Abstract
Dental pulp-derived stem cells (DPSCs) have the potential to regenerate dentin and dental pulp tissue due to their differentiation capacity and angiogenic properties. However, for regenerative approaches to gain regulatory and clinical acceptance, protocols are needed to determine more feasible ways to cultivate DPSCs, namely without the use of xenogeneic derived components (animal sera) and exogenous growth factors. In this study, human DPSCs were isolated from 3rd molars and expanded in standard culture conditions containing fetal bovine serum (DPSCs-FBS) or conditions containing human serum (DPSCs-HS). Following cell characterization and evaluation of their angiogenic secretome, DPSCs were seeded in tooth slice/scaffolds and implanted subcutaneously into immunodeficient mice. After 30 days, tooth slices were retrieved and evaluated for dental pulp tissue regeneration. Immunohistochemistry and confocal microscopy were used to quantify blood vessel formation and evaluate predentin and dentin formation. Following culture, DPSCs-HS produced concentrations of angiogenic growth factors equivalent to DPSCs-FBS. Additionally, in DPSCs-HS, several angiogenic factors were produced in at least one-fold higher concentrations than in DPSCs-FBS. In vivo, it was determined that DPSCs-HS produced a robust angiogenic response and regeneration of dentin equivalent to DPSCs-FBS. These findings demonstrate that DPSCs can be isolated and expanded to clinical-scale numbers in media devoid of animal serum or exogenous growth factors and still maintain their pulp regenerative properties. The implications of these findings are significant for further development of clinical protocols using DPSCs in cell therapies.
Keywords: dental pulp stem cells, pulp tissue engineering, angiogenesis, dentin, cell therapy
INTRODUCTION
Affecting over 92% of adults, dental caries is globally the most prevalent chronic disease in both adults and children (http://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/DentalCaries). Despite the array of restorative and endodontic modalities used to treat carious lesions, regenerating lost tooth structure and pulp tissue would be the ideal way to treat this disease. In the context of regenerative therapies, dental pulp stem cells (DPSCs) provide promise for regenerating lost dental pulp and tooth tissues due to their ability to differentiate into odontoblasts and vascular cells to form pulp-like tissues (1–9). A variety of protocols have emerged which describe different isolation and expansion methodologies for DPSCs. Though varying degrees of efficacy have been demonstrated in these studies, a limitation to most current protocols is that the expansion of cells requires animal serum or the use of a number of exogenous growth factors. Prior to use in clinical applications, preclinical studies are needed which demonstrate that DPSCs can be predictably and efficiently procured under conditions which are more practical and cost efficient for the clinical setting. We recently demonstrated a clinically feasible means of tooth storage, DPSC isolation, and expansion of DPSCs without the use of animal serum (10). This study demonstrated that saline was a suitable overnight storage media for 3rd molars in order to isolate viable DPSCs using human serum instead of animal serum. However, the influence of these culture conditions on the capacity of DPSCs to regenerate pulp tissue was not determined in vivo.
The tooth slice model has been used to evaluate pulp regeneration using different regenerative approaches involving in vivo cell transplantation (11). Using this model, we have shown that in vivo transplantation of stem cells from exfoliated deciduous teeth (SHEDs) yields a robust angiogenic response and formation of dentin. As such, we utilized this model to determine if DPSCs, isolated in conditions free of animal serum and exogenous growth factors, not only maintain their angiogenic capacity but also their in vivo capacity to regenerate pulp tissue. The hypothesis of the present study was that DPSCs, expanded in human serum, could regenerate dental pulp tissue in this in vivo model of dental pulp regeneration.
METHODS
Tooth Storage and DPSC Isolation
3rd molars were obtained from tooth extractions from patients ranging from ages 18–22 years and were placed in sterile saline solution according to the University of Michigan Institutional Review Board guidelines. Isolation of DPSCs was performed as previously described (2). Briefly, the crown of the tooth was cut just above the cemento-enamel junction to open up the contents of the pulp. The pulp cells in the chamber and canals were cleaned out using various instruments, avoiding nerve tissue, and placed in Iscove’s Modified Dulbecco’s Medium (IMDM) without serum. Following isolation, the cell suspension was placed in a conical tube and centrifuged at 1600 rpm for 5 minutes at room temperature. The supernatant was aspirated and the pellet was re-suspended in a DispaseII-Collagenase solution. The solution was placed at 37 °C for 60 minutes, inverting the tube at 15 minute intervals.
IMDM was added to the cells before the suspension was centrifuged at room temperature for 5 minutes at 1600 rpm. The cell pellet was re-suspended in IMDM without serum. This cell suspension was placed in a T-25 tissue culture flask containing one of the following: 15% fetal bovine serum + α-MEM (DPSCs-FBS) or 15% human serum + α-MEM (DPSCs-HS) (10).
Cell Proliferation-Population Doubling Time
Cells counts were performed at each passage and the population doubling time (PDT) was calculated and compared between conditions. In order to determine PDT, the following calculation was used:
where P0 is the # of cells at the initial passage and P1 is the number of cells at the next passage.
Cell surface marker expression
Flow cytometry was performed to determine the expression levels of the cell surface markers CD90, CD73, CD105, and Stro-1. DPSCs were harvested from T150 flasks, washed and aliquoted equally into tubes. Cells were first incubated with a blocking solution containing CD16/CD32 at 4°C for 10 minutes followed by washing. Cells were then incubated with the specific antibodies conjugated with fluorochromes (Biolegends, San Diego, Ca.) at 4°C for 30 minutes. After washing these cells were analyzed on a Beckman Coulter MoFlo flow cytometer.
Angiogenesis Array
DPSC cells were grown in either 15% FBS or 15% Human Sera containing alpha minimum essential medium (αMEM). At passage 11(DPSC-FBS) and 12(DPSC-HS), cell layers were washed with serum free αMEM, followed by the addition 5 ml of serum free αMEM before the cells were incubated at 37°C for 24 hours. After incubation the cells and conditioned media were harvested. Relative levels of 55 angiogenesis related proteins were detected in this conditioned media using the Human Angiogenesis Antibody Array (R&D Systems; Cat. #ARY007). The DNA concentration was measured from the harvested cells. Array membranes were exposed to xray film. These films were analyzed by quantifying the mean spot pixel densities using the freeware Image J software (NIH (http://rsb.info.nih.gov/ij)). Samples were standardized using DNA concentrations.
Tooth Slice Model
The animal studies outlined conformed to the ARRIVE guidelines for animal research. To generate tooth slices, extracted non carious third molars were used in accordance with the University of Michigan Institutional Review Board guidelines. After being extracted, the teeth were collected in a solution of sodium azide 025%. The periodontal soft tissue was removed with a periodontal scalpel. The molar was then fixed in a transversal sectioning apparatus at pulp chamber level. With a diamond saw, at low speed, a precision cut was made using refrigerated PBS for cooling. 1 mm-thick tooth slices were obtained and pulp tissue gently removed. A sodium chloride sieved (250 to 425 μm) was placed in the pulp chamber to be used as porogen. Medical-grade poly(L-lactide) - PLLA, (Resomer L 207 S, Boehringer Ingelheim, Germany) with molecular weight 250,000 g/mol, was dissolved in chloroform (5%) and dropped over the salt. After solvent volatilization, specimens were immersed in distilled water and stirred for 24 hours in order to obtain the highly porous PLLA scaffold (12) (11).
DPSC cells were cultured in either 15% fetal bovine serum (DPSC-FBS) or 15% human serum (DPSCs-HS) containing alpha minimum essential medium (MEM). At passage 5, DPSCs were harvested and resuspended in the appropriate media at a concentration of 6.7 × 107 cells per ml. Ten microliters of the cell suspension was mixed at a 1:1 ratio with Matrigel (Corning #354248) at 4°C. Approximately 20 ul of the cell-Matrigel suspension was transferred to the tooth slice. Tooth slices were incubated at 37°C for 60 minutes before being surgically implanted.
The tooth slices containing either DPSC-FBS or DPSCs-HS were transplanted into the dorsal subcutaneous space of 6–7 week old (~30 g) severe combined immunodeficient mice (n=8) (CB.17 SCID; Charles River, Wilmington, MA, USA). In two of the mice from each group, three intraperitoneal injections of 41.6 nmol/g of body weight of tetracycline hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) were administered after 15, 20 and 25 days post-surgery, as shown (Sakai et al., 2010). After 30 days, mice were euthanized and implants were retrieved and fixed in 10% neutral buffered formaldehyde for 24 hours at 4°C until histological processing was performed.
Histology and Immunohistochemistry-Vessel Formation
Vessel formation was evaluated as we have previously described (13, 14). Briefly, for total blood vessel counts, vessels were identified in H&E-stained tissues, at ×200 magnification, by histological structures with defined lumens and the presence of red blood cells within their boundaries. For defining vessels comprised of implanted human cells, paraffin embedded tissue sections were deparaffinized in xylene, rehydrated, washed and incubated in antigen retrieval solution (Dakocytomation; Dako, Carpinteria, CA) prior to using a 1:100 dilution of a polyclonal rabbit anti-human Factor VIII antibody (Thermo-Fisher-Lab Vision, Fremont, CA. Color development was performed with a Dako EnVision _system kit (AEC, Dakocytomation) according to the manufacturer’s instructions. This staining protocol enabled the localization of the vessels formed by the implanted human DPSCs. The number of microvessels in 10 random fields per implant was counted under a light microscope (100x).
Confocal laser microscopy- Dentinogenesis
A confocal microscope (Leica SP5X, Leica Microsystems GmbH, Germany) with an ultraviolet laser (405 nm) was used to evaluate dentinogenesis as we have previously described (4). Briefly, following implantation of tooth slices, three intraperitoneal injections of 41.6 nmol/g of body weight of tetracycline hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) were administered 15, 20, and 25 days post-implantation. 30 days post-surgery, animals were euthanized and mice incisors and two tooth slices from each group of mice treated with tetracycline were collected in PBS to examine for arising fluorescent lines indicative of dentinogenesis activity. Tooth slices were examined by confocal microscopy (Olympus FluoView 500) with the following parameters: ultraviolet laser (LD405 nm, 35 mW), 405- to 488-nm excitation filter, and 465- to 495-nm barrier filter.
Statistical Analysis
Statistical analysis was performed with the use of Instat software (GraphPad Software, San Diego, CA, USA). All data were reported as mean ± standard deviation, unless otherwise noted. Statistically significant differences were determined by two-tailed Student t tests, and statistical significance was defined as P < 0.05.
RESULTS
DPSC characterization
DPSCs-HS were isolated and expanded in parallel with DPSCs-FBS. The morphology of DPSCs-HS appeared no different than DPSCs-FBS (Fig. 1A). DPSC population doublings up to 27 and doublings times were calculated and found to be between 1 and 1.5 days for both DPSC-HS and DPSCs-FBS, with no significant difference in PDT between cells expanded in the two growth conditions (Fig. 1B). Beyond 28 PDs and up to 33 PDs, PDTs for DPSCs-HS and DPSCs-FBS ranged from 2 to 2.5 days, again with no difference between the two. Following expansion, cell surface marker expression of CD73, CD90, CD105, and CD45 were evaluated for DPSCs in that the presence or absence (CD45) of these markers are important determinants in defining “stemness” associated with mesenchymal stem cells (15). Both expansion conditions resulted in yielding DPSCs which expressed high levels of CD73 (> 95%), CD90 (> 98%), and CD105 (> 85%) (Fig. 1C). Additionally, between the conditions, there were no differences noted in expression of these cell surface markers.
Figure 1.
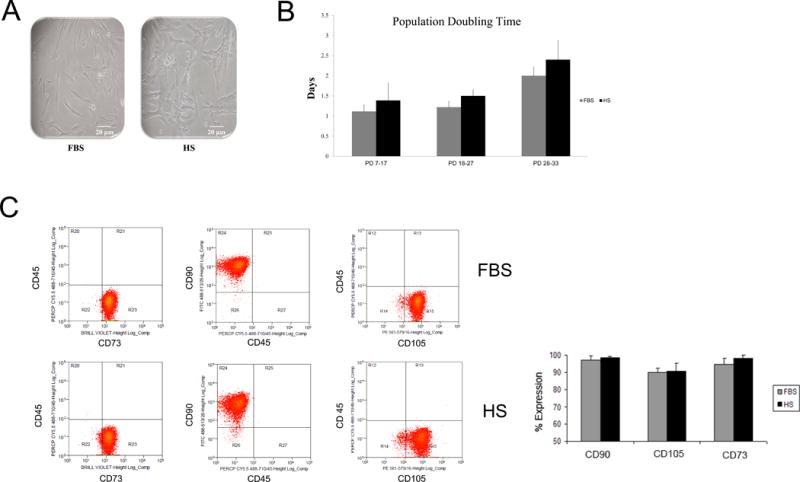
DPSC characterization. (A) Photomicrographs of DPSCs cultured in media containing FBS or HS. (B) Cell proliferation, as measured by population doubling time (PDT), over time between DPSCs expanded in FBS vs. those expanded in HS. (C) Stem cell surface marker expression of passage 5 DPSCs. DPSC populations expanded in media containing FBS or HS consistently yielded high proportions of cells that were positive for the mesenchymal stem cell markers CD73, CD90, and CD105.
Angiogenic factors produced by DPSCs
It has been reported by our group and others that DPSCs have angiogenic capacity in vitro and in vivo. To determine what specific soluble factors play a role in this angiogenic capacity, a proteome profiler array was used to assess secreted molecules from both DPSC-HS and DPSC-FBS. Of the 55 angiogenesis related proteins screened, the array showed that DPSCs produced varying levels of 54 of these analytes (Supplementary Figure 1). In both DPSCs-HS and DPSC-FBS, the angiogenic proteins endothelin, insulin-like growth factor binding protein-3 (IL-3), pentraxin-3, serpin E1(SE1), serpin F1(SF1), thrombospondin-1, tissue inhibitor of MMP-1 (TIMP-1), and vascular endothelial growth factor (VEGF) were most highly produced (Figure 2A). In comparing angiogenic protein production between DPSC-HS and DPSCs-FBS, quantitative analyses showed that DPSC-HS secreted at least one-fold higher concentrations of angiopoietin-1, angiopoietin-2, interleukin-8, persephin, and transforming growth factor beta than DPSC-FBS (Figure 2A, B).
Figure 2.
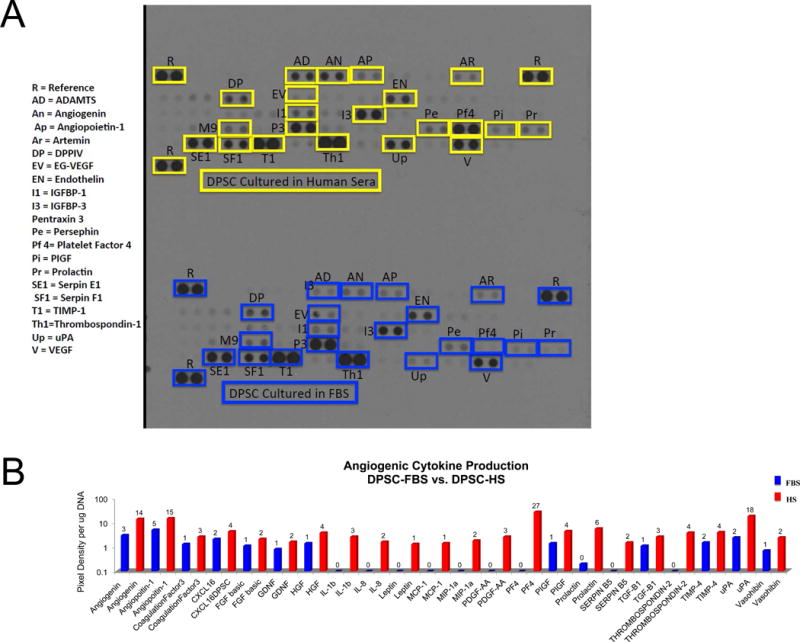
Angiogenic factors produced by DPSCs-HS vs. DPSC-FBS. (A) Representative results of anigogenic proteome profiler arrays probed with serum-free conditioned medium (CM) from DPSCs-HS and DPSCs-FBS; boxes indicate individual factors in duplicate. (B) Quantitative analysis of angiogenic factors and chemokines in CM from DPSC-FBS and DPSC-HS differentially expressed by greater than one-fold. * denotes P < 0.05 relative to FBS condition.
DPSCs production of vascular tissue within tooth slice
Following transplantation of DPSCs-HS and DPSCs-FBS in tooth slice/scaffolds, blood vessel analysis was performed to determine the in vivo vascular potential of these cells relative to their production of soluble angiogenic growth factors. Without transplantation of cells, we have previously reported that scaffolds have very low cellularity and minimal vascularity within the tooth/slice (data not shown). Transplantation of either DPSCs-HS or DPSC-FBS in the present study consistently yielded a robust angiogenic response with the establishment of numerous blood vessels throughout the engineered tissue (Figure 3A). Quantitatively, this response was the same for both cell types (Figure 3B), confirming the vascular capacity of these cells to elicit an in vivo vascular response. In addition to evaluating the overall vascular response, we determined if any of the transplanted cells differentiated into endothelial cells. Through factor VIII staining of human cells, we found that a significant number of vessels were comprised of transplanted cells (Figure 3A). Following quantification, it was observed that up to 50% of the vasculature was comprised of transplanted cells, being either DPSC-HS or DPSC-FBS (Figure 3C). These data indicate that not only are DPSCs-HS and DPSCs-FBS capable of promoting angiogenesis in vivo, but that they are also capable of differentiating into endothelial cells.
Figure 3.
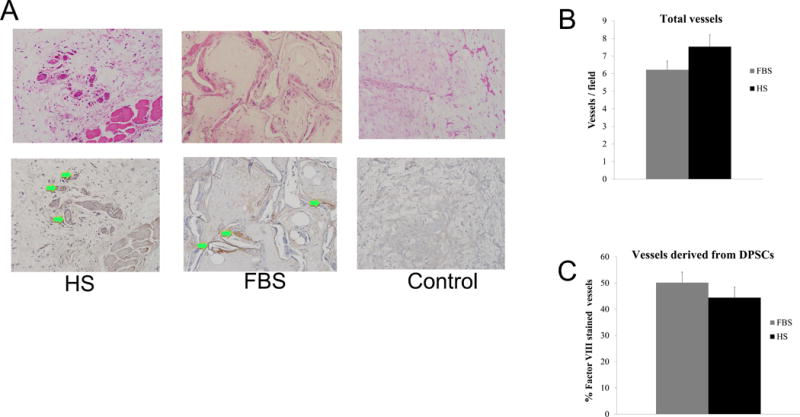
DPSC induced angiogenesis in tooth slice. (A) High magnification histological images of blood vessel formation following hematoxylin and eosin (H&E) staining to evaluate total vessel formation and Factor VIII staining to identify human DPSC derived vessels (magnification, 200x; green arrows delineate vessels with brown anti-human Factor VIII staining). (B) Quantitative analysis of total vessel formation in condition following transplantation of DPSC-HS and DPSCS-FBS. (C) Quantitative analysis of human derived vessels following transplantation of DPSC-HS and DPSCS-FBS.
Formation of Pre-Dentin
We consistently observed that the tissues generated in the pulp chambers of tooth slice/scaffolds seeded with DPSCs-HS and DPSCs-FBS were well organized, showing architectural and histological characteristics that closely resemble those of normal human dental pulps (Figure 4A). In these specimens, the new tissue that was generated centripetally in the pulp chamber of tooth slice/scaffolds seeded with DPSCs contained dentinal tubules and a clearly defined adjacent pre-dentin layer. Under ultrastructural analyses, we have previously shown that in this model, cell transplantation of the appropriate cell types yields cells which present with eccentric polarized nuclei positioned at the basal part of the cell body, well-developed Golgi’s complex, and rough endoplasmic reticulum with multiple cytoplasmic vesicles (Cordeiro et al., 2008). The ability of DPSC-HS and DPSC-FBS to differentiate into odontoblasts to produce dentin was further evaluated with confocal microscopy in tooth slices implanted into mice that were injected with tetracycline, which stains structures actively undergoing mineralization by incorporating into these tissues. Confocal microscopy showed well-defined fluorescent lines in the tooth slice/scaffolds containing DPSC-HS and DPSC-FBS (Figure 4B), coinciding with the timing of injections of tetracycline. Analysis of these data indicates that DPSCs-HS can differentiate into cells that secrete a mineralized dentin matrix at a rate similar to that of DPSCs-FBS and that seen in a normal human dental pulp.
Figure 4.
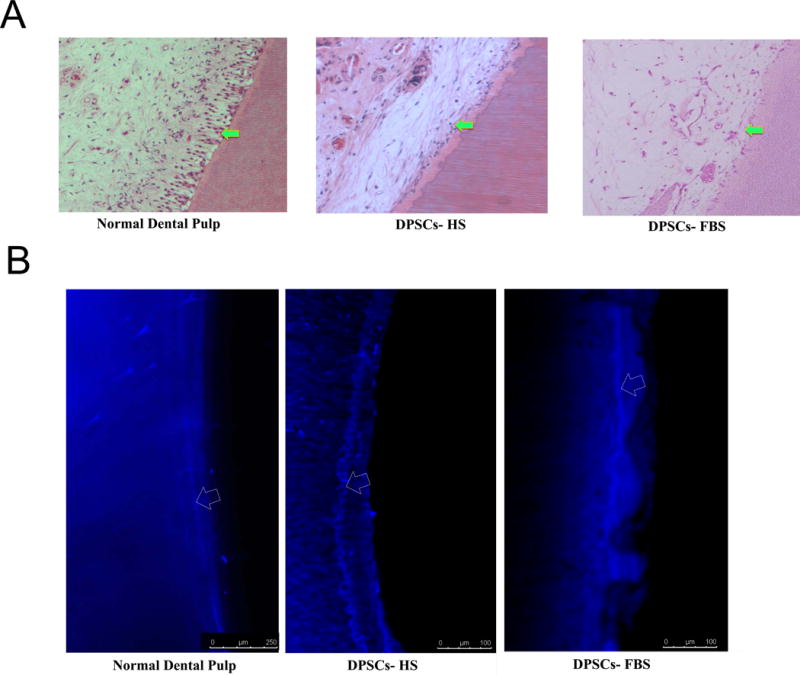
Predentin and dentin formation in tooth slice following transplantation of DPSC-HS and DPSC-FBS. (A) Histologic evaluation shows the cellularity of the tissue lining the predentin of engineered dental pulps in tooth slice/scaffolds seeded with DPSC-HS and DPSC-FBS compared to normal/control dental pulp (magnification, 400x; arrows show predentin region). (B) Representative confocal microscopic photomicrograph showing fluorescent lines (resultant of tetracycline injections) in dental pulp of a mouse incisor, indicating continuous dentin deposition (dentinogenesis) over time. Human tooth slices recovered after 30 days showing the presence of tetracycline lines found either in DPSC-HS or DPSC-FBS groups, indicating the presence of similar dentinogenic activity within engineered pulp tissue of tooth slices (arrows delineate fluorescent lines).
DISCUSSION
Cell therapy offers promise for regeneration of dental pulp and dental-pulp derived tooth tissues. We previously reported on a clinically feasible approach to store teeth, isolate DPSCs, and expand DPSCs without using animal serum; however, the in vivo regenerative capacity of these DPSCs was not determined in these studies (10). In this study, we identified specific soluble angiogenic factors produced by adult DPSCs and compared them to those cultured without animal sera (DPSCs-HS). We then evaluated the angiogenic responses of DPSCs-HS in vivo in a model of dental pulp regeneration. Transplantation of DPSCs-HS in a tooth slice yielded a robust angiogenic response and further, induced the formation of pre-dentin. To our knowledge, this study is the first to demonstrate the in vivo regenerative capacity of adult DPSCs isolated and expanded in conditions completely devoid of animal serum or exogenous growth factors.
In evaluating the angiogenic potential of proteins produced by DPSCs, Bronckaers and others recently reported that over the course of 48 hours, DPSCs secrete high levels of angiogenic molecules (16). Additionally, the level of production of these factors was sufficient to stimulate endothelial cell migration and angiogenesis in vivo. Our findings were consistent with these data in that high levels of proteins were contained in the overnight conditioned medium of DPSCs cultured in both animal and human sera. In fact, as noted in the Results, some of these factors were more highly expressed in the HS condition than in the FBS condition. It is well known that specific conditions of the microenvironment, like hypoxia, can induce the upregulation of stem cell mediated angiogenesis (17, 18). It has more recently been shown that not only can hypoxia induce expression of VEGF specifically from DPSCs (19), but that FGF-2 can directly upregulate VEGF and HGF secretion from pulp cells of deciduous teeth (SHEDs) (20). Through similar mechanisms to FGF-2, there could be components of the human sera which induce or upregulate the production of specific angiogenic proteins from DPSCs. Additional studies are underway to identify what these components of human sera may be.
Our group and others have used various models to investigate the in vivo angiogenic capacity of pulp-derived stem cells from both primary (SHEDs) and adult (DPSCs) teeth and it is clear that they maintain strong angiogenic potential following transplantation (4, 20–23). Through co-transplantation with endothelial cells or transplantation alone, these cells have been shown to induce angiogenesis attributed primarily to their release of trophic factors. Janebodin et. al observed that following transplantation, DPSCs also served as pericytes, surrounding mature vessels (22). In the tooth slice model, we have demonstrated previously with LacZ staining that SHEDS have the capacity to differentiate into endothelial cells and anastomose with the host vasculature to differentiate into endothelial cells (24). Our previous findings with SHEDS are consistent with those in the present study where transplanted adult DPSCs took on an endothelial cell phenotype and formed functional vessels containing red blood cells, which anastomosed with the host vasculature. Additionally, in this study, the capacity of DPSCs-HS to form a vasculature was no different than that of DPSCs-FBS. As such, our current finding that DPSCs maintain their in vivo angiogenic capacity when never being exposed to animal sera has broad-based cell therapy implications for dental pulp regeneration.
Though this study and others have demonstrated that DPSCs can enhance vascular tissue formation in vivo, being able to achieve regeneration of dental pulp tissue is the ultimate in vivo goal. To this end, there are only a few limited reports of in vivo pulp regeneration using DPSCs; yet, these reports utilize animal serum in their isolation and culture of DPSCs (19, 21, 25). Here, we cite the first report of in vivo pulp regeneration using DPSCs isolated in conditions completely devoid of animal serum or exogenous growth factors. In preparation for clinical trials, Nakashima et. al. have recently reported the use of human serum for isolation of human DPSCs mobilized using factors such as G-CSF (MDPSCs) and b-FGF (21, 26, 27). Though they report efficacy in pulp regeneration using these protocols, from the standpoint of feasibility and cost effectiveness, it would be most ideal if exogenous growth factors were not needed to obtain the desired regenerative outcomes. A completely serum-free approach would be ideal and we have previously reported on the development of a serum-free system for periodontal ligament stem cells (PDLSCs) and stem cells from exfoliated deciduous teeth (SHEDs) (28). Though this media supported the growth and expansion of PDLSCs and SHEDs, it did not support the proliferation and expansion of DPSCs. As a result, we evaluated human serum as an alternative to animal serum for these DPSCs and our early results are encouraging using DPSCs-HS without the need for exogenous growth factors or animal serum. However, larger animal models are needed and being planned in immunocompetent hosts to rigorously evaluate the pulp regenerative potential of DPSCs-HS.
In conclusion, this study provides strong evidence that adult DPSCs can be isolated and expanded in media devoid of animal serum or exogenous growth factors, and still maintain potent angiogenic properties. Additionally, following their transplantation, DPSCs-HS yield regeneration of dental pulp tissue evidenced by their differentiation into endothelial cells to participate in forming a functional vasculature and their formation of predentin tissue over time. Further preclinical studies are planned utilizing clinically acceptable conditions (26) to optimize protocols in preparation for clinical trials evaluating DPSC-mediated pulp regeneration.
Supplementary Material
Supplementary Figure 1. Quantitative analysis of all angiogenic factors and chemokines in CM from DPSC-FBS and DPSC-HS.
Acknowledgments
This study was funded by the American Association of Endodontists Foundation, the University of Michigan School of Dentistry Pathways Program, and grant R01-DE21410 from the NIH/NIDCR. The authors would like to acknowledge the University of Michigan Wisdom Tooth clinic for the provision of teeth and to David Adams and Ann Marie Deslauriers at the University of Michigan BRCF Flow Cytometry Core.
Footnotes
Acknowledgement: The authors deny any conflicts of interest
References
- 1.Hara K, Yamada Y, Nakamura S, Umemura E, Ito K, Ueda M. Potential characteristics of stem cells from human exfoliated deciduous teeth compared with bone marrow-derived mesenchymal stem cells for mineralized tissue-forming cell biology. Journal of endodontics. 2011;37(12):1647–1652. doi: 10.1016/j.joen.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, et al. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14(2):149–156. doi: 10.1089/ten.tec.2008.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, et al. SHED differentiate into functional odontoblasts and endothelium. Journal of dental research. 2010;89(8):791–796. doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 5.Osathanon T, Sawangmake C, Nowwarote N, Pavasant P. Neurogenic differentiation of human dental pulp stem cells using different induction protocols. Oral diseases. 2013 doi: 10.1111/odi.12119. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Zhao Y, Jia W, Yang J, Ge L. Preliminary study on dental pulp stem cell-mediated pulp regeneration in canine immature permanent teeth. Journal of endodontics. 2013;39(2):195–201. doi: 10.1016/j.joen.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29(6):532–539. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 8.Saghiri MA, Asatourian A, Sorenson CM, Sheibani N. Role of angiogenesis in endodontics: contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. Journal of endodontics. 2015;41(6):797–803. doi: 10.1016/j.joen.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnamain V, Thinard R, Sergent-Tanguy S, Huet P, Bienvenu G, Naveilhan P, et al. Human dental pulp stem cells cultured in serum-free supplemented medium. Front Physiol. 2013;4:357. doi: 10.3389/fphys.2013.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eubanks EJ, Tarle SA, Kaigler D. Tooth storage, dental pulp stem cell isolation, and clinical scale expansion without animal serum. Journal of endodontics. 2014;40(5):652–657. doi: 10.1016/j.joen.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Sakai VT, Cordeiro MM, Dong Z, Zhang Z, Zeitlin BD, Nor JE. Tooth slice/scaffold model of dental pulp tissue engineering. Advances in dental research. 2011;23(3):325–332. doi: 10.1177/0022034511405325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demarco FF, Casagrande L, Zhang Z, Dong Z, Tarquinio SB, Zeitlin BD, et al. Effects of morphogen and scaffold porogen on the differentiation of dental pulp stem cells. Journal of endodontics. 2010;36(11):1805–1811. doi: 10.1016/j.joen.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Nor JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81(4):453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 14.Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19(6):665–667. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 15.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 16.Bronckaers A, Hilkens P, Fanton Y, Struys T, Gervois P, Politis C, et al. Angiogenic properties of human dental pulp stem cells. PloS one. 2013;8(8):e71104. doi: 10.1371/journal.pone.0071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanacker J, Viswanath A, De Berdt P, Everard A, Cani PD, Bouzin C, et al. Hypoxia modulates the differentiation potential of stem cells of the apical papilla. Journal of endodontics. 2014;40(9):1410–1418. doi: 10.1016/j.joen.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Aranha AM, Zhang Z, Neiva KG, Costa CA, Hebling J, Nor JE. Hypoxia enhances the angiogenic potential of human dental pulp cells. Journal of endodontics. 2010;36(10):1633–1637. doi: 10.1016/j.joen.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Kuang R, Zhang Z, Jin X, Hu J, Shi S, Ni L, et al. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta biomaterialia. 2016 doi: 10.1016/j.actbio.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorin C, Rochefort GY, Bascetin R, Ying H, Lesieur J, Sadoine J, et al. Priming Dental Pulp Stem Cells With Fibroblast Growth Factor-2 Increases Angiogenesis of Implanted Tissue-Engineered Constructs Through Hepatocyte Growth Factor and Vascular Endothelial Growth Factor Secretion. Stem cells translational medicine. 2016 doi: 10.5966/sctm.2015-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, et al. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem cells translational medicine. 2013;2(7):521–533. doi: 10.5966/sctm.2012-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janebodin K, Zeng Y, Buranaphatthana W, Ieronimakis N, Reyes M. VEGFR2-dependent angiogenic capacity of pericyte-like dental pulp stem cells. Journal of dental research. 2013;92(6):524–531. doi: 10.1177/0022034513485599. [DOI] [PubMed] [Google Scholar]
- 23.Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue engineering Part A. 2015;21(3–4):550–563. doi: 10.1089/ten.tea.2014.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. Journal of endodontics. 2008;34(8):962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Dissanayaka WL, Zhu L, Hargreaves KM, Jin L, Zhang C. Scaffold-free Prevascularized Microtissue Spheroids for Pulp Regeneration. Journal of dental research. 2014;93(12):1296–1303. doi: 10.1177/0022034514550040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakashima M, Iohara K. Mobilized dental pulp stem cells for pulp regeneration: initiation of clinical trial. Journal of endodontics. 2014;40(4 Suppl):S26–32. doi: 10.1016/j.joen.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi N, Hayashi Y, Murakami M, Alvarez FJ, Horibe H, Iohara K, et al. Similar in vitro effects and pulp regeneration in ectopic tooth transplantation by basic fibroblast growth factor and granulocyte-colony stimulating factor. Oral diseases. 2015;21(1):113–122. doi: 10.1111/odi.12227. [DOI] [PubMed] [Google Scholar]
- 28.Tarle SA, Shi S, Kaigler D. Development of a serum-free system to expand dental-derived stem cells: PDLSCs and SHEDs. J Cell Physiol. 2011;226(1):66–73. doi: 10.1002/jcp.22304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Quantitative analysis of all angiogenic factors and chemokines in CM from DPSC-FBS and DPSC-HS.


