Abstract
Preventing virally induced liver disease begins with an understanding of the host factors that define susceptibility to infection. Hepatitis C virus (HCV) is a global health issue, with an estimated 170 million infected individuals at risk of developing liver disease including fibrosis and hepatocellular carcinoma. The liver is the major reservoir supporting HCV replication and this hepatocellular tropism is defined by HCV engagement of cellular entry receptors. Hepatocytes are polarized in vivo and this barrier function limits HCV entry. We previously reported that activated macrophages promote HCV entry into polarized hepatocytes via a TNF-α-dependent process; however, the underlying mechanism was not defined. In this study, we show that several TNF superfamily members, including TNF-α, TNF-β, TWEAK and LIGHT, promote HCV entry via NF-κB-mediated activation of myosin light chain kinase (MLCK) and disruption of tight junctions. These observations support a model where HCV hijacks an inflammatory immune response to stimulate infection and uncovers a role for NF-κB-MLCK signalling in maintaining hepatocellular tight junctions.
Keywords: hepatitis C, polarized entry, TNF, MLCK
Introduction
Hepatitis C virus (HCV) is a global health issue with an estimated 170 million infected individuals worldwide. The virus infects hepatocytes in the liver and leads to progressive liver injury, fibrosis and in some cases hepatocellular carcinoma. Infection is initiated by the viral encoded E1E2 glycoproteins interacting with cellular receptors: tetraspanin CD81, scavenger receptor BI (SR-BI), epidermal growth factor receptor, the cholesterol absorption regulator Niemann–Pick disease type C1-like 1 protein, and tight junction proteins claudin-1 and occludin (reviewed in [1, 2]). This multi-step process of receptor engagement primes a clathrin-dependent endocytic uptake of particles into the liver.
Hepatocytes are polarized and HCV particles entering the liver encounter their basolateral surface that expresses the ‘viral attachment’ proteins CD81 and SR-BI [3]. We previously reported the presence of CD81-claudin-1 complexes at the basolateral hepatocellular membrane and live cell imaging demonstrated HCV-dependent CD81 and claudin-1 co-endocytosis and fusion with Rab5 expressing endosomes, supporting a role for this receptor complex in HCV internalization [4]. In contrast, occludin localizes almost exclusively to the apical tight junctions, which are considered inaccessible to virus particles, raising questions on its role in the viral internalization process [5, 6].
Many viruses infect polarized epithelia during host invasion and, given the well-recognized function of the epithelium to restrict macromolecule movement across mucosa, viruses have evolved diverse strategies to overcome this barrier [7, 8]. HCV is associated with liver inflammation during chronic infection, and we previously reported that IL-1β and TNF-α secreted from activated macrophages promote HCV entry by disrupting hepatocyte tight junctions; however, the mechanism by which this occurred was undefined [9]. Here, we demonstrate that the TNF superfamily members TNF-α, TNF-β, TWEAK and LIGHT promote HCV infection through an NF-κB- and myosin light chain kinase (MLCK)-dependent pathway. These data highlight a role for HCV exploiting TNF superfamily signalling responses in the liver to promote viral entry and persistence.
Results
TNF superfamily members promote HCV infection of polarized hepatocytes
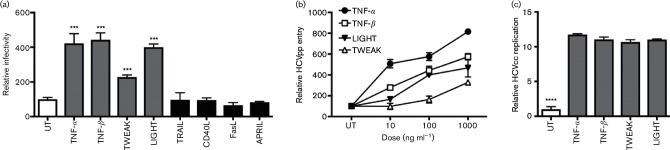
We previously reported that TNF-α promotes HCV entry into polarized hepatocytes [9]. TNF-α is secreted by a wide range of activated immune cells and earlier studies showed that the pro-viral activity of conditioned media from lipopolysaccharide-activate macrophages was only partially inhibited by neutralizing TNF-α, suggesting additional pro-viral factors [9]. To investigate the potential for other TNF superfamily members to promote hepatocellular permissiveness to support HCV entry, we screened a panel of recombinant human TNF superfamily members for their effect on HCV pseudoparticle (HCVpp) infection of polarized HepG2.CD81 cells (Fig. 1a). In addition to TNF-α, we found that TNF-β, TWEAK and LIGHT promote HCVpp infection in a dose-dependent manner (Fig. 1b). In contrast, TNF family members TRAIL, CD40L, FasL and APRIL had no detectable effect on HCVpp infection despite reports of HepG2 cells expressing their cognate receptors [10–13]. We confirmed that TNF-α, TNF-β, TWEAK and LIGHT increased native HCV J6/JFH infection of polarized HepG2 cells and observed a significant increase in viral RNA 48 h post-infection (Fig. 1c).
Fig. 1.
TNF superfamily members promote HCV infection of polarized HepG2 cells. (a) HepG2.CD81 cells were polarized for 5 days and infected with HCVpp in the presence or absence of TNF superfamily members TNF-α, TNF-β, TWEAK, LIGHT, TRAIL, CD40L, FasL or APRIL at 100 ng ml−1 and incubated for 72 h. Cytokines that promote HCV entry are depicted by light grey bars, cytokines that had no detectable effect are depicted by black bars and the untreated control (UT) is depicted by a white bar. (b) Polarized HepG2.CD81 cells were treated with an increasing dose of TNF-α, TNF-β, TWEAK or LIGHT and infected with HCVpp for 72 h. (c) TNF-α, TNF-β, TWEAK and LIGHT (100 ng ml−1) increased cell culture propagated hepatitis C virus (HCVcc) J6/JFH infection of polarized HepG2.CD81 cells, where infection was assessed by qRT (quantitative reverse transcriptase)-PCR quantitation of viral RNA levels after 48 h. Data are presented as mean infectivity ±sd relative to a UT and are representative of three independent experiments where: ****, P<0.0001; ***, P<0.001.
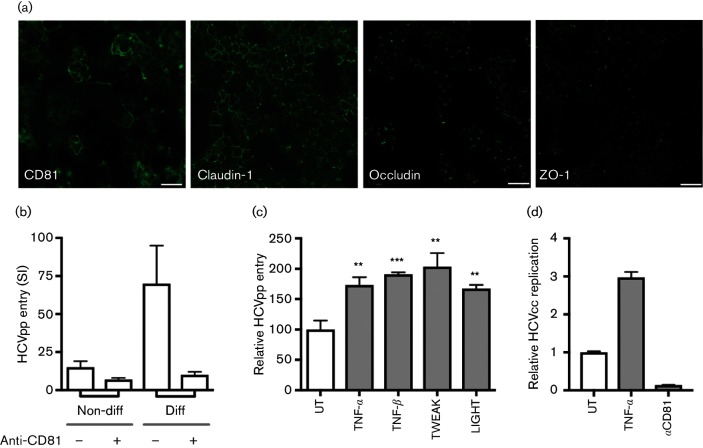
The hepatocyte-derived HepG2 cell line has provided a valuable in vitro model for studying hepatocyte polarity [14]. The bi-potent hepatocyte progenitor HepaRG clone can be differentiated into mixed cultures of polarized hepatocytes and cholangiocytes [15], and provide an independent model to validate our results. Differentiated HepaRG-derived hepatocyte-like cells expressed the essential HCV entry factors CD81, occludin and claudin-1, together with the junctional protein ZO-1, which localize to bile canalicular (BC)-like structures (Fig. 2a). Consistent with this pattern of viral receptor expression, differentiated HepaRG cells supported HCVpp entry that was neutralized with an anti-CD81 antibody, whereas HCVpp failed to infect the non-differentiated progenitor cells (Fig. 2b). Using HCVpp expressing a GFP reporter, we could demonstrate that HCVpp entry was restricted to hepatocyte-like cells, whereas Vesicular Stomatitis Virus encoded G expressing pseudoparticle (VSV-Gpp) infected cells with both hepatocyte and cholangiocyte morphology (data not shown). These observations were consistent with our previous report that primary cholangiocytes were not permissive for HCV infection [16]. Importantly, TNF-α, TNF-β, TWEAK and LIGHT increased the permissiveness of differentiated HepaRG cells to support HCVpp infection (Fig. 2c). Furthermore, pre-treating differentiated HepaRG cells with TNF (100 ng ml−1) induced a significant increase in native HCV RNA levels that was CD81-dependent (Fig. 2d). Collectively, these data demonstrated that several TNF superfamily members promote HCV entry into polarized hepatocytes.
Fig. 2.
TNF promotes HCV infection of differentiated HepaRG cells. (a) HCV receptor staining of differentiated HepaRG cells show basolateral CD81 and claudin-1 expression. The tight junction proteins occludin and ZO-1 were restricted to apical BC-like structures of hepatocyte-like cells. Bars, 10 µm. (b) Non-differentiated and differentiated HepaRG cells were infected with HCVpp in the presence or absence of anti-CD81 2.131 (10 µg ml−1); data is presented as Specific Infectivity (SI) reflecting the mean HCVpp luciferase values relative to an envelope deficient control pseudoparticle. (c) TNF-α, TNF-β, TWEAK and LIGHT (100 ng ml−1) promote HCVpp infection of differentiated HepaRG cells. (d) TNF-α (100 ng ml−1) increased HCVcc J6/JFH infection of differentiated HepaRG cells, where infection was assessed by qRT-PCR determination of viral RNA levels after 72 h. As a control, anti-CD81 2.131 (10 µg ml−1) neutralized HCV infection of differentiated cells is shown. Data are presented as mean infectivity ±sd relative to the untreated control (UT) and are representative of two independent experiments where: ***, P<0.001; **, P<0.01.
TNF superfamily members disrupt tight junction integrity and promote HCV infection through activation of NF-κB p65/RelA
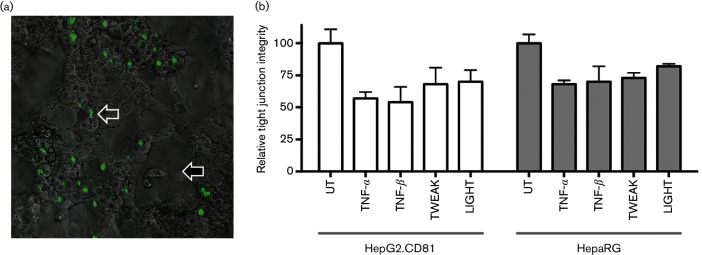
To investigate the mechanism by which TNF superfamily members promote HCV entry, we first assessed their effect on BC tight junction integrity. The fluorescent dye 5-chloromethylfluorescein diacetate (CMFDA), which binds the apical transporter multidrug resistance-associated protein 2 (MRP2), enabled real-time imaging of BC structures in differentiated HepaRG (Fig. 3a) and HepG2 cells, and allowed us to measure the effect of cytokines on tight junction integrity [5]. TNF-α, TNF-β, TWEAK and LIGHT disrupted tight junction integrity in both model systems (Fig. 3b), with no detectable effect on cellular viability. Of note, all cytokine-mediated effects on HepG2 polarity were reversible and tight junction integrity was restored over a 24 h period after their removal, demonstrating the plasticity of the trafficking pathways regulating tight junction proteins.
Fig. 3.
TNF superfamily members disrupt tight junctions. (a) Differentiated HepaRG cultures comprised hepatocytes (open arrow) and cholangiocytes (filled arrow). Magnification, ×400. Cultures were treated with the fluorescent dye CMFDA that binds the apical transporter multidrug resistance-associated protein 2 enabling real time imaging of bile canaliculi (green structures). Treating cultures with CMFDA (30 min) identified BC structures. (b) Tight junction integrity of HepG2.CD81 and HepaRG cells was measured by quantifying the number of BC structures retaining CMFDA following 1 h cytokine treatment (100 ng ml−1) in five independent fields of view at ×10 magnification with data expressed relative to untreated (UT) cells. N=3 independent experiments.
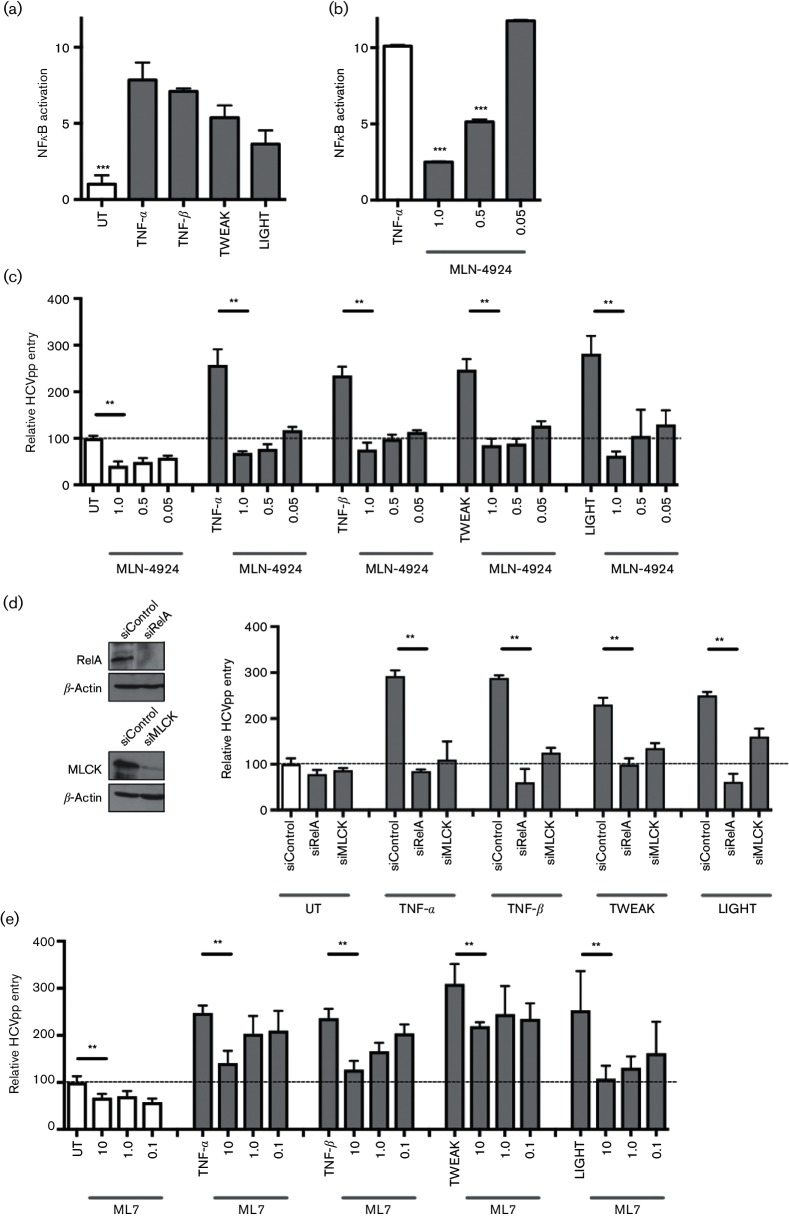
The TNF superfamily are well recognized to activate NF-κB in a range of cell types and using an NF-κB luciferase reporter assay we showed that TNF-α, TNF-β, TWEAK and LIGHT activate NF-κB in HepG2 cells (Fig. 4a). MLN-4924 inhibits the Nedd8-activating enzyme that regulates cullin-RING ubiquitin ligases [17], and has been reported to limit canonical and non-canonical NF-κB signalling pathways [18, 19]. We used MLN-4924 and RelA shortinterfering RNA (siRNA) to investigate the role of NF-κB in TNF-α, TNF-β, TWEAK and LIGHT promoted HCV infection of polarized hepatocytes. We confirmed that MLN-4924 inhibits TNF-α-induced NF-κB reporter activity in a dose-dependent manner (Fig. 4b). Furthermore, MLN-4924 abrogated the pro-viral activity of TNF superfamily members to promote HCVpp entry (Fig. 4c). It is noteworthy that MLN-4924 inhibited basal non-cytokine-mediated HCVpp infection (Fig. 4c), whereas it had no effect on the permissiveness of non-polarized HepG2 or Huh-7 cells to support HCVpp entry, suggesting a role for NF-κB signalling in polarized HCV entry. Silencing RelA abrogated the pro-viral activity of TNF superfamily members for HCVpp infection (Fig. 4d), demonstrating a role for RelA in TNF-boosted HCV entry.
Fig. 4.
TNF superfamily members promote HCV entry in an NF-κB- and MLCK-dependent manner. (a) HepG2.CD81 cells expressing an NF-κB luciferase reporter construct were allowed to polarize for 5 days prior to treating with TNF family members (100 ng ml−1) for 24 h. Luciferase reporter activity is expressed relative to UT cells. (b) Polarized HepG2.CD81 cells expressing NF-κB reporter were treated with an increasing dose of MLN-4924 (µg ml−1) in the presence of TNF-α (100 ng ml−1). (c) Polarized HepG2.CD81 cells were treated with the indicated concentrations of MLN-4924 and 100 ng cytokine ml−1, 15 min prior to infecting with HCVpp; data is presented as mean infectivity ±sd relative to the UT cells (represented by a dotted line). (d) HepG2.CD81 cells were transfected with control siRNA (siControl), siRelA or siMLCK for 48 h prior to treating with 100 ng ml−1 of the indicated cytokines and infecting with HCVpp. Western blots demonstrate knockdown of RelA or MLCK, respectively. (e) Polarized HepG2.CD81 cells were treated with the MLCK inhibitor, ML7, at the indicated concentrations, together with 100 ng cytokine ml−1 for 15 min prior to infecting with HCVpp. Data are presented relative to UT cells. Data are representative of three independent experiments, where: ***, P<0.001; and **, P<0.01. UT, Untreated control.
TNF superfamily members promote HCV entry in a MLCK-dependent manner
TNF-α disruption of gastrointestinal and lung epithelial permeability is dependent on NF-κB activation of MLCK, which regulates occludin and ZO-1 endocytosis [20–22]. We therefore investigated the role of MLCK in basal and TNF-mediated HCVpp infection. Silencing MLCK reduced the pro-viral activity of TNF superfamily members for HCVpp infection of HepG2.CD81 cells (Fig. 4d).
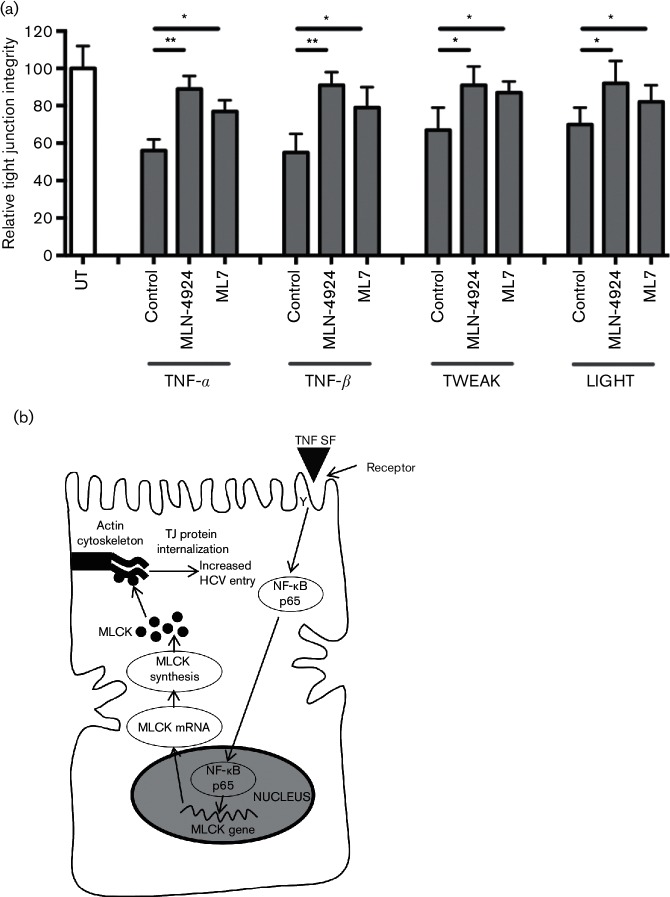
Similar results were obtained by treating cells with the MLCK inhibitor, ML7, demonstrating a role for MLCK in TNF-α-, TNF-β-, TWEAK- and LIGHT-dependent HCVpp infection of polarized to HepG2 (Fig. 4e). We noted that ML7 induced a significant reduction of basal HCVpp infection. Finally, we demonstrated that both ML7 and MLN-4924 treatments abrogated the effects of TNF superfamily members on HepG2 tight junction integrity (Fig. 5a). These data confirm that TNF superfamily members perturb hepatocellular tight junctions via RelA- and MLCK-dependent pathways that most likely play an important role in maintaining hepatocyte polarity.
Fig. 5.
NF-κB- and MLCK-dependent TNF perturbation of tight junction integrity. (a) Polarized HepG2.CD81 cells were incubated with NF-κB RelA inhibitor MLN-4924 (1 µg ml−1) or MLCK inhibitor ML7 (10 µg ml−1) for 15 min prior to treating with TNF-α, TNF-β, TWEAK and LIGHT (100 ng ml−1) for 1 h, and quantifying the number of BC structures retaining CMFDA. Data is presented from a single experiment with triplicate wells and is representative of two independent experiments. UT, Untreated control cells. (b) Proposed model for TNF superfamily members promote HCV uptake into polarized cells. The binding of TNF superfamily members (TNF SF) to their receptors activates NF-κB p65 (RelA), which promotes MLCK transcription and synthesis. We hypothesize that MLCK activates perijunctional actin and trafficking of tight junction (TJ) proteins occludin and ZO-1 to intracellular compartments [20], which facilitate HCV fusion.
Discussion
Our study shows a role for various members of the TNF superfamily to promote HCV infection by activating NF-kB and MLCK-signalling pathways to reduce hepatocellular tight junction integrity. We observed similar results with two polarizing hepatocyte models, the well-established HepG2 hepatoma cell line and the bi-potent HepaRG progenitor that can differentiate into hepatocytes and cholangiocytes [23]. We [9] and others [24, 25] have reported that HCV can activate macrophages to express TNF-α, enabling the virus to hijack this pro-inflammatory responses to promote its own entry. TNF superfamily members are secreted by activated immune cells, including lymphocytes, macrophages and Kupffer cells, and are highly expressed in the inflamed liver [9, 26], providing an environment that potentiates HCV infection of naive hepatocytes. We previously reported that TNF induced a re-organization of occludin and ZO-1 from apical BC structures in HepG2 cells via an undefined mechanism [9]. Using inhibitors and siRNA silencing approaches, we demonstrate a role for NF-κB and MLCK-signalling in TNF-α, TNF-β, TWEAK and LIGHT enhancement of HCV entry via their perturbation of tight junction integrity. The observation that inhibiting NF-κB and MLCK limits HCVpp infection in the absence of inflammatory mediators highlights a role for these signalling pathways in polarized virus entry (Fig. 5b).
Claudin-1 and occludin play an essential role in defining HCV entry and it is of interest to note that these proteins were initially discovered to regulate HCV internalization into non-polarized human embryonal kidney cells [25, 27]. To date, the published literature support a model where claudin-1 and occludin regulation of HCV internalization is independent of their location at tight junctions [2], raising the question of how TNF superfamily members potentiate HCV infection. We reported that HCV interacts with claudin-1-CD81 complexes at the basolateral hepatocellular membrane [3, 4]. At the present time, there is limited evidence to show that HCV trafficks to the tight junction, indeed Blaising and colleagues demonstrated HCV internalization via the basolateral membrane and localization within Rab5-positive intracellular vesicles [28].
Receptor mobility and lateral diffusion speed in the plasma membrane can regulate the movement of viruses at the plasma membrane and can impact on particle internalization rate(s) [29, 30]. We previously reported that hepatocyte polarization limits CD81 mobility via cytoplasmic tail interactions with the cytoskeleton via Ezin-Radixin-Moesin proteins [31]. Furthermore, HepG2 cells expressing a C-terminal truncated CD81 mutant supported comparable levels of HCV entry independent of polarization status, suggesting that membrane protein dynamics on polarized cells may limit pathogen attachment and infection. Indeed, our report showing that TNF-α increased CD81 lateral mobility in polarized HepG2 cells supports this model [9].
Polarized lung and gut epithelia are well recognized to restrict the invasion of microbial pathogens, and many viruses have evolved strategies to modulate the cortical actin network to promote infection of polarized cells [8]. Adenovirus infection of lung epithelia is potentiated by macrophage expressed IL-8, which stimulates the apical expression of an alternate viral receptor CAREx8 to enable the infection of intact epithelium [32]. Our previous report that TNF-α promoted the permissivity of polarized HepG2 cells to support entry of pseudoparticles expressing Vesicular stomatitis virus (VSV), measles or Lassa virus glycoproteins is consistent with a role for cytoskeletal changes associated with cell polarization to alter viral receptor trafficking [9].
Like all epithelial cells, hepatocytes mediate the flow of macromolecules via their polarized surface that comprises tight junctions and cell–cell adhesions. However, our understanding of the molecular pathways and inflammatory mediators regulating hepatocyte polarity in health or disease are not well understood [33]. The tight junction is a dynamic structure, and increasing evidence from studies of gut and lung epithelia highlight the role of MLCK in regulating the tight junction [21]. Our study supports a common pathway for TNF family members to regulate hepatocyte polarity via MLCK, as reported for other epithelial cells. In conclusion, we have demonstrated a role for TNF superfamily signalling in regulating HCV entry and persistence.
Methods
Cell lines and antibodies
HepG2 cells stably expressing CD81 [5] and 293T were propagated in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10 % FBS and 1 % non-essential amino acids. HepaRG cells were obtained from Invitrogen and maintained in Williams E medium supplemented with 10 % FBS, 5 µg insulin ml−1 and 50 µM hydrocortisone. HepaRG cell differentiation was performed over a 4 week period, and the media supplemented with 2 % DMSO for the final 2 weeks of differentiation, as described elsewhere [23]. All cells were maintained at 37 °C and 5 % CO2.
The following primary antibodies were used: anti-CD81 (2.131), anti-claudin-1 mAb (Abnova), anti-claudin-1 polyclonal sera and anti-occludin (Invitrogen). Secondary labelled antibodies, Alexa-488 goat anti-mouse IgG, Alexa-488 goat anti-rabbit IgG, Alexa-594 goat anti-mouse IgG and Alexa-594 goat anti-rat IgG were purchased from Invitrogen. Recombinant human cytokines were obtained from Peprotech, MLN-4924 from Calbiochem and ML7 from Sigma-Aldrich.
Laser scanning confocal microscopy
HepaRG cells were differentiated and fixed with ice-cold methanol (claudin-1, occludin) or 3 % paraformaldehyde (CD81) 24 h after seeding. Primary antibodies were applied for 1 h at room temperature. After washing twice with PBS, anti-mouse, anti-rabbit or anti-rat Alexa Fluor 488 (Invitrogen) secondary antibody was applied for 1 h at room temperature. Cells were counterstained with DAPI (Invitrogen) for nuclei visualization and mounted with ProLong Gold antifade (Invitrogen). Cells were viewed by laser-scanning confocal microscopy on a Zeiss META head confocal microscope with a 100× oil-immersion objective.
Quantifying tight junction barrier function
To determine the functionality of tight junctions and whether they restricted paracellular diffusion of solutes from the BC lumen to the basolateral medium, HepG2 or HepaRG cells were incubated with 5 mM CMFDA (Invitrogen) at 37 °C for 10 min to allow translocation to the BC lumen. After washing extensively with PBS, the capacity of BC to retain CMFDA was enumerated using a fluorescence microscope.
NF-κB reporter assays
HepG2.CD81 cells were transfected with a NF-κB reporter plasmid expressing a firefly luciferase gene (pNiFty-luc; Invivogen) and polarized for 5 days. Cells were treated with TNF superfamily members (100 ng ml−1) for 24 h, lysed and luciferase activity measured for 10 s in a luminometer (Lumat LB 9507).
HCVpp genesis and infection
Pseudoviruses were generated by transfecting 293T cells with plasmids encoding a human immunodeficiency virus provirus expressing luciferase and HCV strain H77 E1E2 region or a no envelope control (Env−), as previously reported [34]. Virus-containing media was added to target cells for 8 h, unbound virus removed and media replaced with DMEM/3 % FBS. At 72 h post-infection, the cells were lysed, luciferase substrate added and luciferase activity measured for 10 s in a luminometer (Lumat LB 9507). Specific infectivity was calculated by expressing mean HCVpp luciferase values relative to the mean Env-pp RLU signal. Cytokines were added to cells at the same time as pseudovirus, and inhibitors were pre-incubated with cells for 15 min prior to the addition of cytokine and pseudovirus.
Cell culture propagated hepatitis C virus genesis and infection
HCV SA13/JFH was generated and infection quantified by real-time PCR detection of viral RNA, as previously described [9].
RelA and MLCK silencing and Western blotting
HepG2.CD81 cells were transfected with a pool of siRNAs (Smartpool) directed against RelA or MLCK, or a non-specific control siRNA (Dharmacon). Forty-eight hours post-transfection, cells were treated with TNF superfamily members (100 ng ml−1) and infected with HCVpp. In parallel, cells were lysed and RelA and MLCK expression measured as previously described [35] by Western blot using RelA and MLCK antibodies (Cell Signaling Technology).
Statistical analysis
Results are expressed as the mean ±1 sd of the mean. Statistical analyses were performed using Students t-test in Prism 4.0 (GraphPad) with a P<0.05 being considered statistically significant and where required corrected for multiple sampling (Bonferroni).
Funding information
Research in the McKeating laboratory was funded by the MRC programme G1100247 and the EU FP7 funded PathCo HEALTH F3-2012-305578 programme, and in the Clark laboratory by Arthritis Research UK programme grant 19416.
Acknowledgements
We thank T. Wakita of National Institute of Infectious Diseases, Tokyo, Japan for the HCV J6/JFH-1 infectious molecular clone and Ulrike Protzer for advice with the HepaRG culture system. We would also like to thank Professor Rice at Rockefeller University, New York, US.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: BC, bile canalicular; CMFDA, 5-chloromethylfluorescein diacetate; HCV, hepatitis C virus; HCVpp, hepatitis C virus pseudoparticle; siRNA, short interfering RNA.
References
- 1.Meredith LW, Wilson GK, Fletcher NF, McKeating JA. Hepatitis C virus entry: beyond receptors. Rev Med Virol. 2012;22:182–193. doi: 10.1002/rmv.723. [DOI] [PubMed] [Google Scholar]
- 2.Ding Q, von Schaewen M, Ploss A. The impact of hepatitis C virus entry on viral tropism. Cell Host Microbe. 2014;16:562–568. doi: 10.1016/j.chom.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris HJ, Davis C, Mullins JG, Hu K, Goodall M, et al. Claudin association with CD81 defines hepatitis C virus entry. J Biol Chem. 2010;285:21092–21102. doi: 10.1074/jbc.M110.104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farquhar MJ, Hu K, Harris HJ, Davis C, Brimacombe CL, et al. Hepatitis C virus induces CD81 and claudin-1 endocytosis. J Virol. 2012;86:4305–4316. doi: 10.1128/JVI.06996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mee CJ, Harris HJ, Farquhar MJ, Wilson G, Reynolds G, et al. Polarization restricts hepatitis C virus entry into HepG2 hepatoma cells. J Virol. 2009;83:6211–6221. doi: 10.1128/JVI.00246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sourisseau M, Michta ML, Zony C, Israelow B, Hopcraft SE, et al. Temporal analysis of hepatitis C virus cell entry with occludin directed blocking antibodies. PLoS Pathog. 2013;9:e1003244. doi: 10.1371/journal.ppat.1003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mateo M, Generous A, Sinn PL, Cattaneo R. Connections matter — how viruses use cell–cell adhesion components. J Cell Sci. 2015;128:431–439. doi: 10.1242/jcs.159400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delorme-Axford E, Coyne CB. The actin cytoskeleton as a barrier to virus infection of polarized epithelial cells. Viruses. 2011;3:2462–2477. doi: 10.3390/v3122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher NF, Sutaria R, Jo J, Barnes A, Blahova M, et al. Activated macrophages promote hepatitis C virus entry in a tumor necrosis factor-dependent manner. Hepatology. 2014;59:1320–1330. doi: 10.1002/hep.26911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng G, Abdalla M, Li Y, Bai Y. NF-κB mediates the induction of Fas receptor and Fas ligand by microcystin-LR in HepG2 cells. Mol Cell Biochem. 2011;352:209–219. doi: 10.1007/s11010-011-0756-y. [DOI] [PubMed] [Google Scholar]
- 11.Abou El Naga RN, Azab SS, El-Demerdash E, Shaarawy S, El-Merzabani M, et al. Sensitization of TRAIL-induced apoptosis in human hepatocellular carcinoma HepG2 cells by phytochemicals. Life Sci. 2013;92:555–561. doi: 10.1016/j.lfs.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Notas G, Alexaki VI, Kampa M, Pelekanou V, Charalampopoulos I, et al. APRIL binding to BCMA activates a JNK2-FOXO3-GADD45 pathway and induces a G2/M cell growth arrest in liver cells. J Immunol. 2012;189:4748–4758. doi: 10.4049/jimmunol.1102891. [DOI] [PubMed] [Google Scholar]
- 13.Shiraki K, Sugimoto K, Okano H, Wagayama H, Fujikawa K, et al. CD40 expression in HCV-associated chronic liver diseases. Int J Mol Med. 2006;18:559–563. doi: 10.3892/ijmm.18.4.559. [DOI] [PubMed] [Google Scholar]
- 14.Decaens C, Durand M, Grosse B, Cassio D. Which in vitro models could be best used to study hepatocyte polarity? Biol Cell. 2008;100:387–398. doi: 10.1042/BC20070127. [DOI] [PubMed] [Google Scholar]
- 15.Marion MJ, Hantz O, Durantel D. The HepaRG cell line: biological properties and relevance as a tool for cell biology, drug metabolism, and virology studies. Methods Mol Biol. 2010;640:261–272. doi: 10.1007/978-1-60761-688-7_13. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher NF, Humphreys E, Jennings E, Osburn W, Lissauer S, et al. Hepatitis C virus infection of cholangiocarcinoma cell lines. J Gen Virol. 2015;96:1380–1388. doi: 10.1099/vir.0.000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godbersen JC, Humphries LA, Danilova OV, Kebbekus PE, Brown JR, et al. The Nedd8-activating enzyme inhibitor MLN4924 thwarts microenvironment-driven NF-κB activation and induces apoptosis in chronic lymphocytic leukemia B cells. Clin Cancer Res. 2014;20:1576–1589. doi: 10.1158/1078-0432.CCR-13-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang FM, Reyna SM, Granados JC, Wei SJ, Innis-Whitehouse W, et al. Inhibition of neddylation represses lipopolysaccharide-induced proinflammatory cytokine production in macrophage cells. J Biol Chem. 2012;287:35756–35767. doi: 10.1074/jbc.M112.397703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathewson N, Toubai T, Kapeles S, Sun Y, Oravecz-Wilson K, et al. Neddylation plays an important role in the regulation of murine and human dendritic cell function. Blood. 2013;122:2062–2073. doi: 10.1182/blood-2013-02-486373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham KE, Turner JR. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci. 2012;1258:34–42. doi: 10.1111/j.1749-6632.2012.06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen L. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann N Y Acad Sci. 2012;1258:9–18. doi: 10.1111/j.1749-6632.2012.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su L, Nalle SC, Shen L, Turner ES, Singh G, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerec V, Glaise D, Garnier D, Morosan S, Turlin B, et al. Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology. 2007;45:957–967. doi: 10.1002/hep.21536. [DOI] [PubMed] [Google Scholar]
- 24.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 27.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 28.Blaising J, Lévy PL, Gondeau C, Phelip C, Varbanov M, et al. Silibinin inhibits hepatitis C virus entry into hepatocytes by hindering clathrin-dependent trafficking. Cell Microbiol. 2013;15:1866–1882. doi: 10.1111/cmi.12155. [DOI] [PubMed] [Google Scholar]
- 29.Greber UF. Virus and host mechanics support membrane penetration and cell entry. J Virol. 2016;90:3802–3805. doi: 10.1128/JVI.02568-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burckhardt CJ, Suomalainen M, Schoenenberger P, Boucke K, Hemmi S, et al. Drifting motions of the adenovirus receptor CAR and immobile integrins initiate virus uncoating and membrane lytic protein exposure. Cell Host Microbe. 2011;10:105–117. doi: 10.1016/j.chom.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Harris HJ, Clerte C, Farquhar MJ, Goodall M, Hu K, et al. Hepatoma polarization limits CD81 and hepatitis C virus dynamics. Cell Microbiol. 2013;15:430–445. doi: 10.1111/cmi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotha PL, Sharma P, Kolawole AO, Yan R, Alghamri MS, et al. Adenovirus entry from the apical surface of polarized epithelia is facilitated by the host innate immune response. PLoS Pathog. 2015;11:e1004696. doi: 10.1371/journal.ppat.1004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol. 2015;63:1023–1037. doi: 10.1016/j.jhep.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flint M, Logvinoff C, Rice CM, McKeating JA. Characterization of infectious retroviral pseudotype particles bearing hepatitis C virus glycoproteins. J Virol. 2004;78:6875–6882. doi: 10.1128/JVI.78.13.6875-6882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farquhar MJ, Harris HJ, Diskar M, Jones S, Mee CJ, et al. Protein kinase A-dependent step(s) in hepatitis C virus entry and infectivity. J Virol. 2008;82:8797–8811. doi: 10.1128/JVI.00592-08. [DOI] [PMC free article] [PubMed] [Google Scholar]