Abstract
In the swine industry, Lawsonia intracellularis is one of the main enteric pathogens; it causes acute intestinal hemorrhage (proliferative hemorrhagic enteropathy) in naïve adult pigs and a wasting disease (proliferative enteropathy) in growing pigs. Among many kinds of cytokines, interferon-γ (IFN-γ) has previously been reported to play a significant role in limiting intracellular infection and increasing cellular proliferation associated with L. intracellularis. However, the levels of various circulating inflammatory cytokines, including IFN-γ, in animals infected with L. intracellularis is still an area of considerable interest for understanding immunity against this bacterium. In addition, there has been no information on cytokine response in animals infected with any L. intracellularis isolate of South Korean origin or Asian origin. To determine the relationship between the changes in the systemic inflammatory cytokine response in the peripheral blood of the host after L. intracellularis infection, we measured the levels of some pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IFN-γ), anti-inflammatory cytokines (IL-4, IL-10, and transforming growth factor-β (TGF-β)), and a chemokine (IL-8) in pigs infected with L. intracellularis isolated from South Korea. This study demonstrated that a L. intracellularis isolate of South Korean origin induced cytokine (TNF-α, IL-6, and IFN-γ) responses in infected animals within 15 days post-infection although the circulating levels of IL-4, IL-10, IL-8 and TGF-β were induced relatively late.
Keywords: antibody, cytokine response, diagnosis, infection, Lawsonia intracellularis
Proliferative enteropathy (PE) is an infectious intestinal disease characterized by thickening of the distal small and proximal large intestinal mucosa as a result of enterocyte proliferation associated with the presence of an intracellular bacterium [25]. Lawsonia intracellularis is the etiological agent that causes PE in various species, especially pigs, horses, and hamsters. L. intracellularis is a highly fastidious obligate intracellular Gram-negative bacterium. In the swine industry, L. intracellularis is one of the main enteric pathogens; it causes acute intestinal hemorrhage (proliferative hemorrhagic enteropathy, PHE) in naïve adult pigs and a wasting disease (PE) in growing pigs [8, 13]. Variations in microbial virulence, the spectrum of symptoms, and antibody responses have been described for L. intracellularis infection or vaccination [1, 16, 17, 19].
Generally, cytokines are involved in many pathophysiological processes of the body. They act as intercellular messengers and exert potent biological effects at extremely low concentrations. Among various cytokines, interferon-γ (IFN-γ) has been reported to play a significant role in limiting intracellular infection and increasing cellular proliferation associated with L. intracellularis and so far most immunological researches on L. intracellularis infection have focused mainly on IFN-γ [2, 3, 20, 22]. The relevance of IFN-γ in L. intracellularis infection has been assessed mainly using IFN-γ receptor knockout mice [2, 22], normal pigs [3, 16, 17, 20, 21], and horses [18].
Certainly, IFN-γ is one of the most important immunological molecules because IFN-γ is produced in large amounts and is crucial for the control of infectious diseases. Although several papers on mucosal, systemic, or primed cell-mediated immune responses to L. intracellularis have recently been published [1, 16, 17, 21], the levels of various circulating inflammatory cytokines, including IFN-γ, in animals infected with L. intracellularis is still an area of considerable interest for understanding immunity against PE. In addition, there has been no information on cytokine response in animals infected with any L. intracellularis isolate of South Korean origin or Asian origin.
To determine the relationship between the changes in systemic inflammatory cytokine response in the peripheral blood and the immunity of the host after L. intracellularis infection, we measured the levels of pro-inflammatory cytokines (tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IFN-γ), anti-inflammatory cytokines (IL-4, IL-10, and transforming growth factor-β (TGF-β)), and a chemokine (IL-8, also known as NAP-1, AMCF-1, and CXCL8) in pigs infected with L. intracellularis isolated from South Korea. In addition, the results of this study may elucidate the effects of pro-inflammatory and anti-inflammatory cytokine response to L. intracellularis infection.
MATERIALS AND METHODS
Preparation of the pure L. intracellularis culture inoculum
Murine fibroblast-like McCoy cells (American Type Culture Collection [ATCC] CRL 1696) were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Invitrogen Corp., Carlsbad, CA, U.S.A.) with 1% glutamine (Gibco BRL, Invitrogen Corp.) and 5% fetal bovine serum (FBS) (Gibco BRL, Invitrogen Corp.) without antibiotics at 37°C in 5% CO2. The infection of McCoy cells with the L. intracellularis isolate PHE/KK421 (Korean Collection for Type Cultures [KCTC] 10686BP) has been described in detail elsewhere [26]. Briefly, the contents of a 1-ml cryogenic vial containing approximately 105L. intracellularis, at passage five from origin, were added to a 175 cm2 flask containing a 1-day-old, 30% confluent McCoy cell monolayer. The flask was placed in a container, which was then evacuated to 500 mmHg and refilled with medical-grade hydrogen. Half of the medium was replaced 3 days after infection. At 7 days after infection, the monolayer of highly infected cells was harvested, and the infection was passaged by scraping the monolayer with a cell scraper, centrifuging the scraped cells with the supernatant medium for 20 min at 3,400 ×g, resuspending the pellet in fresh medium, and infecting three 175 cm2 flasks containing 1-day-old, 30% confluent McCoy cell monolayers. The monolayer of McCoy cells highly infected with L. intracellularis was harvested, and the infection was passaged weekly, using the same technique described above. The number of flasks containing L. intracellularis-infected McCoy monolayers was tripled weekly from passage six to passage nine. At the last passage, bacteria were released from infected McCoy cells by 0.1% potassium chloride treatment and mechanical rupture by passage through a 20-gauge needle [12]. These bacteria were then combined with the bacteria present in the supernatant, homogenized briefly for 15 sec, and centrifuged for 20 min at 3,400 ×g. The bacteria pellet was resuspended in sucrose-potassium glutamate (pH 7.0) (SPG) solution containing 0.218 M sucrose, 0.0038 M KH2PO4, 0.0072 M K2HPO4 and 0.0049 M potassium glutamate with 5% FBS and kept at 4°C until pigs were inoculated later on the same day.
Quantification and microbiological screening of the inoculum
Quantification of the inoculum was accomplished by making serial 1:10 dilutions of each inoculum in phosphate-buffered saline (PBS; pH 7.2), coating 12-well glass slides (Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany) with 10 µl of each dilution, drying the slides at 37°C for 30 min, fixing with cold acetone, and staining by an indirect immunoperoxidase method using hyperimmune antiserum against L. intracellularis. The number of organisms per well from the highest countable dilution (50 to 500 organisms) was evaluated in duplicate using light microscopy.
Animals and experimental design
All animal procedures carried out in this study were reviewed, approved, and supervised by the Institutional Animal Care and Use Committee (IACUC) of National Veterinary Research and Quarantine Service (NVRQS, Anyang-si, Gyeonggi-do, Republic of Korea) (permit number: 2009-10-01). All procedures were conducted in accordance with the guidelines of the “Animal Care and Use Manual” of NVRQS. Ten 5-week-old L. intracellularis-seronegative pigs that had not been vaccinated for L. intracellularis, weighing between 10 and 15 kg, were obtained from a herd with no history or recorded case of PE; they were divided in 2 groups with 5 pigs per group. All of the pigs were bled prior to inoculation. Two days before challenge, serum and heparinized whole-blood samples were collected from all pigs and tested by an immunoperoxidase monolayer assay (IPMA), as reported in a previous paper [5, 7]. In addition, fecal samples were collected and tested for L. intracellularis DNA by PCR [9, 11, 24] to assure PE negativity. One day before the challenge, the animals were divided in two groups, randomized by weight: 5 pigs in the control group and 5 pigs in the infection group. Each group was housed in a different room in isolation barns at the NVRQS. The pigs were orally inoculated with the PHE/KK421 strain of L. intracellularis at 5.25 × 108 organisms of the L. intracellularis isolate PHE/KK421 in SPG with 5% FBS solution per pig on day 0. Pigs in the control group received the same volume of SPG solution. Animals in the control and infection groups were intra-gastrically dosed with 25 ml of the respective inoculum using a stomach tube. Pigs challenged with L. intracellularis and mock-infected pigs were euthanized on 40 days post-infection (DPI).
Clinical sign, fecal and serum sample analysis
Clinical signs based on the consistency of the feces were evaluated every five days. The clinical conditions of the pigs were observed and recorded for at least 10 min, including appetite, feces, body condition, and changes in behavior. The clinical scores ranged from 0 to 2, i.e., 0=normal; 1=slightly to moderately gaunt, depressed and appetite loss, or listless, but still standing; 2=severely gaunt, depressed, and recumbent. The fecal score was determined based on the following characteristics: 0=no diarrhea; 1=semi-solid feces with no blood; 2=watery without dark or bloody feces; 3=blood-tinged feces, loose or formed; and 4=profuse diarrhea with frank blood or dark tarry feces. PCR analysis was conducted on fecal samples every five days for a period of 40 DPI, as described in a previous paper [9, 11, 24]. In serum, anti-L. intracellularis-specific IgG concentration was measured by IPMA.
Cytokine bioassays
Peripheral blood samples for cytokine and chemokine analyses were obtained from the experimental animals, and sera were frozen at −70°C for batch analyses. The levels of TNF-α, IL-6, IFN-γ, IL-4, IL-10, TGF-β and IL-8 in the sera were determined using commercial ELISA kits (R & D Systems, Minneapolis, MN, U.S.A.) according to the manufacturer’s instructions. A standard curve was constructed using a known standard. All detections were performed in parallel.
Statistical analysis
Statistical significance was set at the 5% level, and two-sided P-values were calculated. Statistical analysis was performed by the paired t-test, and a P-value of <0.05 was accepted as statistically significant. The data are presented as the mean ± standard deviation. All data were analyzed using GraphPad PRISM software (version 6.07 for Windows; GraphPad Software Inc., La Jolla, CA, U.S.A.).
RESULTS
The concentration of the pathogenic L. intracellularis isolate PHE/KK421 in the inoculum was 2.1 × 107 organisms/ml, and the total dose per pig was 5.25 × 108 organisms. Before this exposure, all animals were found to be negative for L. intracellularis by fecal PCR and serology.
The results of the PCR testing of fecal samples are shown in Table 1. All of the pigs inoculated with L. intracellularis had persistent or intermittent diarrhea from 10 to 40 DPI. Fecal shedding was initially detected at 10 DPI and lasted, continuously (4 pigs in the infection group) or intermittently (1 pig in the infection group), up to 40 DPI with L. intracellularis, whereas fecal shedding was not detected in uninfected control pigs. The observation of persistent or intermittent diarrhea in animals in the infection group during the study agrees with the results reported for other experimental studies [7, 10]. In one previous study [23], fecal shedding was detected 10 weeks after experimental challenge of pigs with a pure culture of a European pathogenic L. intracellularis isolate. In a follow-up study at a commercial farm after an outbreak of the acute form of PE in the breeding herd [6], intermittent fecal shedding in growing-finishing pigs was detected for up to 12 weeks after the first positive PCR result.
Table 1. Results of PCR testing of fecal samples from pigs infected with Lawsonia intracellularis.
| DPI | Pig |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 0 | – | – | – | – | – |
| 5 | – | – | – | – | – |
| 10 | + | + | – | + | – |
| 15 | + | + | + | + | – |
| 20 | + | + | + | + | + |
| 25 | + | + | + | + | – |
| 30 | + | + | + | + | + |
| 35 | + | + | + | + | – |
| 40 | + | + | + | + | – |
DPI, days post-infection; +, PCR positive for L. intracellularis DNA; –, PCR negative for L. intracellularis DNA.
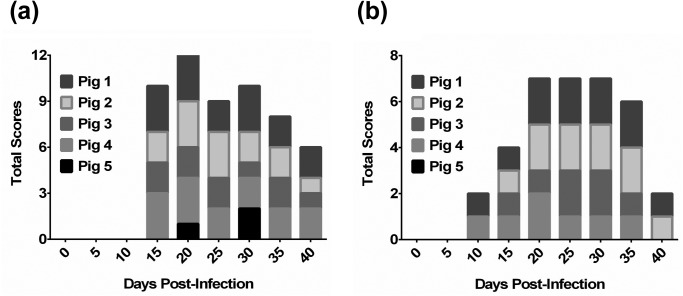
As shown in Fig. 1a, the pigs in the test group inoculated with L. intracellularis showed appetite loss, depression, and significant diarrhea from 10 to 40 DPI. The fecal scores began to rise at 15 DPI, as seen in Fig. 1b. Otherwise, no clinical signs or fecal changes were observed in uninfected control pigs.
Fig. 1.
Total scores of body condition, behavior, and feces in pigs challenged with Lawsonia intracellularis. Clinical signs based on the consistency of the feces were evaluated every five days. The clinical conditions of the pigs were observed and recorded for at least 10 min, including appetite, feces, body condition, and changes in behavior. (a) The clinical scores ranged from 0 to 2, i.e., 0=normal; 1=slightly to moderately gaunt, depressed and appetite loss, or listless, but still standing; 2=severely gaunt, depressed, and recumbent. (b) The fecal score was determined based on the following characteristics: 0=no diarrhea; 1=semi-solid feces with no blood; 2=watery without dark or bloody feces; 3=blood-tinged feces, loose or formed; and 4=profuse diarrhea with frank blood or dark tarry feces.
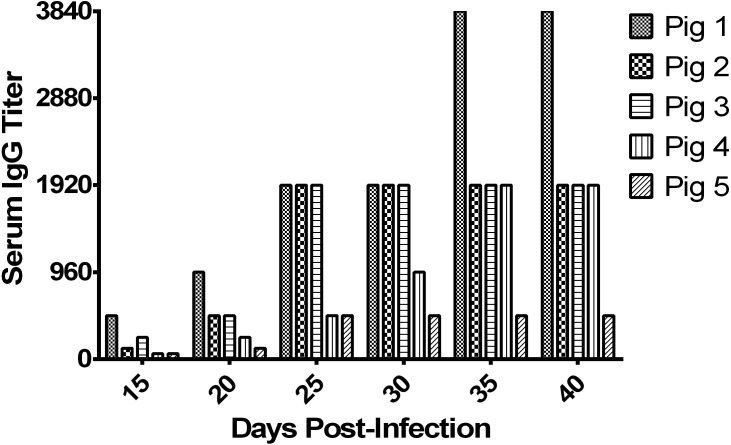
Detection of serum IgG has been shown to be a useful tool to assess previous exposure to L. intracellularis. The IPMA serology test has been shown to be a highly specific (100%) and fairly sensitive (89%) test [5]. Serum IgG antibodies against L. intracellularis began to be detected between 15 and 40 DPI in the challenged pigs. Serum IgG titer elevated progressively during the period of this study, as seen in Fig. 2. Uninfected control pigs had no detectable serum IgG antibody against L. intracellularis throughout the study.
Fig. 2.
Lawsonia intracellularis-specific antibody titer in serum of infected pigs. Sera were collected at the time points indicated from L. intracellularis-inoculated pigs and IgG was measured by an immunoperoxidase monolayer assay (IPMA).
Pro-inflammatory cytokines such as TNF-α, IL-6 and IFN-γ showed the pattern similar to clinical signs, fecal changes, and IgG antibody demonstration. Overall, the cytokine level changes of TNF-α, IL-6, IFN-γ, IL-4, IL-10, TGF-β and IL-8 in blood collected from pigs from 0 to 40 DPI are shown in Fig. 3 and Fig. 4. The increase was statistically significant only for TNF-α, IL-6, IFN-γ, IL-4 and IL-8 in the serum of infected animals (P<0.03, P<0.001, P<0.03 and P<0.02, respectively).
Fig. 3.
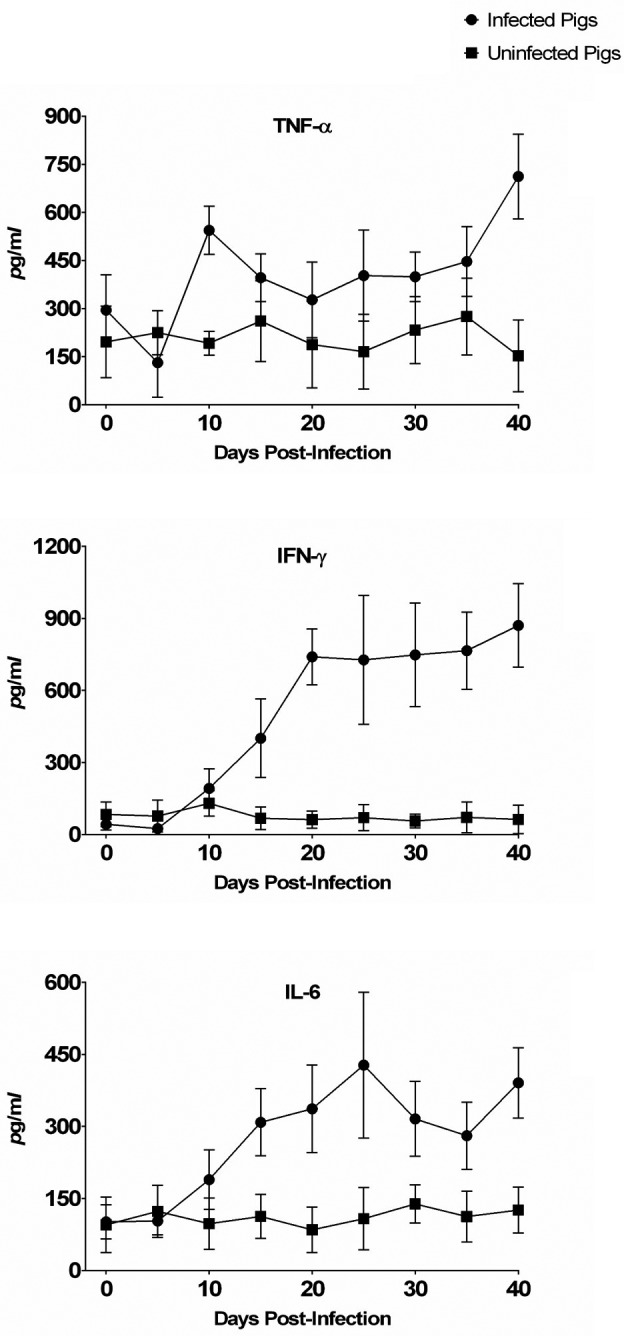
Pro-inflammatory cytokines (TNF-α, IFN-γ and IL-6) level quantitated by ELISA in the sera of control and Lawsonia intracellularis-infected pigs after oral challenge at various time points.
Fig. 4.
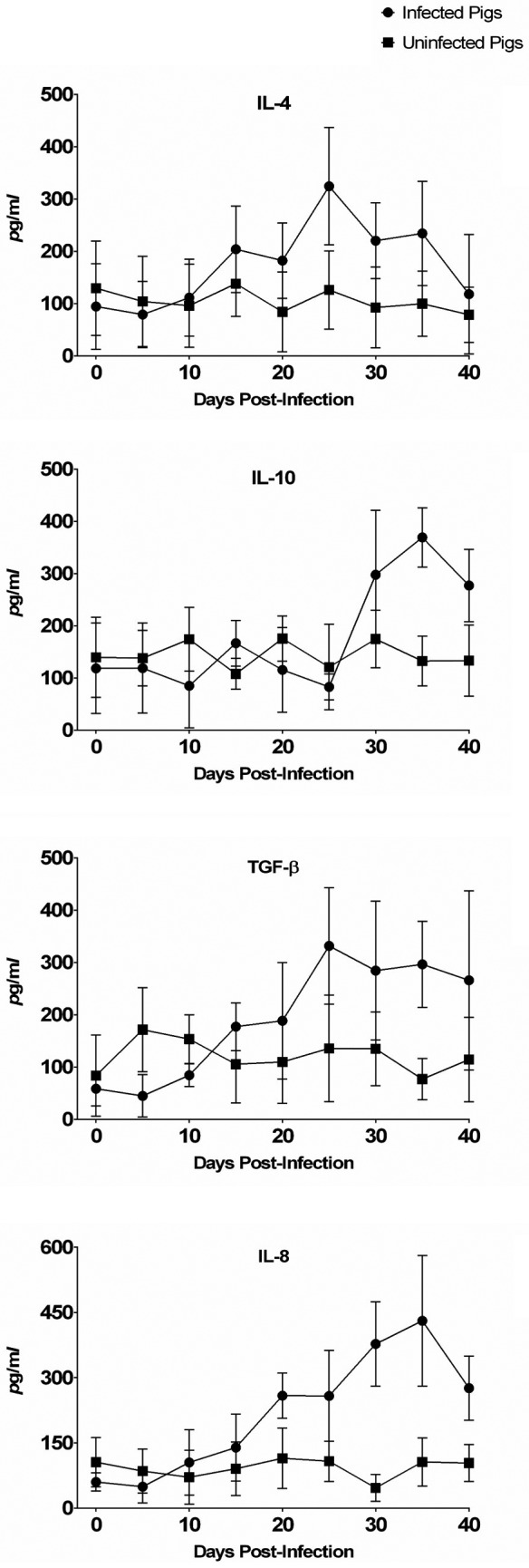
Anti-inflammatory cytokines (IL-4, IL-10 and TGF-β) and a chemokine (IL-8, also known as NAP-1, AMCF-1 and CXCL8) level quantitated by ELISA in the sera of control and L. intracellularis-infected pigs at various time points after oral challenge.
The TNF-α, IL-6 and IFN-γ levels were closely related to L. intracellularis infection, as serum levels of these proteins were higher in L. intracellularis-infected pigs than in uninfected control animals. However, circulating levels of IL-4, IL-8, IL-10 and TGF-β were relatively late affected by the presence of L. intracellularis and were similar between infected and uninfected pigs for the whole experimental period.
The increase was statistically significant for IL-10 and TGF-β and in the serum of infected animals only after 30 DPI and 25 DPI (P<0.0001 and P<0.006, respectively) although IL-10 and TGF-β levels were also similar in L. intracellularis-infected and uninfected pigs (P<0.05) for the whole experimental period.
Infected pigs showed significantly higher TNF-α levels than uninfected pigs. The levels of TNF-α in the infected group quickly reached the first peak at 10 DPI (544.0 ± 75.4 pg/ml). The levels of TNF-α started to rise again at 20 DPI and reached the second peak at 40 DPI (712.0 ± 132.1 pg/ml). The levels of IL-6 and IL-4 for the infected group reached 427.4 ± 151.9 pg/ml and 324.4 ± 112.3 pg/ml, respectively, at 25 DPI and returned nearly to baseline levels at 40 DPI. IL-8 rose progressively from 20 DPI (258.6 ± 52.1 pg/ml) and peaked at 35 DPI (430.4 ± 150.4 pg/ml), then declined to 275.8 ± 73.8 pg/ml. The IL-6, IL-4 and IL-8 levels were increased 2- to 3-fold in sera collected from infected animals as compared with uninfected control animals from 20 DPI to 35 DPI. The TNF-α level began to rise earlier than those of IL-6, IL-4, IL-8, and IFN-γ cytokines investigated in this study.
IFN-γ began to rise at 10 DPI (192.0 ± 81.5 pg/ml) and at 15 DPI (400.4 ± 163.5 pg/ml). The level of IFN-γ continued to rise again at 40 DPI (870.0 ± 174.0 pg/ml). These results support the suggestion of Smith and others that IFN-γ plays a significant role in limiting infection and cell proliferation caused by L. intracellularis [2, 22]. They demonstrated that infection rate of IFN-γ receptor knockout mice was substantially higher than that of wild-type mice in a L. intracellularis challenge model [2, 22].
DISCUSSION
There are numerous in vitro studies of the actions and interactions of cytokines but relatively fewer in vivo studies of cytokines in animal models of infectious disease. The response of cells to cytokines can be markedly affected by the “context”, and the systemic cytokine response in vivo is more valid for immunology than for any other type of research. Although several papers on mucosal, systemic, or primed cell-mediated immune responses to L. intracellularis have recently been published [1, 16, 17, 21], most immunological researches on L. intracellularis have focused mainly on IFN-γ. Here we focused on in vivo research on cytokine involvement in porcine PE caused by L. intracellularis infection using a Korean isolate.
In a previous study [3], the detection of IFN-γ-producing cells purified from the peripheral blood of pigs challenged with L. intracellularis after in vitro specific stimulation with L. intracellularis antigen demonstrated the induction of systemic cell-mediated immune responses against this organism despite the localization and proliferation of L. intracellularis in the cytoplasm of enterocytes. In another study [14], a lymphocyte stimulation assay performed in PBMCs of pigs naturally affected with PE showed an apparently specific relationship between the concentration of L. intracellularis test antigen (3 × 10−5, 3 × 10−4, 3 × 10−3, 3 × 10−2, 0.3 pg/ml) and the lymphocytic response. In the control group, there were no significant changes in the levels of any tested cytokine or chemokine before or during the experiment.
Although this study focused mainly circulating and systemic inflammatory cytokines, actually researches on cytokine response encounter some particular problems. For example, in case of immunological research on mucosal immune response, samples of the gastrointestinal tract, such as intestinal contents in the local area, are needed to study cytokine production, and these are difficult to obtain from living animals. There is a high risk of contamination induced by fecal inhibitors when determining the local cytokine levels, although L. intracellularis-induced cytokine expression within the intestinal mucosa may be more clinically relevant than circulating cytokine levels. The lack of real-time endoscopy or sequential necropsy findings that would allow correlation among local inflammation, gastrointestinal symptoms, and circulating cytokines is also an important consideration and for this reason many previous study did not showed a consistent correlation between the severity of enteritis and local cytokine expression yet [2, 4, 20, 22].
The immune system evolved to resist rapidly proliferating pathogens that, in the absence of an immediate response, have the potential to overwhelm and kill a host. Slowly replicating pathogens, by comparison, pose a less immediate threat and thus require less of an immediate response [15]. When hosts are infected by L. intracellularis, PE may be seen as various proliferating manifestations including acute intestinal hemorrhage or acute to chronic diarrhea with mucosal proliferation or necrosis. The specific mechanisms by which the immune system reacts on the cellular and subcellular levels are still unknown. This is one of the important studies to assess systemic cytokine response in animals infected with L. intracellularis.
Here, we were able to determine the cytokine profiling in pigs challenged with a pathogenic Korean L. intracellularis isolate. L. intracellularis induced cytokine and chemokine responses in infected animals. Detection of pro-inflammatory and anti-inflammatory cytokines may elucidate whether L. intracellularis infection is affected by other aspects of the host immune system. Further studies need to be conducted to better understand the host–pathogen interaction and the importance of cytokines and chemokines in controlling L. intracellularis infection.
Acknowledgments
The authors thank Hyun-Ji Seo, Hee-Soo Park, Mi-Ran Choi, Jin-Hwa, Kyung-Jin Choi, Jin-A Yoon, Ji-Hye Lee and Hyung-Seok Lee for their help with the animal experiment in NVRQS. The investigation and analysis on cytokine profiling was financially supported by a 2013 Incheon National University Research Grant. We are grateful to Yoshitaka Goto, the editor of The Journal of Veterinary Medical Science and an anonymous reviewer that reviewed the first draft of the manuscript for their critical comments and suggestions. The authors declare that they have no conflict of interest.
REFERENCES
- 1.Cordes H., Riber U., Jensen T. K., Jungersen G.2012. Cell-mediated and humoral immune responses in pigs following primary and challenge-exposure to Lawsonia intracellularis. Vet. Res. (Faisalabad) 43: 9. doi: 10.1186/1297-9716-43-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go Y. Y., Lee J. K., Ye J. Y., Lee J. B., Park S. Y., Song C. S., Kim S. K., Choi I. S.2005. Experimental reproduction of proliferative enteropathy and the role of IFN-gamma in protective immunity against Lawsonia intracellularis in mice. J. Vet. Sci. 6: 357–359. [PubMed] [Google Scholar]
- 3.Guedes R. M., Gebhart C. J.2003. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet. Microbiol. 91: 135–145. doi: 10.1016/S0378-1135(02)00301-2 [DOI] [PubMed] [Google Scholar]
- 4.Guedes R. M., Winkelman N. L., Gebhart C. J.2003. Relationship between the severity of porcine proliferative enteropathy and the infectious dose of Lawsonia intracellularis. Vet. Rec. 153: 432–433. doi: 10.1136/vr.153.14.432 [DOI] [PubMed] [Google Scholar]
- 5.Guedes R. M., Gebhart C. J., Deen J., Winkelman N. L.2002. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J. Vet. Diagn. Invest. 14: 528–530. doi: 10.1177/104063870201400618 [DOI] [PubMed] [Google Scholar]
- 6.Guedes R. M., Gebhart C. J., Armbruster G. A., Roggow B. D.2002. Serologic follow-up of a repopulated swine herd after an outbreak of proliferative hemorrhagic enteropathy. Can. J. Vet. Res. 66: 258–263. [PMC free article] [PubMed] [Google Scholar]
- 7.Guedes R. M., Gebhart C. J., Winkelman N. L., Mackie-Nuss R. A., Marsteller T. A., Deen J.2002. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can. J. Vet. Res. 66: 99–107. [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson M., Fellström C., Jensen-Waern M.2010. Porcine proliferative enteropathy: an important disease with questions remaining to be solved. Vet. J. 184: 264–268. doi: 10.1016/j.tvjl.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 9.Jones G. F., Ward G. E., Murtaugh M. P., Lin G., Gebhart C. J.1993. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J. Clin. Microbiol. 31: 2611–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knittel J. P., Jordan D. M., Schwartz K. J., Janke B. H., Roof M. B., McOrist S., Harris D. L.1998. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-exposed pigs. Am. J. Vet. Res. 59: 722–726. [PubMed] [Google Scholar]
- 11.La T., Collins A. M., Phillips N. D., Oksa A., Hampson D. J.2006. Development of a multiplex-PCR for rapid detection of the enteric pathogens Lawsonia intracellularis, Brachyspira hyodysenteriae, and Brachyspira pilosicoli in porcine faeces. Lett. Appl. Microbiol. 42: 284–288. doi: 10.1111/j.1472-765X.2005.01831.x [DOI] [PubMed] [Google Scholar]
- 12.Lawson G. H., McOrist S., Jasni S., Mackie R. A.1993. Intracellular bacteria of porcine proliferative enteropathy: cultivation and maintenance in vitro. J. Clin. Microbiol. 31: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H., Zhang M., Zheng E.2017. Comprehensive miRNA expression profiles in the ilea of Lawsonia intracellularis-infected pigs. J. Vet. Med. Sci. 79: 282–289. doi: 10.1292/jvms.16-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McOrist S., Lawson G. H.1993. Interactions of porcine lymphocytes with Campylobacter-like organism membranes purified from proliferative enteropathy. Vet. Microbiol. 34: 381–388. doi: 10.1016/0378-1135(93)90063-D [DOI] [PubMed] [Google Scholar]
- 15.Murtaugh M. P., Johnson C. R., Xiao Z., Scamurra R. W., Zhou Y.2009. Species specialization in cytokine biology: is interleukin-4 central to the T(H)1-T(H)2 paradigm in swine? Dev. Comp. Immunol. 33: 344–352. doi: 10.1016/j.dci.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 16.Nogueira M. G., Collins A. M., Donahoo M., Emery D.2013. Immunological responses to vaccination following experimental Lawsonia intracellularis virulent challenge in pigs. Vet. Microbiol. 164: 131–138. doi: 10.1016/j.vetmic.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 17.Nogueira M. G., Collins A. M., Dunlop R. H., Emery D.2015. Effect of the route of administration on the mucosal and systemic immune responses to Lawsonia intracellularis vaccine in pigs. Aust. Vet. J. 93: 124–126. doi: 10.1111/avj.12305 [DOI] [PubMed] [Google Scholar]
- 18.Page A. E., Loynachan A. T., Bryant U., Stills H. F., Jr., Adams A. A., Gebhart C. J., Pusterla N., Horohov D. W.2011. Characterization of the interferon gamma response to Lawsonia intracellularis using an equine proliferative enteropathy challenge (EPE) model. Vet. Immunol. Immunopathol. 143: 55–65. doi: 10.1016/j.vetimm.2011.06.023 [DOI] [PubMed] [Google Scholar]
- 19.Resende T. P., Pereira C. E., Gabardo M. P., Haddad J. P., Lobato Z. I., Guedes R. M.2015. Serological profile, seroprevalence and risk factors related to Lawsonia intracellularis infection in swine herds from Minas Gerais State, Brazil. BMC Vet. Res. 11: 306. doi: 10.1186/s12917-015-0618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riber U., Boesen H. T., Jakobsen J. T., Nguyen L. T., Jungersen G.2011. Co-incubation with IL-18 potentiates antigen-specific IFN-γ response in a whole-blood stimulation assay for measurement of cell-mediated immune responses in pigs experimentally infected with Lawsonia intracellularis. Vet. Immunol. Immunopathol. 139: 257–263. doi: 10.1016/j.vetimm.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 21.Riber U., Heegaard P. M., Cordes H., Ståhl M., Jensen T. K., Jungersen G.2015. Vaccination of pigs with attenuated Lawsonia intracellularis induced acute phase protein responses and primed cell-mediated immunity without reduction in bacterial shedding after challenge. Vaccine 33: 156–162. doi: 10.1016/j.vaccine.2014.10.084 [DOI] [PubMed] [Google Scholar]
- 22.Smith D. G., Mitchell S. C., Nash T., Rhind S.2000. Gamma interferon influences intestinal epithelial hyperplasia caused by Lawsonia intracellularis infection in mice. Infect. Immun. 68: 6737–6743. doi: 10.1128/IAI.68.12.6737-6743.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith S. H., McOrist S.1997. Development of persistent intestinal infection and excretion of Lawsonia intracellularis by piglets. Res. Vet. Sci. 62: 6–10. doi: 10.1016/S0034-5288(97)90171-5 [DOI] [PubMed] [Google Scholar]
- 24.Suh D. K., Song J. C.2005. Simultaneous detection of Lawsonia intracellularis, Brachyspira hyodysenteriae and Salmonella spp. in swine intestinal specimens by multiplex polymerase chain reaction. J. Vet. Sci. 6: 231–237. [PubMed] [Google Scholar]
- 25.Vannucci F. A., Gebhart C. J.2014. Recent advances in understanding the pathogenesis of Lawsonia intracellularis infections. Vet. Pathol. 51: 465–477. doi: 10.1177/0300985813520249 [DOI] [PubMed] [Google Scholar]
- 26.Yeh J. Y., Kim T. J., Park S. Y., Song C. S., Yoon Y. D., Kim S. K., Lee J. B., Choi I. S.2006. Isolation of Lawsonia intracellularis in Korea and reproduction of proliferative enteropathy in pigs and hamsters. J. Vet. Med. Sci. 68: 499–501. doi: 10.1292/jvms.68.499 [DOI] [PubMed] [Google Scholar]