Abstract
Impulsive behavior is implicated in the initiation, maintenance, and relapse of drug-seeking behaviors involved in drug addiction. Research shows that changes in impulsive behavior across the lifespan contribute to drug use and addiction. The goal of this review is to examine existing research on the relationship between impulsive behavior and drug use across the lifespan and to recommend directions for future research. Three domains of impulsive behavior are explored in this review: impulsive behavior-related personality traits, delay discounting, and prepotent response inhibition. First, we present previous research on these three domains of impulsive behavior and drug use across developmental stages. Then, we discuss how changes in impulsive behavior across the lifespan are implicated in the progression of drug use and addiction. Finally, we discuss the relatively limited attention given to middle-to-older adults in the current literature, consider the validity of the measures used to assess impulsive behavior in middle-to-older adulthood, and suggest recommendations for future research.
Keywords: impulsive behavior, UPPS-P, delay discounting, prepotent response inhibition, drug use, addiction
1. Introduction
Impulsive behavior is an integral part of the development and maintenance of drug addiction (Evenden, 1999; Ouzir & Errami, 2016; Verdejo-García et al. 2008). Bechara (2005) conceptualized addiction as: “the product of an imbalance between two separate, but interacting, neural systems that control decision making: an impulsive, amygdala system for signaling pain or pleasure of immediate prospects, and a reflective, prefrontal cortex system for signaling pain or pleasure of future prospects” (p. 1458). Research has suggested that differences in drug use across the lifespan are due, in part, to the separate and parallel maturity in these brain systems across different developmental stages that potentially increase the likelihood of impulsive behavior (Blakemore & Robbins, 2012; Crone & Dahl, 2012). The majority of research examining the relationship between impulsive behavior and drug use has predominantly focused on adolescents and young adults. This is not all-together surprising, as these groups are at a particular risk for and have high rates of drug use (Lopez-Quintero et al. 2011; Young et al., 2002) and most individuals “mature out” of drug use throughout middle-to-older adulthood (Fillmore et al., 1988; Littlefield et al., 2009; Littlefield & Sher, 2016; Winick, 1962). Importantly, however, a small percentage of adults fail to mature out of drug use and go on to develop a more severe pattern of drug use, resulting in full-blown substance use disorders (SUDs) in middle-to-older adulthood (Heyman, 2013). Studies using nationally representative samples found that among adults over 50, 60% used alcohol, 3% used illicit drugs, and 1–2% used prescription drugs (Blazer & Wu, 2009; Blazer & Wu, 2011; Moore et al., 2009; Wu & Blazer, 2014). Among users, 7.4% of adults in the 50–64 age group and 3.4% in the 65+ group had a past-year diagnosis of alcohol use disorder, and 10–12% of adults over 50 years had an illicit substance use disorder (Blazer & Wu, 2011; Wu & Blazer, 2014). Additionally, a recent study (Breslow et al., 2017) using 1997–2014 National Health Interview Survey data found an upward trend in alcohol consumption among men (increase 0.7% per year) and women (increase 1.6% per year) age 60+ in the United States. A similar upward trend was found for binge drinking, although only among women (increase 3.7% per year). Despite the clinical relevance of older age groups in drug addiction research, much of the current body of literature examining impulsive behavior and drug use has overlooked middle-to-older adults.
The goal of the current review is to review the relationship between impulsive behavior and drug use across the lifespan and to recommend future research directions. First, we review how impulsive behavior has been defined and measured. Second, we provide an overview, although not exhaustive, of the literature on impulsive behavior and drug use across adolescence and young adulthood. Third, we discuss impulsive behavior across the lifespan and its implication in drug use. Fourth, we review the limited research in middle-to-older adulthood, discuss the relative gap in this literature, and consider the validity of the measures used to assess impulsive behavior in these age groups. Finally, we suggest recommendations for future research concerning the relationship between impulsive behavior and drug use specifically in middle-to-older adults.
2. Impulsive behavior: definition and measurement
Impulsive behavior has been operationalized in a number of ways, including lack of forethought before acting, premature acting, behavioral activation, sensation seeking, motor and cognitive impulsive behavior, and poor capacity to delay gratification (Evenden, 1999; Verdejo-García et al. 2008; Whiteside & Lynam, 2001). In humans, impulsive behavior has been measured via both impulsive behavior-related personality traits (i.e., stable tendencies toward behaviors predominantly measured via self-report questionnaires) and behavioral tasks (i.e., “snapshots” of behavior predominantly measured via behavioral tasks) (e.g., Cyders & Coskunpinar, 2011; Sharma et al., 2014; Verdejo-García et al. 2008), whereas animal models of impulsive behavior mostly only assess behavioral measures of such tendencies due to the inherent difficulty in modeling personality in animal models. Research has shown that in humans there is very little overlap between self-report and behavioral measures, suggesting that these two classes of impulsive behavior assess distinct tendencies (Cyders & Coskunpinar, 2011). Sharma et al. (2014) suggest that self-report measures of impulsive behavior-related personality traits reflect emotional/motivational mechanisms involved in impulsive behavior, whereas behavioral tasks assess cognitive mechanisms involved in such behaviors, further supporting the separation of these two classes. These mechanisms likely interact to affect behavior and the use of both in research is advised for a comprehensive understanding in the role of impulsive behavior in drug use (Sharma et al., 2014).
We describe three distinct, widely-used measures of impulsive behavior, one of which assesses impulsive behavior-related personality traits (the UPPS-P Impulsive Behavior Scale (UPPS-P); Lynam et al., 2007) and two of which assess impulsive behavior-related behaviors (delay discounting and prepotent response inhibition). First, we chose the UPPS-P because 1) the UPPS-P model was created by reviewing and integrating existing self-report measures of impulsive behavior-related traits and, as such, incorporates many of the existing scales and definitions of impulsive behavior-related traits (Whiteside & Lynam, 2001), making it a cumulative assessment of varied definitions and scales; and 2) the UPPS-P is a widely used measure that shows robust and reliable relationships with drug use (e.g., Coskunpinar et al., 2013; Gunn & Smith, 2010; VanderVeen et al., 2016). Second, we chose to include and review delay discounting and prepotent response inhibition because 1) although other types of measures (e.g., Iowa Gambling Task, Risky Gains procedure, and Cambridge Gamble Task) have been used in the literature under the broader term of impulsive behavior (Verdejo-Garcıa et al., 2008), they more accurately reflect risky decision making and are considered to be separate from impulsive behavior (Defoe et al., 2015; Verdejo-Garcıa et al., 2008), 2) delay discounting and prepotent response inhibition are very relevant for addiction (e.g., Khurana et al., 2013; Smith et al., 2014), and 3) both behavioral tasks are assessed in human and animal models, allowing for a translational approach to such research.
2.1. Personality measure of impulsive behavior
The UPPS-P Impulsive Behavior Scale (Lynam et al., 2007) is a multi-dimensional self-report assessment of impulsive behavior-related personality traits in humans. The UPPS-P measures five separate, though related traits: negative urgency (i.e., the tendency to act rashly in response to negative emotion), positive urgency (i.e., the tendency to act rashly in response to positive emotion), lack of premeditation (i.e., the tendency to act without thinking of the consequences), lack of perseverance (i.e., the inability to remain focused on and complete a task), and sensation seeking (i.e., the tendency to seek new and exciting activities). The UPPS-P model was created by subjecting items of existing self-report measures of impulsive behavior-related traits into exploratory factor analysis and, as such, integrates many of the existing scales and definitions of impulsive behavior-related traits (Whiteside & Lynam, 2001). The UPPS-P is a widely used measure that shows robust and reliable relationships with drug use across the lifespan (e.g., Coskunpinar et al., 2013; Gunn & Smith, 2010; VanderVeen et al., 2016), which makes it highly relevant for the purposes of this review. In contrast to human research, animal research in regards to impulsive personality is limited, largely due to issues with translating human personality traits into animal behaviors.
2.2. Behavioral measures of impulsive behavior
Delay discounting describes the tendency to choose an immediate smaller reward over a later larger reward (e.g., Kirby et al., 1999). Among individuals with a high delay discounting rate, the larger reward is discounted as less valuable than its actual worth. This is because of its remoteness in time relative to an immediacy of the smaller reward. Delay discounting in humans has been assessed using various techniques, including a self-report assessment with fixed reward amounts and times (i.e., Monetary Choice Questionnaire; Kirby et al. 1999), a delay discounting task that interactively adjusts reward amounts or times depending on participants’ response, usage of hypothetical rewards, or offering real rewards as a consequence of participants’ discounting behavior during the task (Odum, 2011). When given a choice between an immediate smaller reward and a later larger reward, drug users tend to prefer an immediate smaller reward more over a later larger reward than non-drug users (e.g., Kirby et al., 1999; Bickel et al., 1999; Petry, 2001; Kirby & Petry, 2004; MacKillop et al., 2011). Longitudinal studies have identified delay discounting as a significant predictor for the development of addictive behaviors (Anokhin et al., 2011; Fernie et al., 2013; Khurana et al., 2013) and treatment response (Amlung et al., 2017). This demonstrates the importance of delay discounting in the trajectory of addiction.
In animals, delay discounting is assessed in a similar behavioral manner as in humans: For instance, rodents will learn to associate one response, such as a lever press or a nose poke, with a larger, delayed reward, and one with a smaller, immediate reward. Delay discounting procedures have also been studied using other non-human animals such as pigeons, rhesus monkeys, chimpanzees and bonobos (for review see Vanderveldt, Oliveira, & Green, 2016), and dogs (e.g. Wright, Mills, & Pollux, 2012) with response types such as pecking response keys, pressing response levers, and depressing wooden panels, respectively. There is a large literature concerning differences in delay discounting across mice and rats due to genetic variations (for review of both human and animal literature see MacKillop, 2013), and drug exposure (for review see Setlow, Mendez, Mitchell, & Simon, 2009). As one example, high alcohol preferring and low alcohol preferring rats show different patterns of delay discounting, suggesting that alcohol preference in animals tracks with the tendency to discount larger, delayed rewards (e.g., Wilhelm & Mitchell, 2008).
Prepotent response inhibition is a behavioral measure of impulsive behavior that assesses the ability to suppress an automatic goal response to appropriately respond to a less automatic goal (Snyder et al., 2015). In humans, two main behavioral tasks assess prepotent response inhibition: The Go/NoGo task and the stop-signal task (e.g., Smith et al., 2014). In the Go/NoGo task, participants press a button using one hand in response to a Go stimulus and withhold a button press to a NoGo stimulus. In the stop-signal task, participants use both hands to respective Go stimuli associated with each hand and withhold a button press when a stop-signal is given concurrently with the Go stimuli. Deficits in prepotent response inhibition are associated with an inability to control drug use, resulting in the use of drugs that is excessive in amount and/or frequency (Smith et al., 2014). Past literature demonstrates reliable associations between impaired prepotent response inhibition and different forms of addictive behavior, indicating the relevance of this construct in the development and maintenance of SUDs (Bjork et al., 2004; Ersche et al., 2011; Pandey et al., 2012; Noël et al., 2007; Smith et al., 2014; Sokhadze et al., 2008).
Prepotent response inhibition tasks can be validly used in animal models. For example, Simon, Gregory, Wood, and Moghaddam (2013) describe a cued response inhibition task, which is similar to the stop-signal task described above. This task begins with illuminated nose poke holes in which the animals may respond for a reward, and an inhibitory tone. If the animals nose poke while the tone is playing, they are not rewarded and move to the inter-trial interval. After the tone stops, if the animals have been able to withhold responding, they may respond for a food pellet. Another example is the 5-Choice Serial Reaction Time Task (Robbins, 2002). In this task, animals are presented with five available nose poke holes. When the trial begins, the “correct” hole is illuminated and the animal must respond in this hole to receive the reward. If the animal responds before the trial begins, that is before the hole is illuminated, a time out penalty is triggered. The literature concerning animal models of prepotent response inhibition as a measure of impulsive behavior is large (for translational review see De Wit, 2009). Paradigms such as the 5-Choice Serial Reaction Time Task (e.g. Boutros, Der-Avakian, Markou, & Semenova, 2017; Dalley et al., 2007; Chudasama et al., 2003), stop-signal tasks (e.g. Eagle & Robbins, 2003; Bari, Eagle, Mar, Robinson, & Robbins, 2009, Eagle et al., 2011) and Go/NoGo tasks (e.g. Anker, Gliddon, & Carroll, 2008; Masaki et al., 2006; Tremblay & Schultz, 2000) have been used to examine prepotent response inhibition across substance types and substance use correlates. The research generally suggests a deficit in prepotent response inhibition among animals that prefer or have been exposed to drugs. As one example, mice bred to drink high levels of alcohol show impairments in the ability to inhibit a response (Wilhelm, Reeves, Phillips, & Mitchell, 2007).
3. Review of impulsive behavior and drug use in adolescence and young adulthood
3.1. Impulsive personality
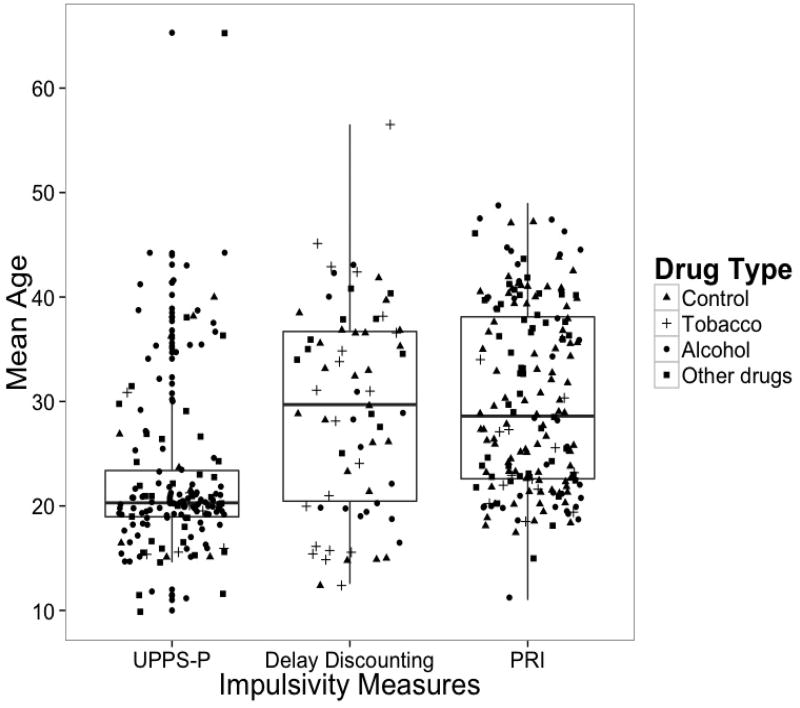
The majority of the research with the UPPS-P Impulsive Behavior Scale has primarily sampled adolescents and young adults (see Figure 1). Findings from this research suggests that impulsive personality is an important risk factor for a wide range of drug use behaviors, but that the strength and nature of the relationship varies across the trait assessed (Coskunpinar et al., 2013; Stautz & Cooper, 2013).
Figure 1.
Mean age distribution of studies examining impulsive behavior and drug use/addiction in humans by impulsive behavior measure type. Figure 1 aggregated 231 studies identified as studies of impulsive behavior and drug use/addiction in four meta-analyses (i.e., Coskupinar et al. (2013); Cross, Copping & Campbell (2011); MacKillop et al. (2011); Smith et al. (2014)). Research studies were included when information about mean age, type of impulsive behavior measures, and type of drugs was available. The figure included studies once when they were reported by more than one meta-analysis, and the mean age by group when the studies reported mean ages separately for each group. Some of the studies are represented by multiple dots because of multi-method approach (e.g., behavioral task and self-report) or examination of various drug use behaviors (e.g., cigarette and alcohol use). The drug legend comprises group comparison studies (e.g., alcohol users vs. controls) and studies with drug use as continuous variables. UPPS-P includes any impulsive behavior self-report measures that fall under the UPPS-P framework (Coskupinar et al., 2013). PRI = Prepotent Response Inhibition
Sensation seeking was associated with tobacco and drug use frequency in both adolescence (Crawford et al., 2003; Spillane et al., 2012; Stautz & Cooper, 2014) and adulthood (Cyders et al., 2009; Krank et al. 2011; Perkins et al., 2008), although some research has failed to find a significant relationship (Adams et al., 2012; Wardell et al., 2016; Albein-Urios et al., 2012; Dvorak & Day, 2014). Lack of premeditation was linked to tobacco use, problematic drug use, and SUDs especially in adulthood (Adams et al., 2012; Lee et al., 2015; Moreno-López et al., 2012; Torres et al., 2013; Wardell et al., 2016). The research on lack of perseverance has produced mixed results with most studies failing to demonstrate a strong relationship (Albein-Urios et al., 2012; Settles et al., 2012). Positive and negative urgency show the most robust relationship with the risk for SUDs and drug-related problems. In adolescents, positive and negative urgency were positively associated with problematic alcohol use (Settles et al., 2012; Stautz & Cooper, 2014; Tomko et al., 2016; Wardell et al., 2016; Wolitzky-Taylor et al., 2016) and marijuana use (Robinson, Ladd, & Anderson, 2014; Stautz & Cooper, 2014; Wardell et al., 2016). Further, positive urgency was related to drug use (Stautz & Cooper, 2013), and negative urgency was related to problematic cannabis use (Wardell et al., 2016; Wolitzky-Taylor et al., 2016). In young adults, positive urgency was related to tobacco dependence (Pang et al., 2014; Spillane et al., 2010), alcohol quantity (Cyders et al., 2009), illegal drug use (Zapolski et al., 2009), alcohol problems (Cyders et al., 2009; Shishido, Gaher, & Simons, 2013), and cocaine addiction (Albein-Urios et al., 2012; Moreno-López et al., 2012). Also, negative urgency was associated with tobacco use (Lee et al., 2015), craving (Billieux, Van der Linden, & Ceschi, 2007) and dependence (Pang et al., 2014; Spillane et al., 2010), problematic alcohol use (Kaiser et al., 2012; King et al., 2011; Martens et al., 2010; Shishido et al., 2013; Settles et al., 2012), alcohol dependence (Settles et al., 2012), and problematic marijuana use (Dvorak & Day, 2014).
Animal research in regards to impulsive personality is limited, largely due to issues with translating human personality traits into animal behaviors. However, there has been some examination of sensation seeking and negative urgency using rodent models. Piazza (1989) developed a model of sensation seeking by exposing rats to a novel environment (a circular open field) and separating them into two groups based on their locomotor activity, high responders who moved above the median of the group, and low responders who moved below. High responders also showed increased sensitivity to the reinforcing effects of psychostimulants as measured by acquisition of amphetamine self-administration, suggesting a relationship between the sensation seeking trait and the development of substance use behaviors. When compared to young adult rats, adolescents exhibited more sensation seeking behaviors (Philpot & Wecker, 2008). Gipson et al. (2012) developed a translational behavioral model of negative urgency to be used in both humans and rats. Rats with greater scores on this measure increased administration of amphetamine after induction of negative mood, supporting the robust relationship between negative urgency and drug use found in humans.
3.2. Delay discounting
Delay discounting is manifested in individuals with SUDs through their tendency to value immediate effects of drug (i.e., the “high”), while disregarding long-term benefits (e.g., health, family relationships) from not using it (MacKillop et al., 2011). Accumulating research substantiates delay discounting as a significant predictor across drug use outcomes. For example, a meta-analysis by MacKillop et al. (2011) suggested a medium effect between delay discounting and addictive behaviors (d = 0.58) in humans, with more robust effects in clinical (d = 0.61) than sub-clinical (d = 0.45) samples. It is worth mentioning that the vast majority of the studies included in this meta-analysis had samples with age ranges that rarely surpass the age of 40. The scarcity of research in older samples is further shown in Figure 1, which demonstrates that there is no research using samples with a mean age older than 55.
Support for the relationship between delay discounting and drug use in human adolescence is mixed. For example, Fernie et al. (2013) found that performance in the delay discounting task in early adolescence predicted six-month post-task alcohol involvement (i.e., frequency, quantity and problematic use). A study by Khurana et al. (2013) produced similar results for the frequency of alcohol use. Field et al. (2007) showed that heavy drinkers in late adolescence were more likely to discount hypothetical monetary and alcohol rewards than light drinkers in late adolescence. However, another longitudinal study failed to identify a significant association between early adolescence delay discounting rates and drug use problems in late adolescence (Isen et al., 2014). Regarding tobacco use, several studies have shown steeper discounting rates in adolescent cigarette smokers (Audrain-McGovern et al., 2004; Audrain-McGovern et al., 2009; Reynolds et al., 2007), although the effect seems to be smaller than adults (Reynolds et al., 2004). Additionally, research shows that delay discounting is predictive of progression to a more regular smoking pattern, although it does not appear to discriminate between smoking trajectories of different onset and magnitude (Audrain-McGovern et al., 2009).
Research on young-to-middle adult samples have revealed a more consistent pattern of relationship between delay discounting and drug use outcomes. Alcohol dependence has been related to higher delay discounting rates in young-to-middle adulthood (Petry, 2001; Vuchinich & Simpson, 1998); although Kirby & Petry (2004) revealed no significant difference in delay discounting between alcohol users and controls. A significant association of delay discounting with different tobacco outcomes has been substantiated in the literature, including tobacco use frequency and quantity (Amlung et al., 2017; Ohmura, Takahashi, & Kitamura, 2005; with some exceptions, e.g., Sweitzer et al., 2008), and nicotine dependence (Amlung & MacKillop, 2014; Mackillop & Tidey, 2011; Sweitzer et al., 2008). Opioid use was also related to delay discounting, with heroin users demonstrating significantly higher delay discounting rates than non-users (Kirby et al., 1999; Madden et al., 1997; MacKillop et al., 2011). The literature shows comparable preference for immediate rewards between opioid and cocaine dependent individuals and this effect seem to be higher than that in alcohol users (Karakula et al., 2016; Kirby & Petry, 2004; MacKillop et al., 2011). Delay discounting was also related to greater addiction severity (Amlung & MacKillop, 2014; Christiansen et al., 2012), although not consistently (Heyman & Gibb, 2006; Stojek et al., 2014). Finally, a recent meta-analysis examining the relationship between delay discounting and addiction severity and quantity-frequency of drug use, found that steeper discounting rates were more robustly associated with the severity of SUDs than quantity-frequency of use (Amlung et al., 2017).
Delay discounting is one of the more popular measures of impulsive behavior found in the animal literature, and this research largely supports the idea of increased impulsive behavior during adolescence. Adolescent rats of both sexes tend to exhibit more impulsive choices in a delay discounting task when compared to adults (e.g., Doremus-Fitzwater, Barreto, & Spear, 2012; Mejia-Toiber et al., 2014). However, support for the relationship between this increased impulsive behavior and increased drug use is less substantial. In an experiment using mice selectively bred for alcohol consumption, adult mice had a higher preference for alcohol and drank more than adolescents during a 12-day drinking period, and alcohol exposure during adolescence had no effect on impulsive choice (O’Tousa, Matson, & Grahame, 2013). Similar to the human research, experiments assessing delay discounting behavior in older animals is scarce. However, Simon et al. (2010) did examine delay discounting behavior in aged (24-month-old) and young adult (6-month-old) rats. Consistent with their hypothesis, aged rats preferred the larger delayed rewards compared to the younger rats, suggesting that discounting of delayed rewards is attenuated by age.
3.3. Prepotent response inhibition
Measures of prepotent response inhibition (e.g., the Go/NoGo and Stop-Signal tasks) are significantly associated with a wide range of drug use behaviors in humans and are implicated in the development and maintenance of SUDs (Fillmore, 2003; Jentsch & Pennington, 2014; Perry & Carroll, 2008; Smith et al., 2014). Deficits in response inhibition have been shown in individuals with alcohol (Sjoerds et al. 2014) and cocaine dependence (Ersche et al. 2011; Fernández-Serrano et al. 2012; Kaufman et al. 2003). However, as it is depicted in Figure 1, previous research has rarely investigated older adult samples. In adolescence, prepotent response inhibition seems to have a marginal, although significant, effect on drug use. A longitudinal study by Nigg et al. (2006) showed that deficits in response inhibition significantly explained 1% of variance in alcohol use-related problems and illicit drug use in adolescents. Further, no significant relationship was found with illicit drug-related problems in adolescents. Fernie et al. (2013) showed that poor performance in the Stop-Signal task predicted later alcohol involvement, while alcohol involvement did not predict later performance in this task. Mixed results have also been produced with tobacco outcomes, with most studies failing to identify a significant effect (Galvan et al., 2011; Groenman et al., 2015; Harakeh et al., 2012; Smith et al., 2014). Further, most studies with adolescent samples included in the meta-analysis by Smith et al. (2014) showed small and nonsignificant associations between prepotent response inhibition and drug use.
Young-to-middle adult samples show a more consistent relationship between prepotent response inhibition tasks and drug use. In young adult samples, significant associations were found between deficits in prepotent response inhibition and MDMA use (Hoshi et al., 2007), heavy drinking (Murphy & Garavan, 2011; Petit et al., 2012; Rubio et al., 2008; Smith & Mattick, 2013), methamphetamine (Monterosso et al., 2005; Tabibnia et al., 2011) and tobacco use (Billieux et al., 2010; Evans et al., 2009; Luijten et al., 2013). Studies in young-to-middle adult samples have also revealed significant relationships between performance in inhibition tasks and alcohol dependence (Bjork et al., 2004; Pandey et al., 2012; Noël et al., 2007), although several studies failed to identify a significant effect (Kamarajan et al., 2005; Karch et al., 2008). Finally, poor inhibition was related to cocaine use and dependence (Colzato et al., 2007; Ersche et al., 2011; Sokhadze et al., 2008). Research in opioid use is more limited and has produced mixed results with a few studies showing an association with prepotent response inhibition (Constantinou et al., 2010; Forman et al., 2004; Liao et al., 2014), and this relationship was insignificant in a recent meta-analysis (Smith et al., 2014). Similar findings exist for cannabis use, although a larger body of research has been conducted, with a few studies demonstrating significant relationships (Grant et al., 2012; Hester et al., 2009; Jutras-Aswad et al., 2012; Moreno et al., 2012; Tamm et al., 2013), and again, this relationship was insignificant in the same meta-analysis (Smith at al., 2014). However, psychostimulants showed a significant association to deficits in prepotent response inhibition in the meta-analysis (Smith et al., 2014). This could be attributed to the chronic damage from these substances to dopaminergic and serotonergic prefrontal-subcortical networks related to motor control (Smith et al., 2014; Volkow et al., 2001) that, as a result, could influence performance on prepotent response inhibition tasks.
In animal research, adolescent rats have shown deficits in cognitive flexibility, but not necessarily impulsivity in stop tasks (Simon, Gregory, & Moghaddam, 2013). Premature responses in the 5-Choice Serial Reaction Time Task were greater in adolescent rats than adults (Burton & Fletcher, 2012). Nicotine increased premature 5-Choice Serial Reaction Time Task responding in rats exposed to nicotine in adolescence, but had no effect on those exposed in adulthood (Counotte et al., 2009).
4. Impulsive behavior across the lifespan: Implications for drug use
4.1. Neural mechanisms of impulsive behavior changes across age
Brain development across the lifespan is thought to underlie changes in decision making processes, which in turn influence the susceptibility toward drug use. Adolescence is a developmental period marked by increased impulsive behavior due to imbalanced neurodevelopmental maturations (Crone & Dahl, 2012). One theory of impulsive behavior in adolescence has suggested a gap in maturation of two brain systems (Ernst & Fudge, 2009; Somerville et al., 2010; Steinberg, 2008), with the affective processing system, including areas of the mesolimbic dopamine circuit, maturing earlier with the onset of puberty, and the cognitive control system, including areas such as the lateral prefrontal cortex and parts of the anterior cingulate cortex, maturing later in young adulthood (Steinberg, 2008). The different impulsive behavior facets are linked to these two neural systems: Delay discounting tasks focus on decisions involving affective and motivational processing that recruit regions of the ‘reward’ pathway (i.e., the mesocorticolimbic system), prepotent response inhibition tasks reflect decisions related to cognitive processing that recruit frontal brain regions including the anterior cingulate cortex and lateral prefrontal cortex, and impulsive behavior traits are underpinned by both of these systems in different levels depending on the trait involved (Blakemore & Robbins, 2012; Karyadi, Coskunpinar, & Cyders, 2012; Samanez-Larkin & Knutson, 2015). These neurodevelopmental changes are theorized to make adolescents more susceptible to rewards (e.g., drug use and peer reinforcement) and less capable to restrain reward seeking behaviors due to immature inhibitory control (Ernst & Fudge, 2009; Somerville et al., 2010; Stautz & Cooper, 2013; Steinberg, 2008).
Another theory of impulsive behavior in adolescence emphasizes the contribution of social and affective processing on a flexible, but not under-developed, cognitive control system (Crone & Dahl, 2012). This flexibility of the prefrontal cortex is thought to result in greater impulsive behavior when social (i.e., peer acceptance) and motivational goals are highly salient. Therefore, with the onset of puberty, cognitive flexibility in combination with a heightened sensitivity of a changing ‘reward system’ related to enhanced responsiveness to incentives likely contributes to the vulnerability of adolescents toward impulsive choice involving drug use (Crone & Dahl, 2012; Galvan, 2010).
Such neuronal vulnerabilities highlight the reasons why much of this research has focused on impulsive behavior and drug use among adolescents and young adults. These groups are considered to be at-risk populations for SUDs and drug use-related problems, which warrants the development of prevention and early intervention strategies to avoid later development of SUDs. Although not as well recognized, the mature adult brain also gradually undergoes structural and functional changes (Samanez-Larkin & Knutson, 2015; Samanez-Larkin et al., 2013). This has been related to changes in decision making (Samanez-Larkin & Knutson, 2015), which may subsequently influence drug use. Healthy aging is linked to a gradual structural decline of the prefrontal cortex (Grady, 2012), although large individual differences exist in the rate of this decline. Additionally, accumulating evidence suggests that changes in affective and motivational brain circuits come with aging and that these changes impact behavior (Samanez-Larkin & Knutson, 2015). For example, glutamatergic projections from prefrontal cortex to striatum enable value integration, and impairment in these projections due to aging may result in choice biases (i.e., suboptimal attributions of value) in tasks requiring more attention and memory (Samanez-Larkin & Knutson, 2015), which might explain why older adults tend to remember positive information (e.g., positive experiences of drug use) more readily than negative information (e.g., negative consequences of drug use; Lim & Yu, 2015), further reinforcing drug use.
However, in older adult substance users, it is hard to distinguish whether aging-related deficits related to impulsive behavior pre-exist or follow the chronic exposure to drugs. For example, Ersche et al. (2013) suggested that cocaine abuse may accelerate cognitive decline accompanying normal aging which was evident by a significant difference in gray matter volume loss between cocaine-dependent and healthy individuals. The psychopharmacology of certain substances, especially that of psychostimulants, has the potential to cause prefrontal cortical network alterations that increase impulsive behavior (Badiani et al., 2011; Ersche et al., 2013; Volkow et al., 2001). Such pharmacological effects would confound impulsive behavior research in older adult drug users as parsing out the causality is challenged (e.g., higher impulsive behavior in older adult drug users as a cause, as often assumed, or a result of chronic use). However, although substances do contribute to the alterations in brain function and structure, longitudinal studies following individuals before the onset of drug use provide compelling evidence that elevated impulsive behavior marks the vulnerability for future drug-related problems (Nigg et al., 2006; King & Chassin, 2004; Kirisci, Vanyukov, & Tarter, 2005; Verdejo-Garcıa, et al., 2008), which may later aggravate impulsive behavior-related cognitive deficits.
4.2. Impulsive personality across age
Impulsive personality traits in humans change with age. Adolescence is strongly associated with a heightened instability in these traits, potentially influenced by neurodevelopmental changes in this developmental stage (Crone & Dahl, 2012; Ernst & Fudge, 2009; Steinberg, 2008; Steinberg et al., 2008). Sensation seeking increases in early adolescence, with a peak in middle adolescence, and decreases closer to young adulthood (Littlefield et al., 2016; Romer & Hennessy, 2007; Steinberg et al., 2008); however, some studies have found an increase in sensation seeking moving toward young adulthood (Collado et al., 2014; Harden & Tucker-Drob, 2011; Pedersen et al., 2012). Negative urgency and positive urgency increase during early adolescence (i.e., 11–13 years) and decline across later adolescence (Littlefield et al., 2016). Lack of premeditation and perseverance show a linear decline from the age of 10 (Steinberg et al., 2008). These findings align with results from neuroimaging studies that support a process of brain maturation with age (Blakemore & Robbins, 2012); however, no study to our knowledge has examined these changes across adulthood (younger vs. older), despite its potential clinical significance for older adults with SUDs. To our knowledge, no research has been conducted examining age-related effects on these tendencies in animals.
4.3. Delay discounting across age
Research generally supports that the degree to which humans devalue delayed rewards decreases from adolescence to middle adulthood, while mixed results exist for older adulthood (Drobetz et al., 2012; Read & Read, 2004; Whelan & McHugh, 2009). Specifically, discounting of delayed rewards is at its highest levels during adolescence and levels off while moving toward adulthood (mean ages of 12.1- vs. 20.3 years; Green et al., 1994; age range of 10–30 years; Steinberg et al., 2009; age range of 9–23 years; Olson et al., 2007; age range of 8–15 years; Prencipe et al., 2011). A particularly sharp decline in discounting rate was observed in the ages of 15–16 (age range of 12–27 years; de Water et al., 2014; Steinberg et al. 2009). Findings are less consistent regarding delay discounting in older adulthood. For example, some studies found higher delay discounting rate among young adults than older adults (mean ages of 20.9 vs. 69.7 olds; Eppinger et al., 2012; mean ages of 20.3 vs. 67.9 olds; Green et al., 1994). One study reported an increase in delayed discounting rates from middle-to-older adulthood (mean ages of 44.0- vs. 75.0 olds; Read & Read, 2004), whereas other studies demonstrated stable rates of discounting in both age groups (mean ages of 33.3 vs 70.7 olds; Green et al., 1996; mean ages of 46.0- vs. 73.0 olds; Whelan & McHugh, 2009). Another study compared discounting rates of young and older adults in different types of rewards (hypothetical monetary and real liquid rewards) and found that young adults discounted monetary rewards more steeply than older adults, while older adults discounted liquid rewards more steeply than young adults (Jimura et al., 2011). This means that the type of the reward may affect the motivation of different age groups to discount larger rewards.
One study directly compared delay discounting between adolescents (mean age = 15.8, SD = 1.3) and young-to-middle adults (mean age = 34, SD = 10.2) in drug use treatment (Lee, Stanger, & Budney, 2015). In this study, discounting rates of the two age groups in two types of rewards were compared (i.e., commodity (money and cannabis) and the magnitude of the monetary reward). The study suggested that adolescents were less sensitive to changes in the magnitude of the rewards, were more likely to discount money than cannabis, and showed less improvement in discounting with treatment compare to adults (Lee et al., 2015). These results are consistent with adolescence being characterized by heightened impulsive behavior, which reduces their ability to discount later larger rewards related to drug use.
In general, the rodent literature seems to show that adolescents discount more steeply than adults, in agreement with the idea in humans that delay discounting declines with age. For example, adolescent and adult Sprague-Dawley rats were trained on a delay discounting task, and adolescents of both sexes exhibited higher discounting rate when compared to adults (Doremus-Fitzwater, Barreto, & Spear, 2012). These age effects might vary across sex. Lukkes et al. (2016) examined locomotor activity, preference for novelty, and delay discounting in juvenile, adolescent, and adult Sprague-Dawley rats of both sexes. Early adolescent males showed higher discounting than their late adolescent and adult counterparts, but early adolescent females showed lower discounting than the older females. In the impulsivity literature in general, there is a lack of investigation using aged animals due to various issues that are discussed in more detail below. However, Simon et al. (2010) did examine delay discounting behavior in aged (24-month-old) and young adult (6-month-old) rats. Consistent with their hypothesis, aged rats preferred the larger delayed rewards compared to the younger rats, suggesting that discounting of delayed rewards is attenuated by age. The authors interpreted these results to mean that regardless of life experience (all rats were treated similarly and raised in the same environments) there are underlying neurobiological factors that may contribute to age-related changes in delay discounting.
4.4. Prepotent response inhibition across age
Research assessing impulsive behavior in humans through prepotent response inhibition tasks shows that performance in these tasks steadily improves (reduction in reaction times and commission errors rates) from childhood to young adulthood (Jaeger, 2013; López-Caneda et al., 2014) and starts to decrease in later adulthood (Votruba & Langenecker, 2013). Inhibitory control seems to start maturing at around age 14 (Fischer et al., 1997; Luna et al., 2004; Munoz et al., 1998). Although the ability to suppress an automatic response begins to develop early in life, efficiency in the process continues to refine through the end of adolescence (Luna et al., 2004). The maturational changes on the adolescent brain, especially the prefrontal cortex, which is linked to prepotent response inhibition, may explain the maintenance of drug-seeking behaviors (López-Caneda et al., 2014). After a period of stabilization in young-to-middle adulthood, performance in prepotent response inhibition tasks starts decreasing in older adulthood. Normal aging has been associated with declined performance in prepotent response inhibition tasks (Sebastian et al., 2013; Van der Lubbe & Verleger, 2002). Additionally, the performance of older adults on these tasks is more variable and more extreme than that of adolescents and young adults (Sebastian et al., 2013; Van der Lubbe & Verleger, 2002). One source of this variability may be the high levels of intra-individual differences in normal aging. There is no uniform trend of decline accompanied by neuronal changes in this age; therefore, there is likely considerable variability in impulsive behavior in older adulthood.
Prepotent response inhibition tasks can be learned and performed in animal studies; however, there is a lack of literature on performance as related to age. For example, Simon et al. (2013) found that although both age groups showed similar response inhibition behaviors, adolescents were slower to respond after the inhibitory cue terminated, suggesting possible deficits in prepotent response inhibition during adolescence in rodents. Counotte et al. (2009) conducted a study in which rats were exposed to nicotine in either adolescence or adulthood; nicotine increased premature responding in only those who were exposed in adolescence, not adulthood. These results indicate there may be a period of vulnerability to nicotine and a relation between nicotine use at a young age and later problems with impulsive behaviors.
5. Little focus after middle adulthood: “Maturing-out” Phenomenon
There are limited studies examining impulsive behavior in middle-to-older adulthood across human (see Figure 1) and animal research. Studies in humans have predominantly focused on adolescence and young adults due to these age groups being in a critical maturational period for the development of drug use and related problems. Although it is certainly a worthwhile goal to identify those at risk early in the developmental trajectory to prevent the onset of drug use, this has led to an under-focus on the middle-to-older adulthood, where the onset of clinical levels of drug use often occurs. Middle-to-older adults with prolonged drug use likely exhibit a greater severity of drug use and related problems compared to younger counterparts, making this group of prime clinical interest. As demonstrated in Figure 1, research across all three domains is limited in age range (mostly not exceeding ages in the mid-40s), although the omission is particularly notable with research using the UPPS-P, where although the age range is similar, the mean age of research participants is in the early 20s (mean age of research participants in research using delay discounting and prepotent response inhibition falls in the mid-30s).
The limited focus on middle-to-older adulthood in humans can be explained by the “maturing-out” phenomenon. Adolescents and young adults are considered to be at higher risk for impulsive behavior related to the engagement in drug use. With age, this increased risk for drug use gradually reduces with the progressive change of certain personality factors and the adoption of adult roles (Littlefield & Sher, 2016; Vergés et al., 2012). Personality traits become more “adaptive” in upholding societal demands associated with adulthood (Caspi et al., 2005). Specifically, shifts to greater agreeableness, lower neuroticism, lower impulsive behavior and higher self-control is seen with the transition to adulthood (Caspi et al., 2005; Littlefield et al., 2009). This shift converges with developmental changes in problematic drug use, where an abrupt decline in prevalence of drug use is observed in the third decade of life, referred as “maturing out” (Bachman et al., 2002; Fillmore et al., 1988; Littlefield et al., 2009; Littlefield & Sher, 2016; Winick, 1962). The relationship between developmental changes in personality and drug use has been empirically supported. For example, Littlefield et al. (2009) reported that a decrease in problematic alcohol involvement between the ages of 18 to 35 was significantly correlated with the sharp decrease in neuroticism and impulsive behavior, reflecting a mechanism in the maturing out phenomenon.
However, research has also indicated individual differences in these trends, with some individuals not experiencing such changes in personality or the decline in drug use (Fillmore, 1988; Littlefield, Sher, & Wood, 2009; Littlefield & Sher, 2016; Vergés et al., 2012). Research in representative samples suggests that typical drug users quit after six to eight years from the onset of drug use (e.g., NESARC project; Lopez-Quintero et al. 2011); however, a portion of these drug users continue the pattern of problematic drug use for a significantly longer time, developing SUDs (Heyman, 2013). Further, persistent drug use patterns increase as a function of life transitions in different developmental stages (Vergés et al., 2012). Different challenges in middle and later stages of life can produce new risk factors for problematic drug use than those found in adolescence and young adulthood. Therefore, studying impulsive behavior across all developmental stages is essential to understand patterns of stability and change in drug use-related conditions (Vergés et al., 2012). Growing literature recognizes impulsive behavior as a prime target for drug use intervention; however, many studies, especially those assessing impulsive behavior, have primarily focused their investigation on adolescents and young adults. Applying the findings from adolescents and young adults to middle-to-older adults can be an over-generalization that can incorrectly characterize risk factors and prime points of intervention among middle-to-older adulthood.
Although middle-to-older adults are a clinically relevant group to study, only a few studies on impulsive behavior and drug use are available that include adults in these age groups. Regarding impulsive personality, only one study has been conducted with the UPPS-P Impulsive Behavior scale with a mean age higher than 40 years (mean age = 40.24, SD = 11.6; Whiteside et al., 2005). This study found a significant effect of negative urgency and sensation seeking on alcohol abuse, while no effect was found for lack of premeditation and lack of perseverance. Similarly, impaired performance in delay discounting tasks has been linked to SUDs (Moody et al., 2016a), heroin and cocaine abuse (Kirby & Petry, 2004), and polysubstance use (Moody et al., 2016b) in middle adult samples with a mean age more than 40 years. However, most of these studies did not include participants older than ages of 55–60 years. More studies have been conducted in participants with a mean age greater than 40 years in the area of prepotent response inhibition, although there is scarcity in research in samples older than age of 50. In these studies, a significant association has been shown between performance in the prepotent response inhibition tasks and cocaine (Parvaz et al., 2012; Hester et al., 2007) and alcohol dependence (Sjoerds et al., 2014; Thoma et al., 2007; Lawrence et al., 2009; Goudriaan et al., 2006), and no significant effects have been found for heroin use (Liang et al., 2014; Yang et al., 2009). Research in older samples exists to a very limited extent, warranting future research about how impulsive behavior changes with normal aging and its effects on drug use.
6. Applicability of measures of impulsive behavior among older adults
Despite large individual variability, normal aging is related to gradual declines in sensorimotor processing and brain resources that could influence impulsive behavior processes and drug use (Samanez-Larkin & Knutson, 2015; Samanez-Larkin et al., 2013). However, it is important to bear in mind that these changes might influence not only the actual impulsive behavior-related processes of older adults, but also their capability to validly complete measures assessing them. Whether these measures can be validly applied to older age groups in both humans and animals remains of question.
The UPPS-P Impulsive Behavior Scale was developed using young adults and validated among children and adolescent populations (e.g., Tomko et al., 2016), but little is known about its validity for older adults. The items measuring impulsive personality traits may not have the same meaning among older adults compared to adolescents or younger adults. The UPPS-P items are generally neutral statements (e.g., “I am a cautious person,” “I often make matters worse because I act without thinking when I am upset,” “It is hard for me to resist acting on my feelings.”) that should carry same meanings across different age groups; however, some statements could have age-sensitive interpretations. For example, items such as “I would enjoy fast driving,” “I would like to go scuba diving,” or “I would enjoy parachute jumping” may not carry the same meanings or social desirability across different age groups. Younger age groups are active participants in such activities and engagement in these activities is more likely, normative, and socially acceptable/desirable. Therefore, these groups’ responses to how much they would “enjoy” these activities could more closely reflect their impulsive personality traits. However, older age groups’ responses on these items are likely confounded by health or physical restrictions that come with aging. Therefore, their response to how much they would “enjoy” these activities can have different meaning, reflecting more their wish to engage in (but not actual engagement in) such activities. Alternatively, they might respond that they would not enjoy such activities because of health outcomes likely with such engagement (and not because they would not ideally like to engage in such activities). This warrants further validation of the UPPS-P Impulsive Behavior Scale in older adults, as the questions might not validly measure impulsive personality in middle-to-older adulthood as they do in young adulthood and adolescence.
Delay discounting has the most evidence across the lifespan in humans, and, as discussed in the previous section, research generally supports a decrease in discounting rate from adolescence to young adulthood. However, inconsistent results exist for the discounting rate in middle to older adulthood. One potential explanation for this inconsistency in older adults might be associated with the tangibility of rewards (de Water et al., 2014). Delay discounting tasks using hypothetical rewards require more cognitive control abilities, whereas others using real monetary rewards activates more affective or motivational processes. Further, research showed that accumulating life experience and the shift in motivational goals accompanying normal aging change participants’ subjective value of rewards and their motivation to discount them (Samanez-Larkin et al., 2013). Therefore, the choices in time frame of discounting might have very different meanings across the lifespan.
Little is known about the validity of prepotent response inhibition tasks among older adult populations, questioning its generalizability. The prepotent response inhibition tasks measure processes, such as motor and processing speed, in addition to response inhibition, known as the task impurity problem (Argyriou et al., 2017; Miyake & Friedman, 2012). Therefore, it is not clear whether slower reaction times in older adults reflect reduced prepotent response inhibition or aging-related slower motoric dexterity or processing speed (Charlton et al., 2008; Sebastian et al., 2013). Another important consideration is the familiarity of older adults with computer technology where computer-based tasks are presented to the participants. Although these tasks are relatively simple, the lack of familiarity with computers might affect older adult’s performance (Iverson et al., 2009). Finally, phenomena such as stereotype threat (i.e., the fear that one’s performance will conform with a negative stereotype) are found to impair the performance of older adults in behavioral tasks (Mazerolle et al., 2012).
Similar to work in human research, there is little work examining age-related changes in impulsive behavior and drug use in the animal literature. This is partially due to the shorter lifespans of the animals used, and thus shorter periods of adolescence and adulthood. Spear (2000) defines the adolescent period in rats generally from postnatal day 28–42, based on the animals’ physical growth, neurological changes like synapse pruning, and behavioral changes, such as increased peer interaction and exploratory behavior. Based on this definition, all adolescent training and testing must occur within only fifteen days. However, unlike in human research, animal tasks such as delay discounting or reward omission tasks require extensive training. For example, in the delay discounting study conducted by Mejia-Toiber et al. (2014), rats were exposed to almost 70 days of training, followed by about 20 days of actual testing. In order to examine differences in this behavior between adolescents and adults, both training and testing time would have to be drastically reduced, which may impact the animals’ ability to learn the task and, thus, perform adequately.
There is a similar gap in the literature in regards to aged animals. Whereas in adolescents there is a short window of time before animals move on from this developmental period, when attempting to examine old age in animals, older animals have a greater risk of attrition due to health-related complications and death. Additionally, there are greater costs related to animal care and upkeep until they reach the desired age.
7. Recommendations for future research
In sum, although the majority of impulsive behavior research has focused on adolescence and young adulthood due to the development of both impulsive behavior and drug use in this period, much research has failed to examine this relationship in middle-to-older adulthood, despite the clinical relevance of the development of SUDs in this group. We recommend that research in this area begin to better examine and characterize how impulsive behavior might be implicated in these older adult groups, as it is important for determining 1) those who are likely to not “mature out” of drug use, 2) those who will go on to develop full blown SUDs, 3) those who might not be able to abstain from or reduce their drug use, and 4) how best to intervene in older adults (as risk factors found to apply for adolescence and early adulthood may or may not generalize to older adults). These limitations and recommendations apply to both human and animal work.
We propose that one significant concern is the lack of validity data concerning how impulsive behavior is assessed in older adults. Many of the self-report items might have very different meaning among younger and older individuals, and the relationship of these traits with substance use might vary as a function of age. Additionally, many behavioral tasks are confounded with other cognitive processes or physical abilities, which are known to decline with age. Therefore, two, not mutually exclusive, prime first steps in this future line of research are to 1) examine the validity and reliability of existing measures of impulsive behavior in older adults and 2) develop new measures that are specifically designed to measure impulsive behavior in this age group. The lack of data concerning the validity and reliability of these measures in older adulthood limits our ability to fully understand how impulsive behavior might contribute or perpetuate drug use in this group. This inability to accurately capture this tendency makes it difficult to determine the most appropriate interventions in older adults. Valid measurement of impulsive behavior in middle-to-older adulthood would enable the identification of risk and protective factors in these clinically relevant age groups. This would contribute to the development of more targeted interventions which take advantage of the strengths and minimize the weaknesses accompanying normal aging that are likely to affect drug use.
Supplementary Material
Highlights.
The relationship between impulsive behavior and drug use across the lifespan is reviewed.
Age differences in impulsive behavior across the lifespan exist and influence drug use.
Impulsive behavior and drug use in middle-to-older adulthood is often overlooked.
Recommendations for future research are discussed.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Adams ZW, Kaiser AJ, Lynam DR, Charnigo RJ, Milich R. Drinking motives as mediators of the impulsive behavior-substance use relation: Pathways for negative urgency, lack of premeditation, and sensation seeking. Addictive Behaviors. 2012;37:848–855. doi: 10.1016/j.addbeh.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albein-Urios N, Martinez-González JM, Lozano Ó, Clark L, Verdejo-García A. Comparison of impulsive behavior and working memory in cocaine addiction and pathological gambling: implications for cocaine-induced neurotoxicity. Drug and Alcohol Dependence. 2012;126:1–6. doi: 10.1016/j.drugalcdep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Amlung M, MacKillop J. Understanding the effects of stress and alcohol cues on motivation for alcohol via behavioral economics. Alcoholism: Clinical and Experimental Research. 2014;38:1780–1789. doi: 10.1111/acer.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. Steep Delay Discounting and Addictive Behavior: A Meta-Analysis of Continuous Associations. Addiction. 2017;112:51–62. doi: 10.1111/add.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behavioural pharmacology. 2008;19(5–6):615–629. doi: 10.1097/FBP.0b013e32830dc0ae. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behavior Genetics. 2011;41:175–183. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou E, Davison CB, Lee TTC. Response Inhibition and Internet Gaming Disorder: A Meta-analysis. Addictive Behaviors. 2017;71:54–60. doi: 10.1016/j.addbeh.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, Wileyto EP. Applying a behavioral economic framework to understanding adolescent smoking. Psychology of Addictive Behaviors. 2004;18:64–73. doi: 10.1037/0893-164X.18.1.64. [DOI] [PubMed] [Google Scholar]
- Bachman JG, O’Malley PM, Schulenberg JE, Johnston LD, Bryant AL, Merline AC. Why substance use declines in young adulthood: Changes in social activities, roles, and beliefs. Mahwah, NJ: Erlbaum; 2002. [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nature Reviews Neuroscience. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology. 2009;205(2):273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsive behavior and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacoloyg. 1999;146(4):447–454. doi: 10.1007/PL00005490. [DOI] [PubMed] [Google Scholar]
- Billieux J, Gay P, Rochat L, Khazaal Y, Zullino D, Van der Linden M. Lack of inhibitory control predicts cigarette smoking dependence: evidence from a non-deprived sample of light to moderate smokers. Drug and Alcohol Dependence. 2010;112:164–167. doi: 10.1016/j.drugalcdep.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Billieux J, Van der Linden M, Ceschi G. Which dimensions of impulsivity are related to cigarette craving? Addictive Behaviors. 2007;32:1189–1199. doi: 10.1002/acp.1289. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Robbins TW. Decision-making in the adolescent brain. Nature. Neuroscience. 2012;15(9):1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Wu LT. The epidemiology of substance use and disorders among middle aged and elderly community adults: national survey on drug use and health. The. American Journal of Geriatric Psychiatry. 2009;17:237–245. doi: 10.1097/JGP.0b013e318190b8ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer DG, Wu LT. The epidemiology of alcohol use disorders and subthreshold dependence in a middle-aged and elderly community sample. The American Journal of Geriatric Psychiatry. 2011;19:685–694. doi: 10.1097/JGP.0b013e3182006a96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsive behavior in abstinent alcohol-dependent patients: relation to control subjects and type 1–/type 2–like traits. Alcohol. 2004;34(2):133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Boutros N, Der-Avakian A, Markou A, Semenova S. Effects of early life stress and adolescent ethanol exposure on adult cognitive performance in the 5-choice serial reaction time task in Wistar male rats. Psychopharmacology. 2017:1–8. doi: 10.1007/s00213-017-4555-3. [DOI] [PubMed] [Google Scholar]
- Breslow RA, Castle I-JP, Chen CM, Graubard BI. Trends in Alcohol Consumption Among Older Americans: National Health Interview Surveys, 1997 to 2014. Alcoholism: Clinical and Experimental Research. 2017;41:976–986. doi: 10.1111/acer.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behavioural Brain Research. 2012;230(1):21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: stability and change. Annual Review of Psychology. 2005;56:1–659. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiology of Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Christiansen P, Cole JC, Goudie AJ, Field M. Components of behavioural impulsive behavior and automatic cue approach predict unique variance in hazardous drinking. Psychopharmacology. 2012;219:501–510. doi: 10.1007/s00213-011-2396-z. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behavioural brain research. 2003;146(1):105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Collado A, Felton JW, Macpherson L, Lejuez CW. Longitudinal trajectories of sensation seeking, risk taking propensity, and impulsive behavior across early to middle adolescence. Addictive Behaviors. 2014;39:1580–1588. doi: 10.1016/j.addbeh.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WP, Hommel B. Impaired inhibitory control in recreational cocaine users. PLoS One. 2007;2(11):e1143. doi: 10.1371/journal.pone.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskunpinar A, Dir AL, Cyders MA. Multidimensionality in impulsive behavior and alcohol Use: a meta-analysis using the UPPS model of impulsive behavior. Alcoholism: Clinical and Experimental Research. 2013;37(9):1441–1450. doi: 10.1111/acer.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou N, Morgan CJ, Battistella S, O’Ryan D, Davis P, Curran HV. Attentional bias, inhibitory control and acute stress in current and former opiate addicts. Drug and alcohol dependence. 2010;109:220–225. doi: 10.1016/j.drugalcdep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Crawford AM, Pentz MA, Chou CP, Li C, Dwyer JH. Parallel developmental trajectories of sensation seeking and regular substance use in adolescents. Psychology of Addictive Behaviors. 2003;17:179–192. doi: 10.1037/0893-164X.17.3.179. [DOI] [PubMed] [Google Scholar]
- Cross CP, Copping LT, Campbell A. Sex differences in impulsive behavior: A meta-analysis. Psychological Bulletin. 2011;137(1):97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsive behavior? Clinical Psychology Review. 2011;31:965–982. doi: 10.1016/j.cpr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Flory K, Rainer S, Smith GT. The role of personality dispositions to risky behavior in predicting first-year college drinking. Addiction. 2009;104:193–202. doi: 10.1111/j.1360-0443.2008.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. Integration of impulsive behavior and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychological Assessment. 2007;19:107–118. doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Abakumova I. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoe IN, Dubas JS, Figner B, van Aken MA. A meta-analysis on age differences in risky decision making: Adolescents versus children and adults. Psychological Bulletin. 2015;141:48–84. doi: 10.1037/a0038088. [DOI] [PubMed] [Google Scholar]
- de Water E, Cillessen AH, Scheres A. Distinct Age-Related Differences in Temporal Discounting and Risk Taking in Adolescents and Young Adults. Child Development. 2014;85:1881–1897. doi: 10.1111/cdev.12245. [DOI] [PubMed] [Google Scholar]
- De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction biology. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Barreto M, Spear LP. Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behavioral Neuroscience. 2012;126:735–741. doi: 10.1037/a0029697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobetz R, Maercker A, Forstmeier S. Delay of gradification in old age: assessment, age-related effects, and clinical implications. Aging Clinical and Experimental Reseach. 2012;24(1):6–14. doi: 10.3275/8178. [DOI] [PubMed] [Google Scholar]
- Dvorak RD, Day AM. Marijuana and self-regulation: Examining likelihood and intensity of use and problems. Addictive Behaviors. 2014;39:709–712. doi: 10.1016/j.addbeh.2013.11.001. 0.1016/j.addbeh.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Wong JC, Allan ME, Mar AC, Theobald DE, Robbins TW. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. Journal of Neuroscience. 2011;31(20):7349–7356. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behavioral neuroscience. 2003;117(6):1302. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Nystrom LE, Cohen JD. Reduced sensitivity to immediate reward during decision-making in older than younger adults. PloS One. 2012;7:e36953. doi: 10.1371/journal.pone.0036953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience & Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsive behavior and compulsivity in cocaine dependence. Brain. 2011:awr138. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET. Cocaine dependence: a fast-track for brain ageing? Molecular psychiatry. 2013;18:134–135. doi: 10.1038/mp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Park JY, Maxfield N, Drobes DJ. Neurocognitive variation in smoking behavior and withdrawal: genetic and affective moderators. Genes, Brain and Behavior. 2009;8:86–96. doi: 10.1111/j.1601-183X.2008.00445.x. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsive behavior. Psychopharmacology. 1999;146:348–361. doi: 10.1007/PL00005481. [DOI] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Perales JC, Moreno-López L, Pérez-García M, Verdejo-García A. Neuropsychological profiling of impulsive behavior and compulsivity in cocaine dependent individuals. Psychopharmacology. 2012;219:673–683. doi: 10.1007/s00213-011-2485-z. [DOI] [PubMed] [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, Field M. Multiple behavioural impulsive behavior tasks predict prospective alcohol involvement in adolescents. Addiction. 2013;108:1916–1923. doi: 10.1111/add.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behavioral and Cognitive Neuroscience Reviews. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research. 1997;754(1):285–297. doi: 10.1016/S0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Fillmore KM. Alcohol use across the life course. Toronto, Ontario, Canada: Alcoholism and Drug Addiction Research Foundation; 1988. [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biological Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:1–9. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Poldrack RA, Baker CM, McGlennen KM, London ED. Neural correlates of response inhibition and cigarette smoking in late adolescence. Neuropsychopharmacology. 2011;36(5):970–978. doi: 10.1038/npp.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, Adams ZW, Marusich JA, Nesland TO, Yates JR, Bardo MT. A translational behavioral model of mood-based impulsivity: Implications for substance abuse. Drug and Alcohol Dependence. 2012;122(1):93–99. doi: 10.1016/j.drugalcdep.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Schreiber L, Odlaug BL. Neuropsychological deficits associated with cannabis use in young adults. Drug and Alcohol Dependence. 2012;121:159–162. doi: 10.1016/j.drugalcdep.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenman AP, Oosterlaan J, Greven CU, Vuijk PJ, Rommelse N, Franke B, Buitelaar J. Neurocognitive predictors of substance use disorders and nicotine dependence in ADHD probands, their unaffected siblings, and controls: a 4-year prospective follow-up. Journal of Child Psychology and Psychiatry. 2015;56:521–529. doi: 10.1111/jcpp.12315. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, De Beurs E, Van Den Brink W. Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101:534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nat. Rev. Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychological Science. 1994;5:33–36. http://dx.doi.org/10.1111/j.1467-9280.1994.tb00610.x. [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: the role of age and income. Psychology and Aging. 1996;11:79–84. doi: 10.1037/0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Gunn RL, Smith GT. Risk factors for elementary school drinking: Pubertal status, personality, and alcohol expectancies concurrently predict fifth grade alcohol consumption. Psychology of Addictive Behaviors. 2010;24:617–627. doi: 10.1037/a0020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harakeh Z, de Sonneville L, van den Eijnden RJ, Huizink AC, Reijneveld SA, Ormel J, Vollebergh WA. The association between neurocognitive functioning and smoking in adolescence: The TRAILS study. Neuropsychology. 2012;26:541–550. doi: 10.1037/a0029217. [DOI] [PubMed] [Google Scholar]
- Harden K, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsive behavior during adolescence: Further evidence for a dual systems model. Developmental Psychology. 2011;47:739–746. doi: 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H. Post-error behavior in active cocaine users: poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Quitting drugs: quantitative and qualitative features. Annual review of Clinical Psychology. 2013;9:29–59. doi: 10.1146/annurev-clinpsy-032511-143041. [DOI] [PubMed] [Google Scholar]
- Heyman GM, Gibb SP. Delay discounting in college cigarette chippers. Behavioural Pharmacology. 2006;17:669–679. doi: 10.1097/FBP.0b013e3280116cfe. [DOI] [PubMed] [Google Scholar]
- Hoshi R, Mullins K, Boundy C, Brignell C, Piccini P, Curran HV. Neurocognitive function in current and ex-users of ecstasy in comparison to both matched polydrug-using controls and drug-naive controls. Psychopharmacology. 2007;194(3):371–379. doi: 10.1007/s00213-007-0837-5. [DOI] [PubMed] [Google Scholar]
- Isen JD, Sparks JC, Iacono WG. Predictive validity of delay discounting behavior in adolescence: A longitudinal twin study. Experimental and clinical psychopharmacology. 2014;22(5):434–443. doi: 10.1037/a0037340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL, Brooks BL, Ashton VL, Johnson LG, Gualtieri CT. Does familiarity with computers affect computerized neuropsychological test performance? Journal of Clinical and Experimental Neuropsychology. 2009;31:594–604. doi: 10.1080/13803390802372125. [DOI] [PubMed] [Google Scholar]
- Jaeger A. Inhibitory control and the adolescent brain: A review of fMRI research. Psychology & Neuroscience. 2013;6:23–30. doi: 10.3922/j.psns.2013.1.05. [DOI] [Google Scholar]
- Jentsch JD, Pennington ZT. Reward, interrupted: inhibitory control and its relevance to addictions. Neuropharmacology. 2014;76:479–486. doi: 10.1016/j.neuropharm.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Keighley J, Braver TS, Green L. Domain independence and stability in young and older adults’ discounting of delayed rewards. Behavioural Processes. 2011;87:253–259. doi: 10.1016/j.beproc.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras-Aswad D, Jacobs MM, Yiannoulos G, Roussos P, Bitsios P, Nomura Y, Liu X, Hurd YL. Cannabis-dependence risk relates to synergism between neuroticism and proenkephalin SNPs associated with amygdala gene expression: case-control study. PLoS One. 2012;7:e39243. doi: 10.1371/journal.pone.0039243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser AJ, Milich R, Lynam DR, Charnigo RJ. Negative urgency, distress tolerance, and substance abuse among college students. Addictive Behaviors. 2012;37:1075–1083. doi: 10.1016/j.addbeh.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biological psychology. 2005;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. 0.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakula SL, Weiss RD, Griffin ML, Borges AM, Bailey AJ, McHugh RK. Delay discounting in opioid use disorder: Differences between heroin and prescription opioid users. Drug and Alcohol Dependence. 2016;169:68–72. doi: 10.1016/j.drugalcdep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch S, Jäger L, Karamatskos E, Graz C, Stammel A, Flatz W, Pogarell O. Influence of trait anxiety on inhibitory control in alcohol-dependent patients: simultaneous acquisition of ERPs and BOLD responses. Journal of Psychiatric Research. 2008;42:734–745. doi: 10.1016/j.jpsychires.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Karyadi K, Coskunpinar A, Cyders MA. Neurobiological underpinnings of dispositions to rash action. In: Cyders M, editor. Psychology of Impulsive behavior. New York: Nova Science Publishers; 2012. pp. 95–110. Invited chapter. [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. The Journal of Neuroscience. 2003;23(21):7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsive behavior. Addiction. 2013;108:506–515. doi: 10.1111/add.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee D. Prefrontal cortex and impulsive decision making. Biological Psychiatry. 2011;69(12):1140–1146. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, Chassin L. Mediating and moderated effects of adolescent behavioral undercontrol and parenting in the prediction of drug use disorders in emerging adulthood. Psychology of Addictive Behaviors. 2004;18:239–249. doi: 10.1037/0893-164X.18.3.239. [DOI] [PubMed] [Google Scholar]
- King KM, Karyadi KA, Luk JW, Patock-Peckham JA. Dispositions to rash action moderate the associations between concurrent drinking, depressive symptoms, and alcohol problems during emerging adulthood. Psychology of Addictive Behaviors. 2011;25:446–454. doi: 10.1037/a0023777. [DOI] [PubMed] [Google Scholar]
- Kirby K, Petry N, Bickel W. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology. 1999;128:78–87. doi: 10.1037/0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Vanyukov M, Tarter R. Detection of youth at high risk for substance use disorders: A longitudinal study. Psychology of Addictive Behaviors. 2005;19:243–252. doi: 10.1037/0893-164X.19.3.243. [DOI] [PubMed] [Google Scholar]
- Krank M, Stewart SH, O'Connor R, Woicik PB, Wall AM, Conrod PJ. Structural, concurrent, and predictive validity of the Substance Use Risk Profile Scale in early adolescence. Addictive behaviors. 2011;36:37–46. doi: 10.1016/j.addbeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Lawrence A, Luty J, Bogdan N, Sahakian B, Clark L. Impulsive behavior andresponse inhibition in alcohol dependence and problem gambling. Psychopharmacology. 2009;207:163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Peters JR, Adams ZW, Milich R, Lynam DR. Specific dimensions of impulsive behavior are differentially associated with daily and non-daily cigarette smoking in young adults. Addictive Behaviors. 2015;46:82–85. doi: 10.1016/j.addbeh.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Stanger C, Budney AJ. A comparison of delay discounting in adolescents and adults in treatment for cannabis use disorders. Experimental and Clinical Psychopharmacology. 2015;23:130. doi: 10.1037/a0038792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C-W, Zhong RY-X, Chung Y-C, Pan C-H, Yen M-Y, Cheng C-P, Hsu W-Y. Using cognitive modeling to investigate the psychological processes of the Go/NoGo discrimination task in male abstinent heroin misusers. Addiction. 2014;109:1355–1362. doi: 10.1111/add.12591. [DOI] [PubMed] [Google Scholar]
- Liao DL, Huang CY, Hu S, Fang SC, Wu CS, Chen WT, Chiang-shan RL. Cognitive control in opioid dependence and methadone maintenance treatment. PloS One. 2014;9:e94589. doi: 10.1371/journal.pone.0094589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KTK, Yu R. Aging and wisdom: age-related changes in economic and social decision making. Frontiers in Aging Neuroscience. 2015;7:1–11. doi: 10.3389/fnagi.2015.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Sher KJ. 10 Personality and Substance Use Disorders. The Oxford. Handbook of Substance Use and Substance Use Disorders: Two-Volume Set. 2016:351. [Google Scholar]
- Littlefield AK, Sher KJ, Wood PK. Is “maturing out” of problematic alcohol involvement related to personality change? Journal of Abnormal Psychology. 2009;118:360–374. doi: 10.1037/a0015125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Stevens AK, Ellingson JM, King KM, Jackson KM. Changes in negative urgency, positive urgency, and sensation seeking across adolescence. Personality and Individual Differences. 2016;90:332–337. doi: 10.1016/j.paid.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Caneda E, Holguín SR, Cadaveira F, Corral M, Doallo S. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol and Alcoholism. 2014;49(2):173–181. doi: 10.1093/alcalc/agt168. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Hasin DS, de Los Cobos JP, Pines A, Wang S, Grant BF, Blanco C. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: Results from the national epidemiologic survey on alcohol and related conditions. Addiction. 2011;106(3):657–669. doi: 10.1111/j.1360-0443.2010.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, Hester R, Smits M, Nijs IM, Pepplinkhuizen L, Franken IH. The role of dopamine in inhibitory control in smokers and non-smokers: a pharmacological fMRI study. European Neuropsychopharmacology. 2013;23:1247–1256. doi: 10.1016/j.euroneuro.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Thompson BS, Freund N, Andersen SL. The developmental inter-relationships between activity, novelty preferences, and delay discounting in male and female rats. Developmental Psychobiology. 2016;58:231–242. doi: 10.1002/dev.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Lynam D, Smith G, Cyders M, Fischer S, Whiteside S. The UPPS-P questionnaire measure of five dispositions to rash action. Unpublished technical report, Purdue University 2007 [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216(3):305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology. 2011;216:91–99. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control patients: Drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Martens M, Hatchet ES, Martin JL, Fowler RM, Fleming KM, Karakashian MA, Cimini MD. Does trait urgency moderate the relationship between parental alcoholism and alcohol use? Addiction Research & Theory. 2010;18:479–488. doi: 10.3109/16066350903145064. [DOI] [Google Scholar]
- Masaki D, Yokoyama C, Kinoshita S, Tsuchida H, Nakatomi Y, Yoshimoto K, Fukui K. Relationship between limbic and cortical 5-HT neurotransmission and acquisition and reversal learning in a go/no-go task in rats. Psychopharmacology. 2006;189(2):249–258. doi: 10.1007/s00213-006-0559-0. [DOI] [PubMed] [Google Scholar]
- Mazerolle M, Régner I, Morisset P, Rigalleau F, Huguet P. Stereotype threat strengthens automatic recall and undermines controlled processes in older adults. Psychological Science. 2012;23:723–727. doi: 10.1177/0956797612437607. [DOI] [PubMed] [Google Scholar]
- Mejia-Toiber J, Boutros N, Markou A, Semenova S. Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behavioural Brain Research. 2014;266:19–28. doi: 10.1016/j.bbr.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Estevez AF, Zaldivar F, Montes JMG, Gutiérrez-Ferre VE, Esteban L, Flores P. Impulsive behavior differences in recreational cannabis users and binge drinkers in a university population. Drug and alcohol dependence. 2012;124(3):355–362. doi: 10.1016/j.drugalcdep.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M, Verdejo-García A. Trait impulsive behavior and prefrontal gray matter reductions in cocaine dependent individuals. Drug and Alcohol Dependence. 2012;125:208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Moody L, Franck C, Bickel WK. Comorbid depression, antisocial personality, and substance dependence: Relationship with delay discounting. Drug and Alcohol Dependence. 2016a;160:190–196. doi: 10.1016/j.drugalcdep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody L, Franck C, Hatz L, Bickel WK. Impulsive behavior and polysubstance use: A systematic comparison of delay discounting in mono-, dual-, and trisubstance use. Experimental and Clinical Psychopharmacology. 2016b;24:30–37. doi: 10.1037/pha0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Karno MP, Grella CE, Lin JC, Warda U, Liao DH, Hu P. Alcohol, tobacco, and nonmedical drug use in older US adults: Data from the 2001/02 National Epidemiologic Survey of Alcohol and Related Conditions. Journal of the American Geriatrics Society. 2009;57:2275–2281. doi: 10.1111/j.1532-5415.2009.02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Murphy P, Garavan H. Cognitive predictors of problem drinking and AUDIT scores among college students. Drug and Alcohol Dependence. 2011;115(1):94–100. doi: 10.1016/j.drugalcdep.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Noël X, Van der Linden M, d’Acremont M, Bechara A, Dan B, Hanak C, Verbanck P. Alcohol cues increase cognitive impulsive behavior in individuals with alcoholism. Psychopharmacology. 2007;192(2):291–298. doi: 10.1007/s00213-006-0695-6. [DOI] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: I’m a k, you’re a k. Journal of the Experimental Analysis of Behavior. 2011;96(3):427–439. doi: 10.1901/jeab.2011.96-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology. 2005;182(4):508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Olson EA, Hooper CJ, Collins P, Luciana M. Adolescents’ performance on delay and probability discounting tasks: Contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Personality and Individual Differences. 2007;43:1886–1897. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tousa DS, Matson LM, Grahame NJ. Effects of Intoxicating Free-Choice Alcohol Consumption During Adolescence on Drinking and Impulsivity During Adulthood in Selectively Bred High-Alcohol Preferring Mice. Alcoholism: Clinical and Experimental Research. 2013;37:141–149. doi: 10.1111/j.1530-0277.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzir M, Errami M. Etiological theories of addiction: A comprehensive update on neurobiological, genetic and behavioural vulnerability. Pharmacology Biochemistry and Behavior. 2016;148:59–68. doi: 10.1016/j.pbb.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Tang Y, Chorlian DB, Roopesh BN, Manz N, Porjesz B. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biological Psychology. 2012;89(1):170–182. doi: 10.1016/j.biopsycho.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang RD, Hom MS, Geary BA, Doran N, Spillane NS, Guillot CR, Leventhal AM. Relationships between trait urgency, smoking reinforcement expectancies, and nicotine dependence. Journal of Addictive Diseases. 2014;33:83–93. doi: 10.1080/10550887.2014.909695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Maloney T, Moeller SJ, Woicik PA, Alia-Klein N, Telang F, Goldstein RZ. Sensitivity to monetary reward is most severely compromised in recently abstaining cocaine addicted individuals: a cross-sectional ERP study. Psychiatry Research: Neuroimaging. 2012;203(1):75–82. doi: 10.1016/j.pscychresns.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SL, Molina BS, Belendiuk KA, Donovan JE. Racial differences in the development of impulsive behavior and sensation seeking from childhood into adolescence and their relation to alcohol use. Alcoholism: Clinical and Experimental Research. 2012;36:1794–1802. doi: 10.1111/j.1530-0277.2012.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology. 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Petit G, Kornreich C, Noël X, Verbanck P, Campanella S. Alcohol-related context modulates performance of social drinkers in a visual Go/No-Go task: a preliminary assessment of event-related potentials. PLoS One. 2012;7(5):e37466. doi: 10.1371/journal.pone.0037466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L. Dependence of adolescent novelty-seeking behavior on response phenotype and effects of apparatus scaling. Behavioral Neuroscience. 2008;122(4):861–875. doi: 10.1037/0735-7044.122.4.861. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1514. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Prencipe A, Kesek A, Cohen J, Lamm C, Lewis MD, Zelazo PD. Development of hot and cool executive function during the transition to adolescence. Journal of Experimental Child Psychology. 2011;108:621–637. doi: 10.1016/j.jecp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Read D, Read NL. Time discounting over the lifespan. Organizational Behavior and Human Decision Processes. 2004;94:22–32. doi: 10.1016/j.obhdp.2004.01.002. [DOI] [Google Scholar]
- Reynolds B, Patak M, Shroff P, Penfold RB, Melanko S, Duhig AM. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2007;15:264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65(1):35–42. doi: 10.1016/S0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Robbins T. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163(3):362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson JM, Ladd BO, Anderson KG. When you see it, let it be: Urgency, mindfulness and adolescent substance use. Addictive Behaviors. 2014;39(6):1038–1041. doi: 10.1016/j.addbeh.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Hennessy M. A biosocial-affect model of adolescent sensation seeking: The role of affect evaluation and peer-group influence in adolescent drug use. Prevention Science. 2007;8:89–101. doi: 10.1007/s11121-007-0064-7. [DOI] [PubMed] [Google Scholar]
- Rubio G, Jiménez M, Rodríguez-Jiménez R, Martínez I, Ávila C, Ferre F, Palomo T. The Role of Behavioral Impulsive behavior in the Development of Alcohol Dependence: A 4-Year Follow-Up Study. Alcoholism: Clinical and Experimental Research. 2008;32(9):1681–1687. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Knutson B. Decision making in the ageing brain: changes in affective and motivational circuits. Nature Reviews Neuroscience. 2015;16(5):278–289. doi: 10.1038/nrn3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Li SC, Ridderinkhof KR. Complementary approaches to the study of decision making across the adult life span. Frontiers in Neuroscience. 2013;7:1–4. doi: 10.3389/fnins.2013.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A, Baldermann C, Feige B, Katzev M, Scheller E, Hellwig B, Klöppel S. Differential effects of age on subcomponents of response inhibition. Neurobiology of Aging. 2013;34(9):2183–2193. doi: 10.1016/j.neurobiolaging.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Setlow B, Mendez IA, Mitchell MR, Simon NW. Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behavioural pharmacology. 2009;20(5–6):380. doi: 10.1097/FBP.0b013e3283305eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles RE, Fischer S, Cyders MA, Combs JL, Gunn RL, Smith GT. Negative urgency: a personality predictor of externalizing behavior characterized by neuroticism, low conscientiousness, and disagreeableness. Journal of Abnormal Psychology. 2012;121:160–172. doi: 10.1037/a0024948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma L, Markon KE, Clark LA. Toward a theory of distinct types of"impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychological Bulletin. 2014;140:374–408. doi: 10.1037/a0034418. [DOI] [PubMed] [Google Scholar]
- Shishido H, Gaher RM, Simons JS. I don't know how I feel, therefore I act: alexithymia, urgency, and alcohol problems. Addictive Behaviors. 2013;38:2014–2017. doi: 10.1016/j.addbeh.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Simon NW, Gregory TA, Wood J, Moghaddam B. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behavioral Neuroscience. 2013;127:23–32. doi: 10.1037/a0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiology of Aging. 2010;31:853–862. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, Van Den Brink W, Beekman ATF, Penninx BWJH, Veltman DJ. Response inhibition in alcohol-dependent patients and patients with depression/anxiety: a functional magnetic resonance imaging study. Psychological Medicine. 2014;44:1713–1725. doi: 10.1017/S0033291713002274. [DOI] [PubMed] [Google Scholar]
- Smith JL, Mattick RP. Evidence of deficits in behavioural inhibition and performance monitoring in young female heavy drinkers. Drug Alcohol Dependence. 2013;133:398–404. doi: 10.1016/j.drugalcdep.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug and Alcohol Dependence. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Stewart C, Hollifield M, Tasman A. Event-related potential study of executive dysfunctions in a speeded reaction task in cocaine addiction. Journal of Neurotherapy. 2008;12(4):185–204. doi: 10.1080/10874200802502144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Neurobehavioral changes in adolescence. Current Directions in Psychological Science. 2000;9:111–114. doi: 10.1111/1467-8721.00072. [DOI] [Google Scholar]
- Spillane NS, Muller CJ, Noonan C, Goins RT, Mitchell CM, Manson S. Sensation-seeking predicts initiation of daily smoking behavior among American Indian high school students. Addictive behaviors. 2012;37(12):1303–1306. doi: 10.1016/j.addbeh.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane NS, Smith GT, Kahler CW. Impulsive behavior-like traits and smoking behavior in college students. Addictive Behaviors. 2010;35:700–705. doi: 10.1016/j.addbeh.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stautz K, Cooper A. Impulsive behavior-related personality traits and adolescent alcohol use: a meta-analytic review. Clinical Psychology Review. 2013;33:574–592. doi: 10.1016/j.cpr.2013.03.003. Doi: http://dx.doi.org/10.1016/j.cpr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Stautz K, Cooper A. Urgency traits and problematic substance use in adolescence: Direct effects and moderation of perceived peer use. Psychology of Addictive Behaviors. 2014;28:487. doi: 10.1037/a0034346. http://dx.doi.org/10.1037/a0034346. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. http://dx.doi.org/10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsive behavior as indexed by behavior and self-report: evidence for a dual systems model. Developmental psychology. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Stojek MM, Fischer S, Murphy CM, MacKillop J. The role of impulsive behavior traits and delayed reward discounting in dysregulated eating and drinking among heavy drinkers. Appetite. 2014;80:81–88. doi: 10.1016/j.appet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, Flory JD, Manuck SB. Delay discounting and smoking: Association with the Fagerström Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine & Tobacco Research. 2008;10(10):1571–1575. doi: 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, London ED. Different forms of self-control share a neurocognitive substrate. The Journal of Neuroscience. 2011;31(13):4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Epstein JN, Lisdahl KM, Molina B, Tapert S, Hinshaw SP, Group MN. Impact of ADHD and cannabis use on executive functioning in young adults. Drug and Alcohol Dependence. 2013;133:607–614. doi: 10.1016/j.drugalcdep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma P, Wiebel B, Daum I. Response inhibition and cognitive flexibility in schizophrenia with and without comorbid substance use disorder. Schizophrenia Research. 2007;92:168–180. doi: 10.1016/j.schres.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Tomko RL, Prisciandaro JJ, Falls SK, Magid V. The structure of the UPPS-R-Child impulsive behavior scale and its relations with substance use outcomes among treatment-seeking adolescents. Drug and Alcohol Dependence. 2016;161:276–283. doi: 10.1016/j.drugalcdep.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A, Catena A, Megías A, Maldonado A, Cándido A, Verdejo-García A, Perales JC. Emotional and non-emotional pathways to impulsive behavior and addiction. Frontiers in Human Neuroscience. 2013;7:1–11. doi: 10.3389/fnhum.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. Journal of neurophysiology. 2000;83(4):1864–1876. doi: 10.1152/jn.2000.83.4.1864. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe RH, Verleger R. Aging and the Simon task. Psychophysiology. 2002;39(01):100–110. doi: 10.1017/S0048577202001221. 10.1017.S0048577201020042. [DOI] [PubMed] [Google Scholar]
- VanderVeen JD, Hershberger AR, Cyders MA. UPPS-P model impulsive behavior and marijuana use behaviors in adolescents: A meta-analysis. Drug and alcohol dependence. 2016;168:181–190. doi: 10.1016/j.drugalcdep.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Vanderveldt A, Oliveira L, Green L. Delay discounting: Pigeon, rat, human—does it matter? Journal of Experimental Psychology: Animal Learning and Cognition. 2016;42(2):141. doi: 10.1037/xan0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsive behavior as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Vergés A, Jackson KM, Bucholz KK, Grant JD, Trull TJ, Wood PK, Sher KJ. Deconstructing the age-prevalence curve of alcohol dependence: Why “maturing out” is only a small piece of the puzzle. Journal of Abnormal Psychology. 2012;121(2):511–523. doi: 10.1037/a0026027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. Journal of Neuroscience. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Votruba KL, Langenecker SA. Factor structure, construct validity, and age-and education-based normative data for the Parametric Go/No-Go Test. Journal of Clinical and Experimental Neuropsychology. 2013;35:132–146. doi: 10.1080/13803395.2012.758239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and clinical psychopharmacology. 1998;6(3):292–305. doi: 10.1037/1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wardell JD, Strang NM, Hendershot CS. Negative urgency mediates the relationship between childhood maltreatment and problems with alcohol and cannabis in late adolescence. Addictive Behaviors. 2016;56:1–7. doi: 10.1016/j.addbeh.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes, Brain and Behavior. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcoholism: Clinical and Experimental Research. 2007;31:1839–184. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Winick C. Maturing out of narcotic addiction. Bulletin on Narcotics. 1962;14:1–7. [Google Scholar]
- Whelan R, McHugh LA. Temporal discounting of hypothetical monetary rewards by adolescents, adults, and older adults. The Psychological Record. 2009;59:247–258. [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsive behavior: Using a structural model of personality to understand impulsive behavior. Personality and Individual Differences. 2001;30:669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: a four-factor model of impulsive behavior. European Journal of Personality. 2005;19(7):559–574. doi: 10.1002/per.556. [DOI] [Google Scholar]
- Wolitzky-Taylor K, McBeth J, Guillot CR, Stone MD, Kirkpatrick MG, Zvolensky MJ, Leventhal AM. Transdiagnostic processes linking anxiety symptoms and substance use problems among adolescents. Journal of Addictive Diseases. 2016;35(4):266–277. doi: 10.1080/10550887.2016.1207969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HF, Mills DS, Pollux PM. Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris) Physiology & behavior. 2012;105(3):676–682. doi: 10.1016/j.physbeh.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Wu LT, Blazer DG. Substance use disorders and psychiatric comorbidity in mid and later life: a review. International Journal of Epidemiology. 2014;43(2):304–317. doi: 10.1093/ije/dyt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Yang S, Zhao L, Yin L, Liu X, An S. Event-related potentials in a Go/Nogo task of abnormal response inhibition in heroin addicts. Science in China Series C: Life Sciences. 2009;52:780–788. doi: 10.1007/s11427-009-0106-4. [DOI] [PubMed] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: Prevalence, symptom profiles and correlates. Drug and Alcohol Dependence. 2002;68:309–322. doi: 10.1016/S0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]
- Zapolski TC, Cyders MA, Smith GT. Positive urgency predicts illegal drug use and risky sexual behavior. Psychology of Addictive Behaviors. 2009;23:348–354. doi: 10.1037/a0014684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.