Abstract
A bacterial strain designated Ca6T was isolated from polycyclic aromatic hydrocarbon (PAH)-contaminated soil from the site of a former manufactured gas plant in Charlotte, NC, USA, and linked phylogenetically to the family Rhodocyclaceae of the class Betaproteobacteria. Its 16S rRNA gene sequence was highly similar to globally distributed environmental sequences, including those previously designated ‘Pyrene Group 1’ demonstrated to grow on the PAHs phenanthrene and pyrene by stable-isotope probing. The most closely related described relative was Sulfuritalea hydrogenivorans strain sk43HT (93.6 % 16S rRNA gene sequence identity). In addition to a limited number of organic acids, Ca6T was capable of growth on the monoaromatic compounds benzene and toluene, and the azaarene carbazole, as sole sources of carbon and energy. Growth on the PAHs phenanthrene and pyrene was also confirmed. Optimal growth was observed aerobically under mesophilic temperature, neutral pH and low salinity conditions. Major fatty acids present included summed feature 3 (C16 : 1ω7c or C16 : 1ω6c) and C16 : 0. The DNA G+C content of the single chromosome was 55.14 mol% as determined by complete genome sequencing. Due to its distinct genetic and physiological properties, strain Ca6T is proposed as a member of a novel genus and species within the family Rhodocyclaceae, for which the name Rugosibacter aromaticivorans gen. nov., sp. nov. is proposed. The type strain of the species is Ca6T (=ATCC TSD-59T=DSM 103039T).
Keywords: polycyclic aromatic hydrocarbons, biodegradation, aromatics, Rhodocyclaceae
In a prior experiment that used stable-isotope probing (SIP) to reveal bacteria involved in the removal of the polycyclic aromatic hydrocarbon (PAH) pyrene in an aerobic, slurry-phase bioreactor treating contaminated soil from a former manufactured gas plant (MGP) in Charlotte, NC, USA, we identified three distinct groups of 16S rRNA genes derived from uncharacterized Proteobacteria. One of these groups was a monophyletic cluster associated with the family Rhodocyclaceae of the class Betaproteobacteria designated ‘Pyrene Group 1’ (PG1) [1]. Subsequent culture-independent experiments suggested that members of PG1 could also degrade the three-ring PAH phenanthrene [2]. A metagenome constructed from a mixed culture dominated by members of PG1 contained multiple genes for PAH degradation, some of which were heterologously expressed in Escherichia coli and their products shown to transform multiple PAHs [3].
16S rRNA gene sequences highly similar to those designated as PG1 have since been recovered from a variety of environments, suggesting widespread distribution of organisms associated with the clade in both water and soil. DNA sequences highly similar to both the 16S rRNA gene and functional genes associated with PG1 have also been detected in geographically distant soils contaminated by PAHs [4, 5]. Other PG1 16S rRNA gene sequences have appeared in samples contaminated with trichloroethylene [6], copper [7] and asphalts [8], and from such diverse locations as oilfields [9] to pristine water environments [10, 11]. In our own laboratory, PG1 sequences were among the most abundant in the bacterial community of a contaminated soil recovering from chemical oxidation as part of a remediation scheme [12]. In each of these instances, however, no isolates were obtained to represent the group.
The most closely related characterized isolate to organisms represented by SIP- and environmentally-derived PG1 sequences is Sulfuritalea hydrogenivorans strain sk43HT, a facultatively autotrophic freshwater bacterium [13]. Cultivation efforts in our lab using a modified medium derived from the aerobic growth medium of strain sk43HT resulted in the successful isolation of a strain from the bioreactor-treated soil from the MGP site in Charlotte, NC, representing PG1 and designated Ca6T. The modified sk43HT isolation medium used to isolate strain Ca6T originally contained 5 mM sodium-potassium phosphate buffer (pH 7.0) and 5 mM NH4NO3 (collectively referred to as ‘reactor buffer’), 1 mM MgSO4 . 7H2O, 1 mM CaCl2 . 2 H2O, 1 ml trace element solution l−1 [containing per litre; 12.5 ml HCl (25 %), 2.1g FeSO4 . 7 H2O, 30 mg H3BO3, 100 mg MnCl2 . 4 H2O, 190 mg CoCl2 . 6 H2O, 24 mg NaCl2 . 6 H2O, 2 mg CuCl2 . 2H2O, 144 mg ZnSO4 . 7H2O, 36 mg Na2MoO4 . 2 H2O] and 1 ml selenium-tungsten solution l−1 (containing per litre; 0.4 g NaOH, 6 mg Na2SeO3 . 5H2O, 8 mg Na2WO4 . 2H2O). After autoclaving for 15 min at 250 °C, the following filter-sterilized solutions (0.2 µm pore size) were added aseptically to the cooled media (per l): 1 ml vitamin solution (containing 4 mg p-aminobenzoic acid, 1 mg biotin, 10 mg nicotinic acid, 5 mg calcium pantothenate, 15 mg pyridoxine hydrochloride, in 100 ml of a 10 mM phosphate buffer, pH 7.1), 1 ml thiamine solution (containing 10 mg thiamine in 100 ml of 25 mM sodium phosphate buffer, pH 3.4), 1 ml vitamin B12 solution (containing 5 mg cyanocobalamin in 100 ml distilled water) and 1.5 ml thiosulfate solution (24.8 g Na2S2O3 . 5H2O in 100 ml distilled water, stored under N2 gas). Carbon was added before autoclaving as either 0.02 % pyrene dissolved in acetone to the flask, allowing the solvent to evaporate prior to adding other components, or as 0.2 % sodium pyruvate. Subsequent tests of strain Ca6T grown on the isolation medium excluding individual components indicated that only the reactor buffer, MgSO4, trace element solution and a carbon source were required for growth at a rate equivalent to that of the complete medium. We observed that growth in the liquid medium was slower and to a lower cell density in the absence of amended MgSO4, and that no growth at all occurred in the absence of the trace element solution. All subsequent tests used a medium containing only the required components: reactor buffer, MgSO4 and trace element solution (referred to as sRB1 medium, ‘supplemented reactor buffer’) with sodium pyruvate as a carbon source unless otherwise noted. For solid media (‘sRB1-agar’), 1.5 % agar (Acros Organics) was added to liquid medium prior to autoclaving.
The optimal growth conditions of strain Ca6T in sRB1 medium with pyruvate as a carbon source were determined for a range of temperatures, pH and salinities. Optimal growth was defined as the maximum growth rate during the exponential growth phase as measured by turbidity at OD600 with a HACH DR3000 spectrophotometer. Optimal temperature for growth was determined in triplicate 5 ml cultures in liquid sRB1 medium at pH 7.0 and 225 r.p.m. for temperatures of 23, 26, 28, 30, 32, 34, 35, 36 and 37 °C. Additional temperatures were evaluated on solid sRB1 plates at 20 and 4 °C. The effect of pH was tested in triplicate 5 ml tubes of sRB1 medium buffered to pH values of pH 5.0, 5.5, 6.0, 6.5 [buffered with 2-(N-morpholino)ethanesulfonic acid, 50 mM], 7.0, 7.5, 8.0 [4-(2-hydroxyethyl)−1-piperazineethanesulfonic acid, 50 mM] and 8.5, 9.0 [Tris/HCl, 50 mM] and incubated at 225 r.p.m. and 30 °C. Salinity effects were determined using triplicate 5 ml cultures of sRB1, pH 7.0, incubated at 30 °C and 225 r.p.m., amended with either 0, 0.25, 0.5, 1, 2, 3, 4 or 5 % NaCl (w/v). The optimal temperature for growth was between 30 and 34 °C, with obvious growth between 20 and 35 °C. Strain Ca6T grew between pH 6.5 and 7.5 with an optimum of pH 6.5. No growth was observed at salt concentrations >0.5 %, but strain Ca6T grew equally well with either 0.25 % or no NaCl. Under optimal conditions in sRB1-pyruvate liquid media with constant shaking at 225 r.p.m., strain Ca6T grew on pyruvate to maximum turbidity in approximately 3 days. Colonies on sRB1-agar plates with pyruvate as a carbon source developed over a period of 2 weeks when incubated at 30 °C. Strain Ca6T colonies on sRB1 plates with pyruvate were generally small (0.5–0.8 mm in diameter), circular, with a convex elevation and were pale yellow–white in colour.
The ability of Ca6T to grow under low oxygen conditions was assessed using the GasPak 100 system containing a GasPak EZ Anaerobe Container System sachet (BD Biosciences). Plates comprising sRB1-agar and pyruvate were inoculated and sealed in the system according to the manufacturer’s directions. According to the manufacturer, the system achieves an atmosphere of <1 % oxygen and ≥13 % carbon dioxide within 2.5 h. Low-oxygen conditions were confirmed after 48 h by visual observation of an indicator strip soaked in 1 mM resazurin solution. Plates were incubated at 30 °C and compared with concurrent aerobic controls. Ca6T did not display growth under low oxygen conditions after 4 weeks. Unless otherwise noted, all subsequent tests of Ca6T occurred at 30 °C, pH 7.0 and 0 % salinity under aerobic conditions. Liquid cultures were additionally incubated with constant shaking at 225 r.p.m.
The cellular morphology of strain Ca6T was investigated using transmission and scanning electron microscopy (TEM, SEM). For the preparation of samples, cells were grown in sRB1 with either pyrene or pyruvate. Specimens were observed and images taken using a Zeiss Supra 25 FESEM operating at 5 kV, 5 mm working distance and 10 µm aperture (Carl Zeiss SMT) or a LEO EM910 transmission electron microscope operating at 80kV (Carl Zeiss Microscopy). Meancell sizes were determined from SEM digital micrographs of well-distinguished cells. The cellular morphology of strain Ca6T was rod-shaped with a mean size of 0.96±0.19 µm by 0.25±0.02 µm (n=100 cells). Many cells were observed to be curved (Fig. 1), and in such instances length was measured using the linear distance from end-to-end of the cell. The outer membrane appeared wrinkled and uneven, and potential outer membrane vesicles were evident on some (but not all) cells examined after growth on either pyrene or pyruvate as a carbon source (Fig. 1c, d). Cells grown on pyruvate appeared more often individually or in pairs or small clusters, while cells grown on pyrene were generally densely clumped, presumably around pyrene crystals, with larger amounts of extracellular material apparent (Fig. 1a, b). Electron-transparent inclusions were evident in some pyrene-grown cells examined by TEM, which were absent in cells grown on pyruvate (not shown). Gram staining performed using the standard reaction and visualized by light microscopy indicated strain Ca6T was Gram-type-negative.
Fig. 1.
Scanning electron micrographs (a, b, c) and transmission electron micrograph (d) of strain Ca6T grown on pyruvate (a, c) or pyrene (b, d). Bars, 1 µm (a, b); 100 nm (c); 200 nm (d). Arrows indicate some potential outer membrane vesicles in the micrographs (c, d).
Cellular motility was tested using sRB1-agar stab-tubes with agar added at 0.3 % (w/v) and pyruvate as the carbon source. No outward motility from the stab was observed. No flagella were observed during microscopy, and few genes for flagellar synthesis were indicated in the annotated genome [14].
Metabolism of a variety of carbon substrates was tested using the Biolog GN2 Microplate. For each plate, cells were grown in liquid sRB1 medium amended with pyruvate and washed three times in PBS (pH 7.5) before being resuspended in Biolog GN/GP Inoculating Fluid as per the manufacturer’s instructions. The microplates were incubated overnight at 30 °C and scored through visual examination in comparison with the no-carbon control. In triplicate samples, the only substrates that strain Ca6T actively metabolized were formic acid, methyl pyruvate, monomethyl succinate and β-hydroxybutyric acid. The other 91 substrates, including a variety of sugars, other organic acids, nucleosides and amino acids were not metabolized.
Utilization of nitrogen sources was tested by substituting either 5 mM KNO3 or NH4Cl for NH4NO3 in sRB1 liquid media. Strain Ca6T displayed growth on pyruvate with both nitrogen sources tested. Nitrate reduction was evaluated using a culture grown in sRB1 media with NH4NO3 substituted with 5 mM KNO3. No nitrate reduction to nitrite beyond what was required for assimilation and growth was observed. Starch hydrolysis and cellulase activity were evaluated by supplementing sRB1 plates with either starch (10 g l−1) or cellulose (1 g l−1) (adapted from Kasana et al. [15]). Inoculated plates were incubated for 2 weeks at 30 °C, after which Gram’s iodine was used to identify clear zones around colonies. Protease activity was tested by adding a skimmed milk solution aseptically (10 % skimmed milk powder dissolved in distilled water) to sRB1-agar after autoclaving. Plates were incubated at 30 °C for 1 month and monitored for zones of clearing. Gelatin hydrolysis was assessed using a nutrient gelatin stab method wherein powdered gelatin (120 g l−1) was added to sRB1, gently heated to dissolve and aliquoted into 5 ml tubes. Gelatin tubes containing Ca6T were incubated at 30 °C and monitored for liquefaction of the media. Ca6T was negative for starch hydrolysis, cellulase, skimmed milk protease and gelatinase activities.
Lipase activity was assessed by adding tributyrin (98 %; Acros Organics) to sRB1-agar (1 %, v/v). Urease activity was determined using Stuart’s urea broth, which was prepared by amending phosphate buffer with urea (20 g l−1) and phenol red (10 mg l−1) in addition to pyruvate, MgSO4 and trace element solution. Urea tubes were monitored for 24 h for a colour change indicating urease production. Strain Ca6T was positive for lipase activity and negative for urease activity.
Strain Ca6T was tested for catalase activity by adding a 3 % hydrogen peroxide solution (v/v) to cells freshly scraped from the surface of an sRB1 plate. Oxidase activity was determined by adding a few drops of freshly-prepared 1 % N′,N′,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride (Acros Organics) to cells scraped from the same plate onto filter paper. Strain Ca6T was catalase-negative and oxidase-positive.
Indole production was tested by supplementing sRB1 media with tryptone (10 g l−1). After growth to turbidity, a few drops of Kovac’s reagent were added to each tube. The lack of a colour change indicated no production of indole by strain Ca6T. As indigo production from indole is indicative of activity by some ring-hydroxylating dioxygenases (RHD), enzymes involved in the initial step in the aerobic degradation of PAHs by bacteria [16, 17], the lack of indole production was anticipated. To further investigate this phenotype, indole was added as a small crystal to the lid of inverted Petri plates of sRB1-pyruvate medium 24 h after inoculation and incubated. A blue/purple colour developed by colonies on the plate due to indigo production confirmed the likely presence of at least one active RHD.
Growth of strain Ca6T on select PAHs as sole sources of carbon and energy was tested in liquid sRB1 media amended with individual PAHs (final concentration 0.2 g l−1). PAHs were added to tubes in a solvent (either acetone or dichloromethane), and the solvent was allowed to evaporate prior to adding the remaining media components. Tubes were additionally sonicated after autoclaving to help break up PAH crystals. Ca6T cells were grown in sRB1-pyruvate and washed three times with reactor buffer prior to inoculation, and triplicate PAH-containing tubes were incubated at 30 °C at 225 r.p.m. for up to 19 days. As spectrophotometric measurements of turbidity were not possible due to the presence of PAH crystals in the media, growth of Ca6T on PAHs was defined by both protein accumulation and disappearance of the parent compound. Protein concentrations were calculated from aliquots of culture approximately every 48 h using a Pierce BCA Protein Assay according to manufacturer’s instructions (ThermoFisher Scientific). Disappearance of each PAH was determined using a liquid-liquid extraction with an equal volume of n-hexanes and quantification using a HPLC system with fluorescence detection as previously described [12]. Extraction efficiency was determined from uninoculated tubes with PAH added. For all substrates except naphthalene, this method was able to quantify PAH concentration; naphthalene presumably volatilized from the tubes during either the incubation or extraction. Removal of pyrene (47 % of the initial mass) and phenanthrene (48 %) by Ca6T was correlated with protein accumulation, indicating growth on those substrates. Incubation with benz[a]anthracene resulted in modest protein accumulation and removal of the PAH from the medium compared with phenanthrene and pyrene, and the protein concentration did not display a steady increase typical of exponential growth. This suggests that Ca6T may be capable of weakly positive growth on this compound. Incubation with fluorene and anthracene resulted in PAH removal without protein accumulation, suggesting transformation but not growth on these substrates. Ca6T was negative for growth on or transformation of chrysene, benzo[a]pyrene, fluoranthene, naphthalene and acenaphthene. Growth on the azaarene carbazole was determined by visual turbidity and protein accumulation over the course of the incubation, as the HPLC assay employed for quantifying PAHs was not suitable for this compound. Strain Ca6T was positive for growth on carbazole.
Mineralization of the partially 14C-labelled PAHs phenanthrene, fluoranthene, chrysene, benz[a]anthracene and benzo[a]pyrene was performed as previously described [18]. Ca6T cells grown on sRB1-pyrene and washed three times with reactor buffer were used to inoculate the flasks. The extent of mineralization was measured after 24 and 48 h. Mineralization was assessed as a percentage of added PAH mineralized, and biotic samples were compared with acidified killed control replicates. Statistically significant mineralization occurred for phenanthrene (29 % mineralization, P<0.01) and chrysene (19 %, P<0.05), but did not occur for fluoranthene, benzo[a]pyrene or benz[a]anthracene. Differential results in PAH removal and mineralization for the PAHs chrysene and benz[a]anthracene may be attributable to several factors including growth of the inocula on aromatic versus non-aromatic carbon sources (potentially affecting gene expression), the low concentration of the 14C-labelled compounds and the length of the incubations.
Growth on BTEX compounds (benzene, toluene, ethylbenzene and xylene) was investigated by creating an atmosphere in separate, tightly-sealed metal containers of either benzene (99 %), toluene (99.8 %), ethylbenzene (99.8 %) or a mixture of xylenes (o-xylene 98.5 %, m- 99 %, p- 99 %, in equal volumes) by adding 0.5 ml of the chemical(s) to a piece of filter paper in a glass beaker. Inoculated sRB1-agar plates without additional carbon were incubated in these containers at room temperature for 1 month. Strain Ca6T grew well on benzene, weakly on toluene, but did not grow on either ethylbenzene or mixed xylenes. Inoculated plates of sRB1-agar without amended carbon in normal atmosphere showed no growth over the same time period.
Cellular fatty acid profiling was performed using the MIDI Sherlock Microbial Identification System by Microbial ID on cells grown on sRB1 plates with pyruvate. The dominant fatty acids in Ca6T were summed feature 3 (C16 : 1ω7c or C16 : 1ω6c) (42.5 %) and C16 : 0 (36.7 %). Ca6T also contained C17 : 0 cyclo (6.5 %), C18 : 1ω7c (4.9 %), C10 : 0 3-OH (3.9 %) and C12 : 0 (3.0 %). Trace amounts (<1 % each) of C10 : 0, C14 : 0, C16 : 1ω5c, C17 : 1ω7c, C18 : 2ω6,9c, C18 : 0, C19 : 0 cyclo ω8c and C19 : 0 were also detected. Analysis of respiratory quinones and polar lipids was carried out by the Identification Service, DSMZ, Braunschweig, Germany. Major polar lipids in Ca6T were phosphatidylethanolamine, phosphatidylglycerol, diphosphatidylglycerol and phospholipid, with minor amounts of aminolipid, glycolipid and lipid present (Fig. S1, available in the online Supplementary Material). Respiratory quinones present were Q7 (9 %), Q8 (80 %) and Q9 (11 %).
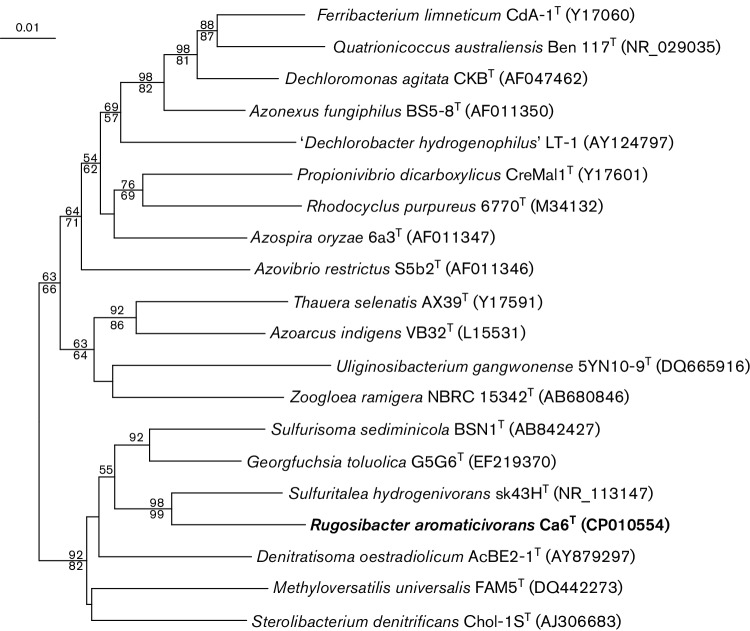
The genome of strain Ca6T was determined as described previously [14] and was composed of a singular, circular chromosome of 2 934 611 bp with a DNA G+C content of 55.14 mol%. No plasmids were detected. The two copies of the 16S rRNA gene detected were identical, and the closest described relatives of Ca6T were Sulfuritalea hydrogenivorans strain sk43HT (93.6 % 16S rRNA gene similarity) [13], Denitratisoma sp. TSA61 (93.2 %) [19], Georgfuchsia toluolica G5G6T (92.0 %) [20], Sulfurisoma sediminicola BSN1T (91.8 %) [21], Denitratisoma oestradiolicum AcBE2-1T (91.6 %) [22] and Sterolibacterium denitrificans ChoI-1ST (91.5 %) [23]; these organisms are all members of genera within the betaproteobacterial family Rhodocyclaceae (Fig. 2).
Fig. 2.
16S rRNA gene neighbour-joining phylogenetic tree of Ca6T with type strains of genera from the family Rhodocyclaceae. Percentage bootstrap values of >50 % based on 1000 iterations are shown for the neighbour-joining [26] and maximum-parsimony algorithms [27] above and below nodes, respectively. The consensus neighbour-joining tree was reconstructed including all sequence information available and without considering positions with gaps. Nitrosomonas europaea strain ATCC 25978T (GenBank accession HE862405) was included as an outgroup (not shown). GenBank accession numbers are included in parentheses to each strain. Bar, 0.01 substitutions per nucleotide position.
In addition to high 16S rRNA gene sequence divergence from described species of phylogenetically related genera, Ca6T was also differentiated by its physiological characteristics (Table 1). Most species from closely related genera within the family Rhodocyclaceae were isolated from aquatic or anoxic environments, while Ca6T was isolated from contaminated soil. Representatives from all closely related genera were also generally capable of facultative or anaerobic growth and nitrate reduction, while Ca6T required an aerobic environment for growth under the growth conditions tested and was not able to reduce nitrate. None of the phylogenetically closest described genera to Ca6T contain species reported to degrade PAHs. Among the genera examined, strain Ca6T was also the only isolate reported to contain the respiratory quinones Q7 and Q9; all isolates for which such information is available, including Ca6T, indicated Q8 as the major or sole respiratory quinone. Similarly, while species from all closely related genera (including strain Ca6T) contained C16 : 1ω7c (generally as part of a summed feature) and C16 : 0 as major fatty acids, strain Ca6T could be distinguished by the presence or absence of other, less abundant fatty acids. A significantly lower genomic DNA G+C content (mol%) further distinguished Ca6T from strains of related genera. A complete genome comparison between Ca6T and Sulfuritalea hydrogenivorans sk43HT (the closest relative by 16S rRNA gene sequence identity) approximated a DNA–DNA hybridization of 19.00 % (16.8–21.4 %) based on a linear regression model [24]. The average nucleotide identity (ANI) between the two genomes was 76.69 % [25]. On the basis of genomic and physiological differences, we propose Ca6T as the type strain of a novel species and genus, for which the name Rugosibacter aromaticivorans gen. nov., sp. nov. is proposed.
Table 1. Differential characteristics of strain Ca6T and closely related taxa within the family Rhodocyclaceae.
Strains: 1, Ca6T; 2, Denitratisoma oestradiolicum AcBE2-1T [22]; 3, Georgfuchsia toluolica G5G6T [20]; 4, species of the genus Methyloversatilis including Methyloversatilis universalis strains FAM5T and Ehg5 [28], Methyloversatilis discipulorum strains FAM1T, RZ18-153 and RZ94 [29], and Methyloversatilis thermotolerans 3tT [30]; 5, Sterolibacterium denitrificans ChoI-1ST [23]; 6, Sulfurisoma sediminicola BSN1T [21]; 7, Sulfuritalea hydrogenivorans sk34HT [13]; 8, species of the genus Rhodocyclus including Rhodocyclus purpureus strain ‘Ames’ 6770T [31, 32] and Rhodocyclus tenuis strain ATCC 25 093T [31, 33, 34]. +, Positive; −, negative; var., variable results among strains; nr, not reported. DNA G+C content (mol%) values for the genus Methyloversatilis based on genomic data presented by Smalley et al. [29].
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Isolation source(s) | Contaminated soil, USA | Activated sludge, Germany | Iron- reducing aquifer, Netherlands |
Lake sediment, USA; hot springs, Russia | Anoxic reactor treating landfill leachate, Uruguay | Freshwater lake sediment, Japan | Freshwater lake, Japan | Swine waste lagoon, USA; freshwater pond, Germany |
| DNA G+C content (mol%) | 55.1 | 61.4 | nr | 65.6–67 | 65.3 | 67 | 67 | 64.1–65.1 |
| Diagnostic fatty acids | ||||||||
| C8 : 0 3-OH | − | + | − | − | + | − | − | − |
| C10 : 0 3-OH | + | − | − | var. | + | + | − | + |
| C17 : 0 cyclo | + | − | + | var. | − | − | − | − |
| C17 : 1ω7c | + | − | − | − | − | − | − | − |
| C19 : 0 cyclo ω8c |
+ | − | − | − | − | − | − | − |
| Respiratory quinones | Q8, Q9, Q7 | Q8 | nr | Q8 | Q8 | nr | nr | Q8, MK8 |
| Catalase | − | − | nr | + | + | + | − | + |
| Motility | − | + | + | − | + | + | nr | var. |
| Oxygen requirement | Aerobic | Facultative | Anaerobic | Facultative | Facultative | Facultative | Facultative | Facultative |
| Nitrate reduction | − | + | + | var. | + | + | + | nr |
Description of Rugosibacter gen. nov.
Rugosibacter (Ru.go.si.bac′ter. L. adj. rugosus wrinkled; N. L. masc. n. bacter a rod; N. L. masc. n. Rugosibacter a wrinkled rod).
Cells are Gram-type-negative, non-motile and grow aerobically. Catalase-negative and oxidase-positive. Heterotrophic growth occurs on a limited number of organic acids. Predominant fatty acids are summed feature 3 (C16 : 1ω7c or C16 : 1ω6c) and C16 : 0. The major polar lipids are phosphatidylethanolamine, phosphatidylglycerol, diphosphatidylglycerol and phospholipid. The major respiratory quinone is Q8. Phylogenetically, the genus is a member of the family Rhodocyclaceae in the class Betaproteobacteria. The type species is Rugosibacter aromaticivorans.
Description of Rugosibacter aromaticivorans sp. nov.
Rugosibacter aromaticivorans [a.ro.ma.ti.ci.vo′rans. L. adj. aromaticus aromatic, fragrant; L. pres. part. vorans devouring; N.L. part. adj. aromaticivorans devouring aromatic (compounds)].
Cells are curved rods (0.77–1.15×0.23–0.27 µm). On solid media, colonies are small, convex and circular with a yellow–white colour. Growth occurs aerobically between 20 and 35 °C (optimum 30–34 °C), at pH 6.5–7.5 (optimum pH 6.5) and with salinity ≤0.5 %. Cells can grow on select monocyclic and polycyclic aromatic compounds as a sole source of carbon, and use both nitrate and ammonia as nitrogen sources. Cells are negative for gelatin hydrolysis, cellulase, skimmed milk protease, starch hydrolysis, nitrate reduction and urease activity, and positive for lipase activity. In addition to those listed in the genus description, fatty acids present in minor amounts are C17 : 0 cyclo, C18 : 0ω7c, C10 : 0 3-OH and C12 : 0. Polar lipids and respiratory quinones are consistent with the genus description.
The type strain, Ca6T (=ATCC TSD-59T=DSM 103039T), was isolated from PAH-contaminated soil from Charlotte, NC, USA. The genomic DNA G+C content of the type strain is 55.14 mol%.
Funding information
This work was supported by the US National Institute of Environmental Health Sciences (NIEHS) as part of the Superfund Research Program (5 P42ES005948).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Data
Footnotes
Abbreviations: MGP, manufactured gas plant; PAH, polycyclic aromatic hydrocarbon; PG1, 'Pyrene Group 1'; SIP, stable-isotope probing.
The GenBank/EMBL/DDBJ accession number for the whole-genome sequence of strain Ca6T is CP010554.
One supplementary figure is available with the online Supplementary Material.
References
- 1.Singleton DR, Sangaiah R, Gold A, Ball LM, Aitken MD. Identification and quantification of uncultivated Proteobacteria associated with pyrene degradation in a bioreactor treating PAH-contaminated soil. Environ Microbiol. 2006;8:1736–1745. doi: 10.1111/j.1462-2920.2006.01112.x. [DOI] [PubMed] [Google Scholar]
- 2.Singleton DR, Hunt M, Powell SN, Frontera-Suau R, Aitken MD. Stable-isotope probing with multiple growth substrates to determine substrate specificity of uncultivated bacteria. J Microbiol Methods. 2007;69:180–187. doi: 10.1016/j.mimet.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Singleton DR, Hu J, Aitken MD. Heterologous expression of polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenase genes from a novel pyrene-degrading betaproteobacterium. Appl Environ Microbiol. 2012;78:3552–3559. doi: 10.1128/AEM.00173-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin F, Torelli S, Le Paslier D, Barbance A, Martin-Laurent F, et al. Betaproteobacteria dominance and diversity shifts in the bacterial community of a PAH-contaminated soil exposed to phenanthrene. Environ Pollut. 2012;162:345–353. doi: 10.1016/j.envpol.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Regonne RK, Martin F, Mbawala A, Ngassoum MB, Jouanneau Y. Identification of soil bacteria able to degrade phenanthrene bound to a hydrophobic sorbent in situ. Environ Pollut. 2013;180:145–151. doi: 10.1016/j.envpol.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Humphries JA, Ashe AM, Smiley JA, Johnston CG. Microbial community structure and trichloroethylene degradation in groundwater. Can J Microbiol. 2005;51:433–439. doi: 10.1139/w05-025. [DOI] [PubMed] [Google Scholar]
- 7.He Z, Xie X, Xiao S, Liu J, Qiu G. Microbial diversity of mine water at Zhong Tiaoshan copper mine, China. J Basic Microbiol. 2007;47:485–495. doi: 10.1002/jobm.200700219. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Crowley DE. Microbial diversity in natural asphalts of the Rancho La Brea Tar Pits. Appl Environ Microbiol. 2007;73:4579–4591. doi: 10.1128/AEM.01372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang YQ, Li Y, Zhao JY, Chi CQ, Huang LX, et al. Microbial communities in long-term, water-flooded petroleum reservoirs with different in situ temperatures in the Huabei Oilfield, China. PLoS One. 2012;7:e33535. doi: 10.1371/journal.pone.0033535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gihring TM, Moser DP, Lin LH, Davidson M, Onstott TC, et al. The distribution of microbial taxa in the subsurface water of the Kalahari shield, South Africa. Geomicrobiol J. 2006;23:415–430. doi: 10.1080/01490450600875696. [DOI] [Google Scholar]
- 11.Zhang L, Gao G, Tang X, Shao K. Impacts of different salinities on bacterial biofilm communities in fresh water. Can J Microbiol. 2014;60:319–326. doi: 10.1139/cjm-2013-0808. [DOI] [PubMed] [Google Scholar]
- 12.Richardson SD, Lebron BL, Miller CT, Aitken MD. Recovery of phenanthrene-degrading bacteria after simulated in situ persulfate oxidation in contaminated soil. Environ Sci Technol. 2011;45:719–725. doi: 10.1021/es102420r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima H, Fukui M. Sulfuritalea hydrogenivorans gen. nov., sp. nov., a facultative autotroph isolated from a freshwater lake. Int J Syst Evol Microbiol. 2011;61:1651–1655. doi: 10.1099/ijs.0.024968-0. [DOI] [PubMed] [Google Scholar]
- 14.Singleton DR, Dickey AN, Scholl EH, Wright FA, Aitken MD. Complete genome sequence of a novel bacterium within the family Rhodocyclaceae that degrades polycyclic aromatic hydrocarbons. Genome Announc. 2015;3:e00251-15. doi: 10.1128/genomeA.00251-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A. A rapid and easy method for the detection of microbial cellulases on agar plates using gram's iodine. Curr Microbiol. 2008;57:503–507. doi: 10.1007/s00284-008-9276-8. [DOI] [PubMed] [Google Scholar]
- 16.Ensley BD, Ratzkin BJ, Osslund TD, Simon MJ, Wackett LP, et al. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science. 1983;222:167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- 17.Schell MA. Cloning and expression in Escherichia coli of the naphthalene degradation genes from plasmid NAH7. J Bacteriol. 1983;153:822–829. doi: 10.1128/jb.153.2.822-829.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singleton DR, Ramirez LG, Aitken MD. Characterization of a polycyclic aromatic hydrocarbon degradation gene cluster in a phenanthrene-degrading Acidovorax strain. Appl Environ Microbiol. 2009;75:2613–2620. doi: 10.1128/AEM.01955-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii S, Ashida N, Otsuka S, Senoo K. Isolation of oligotrophic denitrifiers carrying previously uncharacterized functional gene sequences. Appl Environ Microbiol. 2011;77:338–342. doi: 10.1128/AEM.02189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weelink SA, van Doesburg W, Saia FT, Rijpstra WI, Röling WF, et al. A strictly anaerobic betaproteobacterium Georgfuchsia toluolica gen. nov., sp. nov. degrades aromatic compounds with Fe(III), Mn(IV) or nitrate as an electron acceptor. FEMS Microbiol Ecol. 2009;70:575–585. doi: 10.1111/j.1574-6941.2009.00778.x. [DOI] [PubMed] [Google Scholar]
- 21.Kojima H, Fukui M. Sulfurisoma sediminicola gen. nov., sp. nov., a facultative autotroph isolated from a freshwater lake. Int J Syst Evol Microbiol. 2014;64:1587–1592. doi: 10.1099/ijs.0.057281-0. [DOI] [PubMed] [Google Scholar]
- 22.Fahrbach M, Kuever J, Meinke R, Kämpfer P, Hollender J. Denitratisoma oestradiolicum gen. nov., sp. nov., a 17β-oestradiol-degrading, denitrifying betaproteobacterium. Int J Syst Evol Microbiol. 2006;56:1547–1552. doi: 10.1099/ijs.0.63672-0. [DOI] [PubMed] [Google Scholar]
- 23.Tarlera S, Denner EB. Sterolibacterium denitrificans gen. nov., sp. nov., a novel cholesterol-oxidizing, denitrifying member of the β-Proteobacteria. Int J Syst Evol Microbiol. 2003;53:1085–1091. doi: 10.1099/ijs.0.02039-0. [DOI] [PubMed] [Google Scholar]
- 24.Meier-Kolthoff JP, Auch AF, Klenk HP, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, et al. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 26.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings MP, Hancock JM, Zvelebil MJ. Dictionary of Bioinformatics and Computational Biology. John Wiley & Sons, Ltd; 2004. PAUP* (phylogenetic analysis using parsimony (and other methods) [Google Scholar]
- 28.Kalyuzhnaya MG, De Marco P, Bowerman S, Pacheco CC, Lara JC, et al. Methyloversatilis universalis gen. nov., sp. nov., a novel taxon within the Betaproteobacteria represented by three methylotrophic isolates. Int J Syst Evol Microbiol. 2006;56:2517–2522. doi: 10.1099/ijs.0.64422-0. [DOI] [PubMed] [Google Scholar]
- 29.Smalley NE, Taipale S, De Marco P, Doronina NV, Kyrpides N, et al. Functional and genomic diversity of methylotrophic Rhodocyclaceae: description of Methyloversatilis discipulorum sp. nov. Int J Syst Evol Microbiol. 2015;65:2227–2233. doi: 10.1099/ijs.0.000190. [DOI] [PubMed] [Google Scholar]
- 30.Doronina NV, Kaparullina EN, Trotsenko YA. Methyloversatilis thermotolerans sp. nov., a novel thermotolerant facultative methylotroph isolated from a hot spring. Int J Syst Evol Microbiol. 2014;64:158–164. doi: 10.1099/ijs.0.055046-0. [DOI] [PubMed] [Google Scholar]
- 31.Hiraishi A, Hoshino Y, Satoh T. Rhodoferax fermentans gen. nov., sp. nov., a phototrophic purple nonsulfur bacterium previously referred to as the 'Rhodocyclus gelatinosus-like' group. Arch Microbiol. 1991;155:330–336. doi: 10.1007/BF00243451. [DOI] [Google Scholar]
- 32.Pfennig N. Rhodocyclus purpureus gen. nov. and sp. nov., a ring-shaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Evol Microbiol. 1978;28:283–288. doi: 10.1099/00207713-28-2-283. [DOI] [Google Scholar]
- 33.Imhoff JF. Genus I. Rhodocyclus Pfennig 1978, 285AL. In: Brenner DJ, Staley JT, editors. Bergey's Manual of Systematic Bacteriology, The Proteobacteria Part B, the Betaproteobacteria. vol. 2. New York: Springer; 2005. [Google Scholar]
- 34.Pfennig N. Rhodospirillum tenue sp. n., a new species of the purple nonsulfur bacteria. J Bacteriol. 1969;99:619–620. doi: 10.1128/jb.99.2.619-620.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.