 Holocaust survivors, all of whom had PTSD; the incidence of dementia was 16%. The incidence of dementia, by subtype, was highest for VD (60%) and 20% for both Alzheimer’s disease (AD) and “Other Dementia Subtypes”. [4] In a retrospective observational study of war veterans with dementia, almost all of whom had been diagnosed with PTSD, patients with disruptive nighttime behavior relative to matched subjects without disruptive nighttime behavior were more likely to be diagnosed with subcortical dementia subtypes (i.e., dementia with Lewy bodies [DLB] and Parkinson’s dementia) and less likely to be diagnosed with AD [5]. Though this last study does not help to differentiate the causes of dementia in subjects with PTSD versus without PTSD, it does suggest that dementia in subjects with PTSD might more often be attributable to subcortical causes when it is accompanied by disruptive or agitated nighttime behavior. In sum, based on available evidence, PTSD appears to increase risk for different types of dementia.
Sleep Impairment May Mediate the Relationship Between PTSD and Dementia
Poor sleep is strongly associated with risk of multiple types of dementia [5–9, 10•, 11–13], and as discussed by Germain et al. elsewhere in this special issue, sleep impairment is an extremely common and modifiable symptom of PTSD [14]. Briefly, subjective sleep impairments, including trauma-related nightmares and “sleep disturbance” are listed among the diagnostic criteria for PTSD [1, 15]. Sleep impairments are also among the most commonly reported symptoms of the disorder [2, 16–21]. Nightmares are a common and especially distressing symptom. [22]
With a few notable exceptions [7, 14, 23–27], most studies have found objective sleep differences in subjects with PTSD versus healthy controls, though it should be noted that the sleep impairments associated with PTSD may be different from those observed in chronic short sleep duration and insomnia, two other conditions that have been studied in some detail [21]. A meta-analysis of sleep characteristics associated with PTSD found that, when controlling for age, sex, comorbid depression, and substance use, subjects with PTSD had greater rapid eye movement (REM) sleep density and reduced slow wave sleep. Lower total sleep time was associated with PTSD status only among younger subjects [3, 28–31]. However, overall, the particular sleep differences associated with PTSD have been found inconsistently [32]. Furthermore, the sleep differences seen after a trauma may be different from those seen at later time points, with sleep in the period of time closer to the trauma being characterized by fragmented and decreased REM [32, 33]. Posttraumatic stress disorder is additionally associated with motor disturbances in REM sleep [34]. Though some work suggests that sleep problems are a risk factor for PTSD [1, 35], most work suggests that exposure to trauma and development of PTSD leads to significant sleep problems [4, 14]. Taken together, the literature indicates that PTSD is associated with a variety of sleep differences and that the relationship of sleep disturbances with PTSD is bidirectional.
The sleep problems observed in PTSD may also contribute to dementia risk [7, 24, 26, 27]. One possible mechanism by which this occurs could involve damage to the hippocampus. The hippocampus is one of the few sites in the brain associated with adult neurogenesis and is an essential component of the circuitry of learning and memory and contextual fear conditioning [36]. Damage to the hippocampus by any mechanism could lower the threshold at which cognitive deficits stemming from any mechanism become clinically significant. Numerous studies have determined through imaging that hippocampal volume is reduced in PTSD [37–40]. Although some studies have found no difference in hippocampal volumes in PTSD versus controls [41–44], several recent meta-analyses have reported reduced hippocampal volume in PTSD [43, 45–47]. The causal relationship between hippocampal size and PTSD remains obscure, with some suggesting that smaller hippocampal sizes precede and constitute a risk for PTSD [48–50] and others suggesting that smaller hippocampal size is more likely to be a consequence of the disorder [50]. Studies by Cardenes-Nicholsen et al. [51] and Apfel et al. [37] suggest that hippocampal size in PTSD may increase in subjects who go into remission or decrease among those with worsening of symptoms over time.
Chronic sleep deprivation might lead to changes in the hippocampus as well. Imaging studies have found smaller hippocampal volumes in rodents deprived of sleep [52] and in humans with insomnia or environmental sleep restrictions [53, 54]. Other studies have not found a correlation between the diagnosis of primary insomnia (PI) and hippocampal size, but have found negative correlations between wake time after sleep onset (WASO) and hippocampal volume among those with PI [55, 56]. In what has been called the glucocorticoid vulnerability hypothesis, changes in hippocampal cellular structure or function render the region especially susceptible to permanent damage from acute physiological stressors. In this setting, the hippocampus is less able to regulate the hypothalamic-pituitary-adrenal axis and baseline glucocorticoid levels go up. This situation leads to neuronal cell death under conditions of chronic stress when such stress is punctuated by an additional metabolic challenge (e.g., stroke, ischemia, hyperglycemia, or hypoxia) and damage accumulates over time [57]. An extensive discussion of this topic as it relates to PTSD is beyond the scope of this article.
Few studies have systematically examined the role of sleep in the relationship between PTSD and hippocampal volume [24, 58, 59]. However, there are important reasons to suspect that it could play a key role. Sleep restriction in rats promotes changes in cellular and molecular structures that are crucial to the process of memory formation through long-term potentiation [60]. These include decreases in actin-binding cortactin [61], reduced α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor phosphorylation [62], diminished cell proliferation [63–66], impaired calcium signaling [67, 68], and reduced hippocampal N-methyl-D-aspartate (NMDA) receptor quantity [67, 69]. Sleep disruption in rodents leads to decreased long-term potentiation and impaired learning and memory [62, 66, 70–75], processes that clearly depend on the hippocampus [76]. Evidence from humans is mixed. With important exceptions, the bulk of studies of insomnia show decreased performance compared to healthy controls on measures of learning and memory [77]. Long and short sleep duration has likewise been associated with impaired learning, with an inverted U shape relationship [78]. PTSD is also associated with decreased verbal and visual memory [79], attributable to impairments in encoding [80, 81]. In sum, both PTSD and impaired sleep are related to impairments in learning and memory and all of these entities are related to decreased hippocampal volume. The cognitive reserve model holds that damage to the hippocampus from any cause could elevate the risk of dementia [82]. Thus, available evidence raises the distinct possibility that the sleep problems associated with PTSD damage the hippocampus, leading to impairments in learning and memory and increased risk of dementia.
Sleep impairment in PTSD may also lead to abnormal protein processing and the buildup of amyloid beta (Aβ), tau, and phosphorylated tau (p-tau). These proteins are highly associated with AD [83] .and other neurodegenerative diseases [84]. Based on histopathological findings and the fact that genetic disorders that increased amyloid precursor protein (APP) cleavage conferred an increased risk of AD, the amyloid cascade hypothesis holds that the buildup of Aβ is an essential causal step in the development of AD [83].
In healthy volunteers of various ages, Huang et al. found decreases in diurnal variations of cerebrospinal fluid (CSF) Aβ levels in older age, regardless of various activities performed during wakefulness, possibly indicating impaired sleep-dependent Aβ clearance in older age [85]. Other data from humans indicate that more frequent nighttime awakenings are associated with increased amyloid pathology in CSF and positron emission tomography (PET) imaging [13]. One study of 23 participants found that amyloid burden, as assessed by CSF Ab42 levels, and sleep efficiency interacted in their effects on memory performance [86].
Alternatively, sleep may promote clearance of Aβ by increasing the transfer of fluid to the interstitial space in the brain parenchyma, thereby facilitating CSF flow across neurons [87]. Thus, decreased sleep may lead to buildup of brain Aβ by decreasing its rate of clearance from the brain. Consistent with this hypothesis, self-reported shorter sleep duration is associated with greater brain Aβ burden in older adults [88]. Evidence for this is limited and needs to be extended to human experiments.
Thus, the buildup of Aβ has been associated with lower sleep time and increased awakenings. Therefore, it may be that these features of sleep impairment seen in PTSD lead to increased Aβ buildup in individuals with the disorder. However, a recent interim analysis of a large ongoing multisite study showed that military veterans with lifetime history of PTSD resulting from service in the Vietnam War had a higher prevalence of mild cognitive impairment (MCI), but lower brain amyloid PET levels and a much lower prevalence of amyloid positivity on PET scans [89]. These results support the hypothesis that in many cases, MCI and possibly future dementia that is associated with PTSD may not involve amyloid deposition. On the other hand, another recent study found self-reported poor sleep to be associated with lower levels of cerebrospinal fluid Aβ, a state that is paradoxically associated with increased amyloid in the brain [90]. More work needs to be done in this area.
The Role of the Immune System and Inflammation
The inflammatory response of the immune system could also play an important role in the development of dementia in individuals with PTSD. Several lines of evidence now indicate that trauma exposure and PTSD are associated with elevated inflammation as indexed by higher levels of peripheral systemic inflammatory markers such as high sensitivity C-reaction protein and the pro-inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) [91–93] and by elevated pro-inflammatory signaling to immune cells through the transcription factor nuclear factor-κB (NF-κB) [94–96]. Notably, however, not all individuals with PTSD display elevated inflammation [97•, 98, 99]. A recent meta-analysis including 20 studies of inflammatory markers in PTSD found that PTSD was associated with higher levels of IL-6, IL-1β, and interferon γ, whereas TNF-α was higher in subjects with PTSD only in a subgroup analysis of patients who were not on psychotropic medications. Univariate meta-regression analyses showed IL-6 to be associated with illness severity as assessed by the Clinician-Administered PTSD Scale (CAPS) score but not with illness duration. Illness duration was associated with IL-1β levels, though available studies of IL-1β could have been affected by publication bias, as determined by a separate linear regression test [100••].
The relationship between PTSD and inflammation is likely bidirectional. On the one hand, elevated CRP levels have been linked to the development of PTSD [101]. Additionally, elevated inflammation can manifest as symptoms of PTSD [102–106]. On the other hand, chronic stress is associated with sustained levels of inflammation and acute stressors with increases in inflammatory activity [91, 107–111]. Remitted PTSD does not appear to be associated with above-normal levels of inflammation, though data on this topic is very sparse [112].
Elevated inflammation may in turn be a direct mechanism of increased dementia risk in PTSD. There are at least two lines of clinical evidence linking inflammation with dementia. First, elevated inflammation, as indexed by circulating levels of systemic inflammatory proteins or spontaneous production of pro-inflammatory cytokines, is a risk factor for the development of dementia [113, 114]. Second, there is some evidence that long-term use of some non-steroidal anti-inflammatory drugs (NSAIDS) actually protects against the development of Alzheimer’s and Parkinson’s disease [115, 116] and that administration of the TNF-α antagonist etanercept improves functioning in patients with Alzheimer’s disease [117]. This clinical evidence is bolstered by a large body of basic science research showing that inflammation can promote neurotoxicity across multiple neurodegenerative disorders [118, 119].
Researchers are also now beginning to uncover the mechanistic pathway underlying the association between inflammation and neurodegeneration. Accumulating evidence points to inflammatory proteins called cytokines as key players in this relationship. Cytokines are typically too large to pass through the blood-brain barrier (BBB). However, they can nonetheless influence brain function and structure by transmitting signals through interactions between brain endothelial cells and perivascular macrophages, activating afferent vagal fibers, passing through the BBB when it loses structural integrity due to sepsis, entering brain areas without BBB, and actively transporting circulating cytokines into brain parenchyma via cytokine-specific saturable transporters [103, 119, 120].
Cytokines are critical for almost all aspects of brain function, and they play an important facilitative role in learning and memory [121]. However, when markedly elevated, they may also reduce neurogenesis and have toxic effects on neurons [119]. Such effects may explain the inverse relationship between inflammation and hippocampal volume observed in some studies [97•, 122••]. In fact, neuroinflammation has been proposed as a mechanism of dementia, including vascular dementia, Alzheimer’s disease, and all dementias combined [119, 123–125].
Collective Impact of Sleep and Inflammation
Sleep and inflammation are intricately interlinked. While inflammation plays a key role in regulating sleep (in some cases promoting and in others disturbing it), depriving humans and other animals of sleep also profoundly enhances inflammation [126, 127]. In particular, experimentally induced sleep deprivation is associated with increased pro-inflammatory signaling through NF-κB and heightened levels of inflammatory proteins, including IL-6 and TNF-α [128–130]. Moreover, in a sample of 70 military veterans with TBI receiving care for sleep disturbance, improvements in sleep quality were accompanied by reductions in PTSD, depressive symptoms, and improvements in quality of life. Those improvements in turn associated with reductions in levels of the systemic inflammatory marker C-reactive protein (CRP) [131]. Given the causal effects of sleep loss on inflammation, sleep impairments could contribute to the elevated inflammation observed in patients with PTSD.
Conclusions and Future Directions
Given evidence from animal models and the observed associations among PTSD, sleep, inflammation, and dementia in humans, sleep disturbance and inflammatory factors may play a key role in the relationship between PTSD and dementia. In this review, we have identified multiple possible sleep-related mechanisms that might explain this relationship. Firstly, PTSD and sleep impairments are associated with macrostructural changes in the hippocampus as well as microstructural and functional degradation within the limbic system. Thus, the sleep impairments associated with PTSD may cause damage to structures critical for maintaining cognitive reserve and protecting against dementia. Secondly, recent studies have demonstrated that sleep impairment in rodents leads to the accumulation of Aβ and tau. If this relationship holds true in humans as well, and if such accumulation contributes to the development of dementia, then this represents another mechanism by which sleep impairment associated with PTSD would increase the risk of dementia. Finally, PTSD and sleep are also both associated with elevated inflammation, as indicated by increased levels of inflammatory cytokines and pro-inflammatory signaling. Such changes have also been implicated in neurodegeneration and could similarly increase the likelihood of dementia.
These mechanisms are not specific to PTSD, per se. It may be, for example, that decreased total sleep time (discussed above as being inconsistently observed in PTSD) allows for the buildup of Aβ and that this occurs in individuals who have decreased sleep time regardless of PTSD status. In this model, decreased sleep time could either predate PTSD or be caused by it. Similarly, individuals with PTSD have a high rate of high risk health-related behaviors such as smoking [132] and heavy alcohol use [133] and a high prevalence of obstructive sleep apnea (OSA) [134, 135]. These factors could elevate risk of dementia in PTSD via mechanisms separate from or complimentary to those discussed above [94, 136], as described by the glucocorticoid vulnerability hypothesis referenced above [57].
The relationship between PTSD, sleep impairment, and dementia is likely multidirectional, with sleep impairments playing some role in the development of PTSD and PTSD itself precipitating sleep impairments [137]. It is also likely multifactorial, as the mechanisms discussed are not mutually exclusive. An impaired BBB may allow for the inflammatory mediators to inflict damage upon hippocampal cellular circuitry, lowering the threshold at which clinically significant cognitive impairment is observed. Concurrent abnormalities in protein processing could exacerbate this effect and further accelerate cognitive decline. Finally, the mechanisms discussed in this review do not preclude the possibility that, in some cases, mechanisms that lead to dementia predate both PTSD and sleep problems. Since REM behavior disorder has been associated with increased risk of dementia [138] (especially dementia associated with Lewy body pathology) [139, 140] and may even represent the earliest manifestations of dementia in individuals without PTSD [141], it is possible that REM disturbances in PTSD comprise a common early sign of neurodegeneration in this disorder.
There is little direct evidence for a causal mechanism by which the diagnosis of PTSD confers an increased risk of dementia. However, we believe that enough circumstantial evidence exists to merit greater attention to sleep and inflammation in published studies of cognition in PTSD.
Future studies investigating the relationship between PTSD and cellular damage, Aβ and tau processing, and inflammation should include sleep impairments and inflammatory markers as possible mediators. Also, more longitudinal research is needed on the relationship between PTSD and sleep impairments, and among PTSD, inflammatory markers and specific indicators of dementia risk such as brain and cerebrospinal fluid levels of Aβ, tau, and p-tau. More specifically, the field of dementia research awaits confirmation that sleep impairments lead to altered protein processing in humans as well as rodents, and the mechanisms by which inflammation remain incompletely understood. At the behavioral level, it would be extremely valuable to know whether interventions targeted at improving sleep in individuals with PTSD could lower long-term risk of dementia. Such studies should be undertaken in ways that test certain mechanistic possibilities. For instance, it would be crucial to know whether aspects of Aβ processing or immune system functioning fluctuated with the severity of PTSD and whether they normalized with treatment of the disorder or with treatment of associated sleep problems. Finally, studying subjects with and without PTSD who have the same sleep problems and health-related behaviors would help to determine whether PTSD confers unique risks for dementia beyond those other factors.
Acknowledgments
Brian S. Mohlenhoff received salary support from the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment at the San Francisco Veterans Affairs Medical Center and the Department of Veterans Affairs Sierra-Pacific Region Mental Illness Research, Education and Clinical Center (MIRECC).
Aoife O’Donovan received salary support from Society in Science – The Branco Weiss Fellowship and a NIMH Career Development Award (K01-MH109871). This study was supported by the NIH/NIA/National Institute of Mental Health (BSM: R25MH060482; TCN: 5R01MH073978-04, 5R34MH077667-03, 1R56MH107042-01A1; MWW: R01AG10897, 2U01AG024904, 1P41 EB015904, P01 AG19724, R01 AG032306, R01A G03879) and by funding from the US Veterans Health Administration for the Mental Illness Research, Education and Clinical Center, VISN 21 and grant DAMD17–01–1-0764.
Michael W. Weiner has served on the Scientific Advisory Boards for Pfizer, BOLT International, Neurotrope Bioscience, and Eli Lilly. He has provided consulting to Synarc, Pfizer, Janssen, KLJ Associates, Easton Associates, Harvard University, University of California, Los Angeles (UCLA), Alzheimer’s Drug Discovery Foundation (ADDF), Avid Radiopharmaceuticals, Clearview Healthcare Partners, Perceptive Informatics, Smartfish AS, Decision Resources, Inc., Araclon, Merck, Defined Health, and Genentech. The following entities have provided funding for travel: Pfizer, Paul Sabatier University, MCI Group France, Travel eDreams, Inc., Neuroscience School of Advanced Studies (NSAS), Danone Trading, BV, CTAD Ant Congres, Kenes, Intl., ADRC, UCLA, UCSD, Sanofi-Aventis Groupe, University Center Hospital, Toulouse, Araclon, AC Immune, Eli Lilly, New York Academy of Sciences (NYAS), and National Brain Research Center, India for Johns Hopkins Medicine. He served on the Editorial Boards for Alzheimer’s & Dementia and MRI. He received honoraria from Pfizer, Tohoku University, and Danone Trading, BV. He received research support from Merck, Avid, the Veterans Administration (VA) and Department of Defense (DOD). Dr. Weiner additionally received support for his work from the following grants: W81XWH-13-1-0259, W81XWH-12-2-0012, ADNI 2-12-233,036, 20,110,506 and R01 MH098062–01 from the following sources; DOD, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation.
Footnotes
This article is part of the Topical Collection on Disaster Psychiatry: Trauma, PTSD, and Related Disorders
Compliance with Ethical Standards
Conflict of Interest: Thomas C. Neylan declares no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US VeteransPTSD and dementia in US veterans. Arch Gen Psychiatry. 2010;67:608–13. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qureshi SU, Kimbrell T, Pyne JM, Magruder KM, Hudson TJ, Petersen NJ, et al. Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. J Am Geriatr Soc. 2010;58:1627–33. doi: 10.1111/j.1532-5415.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 3.Sha S, Hou C, Viskontas IV, Miller BL. Are frontotemporal lobar degeneration, progressive supranuclear palsy and corticobasal degeneration distinct diseases? Nat Clin Pract Neurol. 2006;2:658–65. doi: 10.1038/ncpneuro0357. [DOI] [PubMed] [Google Scholar]
- 4.Sperling W, Kreil SK, Biermann T. Posttraumatic stress disorder and dementia in Holocaust survivors. J Nerv Ment Dis. 2011;199:196–8. doi: 10.1097/NMD.0b013e31820c71e0. [DOI] [PubMed] [Google Scholar]
- 5.Dallam DL, Mellman TA, Bhatnagar A, Nguyen S, Kurukumbi M. Trauma reenactments in aging veterans with dementia. J Am Geriatr Soc. 2011;59:766–8. doi: 10.1111/j.1532-5415.2011.03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merlino G, Piani A, Gigli GL, Cancelli I, Rinaldi A, Baroselli A, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. 2010;11:372–7. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Osorio RS, Pirraglia E, Agüera-Ortiz LF, et al. Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–62. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley D, Monjan A, Masaki K, Ross W, Havlik R, White L, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49:1628–32. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–56. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Blackwell T, Yaffe K, Laffan A, Ancoli-Israel S, Redline S, Ensrud KE, et al. Osteoporotic Fractures in Men (MrOS) Study Group. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. 2014;37:655–63. doi: 10.5665/sleep.3562. In this study of 2,822 older men, poor sleep quality and quantify, measured subjectively and objectively using wrist actigraphy, predicted subsequent cognitive decline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–32. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology-a bidirectional relationship. Nat Publ Group. 2013;10:115–9. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju Y-E, Duntley S, Fagan A, Morris J, Holtzman D. Sleep disruption and risk of preclinical Alzheimer disease (P01.081) Neurology. 2012;78 P01.081. [Google Scholar]
- 14.Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatr. 2013;170:372–82. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Trauma- and stressor-related disorders. Fifth. American Psychiatric Association; 2014. https://doi.org/10.1176/appi.books.9780890425596.dsm07. [Google Scholar]
- 16.Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry. 2007;68:1257–70. doi: 10.4088/jcp.v68n0813. [DOI] [PubMed] [Google Scholar]
- 17.Maker MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder. CNS Drugs. 2006;20:567–90. doi: 10.2165/00023210-200620070-00003. [DOI] [PubMed] [Google Scholar]
- 18.Babson K, Feldner M, Badour C, Trainor C, Blumenthal H, Sachs-Ericsson N, et al. Posttraumatic stress and sleep: differential relations across types of symptoms and sleep problems. J Anxiety Disord. 2011;25:706–13. doi: 10.1016/j.janxdis.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellman TA, Kulick-Bell R, Ashlock LE, Nolan B. Sleep events among veterans with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:110–5. doi: 10.1176/ajp.152.1.110. [DOI] [PubMed] [Google Scholar]
- 20.Neylan TC, Marmar CR, Metzler TJ, Weiss DS, Zatzick DF, Delucchi KL, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155:929–33. doi: 10.1176/ajp.155.7.929. [DOI] [PubMed] [Google Scholar]
- 21.Inman DJ, Silver SM, Doghramji K. Sleep disturbance in posttraumatic stress disorder: a comparison with non-PTSD insomnia. J Trauma Stress. 1990;3:429–37. [Google Scholar]
- 22.Pigeon WR, Campbell CE, Possemato K, Ouimette P. Longitudinal relationships of insomnia, nightmares, and PTSD severity in recent combat veterans. J Psychosom Res. 2013;75:546–50. doi: 10.1016/j.jpsychores.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry. 2004;61:508–16. doi: 10.1001/archpsyc.61.5.508. [DOI] [PubMed] [Google Scholar]
- 24.Mohlenhoff BS, Chao LL, Buckley ST, Weiner MW, Neylan TC. Are hippocampal size differences in posttraumatic stress disorder mediated by sleep pathology? Alzheimers Dement. 2014;10:S146–54. doi: 10.1016/j.jalz.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavie P, Katz N, Pillar G, Zinger Y. Elevated awaking thresholds during sleep: characteristics of chronic war-related posttraumatic stress disorder patients. BPS. 1998;44:1060–5. doi: 10.1016/s0006-3223(98)00037-7. [DOI] [PubMed] [Google Scholar]
- 26.Keage HAD, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13:886–92. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. doi: 10.5665/sleep.2802. https://doi.org/10.5665/sleep.2802. [DOI] [PMC free article] [PubMed]
- 28.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–9. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 29.Dow BM, Kelsoe JR, Gillin JC. Sleep and dreams in Vietnam PTSD and depression. BPS. 1996;39:42–50. doi: 10.1016/0006-3223(95)00103-4. [DOI] [PubMed] [Google Scholar]
- 30.Fuller KH, Waters WF, Scott O. An investigation of slow-wave sleep processes in chronic PTSD patients. J Anxiety Disord. 1994;8:227–36. [Google Scholar]
- 31.Germain A, Nielsen TA. Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare sufferers. Biol Psychiatry. 2003;54:1092–8. doi: 10.1016/s0006-3223(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 32.Mellman TA, Kobayashi I, Lavela J, Wilson B, Hall Brown TSA. Relationship between REM sleep measures and the duration of posttraumatic stress disorder in a young adult urban minority population. Sleep. doi: 10.5665/sleep.3922. https://doi.org/10.5665/sleep.3922. [DOI] [PMC free article] [PubMed]
- 33.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 34.Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, et al. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17:723–32. doi: 10.1093/sleep/17.8.723. [DOI] [PubMed] [Google Scholar]
- 35.Gehrman P, Seelig AD, Jacobson IG, Boyko EJ, Hooper TI, Gackstetter GD, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. doi: 10.5665/sleep.2798. https://doi.org/10.5665/sleep.2798. [DOI] [PMC free article] [PubMed]
- 36.McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann N YAcad Sci. 1997;821:271–84. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- 37.Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, et al. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry. 2011;69:541–8. doi: 10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–81. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34:1181–8. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Chao L, Weiner M, Neylan T. Regional cerebral volumes in veterans with current versus remitted posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2013;213:193–201. doi: 10.1016/j.pscychresns.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50:952–9. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, et al. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. 2008;162:147–57. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–51. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. BPS. 2002;52:1089–101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 45.Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Acheson DT, Gresack JE, Risbrough VB. Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology. 2012;62:674–85. doi: 10.1016/j.neuropharm.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Admon R, Milad MR, Hendler T. A causal model of posttraumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–47. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Sapolsky RM. Chickens, eggs and hippocampal atrophy. Nat Neurosci. 2002;5:1111–3. doi: 10.1038/nn1102-1111. [DOI] [PubMed] [Google Scholar]
- 50.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardenas VA, Samuelson K, Lenoci M, Studholme C, Neylan TC, Marmar CR, et al. Changes in brain anatomy during the course of posttraumatic stress disorder. Psychiatry Res. 2011;193:93–100. doi: 10.1016/j.pscychresns.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novati A, Hulshof HJ, Granic I, Meerlo P. Chronic partial sleep deprivation reduces brain sensitivity to glutamate N-methyl-D-aspartate receptor-mediated neurotoxicity. J Sleep Res. 2012;21:3–9. doi: 10.1111/j.1365-2869.2011.00932.x. [DOI] [PubMed] [Google Scholar]
- 53.Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30:955–8. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taki Y, Hashizume H, Thyreau B, et al. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. NeuroImage. 2012;60:471–5. doi: 10.1016/j.neuroimage.2011.11.072. [DOI] [PubMed] [Google Scholar]
- 55.Noh HJ, Joo EY, Kim ST, Yoon SM, Koo DL, Kim D, et al. The relationship between hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol. 2012;8:130–8. doi: 10.3988/jcn.2012.8.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winkelman JW, Benson KL, Buxton OM, Lyoo IK, Yoon S, O’Connor S, et al. Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010;11:576–82. doi: 10.1016/j.sleep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neylan TC, Mueller SG, Wang Z, Metzler TJ, Lenoci M, Truran D, et al. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. BPS. 2010;68:494–6. doi: 10.1016/j.biopsych.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chao LL, Mohlenhoff BS, Weiner MW, Neylan TC. Associations between subjective sleep quality and brain volume in gulf war veterans. Sleep. doi: 10.5665/sleep.3472. https://doi.org/10.5665/sleep.3472. [DOI] [PMC free article] [PubMed]
- 60.Zorumski CF, Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci Biobehav Rev. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis CJ, Meighan PC, Taishi P, Krueger JM, Harding JW, Wright JW. REM sleep deprivation attenuates actin-binding protein cortactin: a link between sleep and hippocampal plasticity. Neurosci Lett. 2006;400:191–6. doi: 10.1016/j.neulet.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 62.Hagewoud R, Havekes R, Novati A, Kaijser JN, Van der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2010;19:280–8. doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 63.Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007;148:325–33. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci. 2005;22:2111–6. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- 65.Guzman-Marin R, Suntsova N, Bashir T, Nienhuis R, Szymusiak R, McGinty D. Rapid eye movement sleep deprivation contributes to reduction of neurogenesis in the hippocampal dentate gyrus of the adult rat. Sleep. 2008;31:167–75. doi: 10.1093/sleep/31.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hairston IS. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 67.Chang H-M, Liao W-C, Sheu J-N, Chang C-C, Lan C-T, Mai F-D. Sleep deprivation impairs Ca2+ expression in the hippocampus: ionic imaging analysis for cognitive deficiency with TOF-SIMS. Microsc Microanal. 2012;18:425–35. doi: 10.1017/S1431927612000086. [DOI] [PubMed] [Google Scholar]
- 68.de Souza L, Smaili SS, Ureshino RP, Sinigaglia-Coimbra R, Andersen ML, Lopes GS, et al. Effect of chronic sleep restriction and aging on calcium signaling and apoptosis in the hippocampus of young and aged animals. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39:23–30. doi: 10.1016/j.pnpbp.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 69.Chen C, Hardy M, Zhang J, LaHoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–40. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 70.Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–7. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- 71.Kim EY, Mahmoud GS, Grover LM. REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neurosci Lett. 2005;388:163–7. doi: 10.1016/j.neulet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 72.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–95. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sportiche N, Suntsova N, Methippara M, Bashir T, Mitrani B, Szymusiak R, et al. Sustained sleep fragmentation results in delayed changes in hippocampal-dependent cognitive function associated with reduced dentate gyrus neurogenesis. Neuroscience. 2010;170:247–58. doi: 10.1016/j.neuroscience.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Süer C, Dolu N, Artis AS, Sahin L, Yilmaz A, Cetin A. The effects of long-term sleep deprivation on the long-term potentiation in the dentate gyrus and brain oxidation status in rats. Neurosci Res. 2011;70:71–7. doi: 10.1016/j.neures.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–48. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eichenbaum H. What hm taught us. J Cogn Neurosci. 2013;25:14–21. doi: 10.1162/jocn_a_00285. [DOI] [PubMed] [Google Scholar]
- 77.Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. 2001;5:423–45. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- 78.Brownlow JA, Hall Brown TS, Mellman TA. Relationships of posttraumatic stress symptoms and sleep measures to cognitive performance in young-adult African Americans. J Trauma Stress. 2014;27:217–23. doi: 10.1002/jts.21906. [DOI] [PubMed] [Google Scholar]
- 79.Brewin CR, Kleiner JS, Vasterling JJ, Field AP. Memory for emotionally neutral information in posttraumatic stress disorder: a meta-analytic investigation. J Abnorm Psychol. 2007;116:448–63. doi: 10.1037/0021-843X.116.3.448. [DOI] [PubMed] [Google Scholar]
- 80.Johnsen GE, Kanagaratnam P, Asbjørnsen AE. Memory impairments in posttraumatic stress disorder are related to depression. J Anxiety Disord. 2008;22:464–74. doi: 10.1016/j.janxdis.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 81.Burriss L, Ayers E, Ginsberg J, Powell DA. Learning and memory impairment in PTSD: relationship to depression. Depress Anxiety. 2008;25:149–57. doi: 10.1002/da.20291. [DOI] [PubMed] [Google Scholar]
- 82.Scarmeas N, Stern Y. Cognitive reserve: implications for diagnosis and prevention of Alzheimer’s disease. Curr Neurol Neurosci Rep. 2004;4:374–80. doi: 10.1007/s11910-004-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 84.Buée L, Troquier L, Burnouf S, et al. From tau phosphorylation to tau aggregation: what about neuronal death? Biochem Soc Trans. 2010;38:967. doi: 10.1042/BST0380967. [DOI] [PubMed] [Google Scholar]
- 85.Huang Y, Potter R, Sigurdson W, et al. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol. 2012;69:51–8. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molano JRV, Roe CM, Ju Y-ES. The interaction of sleep and amyloid deposition on cognitive performance. J Sleep Res. 2016;26:288–92. doi: 10.1111/jsr.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013 doi: 10.1001/jamaneurol.2013.4258. https://doi.org/10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed]
- 89.Weiner MW, Veitch DP, Hayes J, et al. Effects of traumatic brain injury and posttraumatic stress disorder on Alzheimer’s disease in veterans, using the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 2014;10:S226–35. doi: 10.1016/j.jalz.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sprecher KE, Koscik RL, Carlsson CM, et al. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology. 2017 doi: 10.1212/WNL.0000000000004171. https://doi.org/10.1212/WNL.0000000000004171-9. [DOI] [PMC free article] [PubMed]
- 91.Donovan AO, Neylan TC, Metzler T, Cohen BE. Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain Behav Immun. 2012;26:642–9. doi: 10.1016/j.bbi.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–55. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 93.Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Pace TWW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Baker DG, Nievergelt CM, O’Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62:663–73. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 96.O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, et al. Transcriptional control of monocyte gene expression in posttraumatic stress disorder. Dis Markers. 2011;30:123–32. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97•.O’Donovan A, Chao LL, Paulson J, Samuelson KW, Shigenaga JK, Grunfeld C, et al. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendo crinology. 2015;51:557–66. doi: 10.1016/j.psyneuen.2014.11.010. Greater PTSD symptom burden was associated in this study with higher levels of serum tumor necrosis factor, which, in turn, was associated with smaller hippocampal volumes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM, et al. C-reactive protein, Interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55:74–8. doi: 10.1016/j.cyto.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 99.Söndergaard HP, Hansson L-O, Theorell T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta. 2004;342:93–8. doi: 10.1016/j.cccn.2003.12.019. [DOI] [PubMed] [Google Scholar]
-
100••.Passos IC, Vasconcelos-Moreno MP, Costa LG. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–12. doi: 10.1016/S2215-0366(15)00309-0. https://doi.org/10.1016/S2215-0366(15)00309-0. This thorough and thoughtful meta-analysis and meta-regression included 20 studies of inflammatory markers in PTSD and found that PTSD was associated with higher levels of IL-6, IL-1 and interferon. Additionally, TNF-α was higher in subjects with PTSD only in a subgroup analysis of patients who were not on psychotropic medications, IL-6 was associated with illness severity but not with illness duration and Illness duration was associated with IL-1
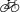 levels. This review includes tables outlining the literature on inflammation and PTSD. [DOI] [PubMed] [Google Scholar]
levels. This review includes tables outlining the literature on inflammation and PTSD. [DOI] [PubMed] [Google Scholar] - 101.Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–19. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J, Song Y, Yang J, Zhang Y, Zhao P, Zhu X-J, et al. The contribution of TNF-α in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci Lett. 2013;541:275–80. doi: 10.1016/j.neulet.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 103.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Engler H, Doenlen R, Engler A, Riether C, Prager G, Niemi M-B, et al. Acute amygdaloid response to systemic inflammation. Brain Behav Immun. 2011;25:1384–92. doi: 10.1016/j.bbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 105.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage. 2012;59:3222–6. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression. JAMA Psychiatry. 2013;70:31–24. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 108.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dekaris D, Sabioncello A, Mažuran R, Rabatić S. Multiple changes of immunologic parameters in prisoners of war: assessments after release from a camp in manjača, bosnia. JAMA. 1993;270:595–9. [PubMed] [Google Scholar]
- 110.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 111.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2011;16:622–53. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 112.Donovan AO, Ahmadian AJ, Neylan TC, Pacult MA, Edmondson D, Cohen BE. Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inflammation in the Mind Your Heart Study. Brain Behav Immun. 2017;60:198–205. doi: 10.1016/j.bbi.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004;61:668–72. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 114.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of Alzheimer disease the Framingham study. Neurology. 2007;68:1902–8. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 115.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–47. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 116.Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology. 2010;74:995–1002. doi: 10.1212/WNL.0b013e3181d5a4a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tobinick E. Deciphering the physiology underlying the rapid clinical effects of perispinal etanercept in Alzheimer’s disease. Curr Alzheimer Res. 2012;9:99–109. doi: 10.2174/156720512799015073. [DOI] [PubMed] [Google Scholar]
- 118.Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006;29:518–27. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 119.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–7. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 120.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 122••.Marsland AL, Gianaros PJ, Kuan DCH, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195–204. doi: 10.1016/j.bbi.2015.03.015. While not directly related to PTSD, this study of 408 subjects links peripheral inflammation to poorer performance in multiple cognitive domains as well as smaller volumes of brain structures crucial to learning and memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–56. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 124.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia aging study. Ann Neurol. 2002;52:168–74. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 125.Rogers J, Griffin WST. Inflammatory mechanisms of Alzheimer’s disease In: Wood P, editor Neuroinflammation: mechanisms and management. New York: Springer Science+Business Media; 1998. pp. 177–93. [Google Scholar]
- 126.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun. 2002;16:503–12. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 128.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 129.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–84. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barr T, Livingston W, Guardado P, Baxter T, Mysliwiec V, Gill J. Chapter 8 military personnel with traumatic brain injuries and insomnia have reductions in PTSD and improved perceived health following sleep restoration: a relationship moderated by inflammation. Annu Rev Nurs Res. 2015;33:249–66. doi: 10.1891/0739-6686.33.249. [DOI] [PubMed] [Google Scholar]
- 132.Fu S, McFall M, Saxon A, Beckham J, Carmody T, Baker D, et al. Post-traumatic stress disorder and smoking: a systematic review. Nicotine Tob Res. 2007;9:1071–84. doi: 10.1080/14622200701488418. [DOI] [PubMed] [Google Scholar]
- 133.Kofoed L, Friedman MJ, Peck R. Alcoholism and drug abuse in patients with PTSD. Psychiatry Q. 1993;64:151–71. doi: 10.1007/BF01065867. [DOI] [PubMed] [Google Scholar]
- 134.Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2014;19:175–82. doi: 10.1007/s11325-014-0984-y. [DOI] [PubMed] [Google Scholar]
- 135.Jaoude P, Vermont LN, Porhomayon J, El-Solh AA. Sleep-disordered breathing in patients with post-traumatic stress disorder. Ann ATS. 2015;12:259–68. doi: 10.1513/AnnalsATS.201407-299FR. [DOI] [PubMed] [Google Scholar]
- 136.O’Hara R, Luzon A, Hubbard J, Zeitzer JM. Sleep apnea, apolipoprotein epsilon 4 allele, and TBI: mechanism for cognitive dysfunction and development of dementia. JRRD. 2009;46:837. doi: 10.1682/jrrd.2008.10.0140. [DOI] [PubMed] [Google Scholar]
- 137.van Liempt S. Sleep disturbances and PTSD: a perpetual circle? Eur J Psychotraumatol. 2012;3:291. doi: 10.3402/ejpt.v3i0.19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zanigni S, Calandra-Buonaura G, Grimaldi D, Cortelli P. REM behaviour disorder and neurodegenerative diseases. Sleep Med. 2011;12:S54–8. doi: 10.1016/j.sleep.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 139.Gagnon J-F, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5:424–32. doi: 10.1016/S1474-4422(06)70441-0. [DOI] [PubMed] [Google Scholar]
- 140.Boeve BF, Saper CB. REM sleep behavior disorder: a possible early marker for synucleinopathies. Neurology. 2006;66:796–7. doi: 10.1212/01.wnl.0000209264.61035.bb. [DOI] [PubMed] [Google Scholar]
- 141.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–51. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]


