Abstract
This study was performed to evaluate the effect of maternal supplementation with seaweed powder (SWP) on the immune status of piglets. Sows were supplementary fed SWP from 85-days of gestation until delactation. Forty-days old piglets were euthanized and lymphocyte subsets were analyzed. The results showed a significantly higher relative population of CD4+CD8+ T cells in the thymus, lymph node, tonsil (P<0.05), peripheral blood mononuclear cells, spleen and liver (P<0.01) of piglets derived from treated sows. A higher relative population of CD8+ T cells was also observed in the liver and spleen (P<0.05) of the piglets. The data suggested the enhancing effects of maternal supplementation with SWP on immune status of piglets.
Keywords: lymphocyte subset, maternal supplementation, piglet, seaweed powder
Irregular and inadequate intake of colostrum is the likely cause of most pre-weaning losses in the swine industry. The placental structure in pigs (diffuse, epitheliochorial) does not allow maternal antibody to transfer through the placenta and neonatal piglets receive their first maternal antibodies from colostrum [11]. The immune stimulatory content of colostrum and milk, including antibodies, antimicrobials, lymphocytes, and a variety of cells, is very important for the neonatal immune system [17, 18]. The colostrum passes through the intestinal epithelium to reach the immune organs through blood circulation and can have both stimulatory and modulatory effects on the neonate’s immune system [1, 4]. Therefore, we hypothesize that food supplements added to the sow’s diet could enrich the colostrum and lead to improved health and immunity in the piglets.
Seaweed has been shown to have a great effect on growth and immunity in piglets [12]. Seaweed and seaweed powder (SWP) are sources of bioactive compounds, containing laminarin and fucoidan [2]. Oral administration of seaweed and licorice enhanced both humoral and cellular immune functions in swine [10]. It is believed that laminarin is ingested through colostrum and milk, internalized by the intestinal epithelium and finally reaches the lymphoid organs in piglets [15].
This study was designed to investigate the effect of maternal SWP supplementation on the immune status of the lymphoid organs and liver of piglets. Immune status of piglets was significantly improved by maternal SWP supplementation compared to that of piglets derived from non-supplemented sows.
Twelve crossbred LWD [crossbred female LW (female Landrace and male Large Yorkshire) crossed with a male Duroc] pregnant sows (Sus scrofa domesticus) were randomly assigned into two groups. The first group (control) was fed a basal diet (Kumiai mixed feed; Minami Nihon Kumiai Siryo Co., Ltd., Kagoshima, Japan) only, but the second group (treated) was fed a basal diet supplemented with 30 g/day SWP (ALGIT®; Shinkyo Industries Inc., Yamaguchi, Japan) from 85-days of gestation up to delactation. The nutrient composition of basal diet was as follows: crude protein, >14.5%; total digestible nutrients (TDN), >75.0%. The diet was given twice a day at 8:00 and 15:00 and individual feed intake was measured. Drinking water was also available ad libitum throughout the study. Sows and piglets were housed together in farrowing pens throughout the lactation period (25 ± 1-days), and then piglets were moved to a weaning barn. From newborn to 21-days old, piglets fed commercial diet (HP kobuta etsuke: crude protein, >22.5%; TDN, >90.0%; Minami Nihon Kumiai Siryo Co., Ltd.) and then fed another commercial diet (HP kobuta hatsurastu: crude protein, >21.0%; TDN, >87.0%; Minami Nihon Kumiai Siryo Co., Ltd.). Sows and piglets were raised at the Miyazaki Livestock Research Institute (Miyazaki, Japan). Twelve piglets derived from each group were euthanized at 40-days old. Piglets were sedated with an intramuscular injection of Mafoprazine mesylate (0.3–0.5 mg/kg, Mafropan® 1% injection; DS Pharma Animal Health Co., Ltd., Osaka, Japan) and intravascular injection of sodium pentobarbital (10 mg/kg, Somnopentyl®; Kyoritsu Seiyaku Co., Ltd., Tokyo, Japan). Cardiac arrest was performed using electric shock and the piglets were exsanguinated soon after the delivery of the electric shock. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miyazaki (No. 2013-009-5). Small chunks of liver, spleen, thymus, mesenteric lymph node (MLN), jejunal Peyer’s patch (JPP) and soft palate tonsil (SPT) were collected for immunohistochemistry (IHC) and flow cytometry (FACS). The specimens for IHC were mounted in OCT embedding compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan) on Cryomold (Sakura Finetek Japan Co., Ltd.), frozen on dry ice, and then stored at −80°C. Cryostat sections were stained with monoclonal antibodies (mAbs), as described below, by using the indirect immunoperoxidase technique [19]. Sections (8 µm thick) were air-dried on slides and fixed with ice-cold acetone for 10 min. To block any nonspecific binding, the sections were rehydrated in phosphate buffer saline (PBS) and incubated with 10% normal horse serum in PBS for 30 min. The sections were then stained with mAbs for 60 min and washed three times with PBS. After incubation with the secondary antibody (biotin labeled horse anti mouse IgG, Vector Lab., Burlingame, CA, U.S.A.) endogenous peroxidase was quenched with 0.3% H2O2 in methanol for 30 min followed by incubation with ABC complex (Vector Lab.) for 15 min. After the sections were rinsed three times in PBS, the reactions were made visible with metal-enhanced diaminobenzidine (DAB, Pierce, Rockford, IL, U.S.A.). All immunohistochemical staining was performed at room temperature in a moist chamber. Control staining, in which the primary antibody was replaced with PBS, was performed simultaneously. No positive staining was found in the control slides (data not shown). For FACS analysis, the cell suspensions were prepared by mincing in ice cold Hank’s balanced salts solution (HBSS, Sigma-Aldrich, St. Louis, MO, U.S.A.) and straining to remove residual tissue. The peripheral blood mononuclear cells (PB) were purified by density gradient centrifugation with Ficoll-Paque (GE Healthcare UK Ltd., Little Chalfont, Buckinghamshire, U.K.). Red blood cells were removed using NH4Cl lysis buffer. For immunofluorescence assays, cells were suspended in PBS supplemented with 0.5% bovine serum albumin and 0.05% sodium azide (BSA-PBS). Viable cells ranging from 1 × 105 to 1 × 106 were incubated with fluorescently labeled mAbs as described below at 4°C for 1 hr. The stained cells were washed three times with BSA-PBS and re-suspended in BSA-PBS containing propidium iodide (1 µg/ml Sigma Aldrich). The relative immunofluorescence intensities were determined by multicolor FACS with a FACS CantoTM II system (Becton Dickinson, Franklin Lakes, NJ, U.S.A.). Anti-CD4 (× 200 dilution, clone number: PT90A, Monoclonal Antibody Center at Washington State University, Pullman, WA, U.S.A.), anti-CD8 (× 200 dilution, clone number: PT36B, Monoclonal Antibody Center at Washington State University), anti-γδ (× 100 dilution, clone number: PGBL22A, Monoclonal Antibody Center at Washington State University) and anti-MHC class II (× 200 dilution, clone number: P-TH81A5, Monoclonal Antibody Center at Washington State University) mAbs were used for IHC and FACS. For fluorescent labeling of mAbs, FITC Labeling Kit-NH2, HiLyteTM Fluor 555 Labeling Kit-NH2, and HiLyteTM Fluor 647 Labeling Kit-NH2 (Dojindo Laboratories, Kumamoto, Japan) were used according to manufacturer’s instructions. Data were analyzed using the statistical software package SPSS for Windows (Version 20.0; SPSS Inc., Chicago, IL, U.S.A.). Student’s t-test was used to determine significant differences between the experimental groups. Results are expressed as mean ± SD. P-values <0.05 were regarded as statistically significant.
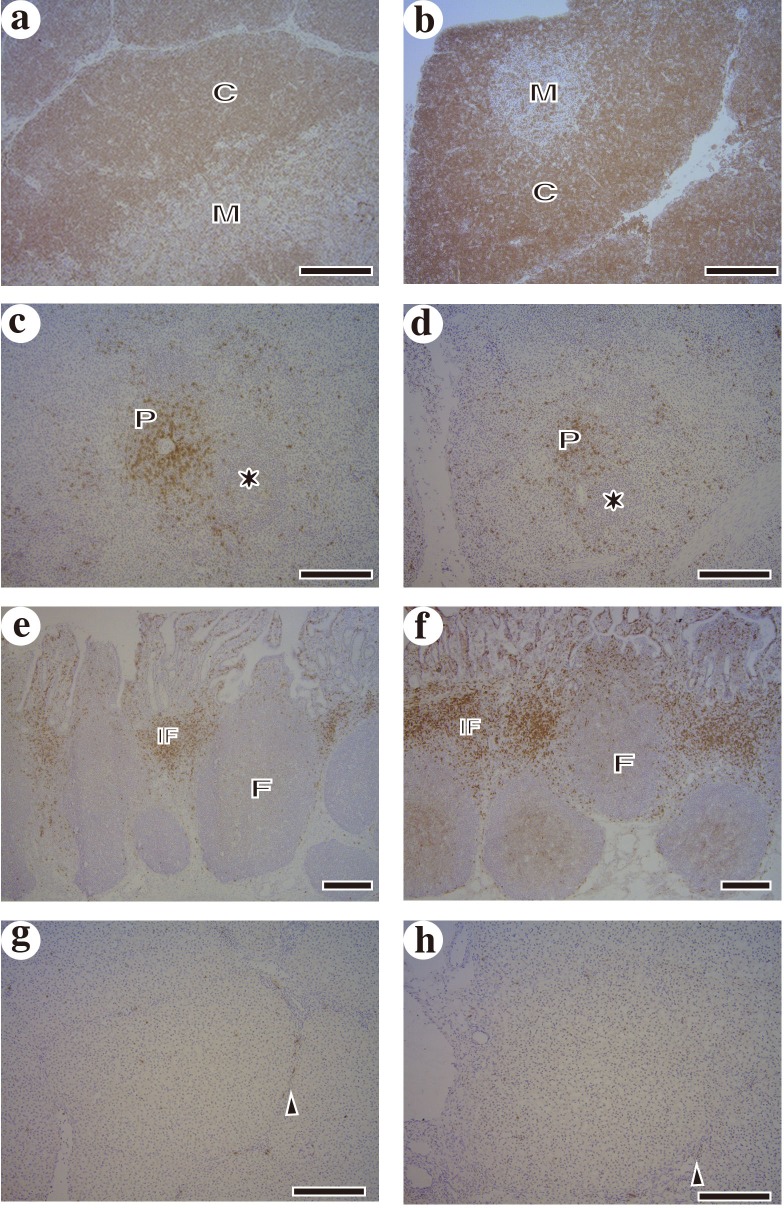
There is no significant difference in the number of offspring and feed intake of sows during lactation between the groups as shown in Table 1. Piglets daily weight gain and body weight of 40-days old did not show significant difference between groups (Table 1). In addition, the size of ileal Peyer’s patch of piglets was also evaluated but there is no significant difference between groups (data not shown). In the immunohistochemical observation, the distributions and numbers of CD8+ T cells in the thymus, spleen, JPP and liver were not significantly different between groups as shown in Fig. 1. In addition, the distributions and numbers of CD8+ T cells, CD4+ T cells, γδ+ T cells and MHC class II+ cells in examined organs were not significantly different between groups (data not shown). However, the relative populations of CD4+CD8+ T cells in the thymus, MLN, SPT (P<0.05), PB, spleen and liver (P<0.01) of piglets derived from treated sows were significantly higher than those of control piglets (Table 2). The relative populations of CD8+ T cells in the liver and spleen (P<0.05) of piglets derived from treated sows were also significantly higher than those of control piglets. Furthermore, the relative population of γδ+ T cells was significantly increased in the JPP (P<0.05) of piglets derived from treated sows. But the relative population of γδ+ T cells in the thymus was decreased (P<0.05) in piglets derived from treated sows. In addition, the CD4/CD8 ratio in these organs was not significantly different between groups.
Table 1. Effects of seaweed powder supplementation on litter size, feed intake of sows and body weight of piglets.
| Control | Treated | |
|---|---|---|
| Litter size (head) | 12.1 ± 4.3 | 12.9 ± 1.6 |
| Feed intake of sows during lactation (kg) | 113.0 ± 22.0 | 130.5 ± 12.2 |
| Piglets daily weight gaina) (kg) | 0.24 ± 0.03 | 0.21 ± 0.03 |
| Piglets body weight on 40-days old (kg) | 16.7 ± 1.1 | 15.5 ± 1.1 |
Data indicate mean ± SD. a) The data analyzed from newborn to 21-days old piglets.
Fig. 1.
Distribution of CD8+ T cells in the thymus (a and b), spleen (c and d), JPP (e and f) and liver (g and h). Left column (a, c, e and g) and right column (b, d, f and h) show control piglets and piglets derived from SWP treated sow, respectively. Distribution of CD8+ T cells in these organs is not significantly different between groups. M: medulla, C: cortex, P: periarterial lymphatic sheath, asterisk: germinal center, F: lymphatic follicle, IF: interfollicular area, arrowhead: perivascular fibrous capsule. Bar=200 µm.
Table 2. Relative T cell and B cell populations isolated from lymphoid organs of maternal supplemented seaweed powder treated and control piglets.
| Organs | Groups | Subsets (%) |
||||
|---|---|---|---|---|---|---|
| CD4 | CD8 | CD4CD8 | γδ | MHC class II | ||
| Thymus | Control | 55.7 ± 22.4 | 64.3 ± 25.4 | 50.6 ± 19.3 | 5.5 ± 6.2a) | 24.5 ± 18.9 |
| Treated | 66.9 ± 13 | 74.0 ± 16.4 | 62.4 ± 11.1a) | 1.2 ± 1.0 | 23.3 ± 13.7 | |
| Spleen | Control | 20.6 ± 6.7 | 25.1 ± 4.1 | 4.5 ± 1.5 | 17.9 ± 6.2 | 40.6 ± 11.8 |
| Treated | 20.0 ± 7.1 | 34.8 ± 12.3a) | 6.5 ± 1.7b) | 20.9 ± 5.2 | 44.6 ± 11.3 | |
| MLN | Control | 33.6 ± 11.8 | 25.1 ± 11 | 2.9 ± 1.6 | 8.9 ± 6.3 | 46.1 ± 10.8 |
| Treated | 34.1 ± 8.4 | 26.5 ± 9.5 | 4.6 ± 1.9a) | 13.5 ± 11.8 | 49.7 ± 15.1 | |
| JPP | Control | 12.8 ± 5.8 | 12.4 ± 6.2 | 2.6 ± 1.1 | 11.1 ± 10.2 | 61.5 ± 12.6 |
| Treated | 13.3 ± 7.5 | 15.2 ± 10.4 | 3.8 ± 2.5 | 24.8 ± 19.3a) | 50.6 ± 20.9 | |
| SPT | Control | 18.4 ± 4.9 | 10.2 ± 4.3 | 4.5 ± 1.9 | 9.1 ± 7.6 | 69.8 ± 13.9 |
| Treated | 20.7 ± 7.6 | 14.4 ± 8.2 | 7.6 ± 4.0a) | 16.1 ± 14.1 | 71.3 ± 10.1 | |
| Liver | Control | 13.5 ± 5.4 | 32.1 ± 5.6 | 3.2 ± 1.4 | 12.5 ± 6.8 | 30.7 ± 12.7 |
| Treated | 19.7 ± 5.5 | 48.2 ± 6.9a) | 8.1 ± 4.2b) | 12.3 ± 2.4 | 30.5 ± 8.5 | |
| PB | Control | 10.8 ± 4.4 | 51.5 ± 8.4 | 2.6 ± 1.7 | 10.6 ± 5.2 | 19.7 ± 5.1 |
| Treated | 14.8 ± 5.4 | 55.1 ± 8.4 | 5.2 ± 1.6 b) | 11.0 ± 3.7 | 16.8 ± 4.5 | |
Data indicate mean ± SD; a) P<0.05, b) P<0.005; MLN: mesenteric lymph node; JPP: jejunal Peyer’s patch; SPT: soft palate tonsil, PB: peripheral blood.
Neonatal piglets are at high risk in the new environment soon after birth and their survival greatly depends on the maternal immunity provided in the colostrum. Therefore, the best way to enhance the immune system of neonates is to ensure that they are given a sufficient volume of good quality colostrum. Seaweed and SWP have previously been shown to be good immune enhancers [12]. Heim et al. has shown that seaweed-derived polysaccharides supplemented in sows fed from 83-days of gestation until weaning improved piglets’ immune status and growth at weaning [8]. In this study, the results indicated that piglets derived from treated sows have an increased relative population of CD4+CD8+ in the thymus, spleen, MLN, SPT, liver and PB. CD8+ T cells were also increased in the liver and spleen. Besides CD4+ as helper and CD8+ as cytotoxic T cells, peripheral CD4+CD8+ are very important T cell populations in swine immunity because they are antigen specific memory T helper cells that result from extra-thymic maturation of naïve T-helper cells [16]. In addition, these cells have been proposed to have several important roles of suppressive, anti-tumor, cytotoxic and anti-viral activity in swine immune system [13, 14, 16, 20]. This population is almost zero in newborn piglets but steadily increases with the age of piglets and comprises between 60 to 80% of the T cell population [5]. Therefore, a significant increase in the population of CD4+CD8+ T cells in the spleen, MLN, SPT and PB of piglets might be a good sign of immune enhancement resulting from maternal supplementation with SWP. In addition, the relative population of γδ+ T cells in JPP of piglets derived from treated sow was significantly higher than those of control piglets. The function of γδ+ T cells is the protection of epithelial surfaces from microorganisms and as mediators of mucosal tolerance [6, 7]. In our previous report, γδ+ T cells were shown to accumulate in the dome region of calf JPP and villi with age [19]. In this study, we evaluated the lymphocyte subsets not only in the lymphoid organ but also in the liver. In the liver of piglets derived from treated sow, the relative populations of CD4+CD8+ T cells and CD8+ T cells were higher than those of control piglets. Liver is the largest organ in animal body and has essential function of protein synthesis and metabolism. However, liver is also responsible for the removal of pathogens and exogenous antigens from circulation via the portal vein. Therefore, the study of the liver immunity which is concerned with immune surveillance and tolerance of animal body has been done [3, 9]. In this study, significant differences of lymphocyte subsets were found in the liver between groups. We speculate that appropriate stimulation by beneficial bacteria or food additives can enhance liver immunity. It needs further comparative study about nutrient and microelement of colostrum between groups for clarification of enhancement of piglet’s immunity.
In conclusion, our data suggested that maternally supplemented SWP could stimulate immune status of piglets. It might be crucial for piglet survival in a new environment, especially during weaning.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (No.16K08052) from the Ministry of Education, Science, Sport and Culture, Japan.
REFERENCES
- 1.Bandrick M., Ariza-Nieto C., Baidoo S. K., Molitor T. W.2014. Colostral antibody-mediated and cell-mediated immunity contributes to innate and antigen-specific immunity in piglets. Dev. Comp. Immunol. 43: 114–120. doi: 10.1016/j.dci.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berteau O., Mulloy B.2003. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 13: 29R–40R. doi: 10.1093/glycob/cwg058 [DOI] [PubMed] [Google Scholar]
- 3.Bogdanos D. P., Gao B., Gershwin M. E.2013. Liver immunology. Compr. Physiol. 3: 567–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans P. A., Newby T. J., Stokes C. R., Bourne F. J.1982. A study of cells in the mammary secretions of sows. Vet. Immunol. Immunopathol. 3: 515–527. doi: 10.1016/0165-2427(82)90017-4 [DOI] [PubMed] [Google Scholar]
- 5.Gerner W., Käser T., Saalmüller A.2009. Porcine T lymphocytes and NK cells--an update. Dev. Comp. Immunol. 33: 310–320. doi: 10.1016/j.dci.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 6.Hänninen A., Harrison L. C.2000. γ δ T cells as mediators of mucosal tolerance: the autoimmune diabetes model. Immunol. Rev. 173: 109–119. doi: 10.1034/j.1600-065X.2000.917303.x [DOI] [PubMed] [Google Scholar]
- 7.Haas W., Pereira P., Tonegawa S.1993. Gamma/delta cells. Annu. Rev. Immunol. 11: 637–685. doi: 10.1146/annurev.iy.11.040193.003225 [DOI] [PubMed] [Google Scholar]
- 8.Heim G., O’Doherty J. V., O’Shea C. J., Doyle D. N., Egan A. M., Thornton K., Sweeney T.2015. Maternal supplementation of seaweed-derived polysaccharides improves intestinal health and immune status of suckling piglets. J. Nutr. Sci. 4: e27. doi: 10.1017/jns.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenne C. N., Kubes P.2013. Immune surveillance by the liver. Nat. Immunol. 14: 996–1006. doi: 10.1038/ni.2691 [DOI] [PubMed] [Google Scholar]
- 10.Katayama M., Fukuda T., Okamura T., Suzuki E., Tamura K., Shimizu Y., Suda Y., Suzuki K.2011. Effect of dietary addition of seaweed and licorice on the immune performance of pigs. Anim. Sci. J. 82: 274–281. doi: 10.1111/j.1740-0929.2010.00826.x [DOI] [PubMed] [Google Scholar]
- 11.Le Dividich J., Rooke J. A., Herpin P.2005. Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Sci. 143: 469–485. doi: 10.1017/S0021859605005642 [DOI] [Google Scholar]
- 12.Makkar H. P. S., Tran G., Heuzè V., Giger-Reverdin S., Lessire M., Lebas F., Ankers P.2016. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 212: 1–17. doi: 10.1016/j.anifeedsci.2015.09.018 [DOI] [Google Scholar]
- 13.Nascimbeni M., Shin E. C., Chiriboga L., Kleiner D. E., Rehermann B.2004. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood 104: 478–486. doi: 10.1182/blood-2003-12-4395 [DOI] [PubMed] [Google Scholar]
- 14.Overgaard N. H., Jung J. W., Steptoe R. J., Wells J. W.2015. CD4+/CD8+ double-positive T cells: more than just a developmental stage? J. Leukoc. Biol. 97: 31–38. doi: 10.1189/jlb.1RU0814-382 [DOI] [PubMed] [Google Scholar]
- 15.Rice P. J., Adams E. L., Ozment-Skelton T., Gonzalez A. J., Goldman M. P., Lockhart B. E., Barker L. A., Breuel K. F., Deponti W. K., Kalbfleisch J. H., Ensley H. E., Brown G. D., Gordon S., Williams D. L.2005. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 314: 1079–1086. doi: 10.1124/jpet.105.085415 [DOI] [PubMed] [Google Scholar]
- 16.Saalmüller A., Werner T., Fachinger V.2002. T-helper cells from naive to committed. Vet. Immunol. Immunopathol. 87: 137–145. doi: 10.1016/S0165-2427(02)00045-4 [DOI] [PubMed] [Google Scholar]
- 17.Salmon H., Berri M., Gerdts V., Meurens F.2009. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 33: 384–393. doi: 10.1016/j.dci.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 18.Wagstrom E. A., Yoon K. J., Zimmerman J. J.2000. Immune components in porcine mammary secretions. Viral Immunol. 13: 383–397. doi: 10.1089/08828240050144699 [DOI] [PubMed] [Google Scholar]
- 19.Yasuda M., Ogawa D., Nasu T., Yamaguchi T., Murakami T.2005. Kinetics and distribution of bovine gammadelta T-lymphocyte in the intestine: gammadelta T cells accumulate in the dome region of Peyer’s patch during prenatal development. Dev. Comp. Immunol. 29: 555–564. doi: 10.1016/j.dci.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 20.Zuckermann F. A.1999. Extrathymic CD4/CD8 double positive T cells. Vet. Immunol. Immunopathol. 72: 55–66. doi: 10.1016/S0165-2427(99)00118-X [DOI] [PubMed] [Google Scholar]