Abstract
Haemorrhagic septicemia (HS) is a contagious disease in cattle with high morbidity and mortality rates. HS vaccine in Thailand is an oil-adjuvant formulation, and is difficult to administer. The present study aimed to formulate and evaluate the protection in dairy calves conferred by immunization with an in-house intranasal HS vaccine. The intranasal vaccine was formulated in a total volume of 500 µl containing either 50 or 100 µg of the recombinant outer membrane protein H (rOmpH) of Pasteurella multocida strain M-1404 (serovar B:2), and 10 µg of Cytosine-phosphate-guanosine oligodeoxynucleotides (CpG-ODN) as a mucosal adjuvant. Intranasal immunizations were conducted three times at three-week intervals. The antibodies post-immunization were detected by indirect ELISA and demonstrated efficient in vitro activity in suppressing a P. multocida strain from the complement-mediated killing assay. An intranasal vaccine induced both the serum IgG and secretory IgA levels that were significantly higher than the level conferred by the parenteral vaccine (P<0.05). Challenge exposure was conducted with a P. multocida strain M-1404 at day 72 of the experiments. The immunized calves had reduced clinical signs after challenge exposure that would normally result in disease proliferation. We conclude that intranasal vaccination of calves with rOmpH with CpG-ODN 2007 stimulated serum and secretory antibodies to rOmpH and whole cells of P. multocida strain M-1404 antigen. Moreover, it would result in protection in calves against artificial P. multocida infection.
Keywords: CpG-ODN 2007, haemorrhagic septicemia, intranasal vaccine, Pasteurella multocida, recombinant outer membrane protein
Pasteurella multocida is recognized as an important veterinary pathogen, being the causative agent in domestic animal diseases such as fowl cholera, swine atrophic rhinitis and bovine Haemorrhagic septicemia (HS) [1]. P. multocida serovar B:2 is the predominant strain in Asia while serovar E:2 predominates in Africa. Both serovars cause HS, a disease of cattle and buffaloes, with high mortality rates [13, 45]. The disease can be controlled by several strategies, including vaccination. There are several candidate immunogens for HS vaccine. The porin protein is a conserved antigen among P. multocida strains and thus has been viewed as a potential vaccine candidate [15, 18, 19, 30, 43]. The outer membrane protein H (OmpH) is a surface antigen of P. multocida and is recognized as an immunodominant porin. The major OmpH of P. multocida (B:2) has been identified, and therefore it has been suggested that this highly antigenic 37 kDa OmpH has protective and immunodiagnostic potential.
Commercial formulations are prepared with alum-precipitated or oil adjuvant killed whole-cell vaccines. However, these vaccines have the disadvantage of providing only short-term immunity [9] and may require annual booster applications [12]. Moreover, the oil-based adjuvant vaccines are often cumbersome to administer due to their high viscosity, although improved oil-adjuvanted vaccines with lower viscosities have been described [35, 43]. Mucosal vaccination is a non-invasive method and has several advantages over traditional systemic vaccines [17, 27, 46]. It is widely considered to be simpler to administer orally or nasally rather than via injection. The mucosal surface is first in line among the host defense mechanisms against invading pathogens [17].
Currently, the commercial HS vaccines are composed of live attenuated or killed bacteria. To our knowledge, the immunogenicity of a killed vaccine is not as great as that of a live attenuated vaccine; however, the recurrent of virulence in a live vaccine must be considered. Modern synthetic vaccines have been developed as veterinary use candidates [36]. Recombinant vaccines rely on the capacity of one or multiple epitopes to induce immunity against the pathogen, when administered in the presence of appropriated adjuvants or with bacterial or viral vectors. The important factors in recombinant vaccine development are the epitope design, a suitable expression vector system, a purification method that protects the immunogenicity of the epitope, and mucosal adjuvant selection [26].
Our objective of this study was to formulate a recombinant OmpH-based intranasal HS vaccine and determine its protectivity against P. multocida serovar B:2 challenge-exposure in dairy calves. Moreover, humoral and cellular immune responses conferred by an intranasal vaccine were also determined.
MATERIALS AND METHODS
Bacterial strains
P. multocida strains M-1404 serotype B:2 (kindly preferred by Prof. Dr. Takuo Sawada, Nippon Veterinary and Life Science University, Tokyo, Japan) were grown in tryptose broth (TB; Difco Laboratories, Detroit, MI, U.S.A.) at 37°C for 6 hr and were then cultured on dextrose starch agar (DSA; Difco) at 37°C for 18 hr. One single colony was selected for crude capsular extract (CCE) and for genomic DNA preparation. E. coli strain INVαF´ (Invitrogen, Carlsbad, CA, U.S.A.), strain M15 [pREP4] (QIAGEN, Valencia, CA, U.S.A.) was cultured at 37°C in Luria broth (LB) or on LB agar plates with the appropriate antibiotics. Plasmid pQE-30 (QIAGEN) was used for cloning and recombinant protein expression.
Preparation of recombinant outer membrane protein H
The recombinant outer membrane protein H (rOmpH) of P. multocida was expressed in an E. coli expression vector system (pQE30, QIAGEN). The PCR was conducted to amplify the ompH of P. multocida strain M-1404 with forward (CAGGATCCGCAACAGTTTACAATCAAGAC) and reverse primers (CACTCGAGTTAGAAGTGTACGCGTAAACC). The primers were designed based on the ompH gene of P. multocida serotype B:2 (GenBank: AJ459785.1). The PCR reaction was performed with 20 ng of P. multocida genomic DNA; 30 pmol each of forward and reverse primers; 0.1 mM dNTP, 1.5 mM MgCl2 and 1.25 units of Taq DNA Polymerase (Takara, Otsu, Japan) in a total volume 50 µl of reaction buffer. The amplification reactions were performed in thin-walled 0.2 ml PCR tubes with GeneAmp PCR System 9700 (AB Applied Biosystems, Foster city, CA, U.S.A.) under the following conditions: 94°C for 5 min; then 35 cycles each at 94°C for 15 sec, 55°C for 30 sec, and 72°C for 1 min; and lastly 72°C for 10 min. PCR products were analyzed in 1.5% agarose gel electrophoresis (Sigma Aldrich, St. Louis, MO, U.S.A.) and stained with ethidium bromide. Pictures were taken under a UV illuminator. The expected size of the PCR product was 960 bp. The purified PCR product was double-digested with BamHI and XhoI (Takara) and purified using QIAquick PCR purification kit (QIAGEN). Purified BamHI and XhoI-digested ompH fragments were ligated into the BamHI and SalI −predigested expression vector pQE-30, and introduced into E. coli strain M15 [pREP4] by transformation. Transformants were plated on selective LB agar plates containing 100 µg/ml ampicillin and 25 µg/ml kanamycin (Sigma). The plasmids of transformants were taken and sequenced by employing sequencing primer Type III/IV forward and reverse sequencing. Transformants were cultured in LB broth with or without the induction of IPTG (Isopropyl-β-D-thiogalactopyranoside; Takara) at the final concentration of 1 mM. The recombinant protein was purified by an electroelution method as described previously [41]. The rOmpH concentration was measured using the BCA protein assay kit (Pierce®, Rockford, IL, U.S.A.), following the manufacturer’s instructions.
Cattle
Fourteen 4–6 month-olds crossbred calves were housed initially in one pen with four separated rooms for each groups at the Bureau of Veterinary Biologics, Department of Livestock Development, Ministry of Agriculture and Cooperatives, Pak Chong, Nakhon Ratchasima, Thailand. The calves had been screened for anti-P. multocida serovar B:2 antibody by indirect ELISA as described previously [40]. Prior and subsequent to challenge, cattle were housed indoors in an Animal Biosafety Level 2 isolation barn. The Institutional Animal Care and Use Committee approved all studies (Approval number R16/2558).
Determination of sera IgG against P. multocida
The heat extract antigen of P. multocida strain M-1404 was prepared by saline extraction method as described previously [40]. Sera were assayed for anti-P. multocida strain M-1404 antibody using ELISA against heat extract antigen as described follows. Microtiter plates (Nunc-Immuno Plate MaxiSorp, Intermed, Roskildes, Denmark) were coated with 160 µg/ml of heat extract antigen diluted in coating buffer. Serum dilutions for the various assays were 1:100 for heat extract antigen in PBS + 1% skim milk, which were in the linear range of established dilution curves. Horseradish peroxidase-conjugated goat anti-bovine IgG (KPL, Gaithersburg, MD, U.S.A.) diluted at 1:2,000 was used as a secondary antibody, and tetramethylbenzidine (TMB; KPL) was used as the substrate. The color reaction was stopped by adding 50 µl of 3M H2SO4. The absorbance of each well was read at a wavelength of 450 nm using an automatic ELISA plate reader (AccuReader, Metertech, Taipei, Taiwan, R.O.C.) and the results were expressed as optical density (OD). The cut-off point of this indirect ELISA was set to 0.100 [40]. Furthermore, the rOmpH was also used to coat onto the immunoplate in order to measure the antibody titer against immunization by indirect ELISA as described above.
Determination of secretory IgA against P. multocida
To confirm that the secretory immunoglobulin A (IgA) was secreted onto the mucosal surface of the upper respiratory tract, nasal secretions of calves were collected as describe previously [10]. To obtain nasal secretions, an absorbent cotton material was placed within the right nostril of dairy calves for 5 min. Then, the absorbent material was flushed with 1 ml of sterile PBS (pH 7.2). Nasal secretions were collected into microcentrifuge tubes. Then, the level of IgA was detected by indirect ELISA as describe previously [10]. The previous indirect ELISA steps were repeated. ELISA plates were coated with rOmpH or heat extract antigen of P. multocida strain M-1404. Nasal secretions were diluted 1:2, and the secondary antibody was HRP-conjugated goat anti-bovine IgA (Bio-Rad Laboratories, Hercules, CA, U.S.A.) at a dilution of 1:400. Tetramethylbenzidine (TMB; KPL) was used as the substrate. The color reaction was stopped by adding 50 µl of 3M H2SO4. The absorbance of each well was read at a wavelength of 450 nm using an automatic ELISA plate reader (AccuReader, Metertech, Taipei, Taiwan, R.O.C.) and the results were expressed as optical density (OD).
Complement-mediated killing assays
The assay was performed as previously described [4, 8, 11] and was repeated at least three times. Briefly, P. multocida cells were grown in BHI broth to OD600=1.0. The cells were harvested by centrifugation at 10,000 × g for 10 min, resuspended in PBS, and decapsulated at 41°C for 1 hr in a water bath. The decapsulated cells were washed three times with sterile PBS and resuspended to an OD600=0.500 and diluted 1:1,000 in PBS (approximately 105 CFU/ml) for use in the assay. Prior to use, bovine sera were incubated at 56°C for 30 min to inactivate the complement. Serum obtained from colostrum-deprived neonatal calves (CDNC) was used as the source of complement for the assay. The assay was performed by mixing 25 µl each of bovine anti-OmpH sera, complement source, decapsulated P. multocida cells and PBS. All mixtures were incubated and plated onto agar as described in Table 1. The experiment was started (T0) and after 30 min of incubation at 37°C (T30), six replicates were plated on BHI blood agar and were then incubated at 37°C and 5% CO2. Viability was determined by counting the number of colonies after 15–16 hr of incubation. In addition, the positive control was hyperimmune serum from a calf vaccinated with commercial HS vaccine, whereas sera from naïve calves were pooled for a negative control. Percent killing was calculated as [(T0 growth−T30 growth)/T0 growth] × 100%.
Table 1. Experiment groups of the complement-mediated killing assay.
| Group | Antibody (µl) | Complement: CDNC (µl) | PBS (µl) |
|---|---|---|---|
| 1 Anti-rOmpH + CDNC | 50 | 50 | - |
| 2 Anti-rOmpH | 50 | - | 50 |
| 3 HS positive serum + CDNC | 50 | 50 | - |
| 4 HS positive serum | 50 | - | 50 |
| 5 Negative serum + CDNC | 50 | 50 | - |
| 6 Negative serum | 50 | - | 50 |
| 7 CDNC | - | 50 | 50 |
Experimental design
The 14 calves were equally divided among 4 groups (Table 2). An intranasal vaccine was freshly prepared using desired concentrations of rOmpH as described in Table 2. Cytosine-phosphate-guanosine oligodeoxynucleotides 2007 (CpG-ODN 2007; Invivogen, San Diego, CA, U.S.A.) was used as an intranasal vaccine adjuvant. Group 1 (n=5) calves were vaccinated with 50 µg rOmpH + CpG-ODN 2007. Group 2 (n=5) calves were vaccinated with 100 µg rOmpH + CpG-ODN 2007, Group 3 (n=2); calves were vaccinated with a commercial HS vaccine (oil-adjuvant bacterin, Bureau of Veterinary Biologics, Department of Livestock Development, Ministry of Agriculture and Cooperative, Pak Chong, Nakhon Ratchasima, Thailand), and Group 4 (n=2) was a non-vaccinated control group. Sera were obtained on days 0, 10, 20, 30, 40, 50, 60 and 72. Vaccinations of calves in Groups 1 and 2 were conducted by dropping from an autopipette, approximately 0.5 ml into the nasal passage on days 0, 21 and 42. Group 3 calves were intramuscularly immunized twice on days 0 and 30. There was no vaccination in Group 4.
Table 2. Vaccine formulations, number of immunization and mean body temperature of cow post challenge-exposure.
| Group | Vaccine formulations | Number of immunization | Inoculum strain c) | Mean body temperature post challenge-exposure (°C) d) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||||
| 1 | 50 µg each rOmpH/rLKT + CpG ODN 10 µg a) | 5 | M-1404 | 37.06 ± 0.04 | 37.63 ± 0.04 | 37.42 ± 0.02 | 37.26 ± 0.03 | 36.84 ± 0.02 | 36.59 ± 0.01 |
| 2 | 100 µg each rOmpH/rLKT + CpG ODN 10 µg a) | 5 | 37.06 ± 0.03 | 37.64 ± 0.02 | 37.44 ± 0.01 | 37.36 ± 0.03 | 36.97 ± 0.03 | 36.83 ± 0.04 | |
| 3 | Commercial hemorrhagic septicemia vaccine b) | 2 | 37.12 ± 0.04 | 37.54 ± 0.03 | 37.52 ± 0.03 | 37.37 ± 0.02 | 37.06 ± 0.02 | 37.27 ± 0.02 | |
| 4 | No vaccination | 2 | 37.04 ± 0.03 | 38.54 ± 0.01e | 38.03 ± 0.02 | 37.49 ± 0.02 | 37.03 ± 0.03 | 37.10 ± 0.02 | |
a) Intranasal administration with 100 µl/dose, b) Intramuscular administration with 1.0 ml/dose. c) Challenge inoculum was 1 ml of PBS containing approximately 103 CFU/ml. d) Daily average body temperature. e) Experiment was terminated due to the clinical signs of cow.
On day 72, all calves were intranasally challenged with P. multocida strain M-1404. After challenge, all calves were observed for 10 days and evaluated for clinical signs by the attending veterinarian following subjective and objective criteria using a commonly used clinical evaluation system; symptoms included depression, appetite, and respiratory signs, and the objective criterion was rectal temperature as described in the Guide for the Care and Use of Agricultural Animals in Research and Teaching, 3rd edition (2010). On day 82, all calves were treated with injectable Synulox™ ready-to-use injection (8.75 mg/kg; Zoetis, Florham Park, NJ, U.S.A.) for 5 days and returned to outside pens for observation. The experiment was terminated depending on the clinical signs of the calves.
Statistical analysis
The level of significance was P<0.05. The ELISA antibody titers between the vaccinated groups and the non-vaccinated control group were analyzed using a repeated measures ANOVA.
RESULTS
SDS-PAGE
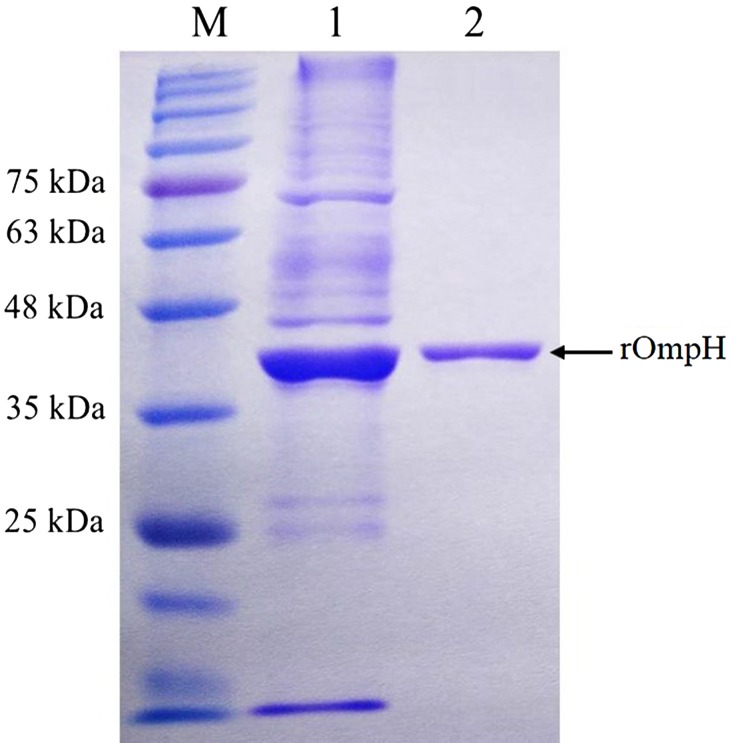
The size, purity, and integrity of the rOmpH were demonstrated by SDS-PAGE and the presence of the rOmpH at approximately 40 kDa was observed (Fig. 1). The predicted molecular weight of rOmpH was 37 kDa. The exact molecular weight on the SDS-PAGE of rOmpH will reflect the fusion with 3 kDa of the 6 His-tag, thus the total molecular weight will be approximately 40 kDa.
Fig. 1.
The rOmpH fractions purified by electroelution were analyzed using SDS-PAGE and stained with Coomassie brilliant blue. Lanes: M: molecular mass standards; 1: unpurified rOmpH; 2: rOmpH fractions purified by electroelution.
Antibody responses
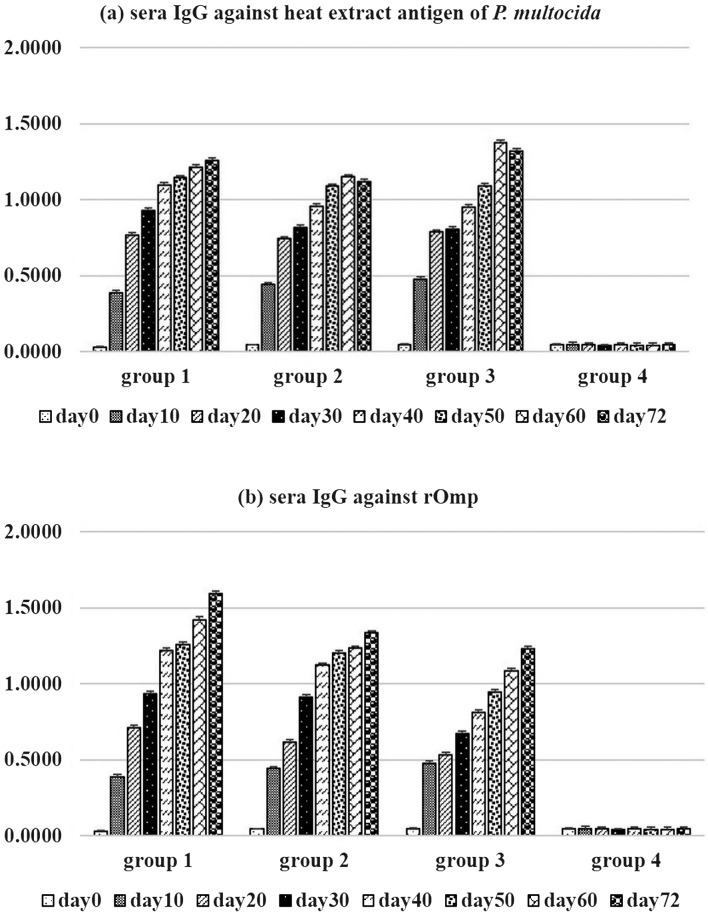
The levels of antibody titers in the calves are shown in Fig. 2. The indirect ELISA confirmed the negative serum level of the calves. The antibody titers against heat extract antigen or rOmpH on day 0 were lower than the baseline (OD450 lower than 0.100). This confirmed that all of the cattle were seronegative to HS (Fig. 2). Primary vaccination with an intranasal vaccine resulted in a slight increase in mean serum antibodies. On day 20, vaccinated calves of groups 1 and 2 had an insignificant increase in mean serum antibodies (Fig. 2). Following the third vaccination, intranasal vaccinated calves of groups 1 and 2 had the highest serum antibody titers. Mean serum antibody titers of groups 1 and 2 calves were significantly greater (P<0.05) than the concentrations for the other groups at day 30. On days 60 and 72, vaccinated Group 3 calves had a greatest mean serum antibody titers than the concentrations for the other groups.
Fig. 2.
Sera IgG against (a) heat extract antigen of P. multocida strain M-1404 and (b) rOmpH by indirect ELISA.
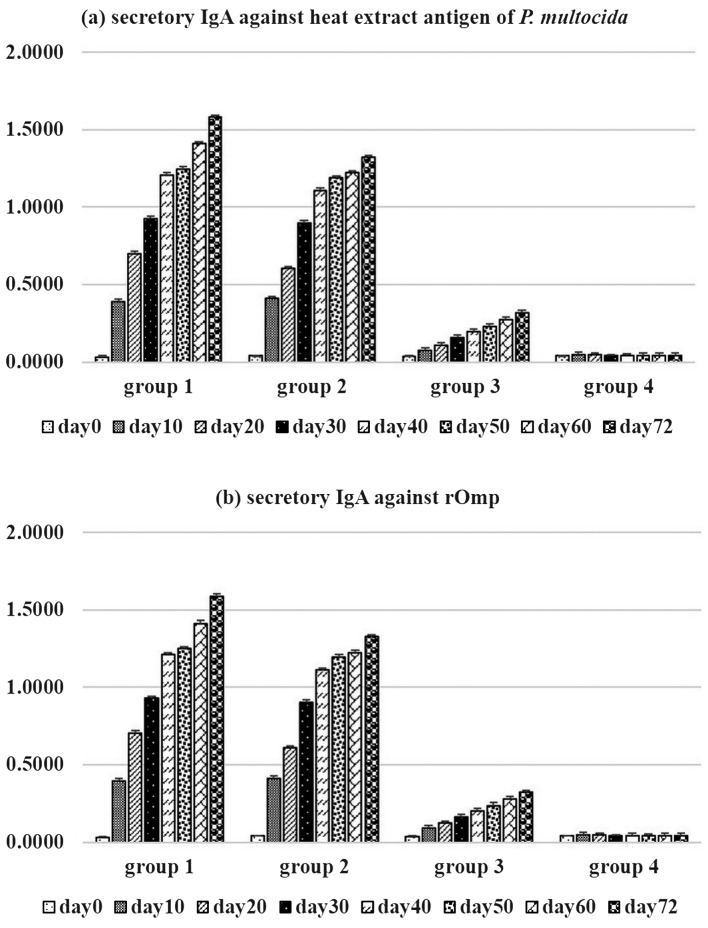
Secretory IgA against rOmpH or heat extract antigen of P. multocida were not detected in calves pre-immunization (Fig. 3). This confirmed that calves were free of secretory IgA at the mucosal surface of the upper respiratory tract. As observed, secretory IgA against rOmpH or heat extract antigen of P. multocida were increased in groups 1 and 2 after the first vaccination. The levels of secretory IgA of groups 1 and 2 were significantly higher than in groups 3 and 4 (P<0.05) along the experiments. In contrast, the levels of secretory IgA in group 3 rarely increased and were lower than in groups 1 and 2. This resulted that the intranasal vaccines were able to induce high levels of the secretory IgA at the mucosal surface of the intranasally immunized calves. There were no secretory IgA responses to rOmpH or heat extract antigen of P. multocida during the experiment for calves in group 4.
Fig. 3.
Secretory IgA against (a) heat extract antigen of P. multocida strain M-1404 and (b) rOmpH by indirect ELISA.
Complement-mediated killing assay with bovine immunized sera
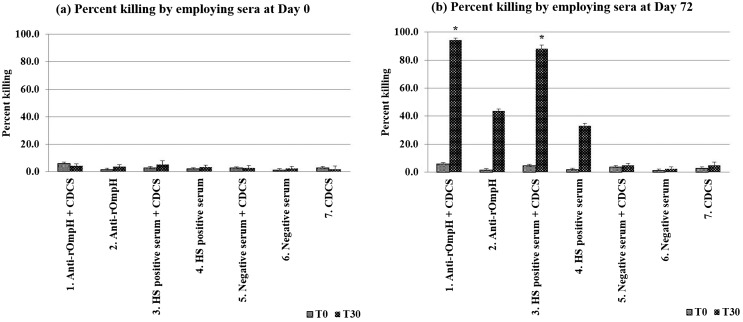
Bovine sera at days 0 and 72 were used to determine the killing assay. After vaccination with either an intranasal vaccine or commercial HS vaccine killed 94.2 and 88.1% of the P. multocida, respectively, whereas day 0 values were 5.9 or 4.7%, respectively, which were significantly higher (P<0.05) than the day 0 values (Fig. 4). In the absence of complement sources, the percent killing of P. multocida was low and there was no significant difference between sera at days 0 or 72.
Fig. 4.
Percent killing of P. multocida strain M-1404 by the complement mediated killing assay. *significantly difference (P<0.05).
P. multocida challenge-exposure
Within 24 hr after P. multocida strain challenge exposed, all calves had moderately elevated rectal temperatures, and the rectal temperatures of the PBS-vaccinated calves remained higher than the other groups throughout the post-challenge period (Table 2). The rectal temperatures of groups 4 were increased and higher those of groups 1–3. The clinical signs of infection in the cattle were depression, loss of appetite, and a rumen contraction rate less than 2 times within 5 min. The experiment was stopped at that point for group 4 via treatment with antimicrobial drugs as described above. The average rectal temperatures of groups 1–3 were slightly increased, but not significantly different among groups. The cattle in these groups still had an appetite, but the rumen contraction rate was less than 2 times within 3 min. The rectal temperatures of calves in group 3 on day 1 of the experiment were higher than those in groups 1 and 2. However, the average temperatures in groups 1–3 showed no increase after day 2 of the experiment. The authors terminated all experiments at day 10 post challenge exposure by administering antimicrobial drugs to all cattle as described above, and observed the clinical signs for 10 days before releasing the calves to the open barn.
DISCUSSION
HS is a fatal infectious disease with a short incubation period. This bacterial disease is very economically important in Asian and African countries. The predominant serovars causing this disease are serovars B:2 and E:2. Since the disease has a short incubation period, treatment options are limited. HS vaccine is recommended in endemic areas, but the conventional vaccines often yield unsatisfactory results, such as side effect tissue damage or conferring only short term immunity [43]. HS bacterin vaccines are cheap but produce only limited protection against heterologous strain infection and are capable to elicit a poor secretory IgA at the mucosal surface of the upper respiratory tract [38]. HS live attenuated vaccines are often the most successful, able to confer prolonged immunity in cattle [43]. However, mucosal vaccine administration can be a useful alternative route of immunization [16, 19]. The mucosal surface acts as the first line of defense against pathogen infection. P. multocida causes HS in cattle and buffaloes, and can achieve catastrophic prevalence during rainy and severe winter months in Asian countries [19]. The bacteria invade through the nasopharyngeal route, and bacterial colonization takes place in the respiratory tract. The mucosal surface is thus important in disease protection. Release of secretory IgA in the upper respiratory tract assists the prevention of invasion and colonization of the bacterium. To ensure that the calves used in the present experiment were free from the disease, the immune responses were determined. The measuring of bovine sera IgG and secretory IgA at pre-immunization can be indicated the immunological status of calves against P. multocida strain in sera or mucous membranes. As the bacterium can be isolated from the upper respiratory tract of healthy cattle with no clinical signs [7], but the antibody profiles in pre-immunized calves of all experiment groups indicated there were no antibody responses to P. multocida strain.
Complement-mediated killing is an important host defense mechanism against microbial infection and is recognized as an important role in controlling P. multocida infection. Level of complement concentrations were found to be lower in stressed cattle [31]. The decreasing in serum complement levels might facilitate the bacterium infection. However, the complement-mediated killing of P. multocida requires sensitization with antibody [22]. The complement-mediated killing assays demonstrated that antibody against rOmpH contribute to the host defense mechanism of dairy cattle in protection against HS. Bovine sera that was depleted of antibody against rOmpH also caused significant percent killing under these assay. These results suggested that antibody against rOmpH also contribute to complement-mediated killing of bacterium. Control complement-mediated killing assays with only complement source without the antibody source indicated that complement alone was unable to kill P. multocida. This suggested that complement was strictly activated through the alternative pathway and may not play a significant role in the killing of the bacterium. These evidence are in agreement with those of previous studies which demonstrated that only activation of the complement cascade through the classical pathway is important in killing P. multocida [5, 22]. Moreover, the role of antibody against rOmpH also include in the host defense mechanisms and may be important in protective immunity against P. multocida infection.
The OmpH are potential targets for HS vaccine development [15, 19]. Kharb and Charan [19] have investigated the effect of boosting immunity via the mucosal route vis-à-vis the parenteral route in a mouse model of HS. Mice were immunized with OmpH-enriched fraction via intranasal and subcutaneous routes. Then, mice were challenged with P. multocida through the intranasal route. This resulted in 88% protection via the intranasal route of immunization, which was higher than the 50% protection observed in mice subjected to subcutaneous immunization. Those authors concluded that an OmpH-enriched fraction provided protection against P. multocida infection in their mouse model. Moreover, the intranasal route can be used as an alternative method of immunization. Ataei et al. [3] have identified the immunogenic proteins of P. multocida serovar B:2 strain associated with protection against HS and demonstrated that a 37 kDa protein separated from OmpH was a strong immunogen. In accordance with our present investigations, the 37 kDa band of OmpH was selected as a candidate immunogen for development of a HS vaccine in cattle. The administration of the present intranasal vaccine was conducted three times at three weeks intervals. The number of immunizations, dosage and antibody levels against P. multocida of this vaccine were determined in preliminary studies (data not shown). The present immunization schedule resulted in better antibody response, and provided appropriate amounts of immunogen per dose. As our results, the intranasal vaccines gave a higher both serum IgG and secretory IgA levels than the parenteral bacterin vaccine. This results supported the benefit of an intranasal vaccine to elicit the secretory IgA at the mucosal surface of calves. The mechanisms of IgA response following the intranasal immunization have been described previously [27, 34, 37]. Intranasal immunization has been shown to induce high levels of IgA response after booster immunization. In addition, intranasal immunization has been shown to induce effective mucosal as well as systemic immune responses and long-lasting protection against nasopharyngeal infections. In particular, efficient protection against bacterial or viral infections of the respiratory tract was directly dependent on mucosal rather than systemic antibody. Moreover, the intranasal immunization was also highly effective against systemic infection following intravenous, intratracheal or intraperitoneal challenge.
Numerous investigators have shown that immunization via mucosal routes using CpG-ODN induces mucosal and systemic immune responses to various antigens [24, 28, 29, 39]. CpG-ODN are unmethylated and present at the expected frequency in bacterial DNA (1/16 bases), whereas they are underrepresented (1/50 to 1/60 bases) and selectively methylated in vertebrate genomes [6]. It has been suggested that because of these differences, a nonself pattern recognition mechanism has evolved in vertebrate immune systems enabling them to counter invading pathogens [20]. Rankin et al. [33] have identified the sequences of synthetic oligonucleotides (ODN) in animal models. Interestingly, the CpG-ODN 2007 has strong immunomodulatory and immunostimulatory activity in sheep, goats, dogs, chickens, and a broad variety of important livestock and companion animals. The adjuvant effects of CpG-ODN have been well documented, particularly in mice and humans. CpG-ODN also has potential as a candidate adjuvant in cattle [23,24,25, 29, 39, 42]. In general, immunostimulatory DNA containing CpG motifs promote Th1 humoral responses for protein-based vaccines [21]. The humoral immune response is an important mechanism against pasteurellosis through the antibody-mediated killing by complement [44] and antibody-mediated opsono-phagocytosis [2, 14, 32]. In HS, the antibody-mediated killing by complement is an important mechanism against infection [44]. In accordance with our present investigation, the intranasal vaccine was able to induce specific antibody against whole cells of P. multocida strain M-1404 and killed the bacteria in vitro. Moreover, immunized calves did not show the severe clinical signs of the disease after challenge exposure to the bacterium. These data suggest that the induced antibody was able to protect the immunized calves from HS. In addition, the rOmpH-based intranasal vaccine against HS added with CpG-ODN 2007 as an adjuvant was able to elicit an efficient antibody response against HS, both in vitro and in vivo. However, the efficacy of this vaccine in buffaloes and other hosts will need to be investigated in future studies.
In conclusions, an intranasal vaccination of dairy calves with rOmpH containing CpG-ODN 2007 stimulated serum and secretory antibodies to rOmpH and whole cells of P. multocida strain M-1404 antigen. Those calves had reduced clinical signs after intranasal challenge exposure. However, additional studies are needed to determine whether an efficacious dosage and schedule of vaccination could be developed in buffaloes or other hosts, and whether these would result in protection against HS.
Acknowledgments
This work was funded by grant # CRP5605010390 from the Agricultural Research Development Agency (Public Administration). The authors thank the Bureau of Veterinary Biologics, Department of Livestock Developments, Ministry of Agriculture and Cooperative staffs, for assistance in animal experiments. This work was also supported through the Chiang Mai University Research Administration Office which provide the budget to the Excellent Center in Veterinary Bioscience, Chiang Mai University, Thailand.
REFERENCES
- 1.Adlam C., Rutter J. M.1989. Pasteurella and Pasteurellosis. Academic Press Limited, London. [Google Scholar]
- 2.Ashfaq M. K., Campbell S. G.1986. The influence of opsonins on the bactericidal effect of bovine alveolar macrophages against Pasteurella multocida. Cornell Vet. 76: 213–221. [PubMed] [Google Scholar]
- 3.Ataei S., Burchmore R., Christopher Hodgson J., Finucane A., Parton R., Coote J. G.2009. Identification of immunogenic proteins associated with protection against haemorrhagic septicaemia after vaccination of calves with a live-attenuated aroA derivative of Pasteurella multocida B:2. Res. Vet. Sci. 87: 207–210. doi: 10.1016/j.rvsc.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 4.Ayalew S., Confer A. W., Payton M. E., Garrels K. D., Shrestha B., Ingram K. R., Montelongo M. A., Taylor J. D.2008. Mannheimia haemolytica chimeric protein vaccine composed of the major surface-exposed epitope of outer membrane lipoprotein PlpE and the neutralizing epitope of leukotoxin. Vaccine 26: 4955–4961. doi: 10.1016/j.vaccine.2008.07.023 [DOI] [PubMed] [Google Scholar]
- 5.Blau K. A., Ward A. C. S., Prieur D. J., Corbeil L. B.1987. Serum susceptibility of bovine pasteurellas. Can. J. Vet. Res. 51: 157–161. [PMC free article] [PubMed] [Google Scholar]
- 6.Cardon L. R., Burge C., Clayton D. A., Karlin S.1994. Pervasive CpG suppression in animal mitochondrial genomes. Proc. Natl. Acad. Sci. U.S.A. 91: 3799–3803. doi: 10.1073/pnas.91.9.3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter G. R., de Alwis M. C. L.1989. Pasteurella multocida and Haemorrhagic septicemia. pp. 37–74, 131–160. In: Pasteurella and Pasteurellosis (Adlam, C., and Rutter, J. M. eds.), Academic Press Limited, London. [Google Scholar]
- 8.Chae C. H., Gentry M. J., Confer A. W., Anderson G. A.1990. Resistance to host immune defense mechanisms afforded by capsular material of Pasteurella haemolytica, serotype 1. Vet. Microbiol. 25: 241–251. doi: 10.1016/0378-1135(90)90081-6 [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekaran S., Kennett L., Yeap P. C., Muniandy N., Rani B., Mukkur T. K.1994. Characterization of immune response and duration of protection in buffaloes immunized with haemorrhagic septicaemia vaccines. Vet. Microbiol. 41: 213–219. doi: 10.1016/0378-1135(94)90102-3 [DOI] [PubMed] [Google Scholar]
- 10.Confer A. W., Ayalew S., Step D. L., Trojan B., Montelongo M.2009. Intranasal vaccination of young Holstein calves with Mannheimia haemolytica chimeric protein PlpE-LKT (SAC89) and cholera toxin. Vet. Immunol. Immunopathol. 132: 232–236. doi: 10.1016/j.vetimm.2009.04.018 [DOI] [PubMed] [Google Scholar]
- 11.Confer A. W., Ayalew S., Panciera R. J., Montelongo M., Whitworth L. C., Hammer J. D.2003. Immunogenicity of recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE and augmentation of a commercial vaccine. Vaccine 21: 2821–2829. doi: 10.1016/S0264-410X(03)00213-5 [DOI] [PubMed] [Google Scholar]
- 12.De Alwis M. C. L.1992. Haemorrhagic septicaemia--a general review. Br. Vet. J. 148: 99–112. doi: 10.1016/0007-1935(92)90101-6 [DOI] [PubMed] [Google Scholar]
- 13.Griffin D.2010. Bovine pasteurellosis and other bacterial infections of the respiratory tract. Vet. Clin. North Am. Food Anim. Pract. 26: 57–71. doi: 10.1016/j.cvfa.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 14.Harmon B. G., Glisson J. R., Nunnally J. C.1992. Turkey macrophage and heterophil bactericidal activity against Pasteurella multocida. Avian Dis. 36: 986–991. doi: 10.2307/1591559 [DOI] [PubMed] [Google Scholar]
- 15.Hatfaludi T., Al-Hasani K., Boyce J. D., Adler B.2010. Outer membrane proteins of Pasteurella multocida. Vet. Microbiol. 144: 1–17. doi: 10.1016/j.vetmic.2010.01.027 [DOI] [PubMed] [Google Scholar]
- 16.Hodgson J. C., Finucane A., Dagleish M. P., Ataei S., Parton R., Coote J. G.2005. Efficacy of vaccination of calves against hemorrhagic septicemia with a live aroA derivative of Pasteurella multocida B:2 by two different routes of administration. Infect. Immun. 73: 1475–1481. doi: 10.1128/IAI.73.3.1475-1481.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgren J., Czerkinsky C.2005. Mucosal immunity and vaccines. Nat. Med. 11 Suppl: S45–S53. doi: 10.1038/nm1213 [DOI] [PubMed] [Google Scholar]
- 18.Joshi S., Tewari S. K., Singh R.2013. Comparative immunogenicity and protective efficacy of different preparations of outer membrane proteins of Pasteurella multocida (B:2) in a mouse model. Vet. Arh. 83: 665–676. [Google Scholar]
- 19.Kharb S., Charan S.2011. Mucosal immunization provides better protection than subcutaneous immunization against Pasteurella multocida (B:2) in mice preimmunized with the outer membrane proteins. Vet. Res. Commun. 35: 457–461. doi: 10.1007/s11259-011-9484-8 [DOI] [PubMed] [Google Scholar]
- 20.Krieg A. M.2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20: 709–760. doi: 10.1146/annurev.immunol.20.100301.064842 [DOI] [PubMed] [Google Scholar]
- 21.Zhang L., Tian X., Zhou F.2007. CpG oligodeoxynucleotides augment the immune responses of piglets to swine Pasteurella multocida living vaccine in vivo. Res. Vet. Sci. 83: 171–181. doi: 10.1016/j.rvsc.2006.11.012 [DOI] [PubMed] [Google Scholar]
- 22.MacDonald J. T., Maheswaran S. K., Opuda-Asibo J., Townsend E. L., Thies E. S.1983. Susceptibility of Pasteurella haemolytica to the bactericidal effects of serum, nasal secretions and bronchoalveolar washings from cattle. Vet. Microbiol. 8: 585–599. doi: 10.1016/0378-1135(83)90007-X [DOI] [PubMed] [Google Scholar]
- 23.Mapletoft J. W., Oumouna M., Townsend H. G., Gomis S., Babiuk L. A., van Drunen Littel-van den Hurk S.2006. Formulation with CpG oligodeoxynucleotides increases cellular immunity and protection induced by vaccination of calves with formalin-inactivated bovine respiratory syncytial virus. Virology 353: 316–323. doi: 10.1016/j.virol.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 24.Mulongo M., Prysliak T., Perez-Casal J.2013. Vaccination of feedlot cattle with extracts and membrane fractions from two Mycoplasma bovis isolates results in strong humoral immune responses but does not protect against an experimental challenge. Vaccine 31: 1406–1412. doi: 10.1016/j.vaccine.2012.12.055 [DOI] [PubMed] [Google Scholar]
- 25.Mutwiri G., Pontarollo R., Babiuk S., Griebel P., van Drunen Littel-van den Hurk S., Mena A., Tsang C., Alcon V., Nichani A., Ioannou X., Gomis S., Townsend H., Hecker R., Potter A., Babiuk L. A.2003. Biological activity of immunostimulatory CpG DNA motifs in domestic animals. Vet. Immunol. Immunopathol. 91: 89–103. doi: 10.1016/S0165-2427(02)00246-5 [DOI] [PubMed] [Google Scholar]
- 26.Nascimento I. P., Leite L. C. C.2012. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 45: 1102–1111. doi: 10.1590/S0100-879X2012007500142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogra P. L., Faden H., Welliver R. C.2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14: 430–445. doi: 10.1128/CMR.14.2.430-445.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okay S., Özcengiz E., Gürsel I., Özcengiz G.2012. Immunogenicity and protective efficacy of the recombinant Pasteurella lipoprotein E and outer membrane protein H from Pasteurella multocida A:3 in mice. Res. Vet. Sci. 93: 1261–1265. doi: 10.1016/j.rvsc.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 29.Parameswaran N., Russell G. C., Bartley K., Grant D. M., Deane D., Todd H., Dagleish M. P., Haig D. M.2014. The effect of the TLR9 ligand CpG-oligodeoxynucleotide on the protective immune response to alcelaphine herpesvirus-1-mediated malignant catarrhal fever in cattle. Vet. Res. (Faisalabad) 45: 59. doi: 10.1186/1297-9716-45-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pati U. S., Srivastava S. K., Roy S. C., More T.1996. Immunogenicity of outer membrane protein of Pasteurella multocida in buffalo calves. Vet. Microbiol. 52: 301–311. doi: 10.1016/S0378-1135(96)00066-1 [DOI] [PubMed] [Google Scholar]
- 31.Purdy C. W., Richards A. B., Foster G. S.1991. Market stress-associated changes in serum complement activity in feeder calves. Am. J. Vet. Res. 52: 1842–1847. [PubMed] [Google Scholar]
- 32.Ramdani X., Adler B.1991. Opsonic monoclonal antibodies against lipopolysaccharide (LPS) antigens of Pasteurella multocida and the role of LPS in immunity. Vet. Microbiol. 26: 335–347. doi: 10.1016/0378-1135(91)90027-D [DOI] [PubMed] [Google Scholar]
- 33.Rankin R., Pontarollo R., Ioannou X., Krieg A. M., Hecker R., Babiuk L. A., van Drunen Littel-van den Hurk S.2001. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev. 11: 333–340. doi: 10.1089/108729001753231713 [DOI] [PubMed] [Google Scholar]
- 34.Ryan E. J., Daly L. M., Mills K. H. G.2001. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 19: 293–304. doi: 10.1016/S0167-7799(01)01670-5 [DOI] [PubMed] [Google Scholar]
- 35.Shah N. H., Jacobs A. A., Shah N. H., de Graaf F. K.2001. Safety and efficacy of an oil-adjuvant vaccine against haemorrhagic septicaemia in buffalo calves: cross-protection between the serotypes B:2,5 and E:2,5. Vet. Rec. 149: 583–587. doi: 10.1136/vr.149.19.583 [DOI] [PubMed] [Google Scholar]
- 36.Shams H.2005. Recent developments in veterinary vaccinology. Vet. J. 170: 289–299. doi: 10.1016/j.tvjl.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 37.Shewen P. E., Carrasco-Medina L., McBey B. A., Hodgins D. C.2009. Challenges in mucosal vaccination of cattle. Vet. Immunol. Immunopathol. 128: 192–198. doi: 10.1016/j.vetimm.2008.10.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shivachandra S. B., Viswas K. N., Kumar A. A.2011. A review of hemorrhagic septicemia in cattle and buffalo. Anim. Health Res. Rev. 12: 67–82. doi: 10.1017/S146625231100003X [DOI] [PubMed] [Google Scholar]
- 39.Snider M., Garg R., Brownlie R., van den Hurk J. V., van Drunen Littel-van den Hurk S.2014. The bovine viral diarrhea virus E2 protein formulated with a novel adjuvant induces strong, balanced immune responses and provides protection from viral challenge in cattle. Vaccine 32: 6758–6764. doi: 10.1016/j.vaccine.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 40.Tankaew P.2016. Development and evaluation of an in-house Enzyme-linked immunosorbent assay kit for detection of antibody to bovine Haemorrhagic septicemia. Graduate School of Veterinary Science, Chiang Mai University, Chiang Mai. [Google Scholar]
- 41.Thanasarasakulpong A., Poolperm P., Tangjitjaroen W., Varinrak T., Sawada T., Pfeiffer D., Sthitmatee N.2016. Comparison of the effect of two purification methods on the immunogenicity of recombinant outer membrane protein h of Pasteurella multocida serovar A:1. Vet. Med. Intern. Article ID 2579345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Drunen Littel-van den Hurk S., Snider M., Thompson P., Latimer L., Babiuk L. A.2008. Strategies for induction of protective immunity to bovine herpesvirus-1 in newborn calves with maternal antibodies. Vaccine 26: 3103–3111. doi: 10.1016/j.vaccine.2008.02.018 [DOI] [PubMed] [Google Scholar]
- 43.Verma R., Jaiswal T. N.1998. Haemorrhagic septicaemia vaccines. Vaccine 16: 1184–1192. doi: 10.1016/S0264-410X(98)80118-7 [DOI] [PubMed] [Google Scholar]
- 44.Wijewardana T. G., Sutherland A. D.1990. Bactericidal activity in the sera of mice vaccinated with Pasteurella multocida type A. Vet. Microbiol. 24: 55–62. doi: 10.1016/0378-1135(90)90050-6 [DOI] [PubMed] [Google Scholar]
- 45.Wijewardana T. G., Wilson C. F., Gilmour N. J. L., Poxton I. R.1990. Production of mouse monoclonal antibodies to Pasteurella multocida type A and the immunological properties of a protective anti-lipopolysaccharide antibody. J. Med. Microbiol. 33: 217–222. doi: 10.1099/00222615-33-4-217 [DOI] [PubMed] [Google Scholar]
- 46.Woodrow K. A., Bennett K. M., Lo D. D.2012. Mucosal vaccine design and delivery. Annu. Rev. Biomed. Eng. 14: 17–46. doi: 10.1146/annurev-bioeng-071811-150054 [DOI] [PubMed] [Google Scholar]