Abstract
This study aims to evaluate the oxidative stress during hot summer season using serum oxidative stress biomarkers and elucidate the effects of serum antioxidant vitamin levels in dairy and beef cows in a daytime grazing system. Blood samples were collected once a month from eight Holstein Friesian (HF) and 10 Japanese Black (JB) cows from November 2013 to October 2014. Serum values of derivatives of reactive oxygen metabolites (d-ROMs) tended to be higher in March in both breeds and those in HF cows were kept at higher (P<0.001) levels than those in JB cows during the study period. Serum levels of biological antioxidant potential (BAP) in both breeds were maintained at almost the same values during study period. The OSI [(d-ROMs/BAP) × 100] values in both breeds showed similar seasonal changes, i. e. increase from December to March and decrease from March to August or September. In addition, the OSI values in HF cows were kept at higher (P<0.01) levels than those in JB cows during the study period. Serum concentrations of α-tocopherol, β-carotene, blood urea nitrogen and total cholesterol showed similar seasonal changes in both breeds, low in the winter and high from spring to summer, which may be attributed to the pasture grass intake. Opposite changes in OSI values and serum concentrations of α-tocopherol and β-carotene indicated that antioxidant vitamin levels could affect oxidative stress status.
Keywords: antioxidant vitamin, biological antioxidant potential (BAP), cow, derivatives of reactive oxygen metabolites (d-ROMs), oxidative stress
The production of a lactating dairy cow is negatively affected by heat stress and heat stress is among the most important factors that affects farm profitability during the warm season [8, 20, 45]. Economic losses due to decreased performance (e.g. feed intake, growth and milk yield), increased mortality and decreased reproduction averaged USD 897 million per year in the United States dairy industry over a decade ago [43]. The decline in milk production depends on the temperature humidity index (THI) and the average milk yield decreases more quickly as THI increases [10]. In addition, the conception rate and pregnancy rate in dairy cattle decrease above THI 72 [19]. Heat stress reduced performance and is a significant animal welfare concern in feedlot cattle during the final phase of the feeding period [28]. Japanese Black (JB) cows showed a decrease in the total number of inseminations and conceptions in the summer season [39]. The conception rates of beef cows decrease when the ambient temperature during the summer breeding season is high compared with the winter breeding season [3]. Also, an elevated ambient temperature during the summer season can affect beef cows by increasing their respiratory rate and rectal temperature [9]. Feed intake and milk production decrease during summer heat stress as a result of increased body temperature and respiratory rate [12, 17].
On the other hand, the total antioxidant status concentrations in serum of heifers were lower in the summer than winter in peri- and postpartum periods [48] and summer hottest period increased plasma values of reactive oxygen metabolite substances (ROMs) and decreased those of total carotenes and vitamin E in mid-lactating cows [13]. Increased oxidant parameters and decreased antioxidant parameters in blood during the hot summer season have been reported in dairy [44] and buffalo cows [37]. In addition, heat stress increases antioxidant enzymes activities, namely superoxide dismutase, catalase and glutathione peroxidase in response to increased reactive oxygen species (ROS) levels [5].
As some regions in the western part of Japan experience hot summer conditions, the occurrence of heat stress in livestock needs to be evaluated. The derivatives of reactive oxygen metabolites (d-ROMs) test exhibits good accuracy as well as linearity and is a simple, reliable and cheap test for the measurement of endogenous hydroperoxides. In addition, it can be easily applied on an automated analyser [46] and is a particularly suitable biomarker for evaluating oxidative stress in different clinical conditions [49]. The plasma biological antioxidant potential [the capacity of a plasma sample to reduce iron from its ferric (Fe3+) to ferrous (Fe2+) form] is measured by the biological antioxidant potential (BAP) test. This test is a photometric test which provides a global measurement of many antioxidants, including uric acid, ascorbic acid, proteins, α-tocopherol and bilirubin [6].
Therefore, it has been hypothesized that heat stress in the summer affects serum values of oxidative stress biomarkers, i.e. d-ROMs and BAP and the abundant grazing from spring to summer also influences them through the alternation in serum antioxidant vitamin levels in cattle in a daytime grazing system. However, studies that have evaluated heat stress by measuring serum d-ROMs and BAP in cattle remain limited. The purpose of this study was to evaluate the oxidative stress during hot summer season using serum oxidative stress biomarkers and elucidate the effects of serum antioxidant vitamin levels in dairy and beef cows in a daytime grazing system.
MATERIALS AND METHODS
Experimental animals
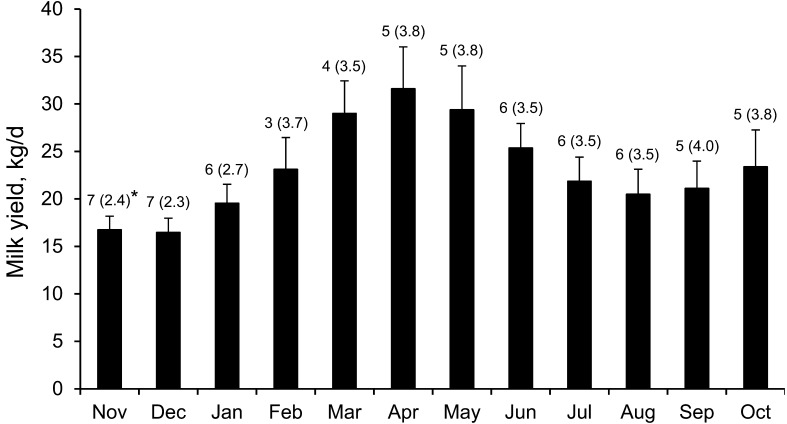
Eight Holstein Frisian (HF) cows [age, 4.95 ± 0.78 years (mean ± SE); number of calving, 2.5 ± 0.5; body weight, 569.8 ± 29.0 kg; body condition score, 2.93 ± 0.16] and 10 JB cows (age, 5.2 ± 0.9 years; number of calving, 3.6 ± 0.7; body weight, 468.7 ± 12.7 kg) raised on the Sumiyoshi Livestock Science Station, an experimental farm of the University of Miyazaki (total area = 50 ha, latitude 31° 59’ north, longitude 131° 28’ east), were enrolled in the experiment from November 2013 to October 2014. HF and JB cows were pastured separately in all seasons from 09:00 to 16:00 hr, on 0.5 to 3.2 ha of Bahia grass (Paspalum notatum Fluegge) and Centipede grass (Eremochloa ophiuroides) pasture, depending on the quantity of available grass, and kept in a freestall barn (19.1 m2/head) from 16:00 to 09:00 hr. During the winter season from November to April, JB cows were fed with wrapped bale silage of Guinea grass or Rhodes grass (13–15 kg/head/day). In addition, they were given wheat bran (2–3 kg), soybean meal (0.2–0.3 kg) and steam rolled corn (0.2–0.3 kg) from one week before the anticipated calving day through seven weeks after calving. HF cows in lactation period were fed with steam rolled barley (3.3 kg), wheat bran (1.7 kg), soybean meal (1.7 kg), steam rolled corn (1.7 kg) and beet pulp (2.5 kg), while those in dry period were given steam rolled barley (1.3 kg), wheat bran (0.7 kg), soybean meal (0.7 kg), steam rolled corn (0.7 kg) and beet pulp (1.0 kg). Additionally, they were fed with corn silage (15.3 kg) from November to April and wrapped bale silage of Italian ryegrass, Guinea grass or Rhodes grass (7.6 kg) all year round. All HF calves were separated from their dams during the first or second days after birth, while all JB dams nursed their calves for nearly four months. On average, the HF cows were dried off 20 weeks before calving. Five HF cows calved in December, January, March, April and June. Two HF cows calved in October. The remaining one HF cow calved in December 2014 after the study period. Figure 1 shows the monthly individual average milk production, the number of milking animals and the average number of calving in milking animals. Four and Five JB cows calved in December and January, respectively. In addition, one JB cow calved in February. None of the enrolled cows experienced any accidents at parturition and also showed no clinical signs during the study period. All the protocols were approved by the Institutional Review Board for animal experiments of the University of Miyazaki (Approval number: 2013-035).
Fig. 1.
Monthly individual average milk production (± SE) in HF cows. *The number and the number in parenthesis indicate the number of milking animals and the average number of calving in milking animals for each month, respectively.
Blood collection
Individual blood samples were collected from the jugular vein into 10 ml vacutainer tubes once a month and brought to the laboratory in chilled ice boxes soon after collection, and centrifuged at 3,000 × g for 15 min to separate the serum from packed erythrocytes. Blood serum was stored at −80°C until d-ROMs, BAP, blood urea nitrogen (BUN), total cholesterol (T-Cho) and vitamin analyses. Additionally, blood samples were collected from five HF and seven JB cows four weeks prepartum, at calving as well as one and four weeks postpartum in order to evaluate the peripartum changes in serum oxidative biomarker values.
Measurement of serum d-ROMs, BAP and biochemical values
The serum was thawed and then stirred thoroughly with a vortex mixer. The d-ROMs and BAP were measured using a free radical analyzer system (FREE carpe diem, Wismerll Co., Ltd., Tokyo, Japan). In addition, an oxidative stress index (OSI) was estimated using the ratio of d-ROMs/BAP multiplied by 100. Celi [15] proposed that when oxidative stress was evaluated using ROMs and BAP, the information of oxidative stress level was more accurate when combining ROMs and BAP data than using them separately. For measurements of serum BUN and T-Cho, a blood biochemistry automatic analyzer (Fuji Dri-Chem 7000V, FUJI FILM Medical Co., Ltd., Tokyo, Japan) was used.
Measurement of vitamin concentration
Serum concentrations of retinol, α-tocopherol and β-carotene were analyzed by high-performance liquid chromatography (HPLC) following the method of Katamoto et al. [33] with some modification. Briefly, 0.5 ml of serum was placed into a centrifuge tube together with 0.5 ml of an ascorbic acid solution (1 mg/ml ethanol) and 0.5 ml of α-tocopherol acetate solution (8 µg/ml ethanol) as an internal standard and mixed. After 5 ml of hexane was added to the tube, the samples were vigorously shaken for 10 min. The mixture was centrifuged at 1,000 × g for 5 min and the hexane layer was removed and filtered through a syringe filter (pore size 0.45 µm, Whatman Inc., NJ, U.S.A.). The filtrate was evaporated under nitrogen at 37°C and the residue was dissolved in 0.5 ml of methanol/chloroform (85:15, v/v) by sonication and 20 µl was injected into the HPLC column. The instruments used in this assay were as follows: the HPLC equipment was a Shimadzu LC-9A (Shimadzu Co., Ltd., Kyoto, Japan) with a COSMOSIL 5C18-MS-II, 4.6 I.D. × 150 mm column (Nacalai Tesque, Inc., Kyoto, Japan). The eluent was methanol/chloroform (90:10, v/v) and the flow rate was 0.9 ml/min. Retinol, α-tocopherol and β-carotene were detected at wavelengths of 325, 292 and 450 nm, respectively, with a Shimadzu SPD-10AV programmable multi-wavelength detector.
Calculation of THI
THI, which is an indicator of heat stress, was calculated by the formula [0.8 T + 0.01 H (T−14.3) + 46.3] where T: temperature (°C) and H: humidity (%). THI was calculated using monthly average maximum temperature and humidity in Miyazaki prefecture from November 2013 to October 2014.
Statistical analysis
All statistical analyses were conducted using R version 3.3.2. Two-way repeated measures analysis of variance (ANOVA) was used to determine the effects of breed and month. When the sphericity was not assumed, Greenhouse-Geisser’s adjustment was conducted. Simple effects for interaction between breed and month were also estimated. If significant interaction was not observed though both breed difference and monthly difference, Welch’s t-test and one-way repeated measures ANOVA were used to compare HF and JB in each month and to compare monthly variables in each breed, respectively. Multiple comparison was performed with Holm’s correction. In addition, peripartum variables in each breed were analyzed using one-way repeated measures ANOVA. To investigate the effect of lactation on OSI level, generalized linear mixed model was used. Results are expressed as the mean ± SE (standard error). A P value <0.05 was considered significant.
RESULTS
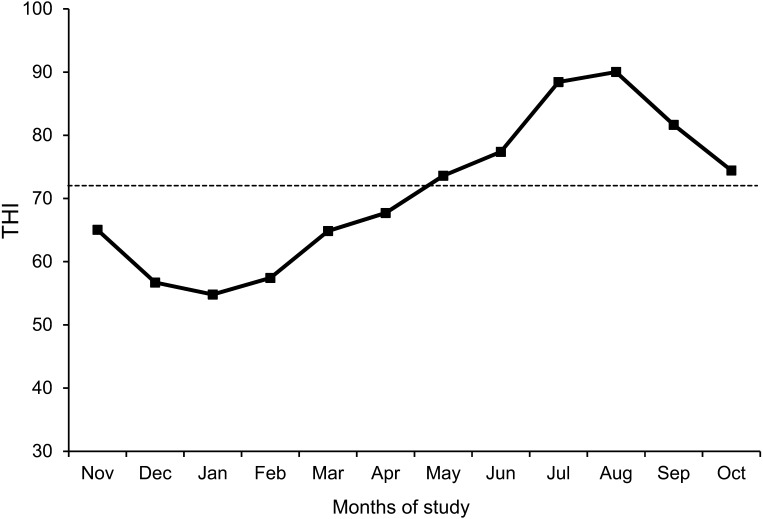
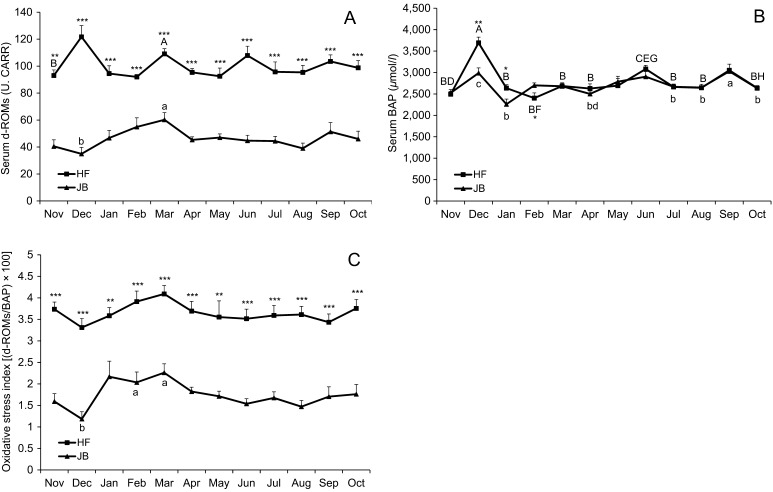
Figure 2 presents THI values from November 2013 to October 2014. The THI was >72 from May to October 2014. The breed × month interactions (P<0.05) were observed for serum d-ROMs, BAP, α-tocopherol, β-carotene and BUN. Serum levels of d-ROMs, a biomarker of oxidative stress, were highest in December in HF cows and also tended to be higher in March in both breeds (Fig. 3A). The d-ROMs values in HF cows were kept at higher (P<0.001) levels than those in JB cows during the study period. Serum levels of BAP in both breeds were maintained at almost the same values during study period except in December, January and February (Fig. 3B). Although there was no breed × month interaction, the breed effect (P<0.001) was observed on OSI. The OSI values increased (P<0.05) from December to March in JB cows (Fig. 3C). Both breeds of cows showed similar seasonal changes, i. e. increase from December to March and decrease from March to August or September. The OSI values in HF cows were kept at higher (P<0.01) levels than those in JB cows during the study period. Table 1 shows serum values of oxidative stress biomarker during peripartum period. There was no significant difference among the mean values in each breed. The results of generalized linear mixed model analysis showed no effect of lactation on OSI level in HF cows.
Fig. 2.
Monthly mean temperature and humidity index (THI) from November 2013 to October 2014. Dashed line represents the THI value of 72.
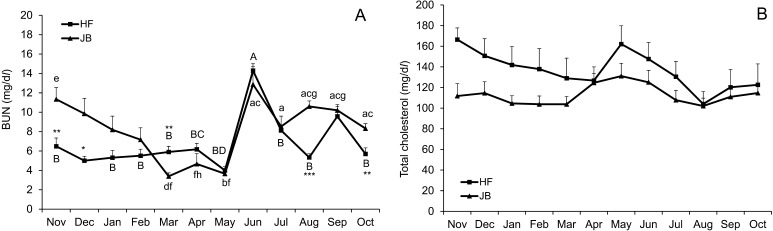
Fig. 3.
(A) Mean values (± SE) of serum d-ROMs, (B) BAP and (C) oxidative stress index [(d-ROMs/BAP) × 100] in HF and JB cows pastured during the daytime from November 2013 to October 2014. Values with different letters are significantly different (A–B, C–D, E–F, G–H; P<0.05) and (a–b, c–d; P<0.05) for HF and JB cows, respectively. *, **, *** Significantly differs from the value in JB cows within a sampling period at P<0.05, P<0.01 and P<0.001, respectively.
Table 1. Serum values of oxidative stress biomarker during peripartum period in HF and JB cows.
| Animal | Parameter | Units | Weeks relative to calving |
|||
|---|---|---|---|---|---|---|
| –4 W | 0 | 1 W | 4 W | |||
| HF (n=5) | d-ROMs | U. CARR | 85.6 ± 6.1 | 89.6 ± 14.1 | 77.2 ± 13.4 | 101.6 ± 6.8 |
| BAP | µmol/l | 3,055.9 ± 294.4 | 2,506.8 ± 150.4 | 2,906.4 ± 275.9 | 2,906.3 ± 166.6 | |
| OSI | 2.89 ± 0.32 | 3.75 ± 0.86 | 2.86 ± 0.59 | 3.53 ± 0.26 | ||
| JB (n=7) | d-ROMs | U. CARR | 46.6 ± 8.6 | 44.1 ± 7.7 | 56.6 ± 12.0 | 56.1 ± 7.8 |
| BAP | µmol/l | 2,641.3 ± 105.5 | 2,755.7 ± 102.7 | 2,746.1 ± 88.1 | 2,734.9 ± 142.9 | |
| OSI | 1.83 ± 0.38 | 1.62 ± 0.30 | 2.11 ± 0.47 | 2.10 ± 0.31 | ||
Data are expressed as mean ± SE.
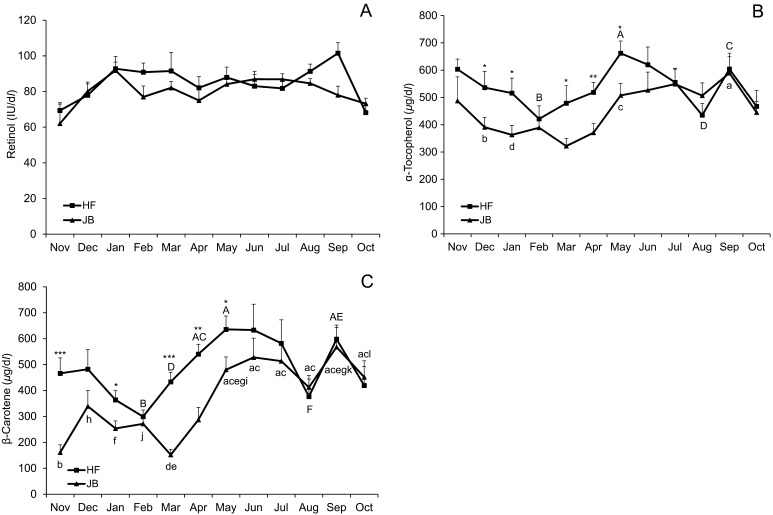
There was no breed × month interaction for serum retinol. Serum retinol concentrations were almost constant and were maintained at the lower reference range limit (83.3–200 IU/dl) [23] in both breeds during the study period (Fig. 4A). Serum α-tocopherol concentrations tended to decrease from November to February and then increased (P<0.05) to May in HF cows (Fig. 4B). Similarly, serum α-tocopherol concentrations tended to decrease from November to March and then tended to increase to July in JB cows. Both HF and JB cows showed a tendency to decrease in α-tocopherol concentrations in August. Serum β-carotene concentrations tended to decrease from December to February and then increased (P<0.05) to May in HF cows (Fig. 4C). Similarly, serum β-carotene concentrations tended to decrease from December to March and then tended to increase to June in JB cows. Similar to serum α-tocopherol concentrations, both HF and JB cows showed a tendency to decrease in β-carotene concentrations in August. The BUN values were low from November to May and increased (P<0.05) to June followed by a decrease (P<0.05) to August in HF cows (Fig. 5A). The BUN concentrations in JB cows decreased (P<0.05) from November to March and increased (P<0.05) from May to June followed by a decreased tendency to July. No breed × month interaction was observed for serum T-Cho. Serum T-Cho concentrations tended to decrease from November to April and then tended to increase to May in HF cows (Fig. 5B). Similarly, serum T-Cho concentrations tended to decrease from November to March and then tended to increase to May in JB cows. In addition, serum T-Cho concentrations tended to decrease from May to August in both breeds.
Fig. 4.
(A) Mean serum concentrations (± SE) of retinol, (B) α-tocopherol and (C) β-carotene in HF and JB cows pastured during the daytime from November 2013 to October 2014. Values with different letters are significantly different (A–B, C–D, E–F; P<0.05) and (a–b, c–d, e–f, g–h, i–j, k–l; P<0.05) for HF and JB cows, respectively. *, **, *** Significantly differs from the value in JB cows within a sampling period at P<0.05, P<0.01 and P<0.001, respectively.
Fig. 5.
(A) Mean concentrations (± SE) of BUN and (B) serum T-Cho in HF and JB cows pastured during the daytime from November 2013 to October 2014. Values with different letters are significantly different (A–B, C–D; P<0.05) and (a–b, c–d, e–f, g–h; P<0.05) for HF and JB cows, respectively. *, **, *** Significantly differs from the value in JB cows within a sampling period at P<0.05, P<0.01 and P<0.001, respectively.
DISCUSSION
The THI has been developed as an indicator of a heat load index, and heat stress is defined as a THI ≥72 [40]. A value of ≤70 is considered comfortable, 75–78 stressful and >78 causes extreme distress with lactating cows unable to maintain thermoregulatory mechanisms or normal body temperature [32]. The monthly mean THI in May (73.6), June (77.4), July (88.4), August (90.0), September (81.6) and October (74.4) were >72; therefore, animals were considered to be exposed to heat stress during these months.
Abuelo et al. [1] found statistical differences in the values of oxidative stress index (reactive oxygen substances/serum antioxidant capacity) between foregoing and subsequent parturition stage, suggesting that dairy cows experienced an oxidative challenge after parturition. In this study, no significant difference was found in serum values of oxidative stress biomarker during peripartum period. This discrepancy could be attributed to the differences in climate condition and diet composition. Additionally, although almost all JB cows (9/10) calved in December or January, OSI values and concentrations of vitamins, BUN and T-Cho exhibited similar seasonal changes in both breeds. Thus, it was considered that parturition did not significantly affect their seasonal changes.
The results of our study showed that serum d-ROMs and OSI values were maintained at higher levels in HF cows compared to JB cows during the study period, suggesting higher levels of oxidative stress. The JB and HF breeds used in this study were bred for different purposes. JB cows are raised as breeding cows for beef cattle, while HF cows are bred as dairy cattle. High milk productivity can be associated with oxidative stress due to the increased cellular metabolism involved [14, 35]. The mean 26 weeks cumulative milk yield was 854 kg for JB cows with single calves [42]. Thus, milk productivity in JB cows was supposed to be lower than that in HF cows. However, statistically significant effect of lactation on OSI level was not observed in this study. In Fig. 1, the individual average milk production in milking animals was highest in April (31.6 ± 4.4 kg) and lowest in December (16.5 ± 1.5 kg). These changes were considered to be due to the different stages of lactation, because three of five and one of seven cows were categorised into early lactation stage in April and in December, respectively. Feeding high levels of starch in the diet to early lactation cows increased oxidative stress due to cellular metabolism probably shifted towards oxidative phosphorylation [24]. HF cows were fed concentrate in all stages including lactation and dry periods, while JB cows were fed concentrate only in peripartum period for eight weeks. Therefore, concentrate feeding in all stages might be the reason for higher oxidative stress in HF cows. Seasonal heat stress is more prominent in HF cows than in other European dairy breeds [25]. Furthermore, high yielding cows are more sensitive to a hot environment than low yielding cows, and body heat production is higher in high producing dairy cows [31, 50]. In addition, Sakatani et al. [41] reported that since the body mass of a HF cow is heavier than that of a JB cow, their heat emission efficiency from their body surface could be lower than in JB cows. Thus, these may be other reasons for higher oxidative stress in HF cows.
Seasonal and nutritional factors can influence oxidative stress biomarker values [7, 22]. In our study, serum d-ROMs and OSI values were higher in March in both breeds. On the other hand, serum α-tocopherol and β-carotene concentrations were lower in February or March. The reason for higher d-ROMs and OSI values could be due to the decrease in serum α-tocopherol and β-carotene concentrations. These observations can be attributed to seasonal dietary changes. Around the winter season, the availability of fresh forage is low and the diet is mostly supplemented with silage which has a poor antioxidant content [4]. In addition, cows are fed with conserved forage and cereal grains during the winter season. According to Kay et al. [34], these kinds of feed have a low α-tocopherol concentration compared to fresh pasture. Descalzo et al. [21] reported that pasture diet contributed to natural antioxidants in sufficient amounts and β-carotene levels in plasma were significantly higher for pasture-fed than for grain-fed animals. Besides that, concentrates and stored forages (hay, haylage and silage) are generally low in vitamin E [38].
Vitamin A, E and selenium are known antioxidants that play important roles in animal health and production. Fresh forage is typically capable of supplying adequate levels of antioxidants for dairy cattle [15]. In the present study, serum α-tocopherol, β-carotene and T-Cho concentrations showed similar seasonal changes; an increase from spring to summer and a dramatic decrease in August in both breeds. An increase in serum α-tocopherol, β-carotene and T-Cho concentrations could be attributed to grazing in a rich pasture and feeding on fresh forage. A pasture-based system could improve the oxidative status of animals due to the elevated antioxidant content of green grass compared with stored fodder [21, 26]. Several studies indicated that grass-based diets were rich in precursors for vitamin A and E [18]. Marino et al. [36] reported that milk α-tocopherol and β-carotene concentrations were significantly higher in the spring and summer compared to the fall. In addition, grass-fed significantly increased α-tocopherol and β-carotene concentrations in the fresh beef of cattle compared to grain-fed [21]. On the other hand, the relationship between cholesterol and vitamin values observed in this study has been documented before. Bouwstra et al. [11] showed that vitamin E supplementation increased blood vitamin E concentrations in HF heifers and blood vitamin E concentrations were strongly correlated to blood cholesterol concentrations. Cholesterol is a component of lipoproteins, and its concentration in serum is an indication of overall lipoprotein concentrations and vitamin E is transported in the very low density lipoproteins. Serum cholesterol concentration has been associated with DMI in healthy transition dairy cows [27]. In addition, BUN is an indicator of protein status and the BUN level has been highly correlated to dietary protein levels under the same level of energy intake [30]. The BUN level also decreased in August or July in both breeds. Therefore, a decreased feed and grass intake due to summer heat stress might be the reason for dramatic decreases in serum α-tocopherol, β-carotene and T-Cho concentrations in August when the THI was at its highest. Cincovic et al. [16] reported that glucose, non-esterified fatty acid and cholesterol levels decrease during heat stress periods. Similar changes in the concentrations of serum retinol and α-tocopherol, low in the summer and high in the winter, were reported by Katamoto et al. [33]. Furthermore, they reported that decreased serum concentrations of retinol and α-tocopherol may be related to a decreased feed intake due to the high ambient temperature in the summer. Although α-tocopherol and β-carotene exhibited seasonal changes in the current study, Trout et al. [47] reported no effect of short exposure to a hot environment on the concentrations of lipid-soluble antioxidants, such as α-tocopherol, β-carotene, retinol and retinyl palmitate, and that heat stress did not appear to increase lipid peroxidation or decrease lipid-soluble antioxidant concentrations in the blood. The reduced production performances in livestock during heat stress were traditionally thought to be due to a decreased feed intake. However, it has recently been recognised that heat stress can enhance ROS production and induce oxidative stress, resulting in both cellular and mitochondrial oxidative damage [5]. Serum α-tocopherol concentrations >400 µg/dl have been considered to indicate adequacy in adult cattle [23] and desirable blood β-carotene concentrations are considered to be in excess of 300 µg/dl in dairy cattle [29]. Serum antioxidant vitamin concentrations in both breeds were above the desirable value in August when the THI was at its highest. In serum α-tocopherol concentrations, HF cows showed decrease in February (421.4 ± 47.8 µg/dl) and August (435.2 ± 42.3 µg/dl), and JB cows showed decrease in March (321.8 ± 27.1 µg/dl) and August (506.8 ± 46.5 µg/dl). While in serum β-carotene concentrations, HF cows exhibited decrease in February (299.3 ± 25.5 µg/dl) and August (376.6 ± 67.6 µg/dl), and JB cows exhibited decrease in March (151.9 ± 21.2 µg/dl) and August (412.2 ± 45.8 µg/dl). Although the values of OSI increased from December to March, those did not change in August in both groups. The reasons for this may be higher vitamin concentrations in August than in February or March in addition to their desirable blood concentrations. The other reason may be that the decreases in both α-tocopherol and β-carotene concentrations were tentative in August. Endogenous antioxidants consist of three major groups: enzymatic antioxidants including superoxide dismutase and glutathione-peroxidase, non-enzymatic protein antioxidants such as sulfhydryl (SH) groups of albumin, l-cysteine and homocysteine, as well as low-molecular weight antioxidants such as glutathione, α-tocopherol, β-carotene and uric acid [15]. Therefore, other antioxidants except α-tocopherol and β-carotene might affect the levels of oxidative stress biomarkers in the current study. Opposite changes in OSI values and concentrations of α-tocopherol and β-carotene indicated that antioxidant vitamin levels could affect oxidative stress status. Supplementation with α-tocopherol acetate during dry period has been found to reduce oxidative stress during peripartum period in cows [2].
In conclusion, this study indicated that the degree of oxidative stress biomarkers was higher in HF than JB cows. The higher oxidative status in HF cows was considered to be due to concentrate feeding in all stages leading to an increase in metabolism. The concentrations of α-tocopherol, β-carotene, BUN and T-Cho showed similar seasonal changes in both breeds, low in the winter and high from spring to summer, which may be attributed to the pasture grass intake. Opposite changes in oxidative stress and antioxidant vitamin levels may show a compensatory regulatory response. It was also found that oxidative stress biomarkers did not change in the hottest period of summer. Further studies are needed to confirm whether sufficient levels of antioxidant vitamin in the blood reduce the acceleration of oxidative stress in the hot summer months.
Acknowledgments
The authors would like to thank the members of the Sumiyoshi Livestock Science Station, an experimental farm of the University of Miyazaki, for their assistance in collecting samples.
REFERENCES
- 1.Abuelo A., Hernández J., Benedito J. L., Castillo C.2013. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal 7: 1374–1378. doi: 10.1017/S1751731113000396 [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal A., Ashutosh, Chandra G., Singh A. K.2013. Heat shock protein 70, oxidative stress, and antioxidant status in periparturient crossbred cows supplemented with α-tocopherol acetate. Trop. Anim. Health Prod. 45: 239–245. doi: 10.1007/s11250-012-0196-z [DOI] [PubMed] [Google Scholar]
- 3.Azzam S. M., Kinder J. E., Nielsen M. K.1989. Conception rate at first insemination in beef cattle: effects of season, age and previous reproductive performance. J. Anim. Sci. 67: 1405–1410. doi: 10.2527/jas1989.6761405x [DOI] [PubMed] [Google Scholar]
- 4.Ballet N., Robert J. C., Williams P. E. V.2000. Vitamins in forages. pp. 399–431. In: Forage Evaluation in Ruminant Nutrition (Givens, D. I., Owen, E., Axford, R. F. E., Omed, H. M. eds), CABI Publishing, Wallingford. [Google Scholar]
- 5.Belhadj Slimen I., Najar T., Ghram A., Abdrrabba M.2016. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. (Berl.) 100: 401–412. doi: 10.1111/jpn.12379 [DOI] [PubMed] [Google Scholar]
- 6.Benzie I. F. F., Strain J. J.1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239: 70–76. doi: 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- 7.Bernabucci U., Ronchi B., Lacetera N., Nardone A.2002. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 85: 2173–2179. doi: 10.3168/jds.S0022-0302(02)74296-3 [DOI] [PubMed] [Google Scholar]
- 8.Bernabucci U., Biffani S., Buggiotti L., Vitali A., Lacetera N., Nardone A.2014. The effects of heat stress in Italian Holstein dairy cattle. J. Dairy Sci. 97: 471–486. doi: 10.3168/jds.2013-6611 [DOI] [PubMed] [Google Scholar]
- 9.Biggers B. G., Geisert R. D., Wetteman R. P., Buchanan D. S.1987. Effect of heat stress on early embryonic development in the beef cow. J. Anim. Sci. 64: 1512–1518. doi: 10.2527/jas1987.6451512x [DOI] [PubMed] [Google Scholar]
- 10.Boonkum W., Duangjinda M.2015. Estimation of genetic parameters for heat stress, including dominance gene effects, on milk yield in Thai Holstein dairy cattle. Anim. Sci. J. 86: 245–250. doi: 10.1111/asj.12276 [DOI] [PubMed] [Google Scholar]
- 11.Bouwstra R. J., Goselink R. M. A., Dobbelaar P., Nielen M., Newbold J. R., van Werven T.2008. The relationship between oxidative damage and vitamin E concentration in blood, milk, and liver tissue from vitamin E supplemented and nonsupplemented periparturient heifers. J. Dairy Sci. 91: 977–987. doi: 10.3168/jds.2007-0596 [DOI] [PubMed] [Google Scholar]
- 12.Brown-Brandl T. M., Eigenberg R. A., Nienaber J. A., Hahn G. L.2005. Dynamic response indicator of heat stress in shaded and no-shaded feedlot cattle, Part 1: analysis of indicators. Biosystems Eng. 90: 451–462. doi: 10.1016/j.biosystemseng.2004.12.006 [DOI] [Google Scholar]
- 13.Calamari L., Maianti M. G., Amendola F., Lombardi G.1999. On some aspects of the oxidative status and on antioxidants in blood of dairy cows during summer. Proceedings of the 13th Associazione Scientifica Produzioni Animali Congress, Pia-cenza.
- 14.Castillo C., Hernandez J., Lopez-Alonso M., Miranda M., Benedito J. L.2003. Values of plasma lipid hydroperoxides and total antioxidant status in healthy dairy cows: preliminary observations. Archiv. Für. Tierzucht. 46: 227–233. [Google Scholar]
- 15.Celi P.2011. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 33: 233–240. doi: 10.3109/08923973.2010.514917 [DOI] [PubMed] [Google Scholar]
- 16.Cincovic M. R., Beli B., Toholj B., Potkonjak A., Stevancevic M., Lako B., Radovic I.2011. Metabolic acclimation to heat stress in farm housed Holstein cows with different body condition scores. Afr. J. Biotechnol. 10: 10293–10303. doi: 10.5897/AJB11.847 [DOI] [Google Scholar]
- 17.Collier R. J., Dahl G. E., VanBaale M. J.2006. Major advances associated with environmental effects on dairy cattle. J. Dairy Sci. 89: 1244–1253. doi: 10.3168/jds.S0022-0302(06)72193-2 [DOI] [PubMed] [Google Scholar]
- 18.Daley C. A., Abbott A., Doyle P. S., Nader G. A., Larson S.2010. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 9: 10. doi: 10.1186/1475-2891-9-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dash S., Chakravarty A. K., Singh A., Upadhyay A., Singh M., Yousuf S.2016. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: A review. Vet. World 9: 235–244. doi: 10.14202/vetworld.2016.235-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rensis F., Garcia-Ispierto I., López-Gatius F.2015. Seasonal heat stress: Clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 84: 659–666. doi: 10.1016/j.theriogenology.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 21.Descalzo A. M., Insani E. M., Biolatto A., Sancho A. M., García P. T., Pensel N. A., Josifovich J. A.2005. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Sci. 70: 35–44. doi: 10.1016/j.meatsci.2004.11.018 [DOI] [PubMed] [Google Scholar]
- 22.Di Trana A., Celi P., Claps S., Fedele V., Rubino R.2006. The effect of hot season and nutrition on the oxidative status and metabolic profile in dairy goats during mid lactation. Anim. Sci. 82: 717–722. doi: 10.1079/ASC200672 [DOI] [Google Scholar]
- 23.Frye T. M., Williams S. N., Graham T. W.1991. Vitamin deficiencies in cattle. Vet. Clin. North Am. Food Anim. Pract. 7: 217–275. doi: 10.1016/S0749-0720(15)30817-3 [DOI] [PubMed] [Google Scholar]
- 24.Gabai G., Testoni S., Piccinini R., Marinelli L., Stradaioli G.2004. Oxidative stress in primiparous cows in relation to dietary starch and the progress of lactation. Anim. Sci. 79: 99–108. [Google Scholar]
- 25.Garcia-Peniche T. B., Cassell B. G., Pearson R. E., Misztal I.2005. Comparisons of Holsteins with Brown Swiss and Jersey cows on the same farm for age at first calving and first calving interval. J. Dairy Sci. 88: 790–796. doi: 10.3168/jds.S0022-0302(05)72743-0 [DOI] [PubMed] [Google Scholar]
- 26.Gatellier P., Mercier Y., Renerre M.2004. Effect of diet finishing mode (pasture or mixed diet) on antioxidant status of Charolais bovine meat. Meat Sci. 67: 385–394. doi: 10.1016/j.meatsci.2003.11.009 [DOI] [PubMed] [Google Scholar]
- 27.Guretzky N. A. J., Carlson D. B., Garrett J. E., Drackley J. K.2006. Lipid metabolite profiles and milk production for Holstein and Jersey cows fed rumen-protected choline during the periparturient period. J. Dairy Sci. 89: 188–200. doi: 10.3168/jds.S0022-0302(06)72083-5 [DOI] [PubMed] [Google Scholar]
- 28.Hagenmaier J. A., Reinhardt C. D., Bartle S. J., Thomson D. U.2016. Effect of shade on animal welfare, growth performance, and carcass characteristics in large pens of beef cattle fed a beta agonist in a commercial feedlot. J. Anim. Sci. 94: 5064–5076. doi: 10.2527/jas.2016-0935 [DOI] [PubMed] [Google Scholar]
- 29.Herdt T. H., Stowe H. D.1991. Fat-soluble vitamin nutrition for dairy cattle. Vet. Clin. North Am. Food Anim. Pract. 7: 391–415. doi: 10.1016/S0749-0720(15)30796-9 [DOI] [PubMed] [Google Scholar]
- 30.Hwang S. Y., Lee M. J., Peh H. C.2001. Diurnal variations in milk and blood urea nitrogen and whole blood ammonia nitrogen in dairy cows. Asian-australas. J. Anim. Sci. 14: 1683–1689. doi: 10.5713/ajas.2001.1683 [DOI] [Google Scholar]
- 31.Igono M. O., Johnson H. D., Steevens B. J., Hainen W. A., Shanklin M. D.1988. Effect of season on milk temperature, milk growth hormone, prolactin, and somatic cell counts of lactating cattle. Int. J. Biometeorol. 32: 194–200. doi: 10.1007/BF01045279 [DOI] [PubMed] [Google Scholar]
- 32.Kadzere C. T., Murphy M. R., Silanikove N., Maltz E.2002. Heat stress in lactating dairy cows: a review. Livest. Prod. Sci. 77: 59–91. doi: 10.1016/S0301-6226(01)00330-X [DOI] [Google Scholar]
- 33.Katamoto H., Yamada Y., Nishizaki S., Hashimoto T.2003. Seasonal changes in serum vitamin A, vitamin E and β-carotene concentrations in Japanese Black breeding cattle in Hyogo prefecture. J. Vet. Med. Sci. 65: 1001–1002. doi: 10.1292/jvms.65.1001 [DOI] [PubMed] [Google Scholar]
- 34.Kay J. K., Roche J. R., Kolver E. S., Thomson N. A., Baumgard L. H.2005. A comparison between feeding systems (pasture and TMR) and the effect of vitamin E supplementation on plasma and milk fatty acid profiles in dairy cows. J. Dairy Res. 72: 322–332. doi: 10.1017/S0022029905000944 [DOI] [PubMed] [Google Scholar]
- 35.Löhrke B., Viergutz T., Kanitz W., Losand B., Weiss D. G., Simko M.2005. Short communication: hydroperoxides in circulating lipids from dairy cows: implications for bioactivity of endogenous-oxidized lipids. J. Dairy Sci. 88: 1708–1710. doi: 10.3168/jds.S0022-0302(05)72843-5 [DOI] [PubMed] [Google Scholar]
- 36.Marino V. M., Schadt I., La Terra S., Manenti M., Caccamo M., Licitra G., Carpino S.2012. Influence of season and pasture feeding on the content of α-tocopherol and β-carotene in milk from Holstein, Brown Swiss and Modicana cows in Sicily. Dairy Sci. Technol. 92: 501–513. doi: 10.1007/s13594-012-0069-2 [DOI] [Google Scholar]
- 37.Megahed G. A., Anwar M. M., Wasfy S. I., Hammadeh M. E.2008. Influence of heat stress on the cortisol and oxidant-antioxidants balance during oestrous phase in buffalo-cows (Bubalus bubalis): thermo-protective role of antioxidant treatment. Reprod. Domest. Anim. 43: 672–677. doi: 10.1111/j.1439-0531.2007.00968.x [DOI] [PubMed] [Google Scholar]
- 38.NRC 2001. Nutrient Requirements of Dairy Cattle, 7th rev edn. National Academy Press, Washington, D. C. [Google Scholar]
- 39.Ogawa K., Nakanishi Y., Tojo H., Yanagita K., Nishi I.1978. An additional field survey in the reproductive activity of the Japanese beef cattle in public breeding farms in Kagoshima prefecture. Bulletin of the Faculty of Agriculture, Kagoshima University 28: 9–18. [Google Scholar]
- 40.Rees A., Fischer-Tenhagen C., Heuwieser W.2016. Effect of heat stress on concentrations of faecal cortisol metabolites in dairy cows. Reprod. Domest. Anim. 51: 392–399. doi: 10.1111/rda.12691 [DOI] [PubMed] [Google Scholar]
- 41.Sakatani M., Balboula A. Z., Yamanaka K., Takahashi M.2012. Effect of summer heat environment on body temperature, estrous cycles and blood antioxidant levels in Japanese Black cow. Anim. Sci. J. 83: 394–402. doi: 10.1111/j.1740-0929.2011.00967.x [DOI] [PubMed] [Google Scholar]
- 42.Shimada K., Izaike Y., Suzuki O., Kosugiyama M., Takenouchi N., Ohshima K., Takahashi M.1992. Effect of milk yield on growth of multiple calves in Japanese Black cattle (Wagyu). Asian-australas. J. Anim. Sci. 5: 717–722. doi: 10.5713/ajas.1992.717 [DOI] [Google Scholar]
- 43.St-Pierre N. R., Cobanov B., Schnitkey G.2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86Suppl: E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- 44.Tanaka M., Kamiya Y., Kamiya M., Nakai Y.2007. Effect of high environmental temperatures on ascorbic acid, sulfhydryl residue and oxidized lipid concentrations in plasma of dairy cows. Anim. Sci. J. 78: 301–306. doi: 10.1111/j.1740-0929.2007.00439.x [DOI] [Google Scholar]
- 45.Tao S., Dahl G. E.2013. Invited review: heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 96: 4079–4093. doi: 10.3168/jds.2012-6278 [DOI] [PubMed] [Google Scholar]
- 46.Trotti R., Carratelli M., Barbieri M.2002. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 44: 37–40. [PubMed] [Google Scholar]
- 47.Trout J. P., McDowell L. R., Hansen P. J.1998. Characteristics of the estrous cycle and antioxidant status of lactating Holstein cows exposed to heat stress. J. Dairy Sci. 81: 1244–1250. doi: 10.3168/jds.S0022-0302(98)75685-1 [DOI] [PubMed] [Google Scholar]
- 48.Turk R., Podpečan O., Mrkun J., Flegar-Meštrić Z., Perkov S., Zrimšek P.2015. The Effect of Seasonal Thermal Stress on Lipid Mobilisation, Antioxidant Status and Reproductive Performance in Dairy Cows. Reprod. Domest. Anim. 50: 595–603. doi: 10.1111/rda.12534 [DOI] [PubMed] [Google Scholar]
- 49.Vassalle C., Boni C., Di Cecco P., Ndreu R., Zucchelli G. C.2006. Automation and validation of a fast method for the assessment of in vivo oxidative stress levels. Clin. Chem. Lab. Med. 44: 1372–1375. doi: 10.1515/CCLM.2006.243 [DOI] [PubMed] [Google Scholar]
- 50.West J. W.2003. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 86: 2131–2144. doi: 10.3168/jds.S0022-0302(03)73803-X [DOI] [PubMed] [Google Scholar]