Abstract
A cross-sectional study was conducted to estimate the prevalence of Fasciola hepatica (F. hepatica) infection in water buffalo (Bubalus bubalis) in Alexandria, Beheira, and Kafr el-Sheikh governorates (provinces) of the Nile Delta in Egypt and to identify the underlying risk factors associated with the infection. A total of 29 farms (10 in Alexandria, 10 in Beheira, and 9 in Kafr el-Sheikh) were randomly selected and all the buffaloes that resided on these farms from 21 February 2015 to 20 February 2016 were included in the study. The sampling approach was target-based where all the buffaloes were examined and screened for clinical signs of Fasciola infection. All suspected buffaloes were then subjected to fecal examination, and those positive for Fasciola eggs underwent antibody testing using indirect hemagglutination test. Consequently, data on 3,356 buffaloes from 29 farms in these governorates was analyzed using a multiple logistic regression model. The final model showed that the age and body condition score of the buffalo, location and type of the farm, application of prophylactic treatment, and temperature and relative humidity of the farm’s location significantly affected the rate of infection. The highest prevalence was observed in buffaloes from Alexandria governorate (19.6%), followed by Beheira and Kafr el-Sheikh governorates (15.5 and 9.1%, respectively).
Keywords: buffalo, cross-sectional study, Egypt, Fasciola hepatica, logistic regression
Fasciolosis is a global zoonotic disease caused by infection with the food- and water-borne trematodes, Fasciola hepatica (F. hepatica) and Fasciola gigantica (F. gigantica). The common liver fluke, F. hepatica, is widely distributed worldwide. It is found chiefly in cattle, sheep, goats, and buffaloes, but it also affects humans. It is estimated that over 17 million people are infected worldwide, and a further 180 million people in endemic areas are at risk of infection [8]. Human infection typically occurs via the consumption of aquatic vegetables such as watercress, or water contaminated with encysted cercariae. In Egypt, fasciolosis is a serious public health and economic issue consistently affecting human and animal health, and livestock production particularly cattle, buffalo, and sheep [32]. The presence of both, F. hepatica and F. gigantica, has been confirmed in local cattle and buffaloes [25].
Estimating the prevalence of fasciolosis and identifying the associated risk factors of F. hepatica infection will provide useful information in establishing effective preventive and control measures against this majorly neglected tropical disease. Fasciolosis in production animals in different regions of Egypt have been widely studied and the estimated prevalence varied greatly from 0.2–33.7% [2, 10, 11, 17, 27]. Various risk factors such as the management system, pasture management, and climatic/environmental factors like temperature, rainfall, and soil type have been identified in terms of F. hepatica infection in farm animals [13, 19, 31, 36]. However, information regarding the risk factors associated with F. hepatica infection in the domestic Asian water buffalo (Bubalus bubalis) in Egypt is very limited. Therefore, the aim of this study is to estimate the prevalence of F. hepatica infection in water buffalo in three governorates of the Nile Delta in Egypt and to identify the underlying risk factors associated with the infection.
MATERIALS AND METHODS
Study population
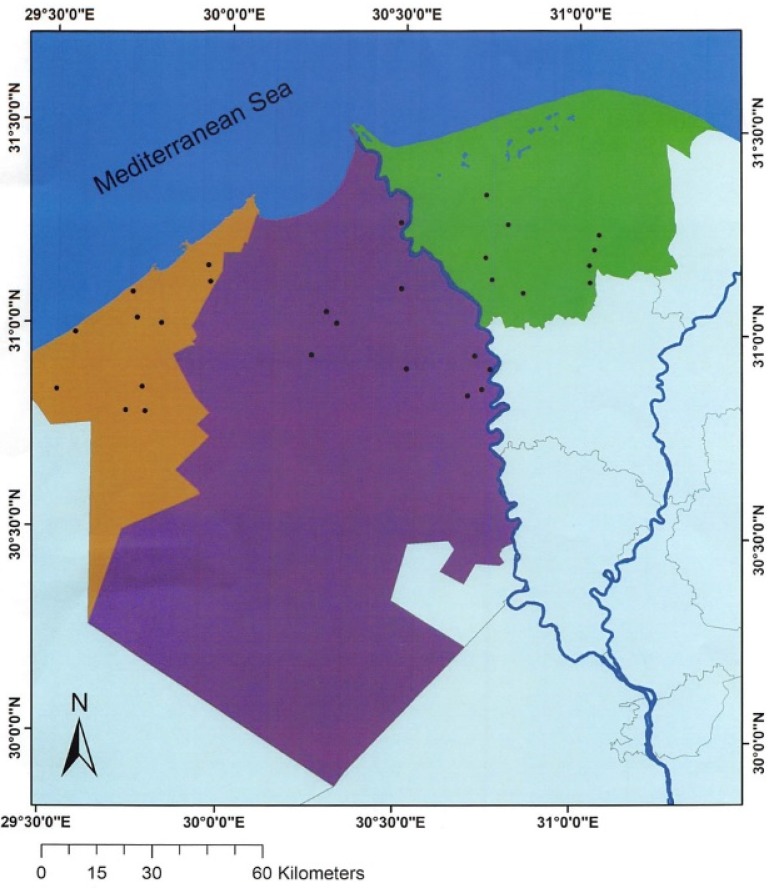
The study population comprised animals from three governorates (provinces), Kafr el-Sheikh, Beheira, and Alexandria, in the Nile Delta of Egypt, where more than 75% of the Egyptian water buffaloes are farmed. A total of 29 farms, including 10 in Alexandria, 10 in Beheira, and 9 in Kafr el-Sheikh, were randomly selected for this study from an exhaustive list of buffalo farms (45 farms in total) provided by the Egyptian government (Fig. 1). Three farm owners declined to participate in the study and were replaced by three other farms from the list. In the selected governorates, female and male buffaloes were usually kept together for dairy and meat production, and Fasciola infection constituted a major economic problem that caused declines in productivity. To mitigate the potential economic loss, it was a common for farmers, particularly those on organic farms, to provide prophylactic treatment to the buffaloes using anthelmintic drugs such as triclabendazole, rafoxanid, and/or albendazole.
Fig. 1.
Geographical distribution of the 29 farms (black dots) included in the current study in Alexandria (brown), Beheira (purple) and Kafr el-Sheikh (green) governorates in the Nile Delta, Egypt.
Case definition and sampling method
All the buffaloes (n=3,356) that resided on the 29 selected farms from 21 February 2015 to 20 February 2016 were included in the current study. Buffaloes that were born, moved out of the farm, or had died within the one-year study period were excluded. Local veterinarians, who assisted with the current study, visited each of the 29 farms from 26 February 2016 to 28 April 2016 and collected all the necessary samples and data. The sampling approach was target-based i.e., all the buffaloes were examined and screened for clinical signs of Fasciola infection including emaciation, rough coat, persistent poor appetite, weight loss and/or diarrhea, icterus of the conjunctiva and/or vulva, decreased milk production, bitter taste of the milk, and/or anestrous features. All the suspected buffaloes were then subjected to fecal examination and those positive for Fasciola eggs underwent antibody testing using the indirect hemagglutination test (IHAT) to diagnose F. hepatica infection. A total of 984 buffaloes, suspected of Fasciola infection, were subjected to coprological sampling with an average of 34 buffaloes (range, 23–50) being sampled from each farm. Three grams of fecal sample obtained from each buffalo was transferred to clean jugs protected with 10% formalin and fecal examination was performed utilizing the sedimentation strategy according to Hansen et al. [16]. The confirmation test for F. hepatica antibody in the serum samples was conducted using the Distomiasis Fumouze® IHAT kit (Fumouze Diagnostics, Strasbourg, France) based on the method detailed by Nossair et al. [29]: Approximately 5 ml of blood was collected aseptically in sterile tubes from each suspected buffalo. The blood samples were left to clot for 30 min at room temperature and centrifuged at 3,000 rpm for 15 min. The serum was then aspirated, labeled, and kept at −20°C until examination. This method was chosen owing to several advantages such as accuracy, short testing time, ready-to-use capability, and high sample stability. Sensitized red blood cells were composed of sheep red blood cells coated with an F. hepatica antigen. Serum antibodies against F. hepatica were revealed by an agglutination of the sensitized red blood cells i.e., a reddish-brown film was observed in the well. These red blood cells precipitated in the absence of specific antibodies, forming a ring at the bottom of the well. The unsensitized red blood cells ensured the reaction specificity and eliminated interferences due to natural anti-sheep agglutinins (Forssman heteroantibodies, infectious mononucleosis antibodies).
Data collection
The following factors (independent variables) were considered for the logistic regression model (see section 2.4) to determine F. hepatica infection (dependent variable):
Age and sex of the buffalo: Information was collected by the attending veterinarians on the day of the visit.
Body condition (good, medium, or poor): Each buffalo was evaluated for its body condition score by the attending veterinarian according to Nicholson et al. [28]. The evaluation was based on a manual assessment of the thickness of fat cover and prominence of bone at the tail. Buffaloes with scores 3 and 4 (the short ribs could be felt by applying slight pressure; the overhanging shelf-like appearance of these bones had disappeared; the backbone was a rounded ridge; and the hook and pin bones were round and smoothed over) were considered to be in good body condition, buffaloes with a score of 2 (the ends of the short ribs could be felt, but they and the individual vertebrae were less visibly prominent; the short ribs did not form an obvious overhang or shelf effect; the hook and pin bones were prominent, but the depression of the thurl region between them was less severe) were considered to be in medium body condition, and buffaloes with scores of 1 (the ends of the short ribs were sharp to the touch and together give a prominent shelf-like appearance to the loin; the individual vertebrae of the backbone were prominent; the hook and pin bones were sharply defined) or 5 (the back bone had a flat appearance and could not be felt even with pressure; folds of fat were apparent over the ribs, thurl, and thighs) were considered to be in poor body condition.
Type of farm (organic or conventional): The farms that satisfied the criteria as per Sorge et al. [33] were considered as organic. Briefly, the criteria for organic production included a ban on the use of artificial fertilizer, grazing of animals, restriction on the use of concentrated feed, organic production of concentrated and roughage feed, and ban or restriction on the use of antibiotics and other hormones. The number of conventional and organic farms in each governorate was as follows: 8 conventional and 2 organic farms in Alexandria governorate, 6 conventional and 4 organic farms in Beheira governorate, and 3 conventional and 6 organic farms in Kafr el-Sheikh governorate.
Age and education level of the farmer (basic or higher): This information was collected by the attending veterinarian via face-to-face interview. Farmers who attended high school and elementary school were considered to have basic education, whereas those that attended university were considered to have higher education.
Application of prophylactic treatment: Buffaloes that received at least one dose of the anthelmintic drugs, triclabendazole, rafoxanide (Rameda Pharmaceuticals, Cairo, Egypt), and/or albendazole (Arabcomed, Obour, Egypt), for prophylactic purposes during the 12-month study period were considered to have received prophylactic treatment. The prophylactic treatment was provided on all the conventional farms and two organic farms within the Alexandria governorate in anticipation of heavy infestation on these farms.
Temperature (°C) and relative humidity (%): The Nile Delta has a hot desert climate as the rest of Egypt, although its northernmost part has relatively moderate temperatures with an average not surpassing 31°C during the summer. Annual rainfall averages only 100–200 mm, most of which falls during the winter season. Average annual temperature and relative humidity were calculated for the 12-month study period based on monthly data of the respective regions where the farms were situated using Egyptian Meteorological Agency websites (http://www.wunderground.com and freemeteo.com).
Statistical analysis
Odds ratios for infection with F. hepatica were estimated using logistic regression analysis. We first conducted a univariate analysis to check for an association between the dependent variable (F. hepatica infection) and independent variables (potential risk factors), and only selected those that affected the dependent variable significantly (P<0.05). All independent variables (listed in the previous section) that passed this first screening were considered for the multiple logistic regression model. We used the Hosmer-Lemeshow goodness-of-fit test to decide the linear or categorical characteristic of the independent variable, and thus the farmer’s age was excluded as a variable due to the poor fit with the final model (P=0.23). The significance level of the final model was set as 0.05. All statistical analyses were performed using SPSS ver 24.0 (IBM, Armonk, NY, U.S.A.).
RESULTS
The within-herd prevalence of F. hepatica infection ranged from 7.4–20% in the 29 farms included in the current study, giving an overall prevalence of 12.9% (95% confidence interval (CI): 11.7–14%) in 3,356 individual buffaloes (Table 1). Further summary statistics of the study buffalo population is presented in Table 2 and the results of the multiple logistic regression model are shown in Table 3. The location of the farm had a significant effect on F. hepatica infection; buffaloes in Beheira and Kafr el-Sheikh governorates had lower risks of infection than those in Alexandria governorate with odds ratios of 0.36 (95% CI: 0.24–0.54) and 0.37 (95% CI: 0.26–0.54), respectively. In terms of individual factors, older buffaloes were less likely to be infected than the younger ones, with an odds ratio of 0.73 (95% CI: 0.65–0.82), and sex was not a significant risk factor. Buffaloes that received prophylactic treatment had a lower risk of infection than those that did not, with an odds ratio of 0.41 (95% CI: 0.28–0.59). In terms of management systems, buffaloes raised on organic farms had a lower risk of infection than those on conventional farms, with an odds ratio of 0.41 (95% CI: 0.28–0.59). In terms of climatic conditions, buffaloes raised in regions with annual average temperatures lower than 26°C and between 26 and 30°C had higher risks of infection than those raised in regions higher than 30°C, with odds ratios of 20.96 (95% CI: 13.42–32.74) and 6.49 (95% CI: 4.23–9.97), respectively. Moreover, buffaloes raised in regions with an annual average relative humidity (RH) lower than 50% and between 50% and 59% had higher risks of infection than those raised in regions with an RH higher than 59%, with odds ratios of 4.63 (95% CI: 3.12–6.85) and 3.21 (95% CI: 2.26–4.54), respectively. Finally, buffaloes with poor and medium body condition scores were more likely to be associated with infection than those with good body condition scores, with odds ratios of 3.71 (95% CI: 2.59–5.33) and 4.95 (95% CI: 3.30–7.43), respectively.
Table 1. Number of buffaloes sampled on each farm and estimated within-herd prevalence of F. hepatica on the 29 farms included in the current study.
| Governorate | Study population (n) | Number of buffaloes tested (proportion of the study population, %) | Number of buffaloes positive for fecal examination and F. hepatica antibody a) | Within-herd prevalence (%) | 95% confidence interval b) (%) |
|---|---|---|---|---|---|
| Alexandria | 85 | 23 (27) | 11 | 12.9 | 5.8–20.1 |
| 96 | 28 (29) | 10 | 10.4 | 4.3–16.5 | |
| 110 | 28 (25) | 12 | 10.9 | 5.1–16.7 | |
| 80 | 28 (35) | 13 | 16.3 | 8.2–24.3 | |
| 113 | 33 (29) | 18 | 15.9 | 9.2–22.7 | |
| 125 | 34 (27) | 19 | 15.2 | 8.9–21.5 | |
| 148 | 35 (24) | 11 | 7.4 | 3.2–11.7 | |
| 111 | 42 (38) | 13 | 11.7 | 5.7–17.7 | |
| 133 | 43 (32) | 15 | 11.3 | 5.9–16.7 | |
| 90 | 45 (50) | 18 | 20.0 | 11.7–28.3 | |
| Beheira | 106 | 23 (22) | 15 | 14.2 | 7.5–20.8 |
| 144 | 26 (18) | 15 | 10.4 | 5.4–15.4 | |
| 150 | 27 (18) | 17 | 11.3 | 6.3–16.4 | |
| 133 | 33 (25) | 17 | 12.8 | 7.1–18.5 | |
| 125 | 33 (26) | 11 | 8.8 | 3.8–13.8 | |
| 120 | 33 (28) | 14 | 11.7 | 5.9–17.4 | |
| 98 | 34 (35) | 19 | 19.4 | 11.6–27.2 | |
| 114 | 40 (35) | 13 | 11.4 | 5.6–17.2 | |
| 124 | 40 (32) | 20 | 16.1 | 9.7–22.6 | |
| 120 | 43 (36) | 17 | 14.2 | 7.9–20.4 | |
| Kafr el-Sheikh | 100 | 22 (22) | 13 | 13.0 | 6.4–19.6 |
| 131 | 27 (21) | 16 | 12.2 | 6.6–17.8 | |
| 107 | 29 (27) | 12 | 11.2 | 5.2–17.2 | |
| 93 | 31 (33) | 13 | 14.0 | 6.9–21.0 | |
| 122 | 33 (27) | 16 | 13.1 | 7.1–19.1 | |
| 118 | 36 (31) | 14 | 11.9 | 6.0–17.7 | |
| 83 | 41 (49) | 15 | 18.1 | 9.8–26.4 | |
| 133 | 44 (33) | 16 | 12.0 | 6.5–17.6 | |
| 144 | 50 (35) | 19 | 13.2 | 7.7–18.7 | |
| Total | 3,356 | 984 (29) | 432 | 12.9 | 11.7–14.0 |
a) All the 432 buffaloes positive for Fasciola eggs during the fecal examination were also positive for the F. hepatica antibody during the indirect hemagglutination test. b) The
95% confidence interval of prevalence (Pre) is given by: 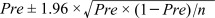 .
.
Table 2. Summary statistics of the buffalo population included in the current study.
| Total population | Number of buffaloes tested in the population | Number of buffaloes that exhibited F. hepatica infection (prevalence in %) | |||
|---|---|---|---|---|---|
| Total | 3,356 | 984 | 432 | (12.8) | |
| Sex | |||||
| Male | 1,810 | 350 | 321 | (17.7) | |
| Female | 1,546 | 634 | 111 | (7.2) | |
| Age (years) | |||||
| 1 | 852 | 355 | 152 | (17.8) | |
| 2 | 1,024 | 385 | 168 | (16.4) | |
| 3 | 537 | 128 | 47 | (8.8) | |
| 4 | 224 | 82 | 46 | (20.5) | |
| 5 | 719 | 34 | 19 | (2.6) | |
| Governorates | |||||
| Alexandria | 685 | 339 | 134 | (19.6) | |
| Beheira | 860 | 332 | 133 | (15.5) | |
| Kafr El-Sheikh | 1,811 | 313 | 165 | (9.1) | |
| Type of farm | |||||
| Conventional | 1,159 | 534 | 384 | (33.1) | |
| Organic | 2,197 | 450 | 48 | (2.2) | |
| Body condition score | |||||
| Good | 2,281 | 290 | 195 | (8.6) | |
| Medium | 309 | 344 | 108 | (35.0) | |
| Poor | 766 | 350 | 129 | (16.8) | |
| Application of prophylactic treatment | |||||
| Not applied | 2,168 | 574 | 359 | (16.6) | |
| Applied | 1,188 | 410 | 73 | (6.1) | |
| Age of farmer (years) | |||||
| <40 | 620 | 234 | 65 | (10.5) | |
| 40–49 | 1,525 | 398 | 222 | (14.6) | |
| >49 | 1,211 | 352 | 145 | (12.0) | |
| Education of farmer | |||||
| Basic | 2,572 | 660 | 407 | (15.8) | |
| Higher | 784 | 324 | 25 | (3.2) | |
| Relative humidity (%) | |||||
| <50 | 596 | 288 | 123 | (20.6) | |
| 50–59 | 509 | 374 | 159 | (31.2) | |
| >59 | 2,251 | 322 | 150 | (6.7) | |
| Temperature (°C) | |||||
| <26 | 494 | 291 | 216 | (43.7) | |
| 26–30 | 872 | 321 | 164 | (18.8) | |
| >30 | 1,990 | 372 | 520 | (2.6) | |
Table 3. Association of age, sex, body condition score and underlying risk factors with F. hepatica infection predicted by multiple logistic regression for the study buffalo population in three governorates in the Nile Delta of Egypt.
| Variable | Coefficient | Odds ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| Constant | −2.84 | - | <0.0001 | ||
| Age (years) | −0.32 | 0.73 | 0.65–0.82 | <0.0001 | |
| Sex | |||||
| Male (reference) | 0.00 | 1.00 | |||
| Female | 0.102 | 1.11 | 0.79–1.55 | 0.55 | |
| Governorate | |||||
| Alexandria (reference) | 0.00 | 1.00 | |||
| Beheira | −1.03 | 0.36 | 0.24–0.54 | <0.0001 | |
| Kafr el-Sheikh | −0.99 | 0.37 | 0.26–0.54 | <0.0001 | |
| Body condition score | |||||
| Good (reference) | 0.00 | 1.00 | |||
| Medium | 1.60 | 4.95 | 3.30–7.43 | <0.0001 | |
| Poor | 1.31 | 3.71 | 2.59–5.33 | <0.0001 | |
| Prophylactic treatment | |||||
| Not applied (reference) | 0.00 | 1.00 | |||
| Applied | −0.89 | 0.41 | 0.28–0.59 | <0.0001 | |
| Type of farm | |||||
| Conventional (reference) | 0.00 | 1.00 | |||
| Organic | −0.89 | 0.41 | 0.28–0.59 | <0.0001 | |
| Education of farmer | |||||
| Basic (reference) | 0.00 | 1.00 | |||
| Higher | −0.42 | 0.66 | 0.37–1.77 | 0.15 | |
| Temperature | |||||
| >30°C (reference) | 0.00 | 1.00 | |||
| 26–30°C | 1.87 | 6.49 | 4.23–9.97 | <0.0001 | |
| <26°C | 3.04 | 20.96 | 13.42–32.74 | <0.0001 | |
| Relative humidity | |||||
| >59% (reference) | 0.00 | 1.00 | |||
| 50–59% | 1.17 | 3.21 | 2.26–4.54 | <0.0001 | |
| <50% | 1.53 | 4.63 | 3.12–6.85 | <0.0001 | |
Model statistics: χ2 (13)=1,290.016, P≤0.0001; Nagelkerke R2=0.595; Hosmer-Lemeshow test: P=0.709.
DISCUSSION
Fasciolosis is one of the most common parasitic diseases severely affecting public health and animal production, especially in developing countries such as Egypt. In terms of cattle production, economic losses associated with fasciolosis includes direct losses from condemnation of the liver and reduced carcass weight at slaughter [9]. Indirect losses in the form of poor feed conversion, weight loss, slow fattening, reduced milk yield, reproductive failure, and sometimes death also affect cattle production [4, 7]. The current study significantly associated F. hepatica infection with buffaloes having medium and poor body condition scores when compared to those with good body condition scores, which is consistent with previous reports on fasciolosis in cattle [5, 23].
The present study showed that the prevalence of F. hepatica infection in buffaloes in three governorates in the Nile Delta of Egypt was 12.87%. National level surveys in the late 1990s reported an infection prevalence of 3.5% for the whole country. Meanwhile, in the southern part of Egypt, the infection prevalence in the Assiut governorate (located about 400 km to the south of Cairo) and the Qena governorate (located about 600 km to the south of Cairo) was estimated to be 24.3 and 33.7%, respectively [2, 17, 27]. Our results revealed that the infection prevalence in Alexandria governorate (19.56%) was higher than that in Beheira (15.47%) and Kafr el-Sheikh (9.11%) governorates, which could be explained by the differences in the climatic/environmental conditions such as rainfall and/or the management systems (i.e., the scale of the farm and grazing habits of the cattle) between these governorates [6]. The farms included in the current study in Alexandria governorate were either situated close to the Mediterranean Sea or in areas filled with water channels having poor water management programs, all of which possibly promoted the development and spread of the intermediate snail hosts of fasciolosis.
Our estimated value for infection prevalence in Alexandria governorate appeared to be higher than the previously reported 13% by El-Sherif et al. [12], possibly due to the increase in contaminated vegetated areas in the governorate over the last 50 years. Contrastingly, most of the water channels running through Beheira and Kafr el-Sheikh governorates were periodically disinfected when compared to the water channels running through several areas in the Alexandria governorate.
In terms of management factors, we demonstrated that buffaloes raised on conventional farms had a higher risk of infection than those on organic farms, possibly due to poorer grassland management on conventional farms where buffaloes had easier access to contaminated pastures. However, this finding differed from the findings of Kantzoora et al. [19] which reported no significant difference in the prevalence of ovine fasciolosis between organic and conventional farms in Greece.
Our results suggested that sex was not a significant risk factor, which was consistent with several previous studies [18, 20,21,22, 30]. However, Yildirim et al. [36] reported that bovine fasciolosis in Turkey was more prevalent in females (70.7%) than in males (47.8%). The higher risk among females could be attributed to the fact that most female cattle are kept for breeding and milk production purposes, thereby increasing stress and reducing their resistance to infections. Furthermore, we demonstrated age to be a relevant risk factor for F. hepatica infection in buffaloes. This result contrasted the findings of Hansen et al. [13], which showed no significant difference between the prevalence of bovine fasciolosis in Ethiopia and young animals (33.3%), old animals (26.11%), or adult animals (24.7%). Nicholson et al. [15] also concluded that age was not a risk factor for bovine fasciolosis in Northern Ethiopia.
In terms of climatic conditions, our analysis revealed that temperatures lower than 30°C combined with a relative humidity (RH) lower than 60% had positive effects on fasciolosis in buffaloes. Abdelhamid et al. [1] investigated the prevalence of ovine fasciolosis in the Beheira governorate at different temperatures and RH, but found no significant difference between the following three conditions: group 1, temperatures <25°C and RH of 72.8%; group 2, temperatures between 25 and 30°C and RH of 70.25%; and group 3, temperatures >30°C and RH of 68.96%. Temperature alone has also been proven as a consistent risk predictor. Torgerson et al. [34] and Urquhart et al. [35] demonstrated 10°C to be the minimum critical temperature for the development of F. hepatica eggs and the infection of the intermediate snail host, while Augot et al. [3] and Lee et al. [24] mentioned that the optimal temperature for metacercariae production in infected snails was between 22 and 25°C. More recently, Moayad et al. [26] revealed that the optimal temperature for metacercarial production in F. gigantica-infected snails was 25 ± 1°C.
Finally, our analysis highlighted that buffaloes that did not receive prophylactic treatment were at a higher risk of fasciolosis than those that did. Likewise, Kantzoora et al. [19] also found a negative association between the use of anthelmintic treatment and the occurrence of F. hepatica infection (in terms of coproantigen detection) on sheep and goat farms. Given the fact that fasciolosis in cattle is mostly subclinical, the use of prophylactic treatment appears to be the most cost-effective control strategy in a developing country setting like Egypt.
The evidence-based information provided in this study could assist local veterinarians in targeting farms having high-risk factors such as conventional farms situated in Alexandria with an average temperature lower than 26°C, and in advising farmers to adopt a prophylactic strategy when necessary. However, anthelmintic resistance is a growing global problem and its development in livestock animals should not be overlooked [14].
A cross-sectional study was conducted to estimate the prevalence of F. hepatica infection and to identify its underlying risk factors in three governorates in the Nile Delta of Egypt. We found that the prevalence of F. hepatica infection was significantly associated with the location and type of the farm, age and body condition of the buffalo, the application of anthelmintic treatment, temperature, and relative humidity. These results are fundamental in formulating appropriate control strategies against bovine fasciolosis in Egypt and other areas with similar farming practices and climatic conditions.
DECLARATION OF INTERESTS
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank the veterinarians in Alexandria, Beheira, and Kafr el-Sheikh governorates who assisted in data collection for this study.
REFERENCES
- 1.Abdelhamid M. S.2009. Zoonotic importance of fascioliasis in sheep and human, Master Thesis, Faculty of Veterinary Medicine, Department of Animal Hygiene and Zoonosis. Menofia University, Egypt. [Google Scholar]
- 2.Abdel-Nasser A., Hussein R., Khalifa M. A.2010. Fascioliasis prevalences among animals and human in Upper Egypt. J. King. Saud. Univ. Sci. 22: 15–19. doi: 10.1016/j.jksus.2009.12.003 [DOI] [Google Scholar]
- 3.Augot D., Rondelaud D., Dreyfuss G., Cabaret J., Bayssade-Dufour C., Albaret J. L.1998. Characterization of Fasciola hepatica redial generations by morphometry and chaetotaxy under experimental conditions. J. Helminthol. 72: 193–198. doi: 10.1017/S0022149X00016436 [DOI] [PubMed] [Google Scholar]
- 4.Ayana D., Gebreab F., Sori H.2009. Prevalence of Ovine and Caprine Fasciolosis in and around Assela Town of Oromia Regional State, Ethiopia. Bull. Anim. Health Prod. Afr. 57: 109–116. [Google Scholar]
- 5.Bekele M., Haftom T., Yehenew G.2010. Bovine fascioliosis: Prevalence and its economic loss due to liver condemnation at Adwa municipal abattoir, North Ethiopia. Ethiop. J. Agri. Sci. Tech. 1: 39–47. [Google Scholar]
- 6.Bennema S. C., Ducheyne E., Vercruysse J., Claerebout E., Hendrickx G., Charlier J.2011. Relative importance of management, meteorological and environmental factors in the spatial distribution of Fasciola hepatica in dairy cattle in a temperate climate zone. Int. J. Parasitol. 41: 225–233. doi: 10.1016/j.ijpara.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 7.Biu A., Paul B., Konto M., Ya’Uba A.2013. Cross sectional and phenotypic studies on fasciolosis in slaughter cattle in Maiduguri. J. Agri. Vet. Sci. 5: 155–162. [Google Scholar]
- 8.Cwiklinski K., O’Neill S. M., Donnelly S., Dalton J. P.2016. A prospective view of animal and human Fasciolosis. Parasite Immunol. 38: 558–568. doi: 10.1111/pim.12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degheidy N., Al-Malki J.2012. Epidemiological Studies of Fasciolosis in Human and Animals at Taif, Saudi Arabia. World Appl. Sci. J. 19: 1099–1104. [Google Scholar]
- 10.Elmonir W., Mousa W., Sultan K.2015. The prevalence of some parasitic zoonoses in different slaughtered animal species at Abattoir in the mid-delta of Egypt; with special reference to its economic implications. Alex. J. Vet. Sci. 47: 97–103. [Google Scholar]
- 11.el-Shazly A. M., el-Wafa S. A., Haridy F. M., Soliman M., Rifaat M. M., Morsy T. A.2002. Fascioliasis among live and slaugthered animals in nine centers of Dakahlia Governorate. J. Egypt. Soc. Parasitol. 32: 47–57. [PubMed] [Google Scholar]
- 12.El-Sherif A. F., Abdou A. H., El-Sawi M. F.1959. The incidence of parasitic infestation among the farm animals of Faculty of Agriculture, University of Egypt. Vet. Med. Assoc. 19: 19–21. [Google Scholar]
- 13.Ephrem B., Wassie M., Abadi A.2012. Prevalence and economic losses of bovine fasciolosis in dessie Municipal Abattoir, South Wollo Zone, Ethiopia. Eur. J. Biol. Sci. 4: 53–59. [Google Scholar]
- 14.Furtado L. F. V., de Paiva Bello A. C., Rabelo E. M. L.2016. Benzimidazole resistance in helminths: from problem to diagnosis. Acta Trop. 162: 95–102. doi: 10.1016/j.actatropica.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 15.Girmay T., Teshome Z., Hailemikael A.2015. Prevalence and economic losses of bovine fasciolosis at Hawzien Abattoir, Tigray Region. Northern Ethiopia. J. Vet. Adv. 5: 945–951. [Google Scholar]
- 16.Hansen J., Perry B.1994. The epidemiology, diagnosis and control of helminth parasites of ruminants. A handbook, Food and Agricultural Organization of the United Nations, Rome. [Google Scholar]
- 17.Haridy F. M., Ibrahim B. B., Morsy T. A., El-Sharkawy I. M.1999. Fascioliasis an increasing zoonotic disease in Egypt. J. Egypt. Soc. Parasitol. 29: 35–48. [PubMed] [Google Scholar]
- 18.Kabir M. H., Eliyas M., Hashem M. A., Miazi O. F.2010. Prevalence of zoonotic parasitic diseases of domestic animals in different abattoir of Comilla and Brahman Baria region in Bangladesh. J. Zool. (Lond.) 28: 21–25. [Google Scholar]
- 19.Kantzoura V., Kouam M. K., Demiris N., Feidas H., Theodoropoulos G.2011. Risk factors and geospatial modelling for the presence of Fasciola hepatica infection in sheep and goat farms in the Greek temperate Mediterranean environment. Parasitology 138: 926–938. doi: 10.1017/S0031182011000436 [DOI] [PubMed] [Google Scholar]
- 20.Kanyari P. W., Kagira J. M., Mhoma R. J.2010. Prevalence of endoparasites in cattle with zoonotic potential within urban and peri-urban areas of Lake Victoria Basin, Kenya. J. Anim. Biomed. Sci. 4: 26–33. [Google Scholar]
- 21.Keyyu J. D., Monrad J., Kyvsgaard N. C., Kassuku A. A.2005. Epidemiology of Fasciola gigantica and amphistomes in cattle on traditional, small-scale dairy and large-scale dairy farms in the southern highlands of Tanzania. Trop. Anim. Health Prod. 37: 303–314. doi: 10.1007/s11250-005-5688-7 [DOI] [PubMed] [Google Scholar]
- 22.Khan M. K., Sajid M. S., Khan M. N., Iqbal Z., Iqbal M. U.2009. Bovine fasciolosis: prevalence, effects of treatment on productivity and cost benefit analysis in five districts of Punjab, Pakistan. Res. Vet. Sci. 87: 70–75. doi: 10.1016/j.rvsc.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 23.Kheider Z. A.2014. Prevalence and risk factors of bovine fasciolosis in North Kordofan State, Sudan, M.Sc., College of Veterinary Medicine, University of Khartoum, Khartoum. [Google Scholar]
- 24.Lee C. G., Cho S. H., Lee C. Y.1995. Metacercarial production of Lymnaea viridis experimentally infected with Fasciola hepatica. Vet. Parasitol. 58: 313–318. doi: 10.1016/0304-4017(94)00725-R [DOI] [PubMed] [Google Scholar]
- 25.Lotfy W. M., El-Morshedy H. N., Abou El-Hoda M., El-Tawila M. M., Omar E. A., Farag H. F.2002. Identification of the Egyptian species of Fasciola. Vet. Parasitol. 103: 323–332. doi: 10.1016/S0304-4017(01)00613-6 [DOI] [PubMed] [Google Scholar]
- 26.Moayad M., Al-jibouri S., Al-Mayah H., Hadi R. H.2011. The factors affecting metacercarial production of Fasciola gigantica from Lymnaeaauricularia snails. J. Basr. Res. 37: 9–16. [Google Scholar]
- 27.Monib M. E. M.1977. Study on some helminth parasites of ruminants in Assiut Governorate, M.Sc. Thesis, Faculty of Veterinary Medicine, Assiut University, Egypt.
- 28.Nicholson M. J., Butterworth M. H.1986. A guide to condition scoring of zebu cattle. International Livestock Center for Africa, Addis Ababa, Ethiopia. [Google Scholar]
- 29.Nossair M. A., Abdella D. E.2014. Serological detection of Fasciola hepatica antibodies among cattle and human in Behera Province, West Delta, Egypt. Alex. J. Vet. Sci. 40: 16–23. [Google Scholar]
- 30.Phiri A. M., Phiri I. K., Sikasunge C. S., Monrad J.2005. Prevalence of fasciolosis in Zambian cattle observed at selected abattoirs with emphasis on age, sex and origin. J. Vet. Med. B Infect. Dis. Vet. Public Health 52: 414–416. doi: 10.1111/j.1439-0450.2005.00872.x [DOI] [PubMed] [Google Scholar]
- 31.Selemetas N., Phelan P., O’Kiely P., Waal T.2014. Weather and soil type affect incidence of fasciolosis in dairy cow herds. Vet. Rec. 175: 371–375. doi: 10.1136/vr.102437 [DOI] [PubMed] [Google Scholar]
- 32.Soliman M. F.2008. Epidemiological review of human and animal fascioliasis in Egypt. J. Infect. Dev. Ctries. 2: 182–189. doi: 10.3855/jidc.260 [DOI] [PubMed] [Google Scholar]
- 33.Sorge U. S. R., Moon R., Wolff L. J., Michels L., Schroth S., Kelton D. F., Heins B.2016. Management practices on organic and conventional dairy herds in Minnesota. J. Dairy Sci. 99: 3183–3192. doi: 10.3168/jds.2015-10193 [DOI] [PubMed] [Google Scholar]
- 34.Torgerson P., Claxton J.1999. Epidemiology and control. In: Fascioliosisb (Dalton, J. P.eds), CABI Publishing, Oxfordshire. [Google Scholar]
- 35.Urquhart G. M., Armour J., Duncan J. L., Dunn A. M., Jennings F. W.1996. Veterinary Parasitology, 2nd ed. Longman Scientific and Technical Press, Oxford. [Google Scholar]
- 36.Yildirim A., Duzlu O., Inci A.2007. Prevalence and risk factors associated with Fasciola hepatica in cattle from Kayseri province, Turkey. Revue. Méd. Vét. 158: 613–617. [Google Scholar]