Abstract
Microglia have recently been recognized as key regulators of synapse development, function, and plasticity. Critical to progressing the field is the identification of molecular underpinnings necessary for microglia to carry out these important functions within neural circuits. Here, we focus a review specifically on roles for microglial cytokine signaling within developing and mature neural circuits. We review exciting new studies demonstrating essential roles for microglial cytokine signaling in axon outgrowth, synaptogenesis and synapse maturation during development, as well as synaptic transmission and plasticity in adulthood. Together, these studies identify microglia and cytokines as critical modulators of neural circuits within the healthy brain, with implications for a broad range of neurological disorders with disruptions in synaptic structure and function.
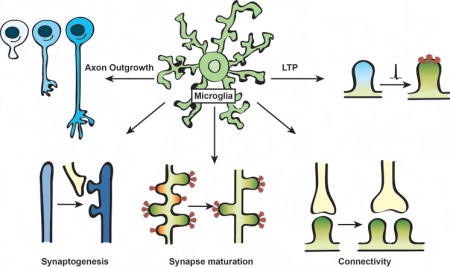
Graphical abstract
Introduction
Nearly 100 years ago, Pío del Río-Hortega stained fixed tissue with silver carbonate to reveal mysterious brain cells that he called “microglia” [1]. From this simple tissue preparation, he made the keen observation that these resident brain macrophages were uniquely dynamic with robust “plasticity of their protoplasm” and a high degree of physical interaction with other nervous system cells. Fast forward to the 21st century, del Río-Hortega’s suspicions are being realized with exciting work defining key functional roles for microglia within neural circuits in the healthy brain.
Some of the first evidence that suggested microglia were playing important functions within the healthy brain were seminal 2-photon live imaging studies in mice, demonstrating that microglial processes in the intact healthy brain were highly dynamic and continuously surveying their extracellular environment [2,3]. This surveillance activity was later shown to be highly sensitive to neural activity whereby microglia modulated the motility of their processes in response to changes in neural activity and sensory experience [4–9]. Further live and static imaging revealed remarkable activity-dependent physical interactions and contact between microglial processes and synaptic elements (dendritic spines and presynaptic boutons) under steady-state conditions [4,5,10]. Indeed, given an estimated 94% of microglial processes are in contact with synaptic elements at any given point in time [4], this begs the question--what is the function(s) of microglia at synapses? It is now increasingly clear that microglia-derived molecules regulate synaptic connectivity including regulation of axon outgrowth, synaptogenesis, synapse maturation, synaptic pruning, basal synaptic transmission, and functional synapse plasticity [11]. Here, we review exciting new work on emerging roles for microglial cytokine signaling necessary for modulating synaptic connectivity and function.
Cytokines: An introduction
Cytokines are an exceptionally large and diverse group of small signaling proteins that, upon binding to their cognate receptors, activate cellular pathways to modulate a large variety of physiological and pathological processes [12]. Based on their structural homology, cytokines are subdivided in different classes: chemokines, lymphokines, tumor necrosis factors (TNFs), colony stimulating factors (CSFs), interferons (IFNs) and interleukins (ILs). These classes are further divided into subgroups based on structural and functional properties. For example, chemokines are classified into four groups based on the presence of conserved cysteine residues in their sequence (C, CC, CXC, CX3C). IFNs are subdivided into three main groups according to their function and the molecular pathways used for signaling (type I IFNs including α- and β-IFN family members, and type II or γ-IFN family members). Moreover, subgroups of CSFs are defined based on their primary target cell types, including macrophages, granulocytes and monocytes, while TNFs and ILs comprise groups with larger diversity.
Each of these cytokine classes have canonical and well know functions in peripheral tissues. Canonically, chemokines and lymphokines enable chemotactic recruitment of various cell types in different tissues, TNFs and CSFs mainly influence growth and survival of cells, and IFNs and ILs primarily regulate aspects of cellular differentiation and inflammation [12]. However, depending on the cellular context and surrounding molecular cues, such as the presence or absence of other cytokines and cytokine receptors, the same cytokine can exert pleiotropic biological effects (e.g. cell proliferation vs. regulation of inflammatory state). Further adding to the complexity, while many of these cytokines and their receptors were identified in the context of innate and adaptive immune cell function, it is now clear that immune and non-immune cell types throughout the body utilize these signaling pathways for homeostatic function. Exemplifying this is the nervous system in which an increasing number of studies demonstrate that cytokines regulate development and function of multiple resident cell types (neurons, astrocytes, myelinating glia, and microglia) in health and disease [13]. While roles for cytokines in the healthy and diseased nervous system now represent a large body of literature, we will focus specifically on those microglial cytokines and cytokine receptors that modulate synaptic connectivity and function in the healthy central nervous system (CNS). Among the molecules that will be reviewed most extensively are the fractalkine receptor (CX3CR1) and it’s canonical either secreted or membrane-bound ligand fractalkine (CX3CL1), tumor necrosis factor alpha (TNFα), and interleukin 1β (IL-1β).
Microglial cytokine signaling: establishing synaptic connectivity
Prior to forming synapses, newborn axons must grow towards their eventual synaptic targets. Recent work in the embryonic brain has suggested a key role for microglia and microglial cytokine signaling in regulating initial axon outgrowth. When microglia were absent in the embryonic brain by genetic targeting the transcription factor PU.1 or pharmacological blockade of the cytokine colony stimulating factor 1 receptor (CSF1R), a trophic factor necessary for microglial survival, there was exuberant outgrowth of dopaminergic axons in the E14.5 and P0 brain [14]. In contrast, reduced dopaminergic axon outgrowth was observed in a mouse model of maternal immune activation (MIA), in which pregnant dams were injected with lipopolysaccharide (LPS), a component of the envelope of Gram-negative bacteria that binds Toll-like receptor 4 (TLR4) on microglia and induces production of a broad range of cytokines. To explore the molecular mechanism(s), mice deficient in microglia-enriched molecules including the adaptor protein DAP12, the engulfment receptor complement receptor 3, or the chemokine receptor CX3CR1 were analyzed [12]. Interestingly, Cx3cr1−/− embryos were the only mutants that had defects in dopaminergic axon outgrowth, which was similar to the decreased outgrowth observed in MIA. Determining how MIA and CX3CR1 signaling may be linked mechanistically to alter axon outgrowth and identifying whether these developmental defects persist into adulthood to affect circuit function are important future directions. Also, CX3CR1 is a receptor that typically binds CX3CL1, which is expressed primarily by neurons. It is unknown if this or another ligand is involved in these developmental effects mediated by CX3CR1.
In addition to axon outgrowth, recent studies have suggested a role for microglia and microglia-derived cytokines in synaptogenesis. One study used a genetic diphtheria toxin strategy to deplete microglia by ~50% and provided evidence that synaptogenesis was impaired in the P11 somatosensory cortex [15]. Another study used a similar strategy to deplete microglia in postnatal and adult mice and showed that learning-induced synaptogenesis in the motor cortex was impaired [16]. While the effects in the motor cortex were largely attributed to microglial brain-derived neurotrophic factor (BDNF), it is unknown whether BDNF regulates synaptogenesis more globally or what molecules regulate synaptogenesis in the somatosensory cortex. Potential soluble synaptogenic factors released by microglia include cytokines. Indeed, an in vitro study in postnatal rat hippocampal neurons demonstrated increased numbers of excitatory and inhibitory synapses upon exposure to microglia-derived IL-10, which was antagonized by the addition of IL-1β, another microglial-derived cytokine [17]. Determining how these cytokines orchestrate downstream signaling cascades to affect synaptogenesis and identifying whether similar mechanisms apply in vivo will be important next steps.
Microglial cytokine signaling: synapse maturation
During development, synaptic connections first form in excess to form immature neural circuits. These nascent circuits then undergo synaptic pruning in which less active synapses are eliminated. The remaining synapses in the circuit are then functionally strengthened and elaborated into mature neural circuits. Some of the first evidence suggesting microglia play roles in these maturation processes was work demonstrating that microglia engulf and prune away less active synapses during brain development [4,5,10]. While complement-dependent phagocytic signaling was identified to regulate microglial synaptic engulfment and pruning [10], cytokine signaling has also been implicated in this developmental process. For example, astrocyte-derived transforming growth factor beta (TGFβ) was shown to modulate complement expression on neurons and consequently influence complement-dependent synaptic engulfment by microglia [18].
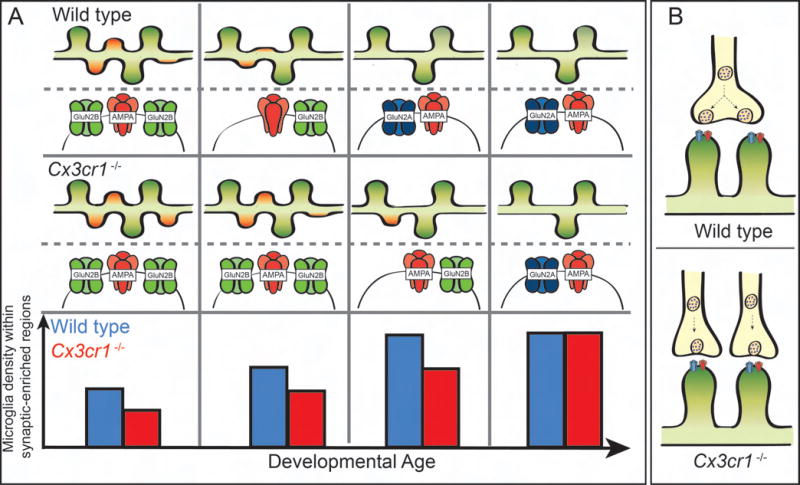
Other work has focused on microglial cytokine signaling more specifically by assessing synaptic maturation in Cx3cr1−/− mice. In the developing hippocampus, Cx3cr1−/− mice had transient increases in dendritic spine density (Figure 1a) [19]. Accompanying this delay in maturation of spine density was a defect in functional maturation of hippocampal synapses as measured by the ratio of spontaneous and miniature excitatory postsynaptic current amplitude (sEPSC/mEPSC amplitude ratio), long term depression (LTD) induction, and seizure susceptibility. Similar defects in functional synapse maturation were also observed within the developing barrel cortex in Cx3cr1−/− mice as measured by the ratio of Nmethyl-D-aspartate (NMDA) receptor subunits GluN2A and GluN2B and the relative ratio of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA receptor content at thalamocortical synapses [20•]. Interestingly, in both studies, the effects on structural and functional maturation of synapses were accompanied by transient reductions in microglial density near synapses in Cx3cr1−/− mice. Once microglial numbers reached wild type levels in older Cx3cr1−/− mice, defects in spine density and/or functional synapse maturation were largely resolved (Figure 1A). Going forward, it will be important to identify whether synaptic defects in Cx3cr1−/− mice are exclusively due to modulation of microglial recruitment to synapses or whether CX3CR1 signaling has direct downstream effects on other microglial molecules that regulate synaptic pruning and maturation.
Figure1. CX3XR1-deficient mice have delayed synapse maturation and persistent defects in synaptic connectivity.
(a) Top panel, during development in wild type animals, a subset of dendritic spines are pruned away (red spines) leaving the remaining subset to strengthen into mature neural circuits (green spines). In addition, there is a developmental shift from GluN2B (green) to GluN2A (blue)-containing postsynaptic NMDA receptors and an increase in AMPA/NMDA ratio. Middle panel, loss of CX3CR1 leads to a transient delay in spine pruning in the hippocampus and maturation of postsynaptic receptors in the hippocampus and barrel cortex. Bottom panel, once microglia density reaches wild type levels in the CX3CR1 deficient brain, spine density and postsynaptic receptor maturation are indistinguishable from wild type brains. (b) Adult animals deficient in CX3CR1 have decreased multi synapse boutons (MSBs) along the same dendrite in the hippocampus.
While the original study in the hippocampus identified transient defects in synapse maturation that were largely attenuated in older animals, one parameter that was not shown to attenuate in Cx3cr1−/− mice was the sEPSC/mEPSC amplitude ratio [21]. In a follow-up study, the same group assessed more long-term effects of loss of CX3CR1 on functional neural circuits [21]. In this later study, persistent defects in sEPSC/mEPSC amplitude ratio were observed in adult Cx3cr1−/− mice, which were indicative of less multisynapse boutons (MSBs) on CA1 pyramidal neurons. This result was confirmed by ultrastructure in which single presynaptic terminals within CA1 contacted multiple spines along the same dendrite in wild type mice and the frequency of these MSBs was reduced in Cx3cr1−/− mice (Figure 1B). This study also further revealed reduced functional connectivity between the hippocampus and prefrontal cortex as measured by fMRI, which were correlated with defects in social interactions and increased repetitive behaviors in Cx3cr1−/− mice. Precisely how CX3CR1 regulates these functional properties of synapses and neural circuits, the relative involvement of CX3CL1, and how changes in synapse function ultimately translate to behavioral alterations in knock-out mice will be critical next steps
Microglial-derived cytokine signaling: regulating synaptic transmission and functional plasticity
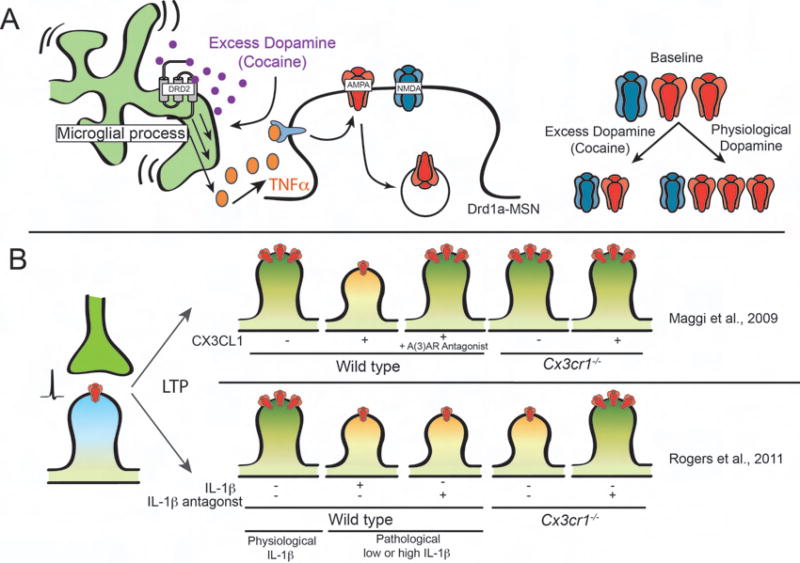
Microglia are in close apposition to and physically interact with synapses, dynamically sense changes in neural activity, and express several molecules known to modulate synaptic transmission [22]. Likewise, there has been a large amount of literature demonstrating that cytokines affect a large array of synaptic properties and physiology [23]. Much of this work has suggested that cytokine signaling from other cell types such as neurons and astrocytes directly affects synapses, although it is intriguing to speculate that these effects are related to changes in microglia. Some of the first evidence suggesting that microglia-derived cytokine signaling could regulate synaptic function was work demonstrating roles for tumor necrosis factor alpha (TNFα) in regulating the amount of surface AMPA receptors, synaptic strength, and homeostatic plasticity [24–26]. While originally proposed to be astrocyte-derived, transcriptomics has revealed little to no TNFα transcripts in astrocytes and near exclusive expression in microglial cells [27,28]. More recently, using cell-specific Cre lines in vivo, it was demonstrated that microglia-derived TNFα drives the internalization of synaptic AMPA receptors, decreases synaptic strength, and suppresses behavioral sensitization following repeated cocaine administration (Figure 2a) [29].
Figure 2. Microglial cytokine signaling regulates functional synapse plasticity.
(a) Summary of microglial-derived TNFα effects on AMPA receptor (red) internalization within the nucleus accumbens following cocaine administration. (b) Summary CX3CL1 and IL-1β effects on LTP. Red receptors represent membrane-associated postsynaptic AMPA receptors necessary for LTP. Note the differing results assessing LTP in Cx3cr1−/− (Maggi et al., 2009, top vs. Rogers et al., 2011, bottom).
In addition to TNFα, one of the most robust microglial cytokine pathways identified to regulate functional synapse plasticity is signaling between microglial CX3CR1 and its ligand CX3CL1 (Figure 2B), which is largely expressed by neurons in a secreted or membrane-bound form [30]. Some of the first evidence that this receptor-ligand signaling modulated synaptic function was work in the hippocampus in which acute application of CX3CL1 depressed glutamatergic transmission, which was blocked in Cx3cr1−/− mice [31,32]. It was later discovered that local application of CX3CL1 to acute hippocampal slices at the time of long-term potentiation (LTP) induction resulted in impaired LTP [33]. While Cx3cr1−/− hippocampus showed no defects in LTP, CX3CL1-induced LTP impairments were blocked in Cx3cr1−/− slices. Further, CX3CL1-induced LTP impairment required signaling through a purinergic receptor, adenosine receptor 3 (A(3)AR) (Figure 2b, top) [33,34]. Interestingly, another chemokine signaling molecule CXCL16, which, similar to CX3CL1, is cleaved by ADAM10 into a soluble form, was also recently implicated in modulating glutamatergic and GABAergic transmission through A(3)AR and microglia [35]. Precisely how purinergic and chemokine receptor signaling are mechanistically linked remains an important open question. One possibility is that chemokine signaling works in the same pathway as purinergic signaling to modulate rapid microglial recruitment to synapses, which has been suggested by two recent students in acute brain slices. For example, CX3CR1-deficient microglia were no longer attracted to a pipette containing 2-MeSADP, a purinergic receptor agonist [36]. Another study demonstrated increased physical contact between microglia and neurons following excitotoxic challenge were increased upon CX3CL1 treatment [37]. Conversely, these contacts were largely inhibited in slices prepared from mice deficient in CX3CR1 or the microglial purinergic receptor P2Y12. Determining how these two pathways converge through downstream signaling to regulate microglial recruitment to synapses will be important to advance our understanding.
In contrast to the studies described above, a very recent study provided in vivo evidence that CX3CR1-deficient microglia within the visual cortex had an amplified response to laser-induced injury while basal microglial dynamics and interactions with synapses were unaltered [65•]. They also provide data that Cx3cr1−/− mice have normal activity-dependent plasticity within the visual cortex. Therefore, CX3CR1-dependent modulation of synaptic plasticity may be context or region-specific. Consistent with this idea, another group has shown that adult Cx3cr1−/− mice have reduced hippocampal LTP accompanied by impairments in learning and memory andhippocampal neurogenesis [38••]. Further, IL-1β was elevated in Cx3cr1−/− mice and LTP and behavioral deficits were attenuated upon treatment with an IL-1β receptor antagonist (Figure 2b, bottom). Similar to CX3CL1/CX3CR1-purinergic receptor signaling, it remains to be determined how CX3CR1 modulates IL-1β. It is also unknown why results differ between this study and the study which showed no LTP defects in Cx3cr1−/− hippocampal slices and acute application of CX3CL1 diminished LTP [33]. Possibilities include differences in LTP induction paradigms and age of animals used for electrophysiological recordings, which may be important for IL-1β expression.
Work demonstrating a link between CX3CL1/CX3CR1 signaling and IL-1β in the regulation of LTP is consistent with past studies demonstrating that acute application of IL-1β impairs LTP [39–41]. This is further supported by data from an early life infection model in which postnatal pups are given a dose of Escherichia coli followed by an immune challenge with LPS in later life. This paradigm elicits increased microglial IL-1β levels and resulted in learning and memory impairments in rats, which are blocked upon inhibition of soluble IL-1β production with a caspase I inhibitor [42,43]. Given recent transcriptomics data sets, at least under basal conditions, IL-1β is nearly exclusively expressed by microglial cells in the CNS. However, this cell type-specific expression of IL-1β may change with age or inflammation [27,28]. It is also important to note that IL-1β-induced LTP impairments are dose dependent. While at higher, more disease-relevant concentrations IL-1β impairs LTP, at lower, more physiological doses it is required for LTP induction and learning and memory [44–47]. Together, these data suggest that maintaining the appropriate balance of physiological levels of IL-1β is critical for learning and memory and subtle alterations may have large effects on functional synapse plasticity. Identifying what cell types express the receptor for IL-1β and determining how IL-1β has differing effects based on concentration are important open questions.
Conclusions
Microglia are now emerging as key regulators of structural and functional synapses in the healthy brain. Among the molecular pathways identified, cytokines and cytokine receptors have emerged as important regulators of microglia function at synapses. During embryogenesis, microglial CX3CR1 signaling has been identified as regulator of axon outgrowth. Microglial IL-10 has been suggested to promote synaptogenesis in vitro. During postnatal development, microglial CX3CR1 signaling regulates microglial density within neural circuits, which, in turn, modulates synaptic pruning and maturation. Throughout the lifespan, microglial CX3CR1, TNFα and IL-1β have been implicated in regulating synaptic transmission and functional plasticity.
Together, this exciting work has opened up a new way of thinking about microglial cytokine signaling within neural circuits during non-pathogenic conditions and several new questions have emerged. First, we need to identify the precise mechanisms, including intercellular signaling, by which cytokines regulate microglia function at synapses. This also includes identifying potential interactions between other microglial-derived molecular pathways shown to modulate synapses such as complement, purinergic signaling, and BDNF. Second, there is a large body of work demonstrating cytokines derived from multiple other cell types affect synapses. For example, there has been recent exciting evidence that neuronal chemokine CCR5 modulates hippocampal synaptic plasticity and learning and memory [48]. Whether cytokine signaling from other cell types, such as neuronal CCR5 signaling, is modulated by or affects microglial function at synapses is an open question. Last, there is a large body of work indicating that abnormally reactive microglia and dysregulated cytokine levels are often concomitant with alterations in synaptic structure and function [49–53]. This includes disorders thought to have a developmental underpinning such as autism and schizophrenia as well as neurodegenerative diseases such as Alzheimer’s disease (AD). For example, altered levels of cytokines such as IL-1β, IL-6, IL-4, IFN-γ, and TGF-β and abnormally reactive microglia have been observed in individuals with autism and in mouse models of autism [54–58]. In the context of neurodegenerative disease, CX3CR1 deficiency inhibits several aspects of pathology in mouse models of AD including neuronal cell loss, betaamyloid accumulation, and cognitive deficits [13,59–61]. In the context of neurodegenerative disease, CX3CR1 deficiency inhibits several aspects of pathology in mouse models of AD including neuronal cell loss, beta-amyloid accumulation, and cognitive deficits [59–61,66].
Important in our progress to tackling these important questions is the development of new tools. One challenge has been measuring cell-type specific protein localization and levels of cytokines, particularly those that are secreted, as well as their receptors in tissue. Another challenge has been achieving cell-specific gene ablation. Mice expressing floxed alleles for cytokines and their receptors are often lacking. Similarly, while the emergence of new mice expressing CX3CR1-driven Cre and CreER expression have been invaluable for the field [16,62], these mice are a knock-in at the CX3CR1 locus and, therefore, either heterozygous are homozygous null for CX3CR1. This is further complicated by CX3CR1 expression by, not only microglia, but also a subset of peripheral immune cells. Additional tools to specifically manipulate microglia such as Cre/CreER driven by microglia-specific promoters such as TMEM119 and P2RY12 would be tremendous resources for the field [27,63,64]. Going forward, developing these new tools, determining cell-type specific effects, and identifying downstream signaling by which microglial cytokine signaling modulates synapses will be profoundly important. Elucidating these basic biological mechanisms will be important for understanding microglia function within neural circuits and for developing novel, microglia-based therapeutics for neurological disorders.
Highlights.
Microglial cytokine signaling regulates axon outgrowth and synaptogenesis
CX3CR1 regulates microglial recruitment to synapses necessary for synaptic maturation
Microglial CX3CR1, TNFα, and IL-1β modulate synaptic transmission and plasticity
Microglial cytokine signaling at synapses has important implications for disease
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health (NIMH R00MH102351; DS, NIMH R01MH113743; DS, NIGMS T32GM107000; PF); The Charles H. Hood Foundation (DS); The Brain and Behavior Research Foundation (DS); The Worcester Foundation (DS); the German Research Foundation (DFG; WE 6170/1-1; SW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• Special Interest
•• Outstanding Interest
- 1.del Rio-Hortega P. Microglia. In: Penfield W, Hoeber, editors. Cytology and cellular pathology of the nervous system. 1932. [Penfield W (Series Editor), vol 2.] [Google Scholar]
- 2.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 3.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dissing-Olesen L, LeDue JM, Rungta RL, Hefendehl JK, Choi HB, MacVicar BA. Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J Neurosci. 2014;34:10511–10527. doi: 10.1523/JNEUROSCI.0405-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci. 2014;34:10528–10540. doi: 10.1523/JNEUROSCI.0416-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011;6:e15973. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015;36:605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson AW, Lotze MT. The Cytokine Handbook. Fourth. Academic Press, An Imprint of Elsevier Science; 2003. [Google Scholar]
- 13.Ransohoff RM, Benveniste EN. Cytokines and the CNS. Second. Taylor and Francis Group; 2006. [Google Scholar]
- 14••.Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. doi: 10.1016/j.celrep.2014.07.042. Using multiple mouse models, the authors show that the outgrowth of dopaminergic axons and the laminar positioning of neocortical interneurons during embryonic development is regulated by microglia and cytokine signaling. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim SH, Park E, You B, Jung Y, Park AR, Park SG, Lee JR. Neuronal synapse formation induced by microglia and interleukin 10. PLoS One. 2013;8:e81218. doi: 10.1371/journal.pone.0081218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19••.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. This study demonstrated that microglial CX3CR1 signaling regulates synaptic maturation in the hippocampus. Mice deficient in CX3CR1 displayed transiently reduced numbers of microglia accompanied by increased numbers of dendritic spines and immature synapses. [DOI] [PubMed] [Google Scholar]
- 20•.Hoshiko M, Arnoux I, Avignone E, Yamamoto N, Audinat E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J Neurosci. 2012;32:15106–15111. doi: 10.1523/JNEUROSCI.1167-12.2012. This study further demonstrated a role for CX3CR1 in regulating microglia numbers and synapse maturation in the rodent somatosensory cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. This study assessed long-term effects of CX3CR1 deficency on functional neural networks. The authors describe defects in hippocampal synaptic multiplicity, functional connectivity within the prefrontal cortex, and impaired social and repetitive behaviors in adult CX3CR1-deficient mice. [DOI] [PubMed] [Google Scholar]
- 22.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 24•.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. Using mice that lack TNFα in neurons or glia, the authors demonstrate that glial-derived TNFα mediates synaptic scaling in response to prolonged blockade of activity by regualting th surface expression of AMPA receptors. [DOI] [PubMed] [Google Scholar]
- 25.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 26.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K, Stellwagen D. Microglial TNF-alpha Suppresses Cocaine-Induced Plasticity and Behavioral Sensitization. Neuron. 2016;90:483–491. doi: 10.1016/j.neuron.2016.03.030. This study demonstrates that microglial TNFα regulates drug-induced behaviors. In response to repeated cocaine administration striatal microglia are transiently activated and release TNFα, that drives the internalization of AMPA receptor from the surface of medium spiny neurons, decreases synaptic strength, and limits the development of behavioural sensitivation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 31•.Bertollini C, Ragozzino D, Gross C, Limatola C, Eusebi F. Fractalkine/CX3CL1 depresses central synaptic transmission in mouse hippocampal slices. Neuropharmacology. 2006;51:816–821. doi: 10.1016/j.neuropharm.2006.05.027. One of the first studies providing evidence that CX3CL1/CX3CR1 signaling modulates synaptic functions in organotypic slice cultures. The authors demonstrate that CX3CL1 treatment of hippocampal slices reduces the efficacy of glutamatergic synaptic transmission between Schaffer collateral fibers and pyramidal neurons in CA1 in a CX3CR1-dependent manner. [DOI] [PubMed] [Google Scholar]
- 32•.Ragozzino D, Di Angelantonio S, Trettel F, Bertollini C, Maggi L, Gross C, Charo IF, Limatola C, Eusebi F. Chemokine fractalkine/CX3CL1 negatively modulates active glutamatergic synapses in rat hippocampal neurons. J Neurosci. 2006;26:10488–10498. doi: 10.1523/JNEUROSCI.3192-06.2006. The authors show that application of CX3CL1 modulates synaptic activity in the hippocampus and results in diminished EPSC amplitude in a CX3CR1-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Maggi L, Trettel F, Scianni M, Bertollini C, Eusebi F, Fredholm BB, Limatola C. LTP impairment by fractalkine/CX3CL1 in mouse hippocampus is mediated through the activity of adenosine receptor type 3 (A3R) J Neuroimmunol. 2009;215:36–42. doi: 10.1016/j.jneuroim.2009.07.016. These manuscript provides evidence that purinergic receptor signaling is involved in CX3CL1-dependent changes in LTP. The authors show that LTP is impaired in the hippocampus upon application of CX3CL1 during the critical induction period, which was dependent upon adenosine receptor 3. [DOI] [PubMed] [Google Scholar]
- 34.Piccinin S, Di Angelantonio S, Piccioni A, Volpini R, Cristalli G, Fredholm BB, Limatola C, Eusebi F, Ragozzino D. CX3CL1-induced modulation at CA1 synapses reveals multiple mechanisms of EPSC modulation involving adenosine receptor subtypes. J Neuroimmunol. 2010;224:85–92. doi: 10.1016/j.jneuroim.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Di Castro MA, Trettel F, Milior G, Maggi L, Ragozzino D, Limatola C. The chemokine CXCL16 modulates neurotransmitter release in hippocampal CA1 area. Sci Rep. 2016;6:34633. doi: 10.1038/srep34633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagani F, Paolicelli RC, Murana E, Cortese B, Di Angelantonio S, Zurolo E, Guiducci E, Ferreira TA, Garofalo S, Catalano M, et al. Defective microglial development in the hippocampus of Cx3cr1 deficient mice. Front Cell Neurosci. 2015;9:111. doi: 10.3389/fncel.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eyo UB, Peng J, Murugan M, Mo M, Lalani A, Xie P, Xu P, Margolis DJ, Wu LJ. Regulation of Physical Microglia-Neuron Interactions by Fractalkine Signaling after Status Epilepticus. eNeuro. 2016;3 doi: 10.1523/ENEURO.0209-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. This study demonstrated that CX3CR1-deficient mice have deficits in LTP, contextual fear conditioning, and motor learning, which were reversed upon infusion of an IL1β antagonist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham AJ, Murray CA, O'Neill LA, Lynch MA, O'Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 41•.Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. This study provides early evidence that IL-1s can regulate LTP. The authors show that acute application of IL-1s in the hippocampus inhibits LTP, which is blocked when co-applied with an IL-1s analog inhibitor. [DOI] [PubMed] [Google Scholar]
- 42.Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. A study showing that IL-1 has concentration-dependent effects on LTP. While at low and physiological levels IL-1 is necessary for LTP, increased, pathological amounts of IL-1 inhibit LTP. [DOI] [PubMed] [Google Scholar]
- 45••.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. This study demonstrates that IL-1s gene expression increases in response to LTP and is dependent on NMDA receptor activity. It further demonstrated that while IL1-β is needed for maintaining LTP in the hippocampus, it is not involved in LTP induction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. Using genetic and pharmacological approaches, the authors show that IL-1 is necessary for memory. Using intracerebroventricular injection of IL-1 or its antagonist, the authors show that low, physiological values of IL-1 can improve memory while increased levels impair hippocampal-dependent memory. [DOI] [PubMed] [Google Scholar]
- 47.Spulber S, Mateos L, Oprica M, Cedazo-Minguez A, Bartfai T, Winblad B, Schultzberg M. Impaired long term memory consolidation in transgenic mice overexpressing the human soluble form of IL-1ra in the brain. J Neuroimmunol. 2009;208:46–53. doi: 10.1016/j.jneuroim.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Zhou M, Greenhill S, Huang S, Silva TK, Sano Y, Wu S, Cai Y, Nagaoka Y, Sehgal M, Cai DJ, et al. CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory. Elife. 2016;5 doi: 10.7554/eLife.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2017;17:49–59. doi: 10.1038/nri.2016.123. [DOI] [PubMed] [Google Scholar]
- 50.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong S, Dissing-Olesen L, Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol. 2016;36:128–134. doi: 10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 53.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 54.Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- 55.Careaga M, Van de Water J, Ashwood P. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goines P, Van de Water J. The immune system's role in the biology of autism. Curr Opin Neurol. 2010;23:111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koyama R, Ikegaya Y. Microglia in the pathogenesis of autism spectrum disorders. Neurosci Res. 2015;100:1–5. doi: 10.1016/j.neures.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. 2015;20:440–446. doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- 59.Ransohoff RMaEKJ. Microglia in Health and Disease. Cold Spring Harb Perspect Biol. 2015;8 doi: 10.1101/cshperspect.a020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer's disease mouse models. Am J Pathol. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, Ransohoff RM, Gan L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem. 2011;286:32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113:E1738–1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Lowery RL, Tremblay ME, Hopkins BE, Majewska AK. The microglial fractalkine receptor is not required for activitydependent plasticity in the mouse visual system. Glia. 2017;65:1744–1761. doi: 10.1002/glia.23192. The authors provide in vivo evidence that CX3CR1-deficiency results in amplified response to laser ablation-induced injury without affect basal microglial dynamics. They also demonstrate no defect in activity-dependent plasticity within the visual cortex in Cx3CR1-deficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S, Xu G, Jay TR, Bhatta S, Kim KW, Jung S, Landreth GE, Ransohoff RM, Lamb BT. Opposing effects of membrane anchored CX3CL1 on amyloid and tau pathologies via the p38MAPK pathway. J Neurosci. 2014;34:12538–12546. doi: 10.1523/JNEUROSCI.0853-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]