Abstract
Objective
To describe outcomes of HIV-infected pediatric patients with drug-resistant tuberculosis (DR TB).
Methods
Demographic, clinical, and laboratory data from pediatric patient charts treated for DR TB during 2005–2008 were collected retrospectively from five MDR TB hospitals in South Africa. Data were summarized and Pearson’s chi-squared test or Fisher’s exact test were used to assess differences in variables of interest by HIV status. A time-to-event analysis was conducted using days from start of treatment to death. Variables of interest were first assessed using the Kaplan-Meier method. Cox proportional hazard models were fit to estimate crude and adjusted hazard ratios.
Results
Of 423 eligible participants, 398 (95%) had culture-confirmed DR-TB and 238 (56%) were HIV-infected. A total of 54% were underweight, 42% were male and median age was 10.7 years (IQR: 5.5–15.3). Of the 423 participants, 245 (58%) were successfully treated, 69 (16%) died, treatment failed in 3 (1%), 36 (9%) were lost to follow-up, and 70 (17%) were still on treatment, transferred or had unknown outcomes. Time to death differed by HIV status (p=0.008), sex (p<0.001), year of TB diagnosis (p=0.05) and weight status (p=0.002). Over the two-year risk period, the adjusted rate of death was 2-fold higher among participants with HIV compared to HIV-negative participants (aHR=2.28; 95% CI: 1.11, 4.68).
Conclusions
Male, underweight, and HIV-infected children with DR TB were more likely to experience death when compared to other children with DR TB within this study population.
Keywords: HIV, pediatric, drug resistance, tuberculosis
Introduction
In 2015 there were an estimated 480,000 new cases of multidrug-resistant tuberculosis (MDR TB), defined as TB having resistance to at least isoniazid (INH) and rifampicin (RIF) (1, 2). An estimated one million children develop TB each year (1). Pediatric TB results from recent transmission (as opposed to reactivation), especially in young children (3–7). Attempts to quantify the incidence of MDR TB in children estimate that 3.2% of new pediatric TB cases are MDR TB and indicate that the risk of MDR TB is generally similar among children and adults (3, 8). As expected, estimates of drug resistance in children are often higher in high-burden countries. In South Africa, it is estimated that 15% of pediatric patients with culture-confirmed TB have a strain resistant to INH and 8.9% have MDR TB, similar to adults (1, 7, 9–11).
Approximately 12% of the 9.6 million incident cases of TB around the world in 2014 were also infected with human immunodeficiency virus (HIV) (1). South Africa has one of the highest burdens of HIV-infected TB cases worldwide. In 2014, 61% of incident TB cases in South Africa were also infected with HIV (1). HIV-infected TB cases present additional challenges in treating DR TB and are associated with worse treatment outcomes and survival (12–16). In South Africa, data from a variety of cross sectional studies indicate that 11%–64% of children with TB are HIV-infected (17). Although there is conflicting evidence about the association between HIV infection and drug-resistant TB (7, 18, 19), once infected with TB, children who are younger or HIV-infected are more likely to progress to TB disease (20). In South Africa, each adult with MDR TB has, on average, nearly two contacts who are younger than five years (4).
Outcomes and survival in adults co-infected with TB and HIV can be improved with simultaneous treatment of both diseases (21–23), but few studies (small cohort studies and case series) describe treatment outcomes of co-infected pediatric patients (10, 24–27). Previous studies have demonstrated that mortality among children with DR TB in South Africa differs by province (12%–31%), but overall treatment success rates (70%–83%) can be similar to, if not better than, those seen in adults (28–30). The objective of this analysis was to assess the association between treatment outcomes with HIV status and other risk factors among children and adolescents with MDR TB.
Methods
Ethics
Ethics approval for this project was obtained from the South African National Department of Health (NDOH), each provincial Department of Health, the City of Johannesburg and all participating hospitals. Institutional Review Board approval was obtained from the South African Medical Research Council Ethics Committee and the Human Research Ethics Committee of University of the Witwatersrand in Johannesburg. This project was reviewed by the Centers for Disease Control and Prevention, Atlanta, GA, USA and determined to be routine disease surveillance.
Data Collection
The setting and data collection process for this analysis have been previously described (29). Briefly, this was a retrospective cohort study of abstracted data from the records of all children (<13 years) and adolescents (13–17 years) diagnosed with DR TB between January 1, 2005, and June 30, 2010, at five MDR TB hospitals located in Eastern Cape, Gauteng, Kwazulu-Natal and Limpopo provinces. Eligibility criteria included patients younger than 18 years of age with laboratory-confirmed DR TB, and/or treatment for drug-resistant TB during the study period. Demographic, clinical, and laboratory data were abstracted from medical records using a standardized form. When patient records at the MDR TB hospitals were incomplete, data were abstracted from alternate sources including TB surveillance systems, hospital databases, the National Health Laboratory Service (NHLS) database and patient charts from referring facilities.
Definitions
We used standard anti-TB drug resistance category definitions for INH and RIF mono-resistance, poly-resistance, MDR, and extensive drug resistance (XDR) (31). Pre-extensively drug-resistant TB (Pre XDR TB) was defined as MDR TB plus resistance to any fluoroquinolone or second-line injectable drug (capreomycin, kanamycin or amikacin), but not both.
A DR TB treatment regimen was defined as any TB treatment regimen that differed from standard first-line therapy and was started after registration at a MDR TB hospital. WHO recommends that MDR TB treatment regimens include at least four effective second-line anti-TB drugs, including a fluoroquinolone and second-line injectable drug (32). For the time period during which this cohort was diagnosed and treated, there were no standard national guidelines for treatment of drug-resistant TB in children in South Africa.
Weight-for-age z-scores were calculated as a marker of malnutrition. Underweight was defined according to WHO definition, as below two standard deviations below the mean. Severely underweight was defined as below three standard deviations below the mean. Standard WHO definitions for MDR TB treatment outcome were used: cure/complete, died, failed, lost to follow up, unknown/on treatment /transfer (33). Death was the outcome of interest for the survival analysis.
Statistical Methods
Data were analyzed using SAS 9.4 (SAS Institute, Cary, North Carolina, USA). This analysis was limited to participants diagnosed before 2009 (to allow time to ascertain treatment outcome) with a documented HIV status and treatment outcome. Observations from Limpopo province were excluded because of the small number of participants (n=5) from that province that were diagnosed before 2009. The very small number of participants with isoniazid mono-resistance or poly-resistance were excluded from the analysis of outcomes in order to satisfy convergence criteria. Participants with mono-resistance to rifampicin were included with the MDR participants. Frequencies and proportions were reported for all categorical variables. Pearson’s chi-squared test, Fisher’s exact test or Wilcoxon-Mann-Whitney tests were used to assess bivariate associations of categorical variables of interest with HIV status. An alpha of 0.05 was used to test the statistical significance of all associations.
To assess the effect of HIV infection on the rate of poor outcomes, a time-to-event analysis was conducted. The event of interest was death. Participants contributed person-time from the start of DR TB treatment (T0) until the date of event or the date they were censored (T1). Participants who were cured, completed treatment or failed treatment were censored on the date they reached their treatment outcome. Participants who were still on treatment, transferred or were lost to follow up were censored on the last date of contact. Participants who had not experienced the event of interest after 2 years of follow-up were censored at 730.5 days. Those who were not on treatment for at least 15 days (T1−T0 < 15) were excluded from the survival analysis.
Variables of interest (HIV status, sex, age, province, year of TB diagnosis, resistance profile, presence of extra pulmonary TB and weight status) were first assessed independently using the Kaplan-Meier method and log-rank tests. In order to calculate hazard ratios and corresponding confidence intervals as a measure of effect, statistically significant variables (and variables that are known to influence survival) were considered for inclusion in bivariate and multivariable Cox proportional hazard models.
The proportional hazard assumption was assessed visually win unadjusted –ln-ln curves and tested statistically by looking at the significance of time dependent covariates and goodness of fit methods that assess the correlation between Schoenfeld residuals and ranked failure times. In order to satisfy the proportional hazards assumption for the time to event analysis, age was collapsed into a dichotomous variable indicating 0–7 years old and 8–17 years old. PreXDR TB and XDR TB were also collapsed to create a dichotomized variable that indicated PreXDR/XDR TB versus MDR TB. Cox proportional hazard bivariate models were fit with each individual predictor that did not violate the proportional hazards assumption. Variables that were significant in the bivariate models were then included in the multivariable analysis. A Cox proportional hazards model, stratified on province and year of diagnosis, was fit to determine adjusted hazard ratios (aHR).
Results
Demographic and Clinical Information
There were 774 eligible children and adolescents diagnosed with DR TB at MDR-TB hospitals in the four areas. A total of 423 were eligible for this analysis and slightly more than half (n=238, 56%) were HIV-infected (Table 1). Culture results were available for 419 (99.1%) participants, 398 (95.0%) of which were positive. The majority of participants were cared for in KwaZulu-Natal (n=252, 59.6%), had a median age of 10.7 years (IQR: 5.5–15.3) and less than half were male (n=177, 41.9%). Age differed by HIV status (p<0.001), with HIV-negative participants tending to be older than HIV-infected patients. More HIV-infected participants were male (47.7%) than HIV negative participants (34.6%, p=0.007). A significantly higher proportion of HIV-infected participants were severely underweight compared to those who were HIV-negative (n=86, 38.7% vs. n=30, 17.1%, p<0.001).
Table 1.
Demographic and clinical description of children and adolescents with drug-resistant tuberculosis from three provinces, South Africa, 2005–2008.
| Total N=423 |
HIV− n=185 |
HIV+ n=238 |
|||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Demographic | n | (%) | n | (%) | n | (%) | P valuea |
| Age in years, median (IQR) | 10.7 | (5.5–15.3) | 14.8 | (8.7–16.6) | 8.7 | (5.0–11.8) | <0.001 |
| Age Categories | <0.001 | ||||||
| 0–1 | 45 | (10.6) | 23 | (12.4) | 22 | (9.2) | |
| 2–7 | 106 | (25.1) | 18 | (9.7) | 88 | (37.0) | |
| 8–12 | 113 | (26.7) | 29 | (15.7) | 84 | (35.3) | |
| 13–17 | 159 | (37.6) | 115 | (62.2) | 44 | (18.5) | |
|
| |||||||
| Sex | 0.007 | ||||||
| Male | 177 | (41.9) | 64 | (34.6) | 113 | (47.7) | |
| Female | 245 | (58.1) | 121 | (65.4) | 124 | (52.3) | |
| Missing | 1 | ||||||
|
| |||||||
| Province | <0.001 | ||||||
| Eastern Cape | 78 | (18.4) | 52 | (28.1) | 26 | (10.9) | |
| Gauteng | 93 | (22.0) | 40 | (21.6) | 53 | (22.3) | |
| KwaZulu-Natal | 252 | (59.6) | 93 | (50.3) | 159 | (66.8) | |
|
| |||||||
| Year of TB Diagnosis | <0.001 | ||||||
| 2005 | 64 | (15.1) | 26 | (14.1) | 38 | (16.0) | |
| 2006 | 79 | (18.7) | 24 | (13.0) | 55 | (23.1) | |
| 2007 | 138 | (33.6) | 54 | (29.2) | 84 | (35.3) | |
| 2008 | 142 | (33.6) | 81 | (43.8) | 61 | (25.6) | |
|
| |||||||
| Clinical | |||||||
|
| |||||||
| Weight known | 397 | (93.9) | 175 | (94.6) | 222 | (93.3) | 0.6 |
| Normal weight | 184 | (46.4) | 100 | (57.1) | 84 | (37.7) | |
| Underweightb | 97 | (24.4) | 45 | (25.7) | 52 | (23.4) | |
| Severely underweightc | 116 | (29.2) | 30 | (17.1) | 86 | (38.7) | <0.001 |
|
| |||||||
| Documented TB contact history | 249 | (58.9) | 106 | (57.3) | 143 | (60.1) | 0.6 |
| Previous contact with a TB case | 198 | (79.5) | 89 | (84.0) | 109 | (76.2) | 0.1 |
| Previous contact with MDR/XDR TB case | 44 | (17.7) | 24 | (22.6) | 20 | (14.0) | 0.08 |
|
| |||||||
| Documented TB treatment history | 341 | (80.6) | 140 | (75.7) | 201 | (84.5) | 0.02 |
| Retreatment | 169 | (49.6) | 51 | (36.4) | 118 | (58.7) | <0.001 |
| One previous TB episode | 149 | (88.2) | 46 | (90.2) | 103 | (87.3) | |
| Two previous TB episodes | 16 | (9.5) | 4 | (7.8) | 12 | (10.2) | |
| Three previous TB episodes | 4 | (2.4) | 1 | (2.0) | 3 | (2.5) | 0.09 |
|
| |||||||
| Known anatomic site of TB | 408 | (96.5) | 177 | (95.7) | 231 | (97.1) | 0.4 |
| Pulmonary only | 316 | (77.5) | 161 | (91.0) | 155 | (67.1) | |
| Extrapulmonary (EP) only | 13 | (3.2) | 7 | (4.0) | 6 | (2.6) | |
| Pulmonary and EP | 79 | (19.4) | 9 | (5.1) | 70 | (30.3) | <0.001 |
|
| |||||||
| Documented chest x-ray result | 389 | (92.0) | 165 | (89.2) | 224 | (94.1) | 0.06 |
| Abnormal chest x-ray result | 378 | (97.2) | 161 | (97.6) | 217 | (96.9) | 0.8 |
| Cavity lesions | 145 | (38.4) | 84 | (52.2) | 61 | (28.1) | 0.06 |
|
| |||||||
| Number of complaints/symptoms | |||||||
| Asymptomatic | 17 | (4.0) | 10 | (5.4) | 7 | (2.9) | |
| 1–2 | 170 | (40.2) | 76 | (41.1) | 94 | (39.5) | |
| 3–4 | 143 | (33.8) | 60 | (32.4) | 83 | (34.9) | |
| >4 | 93 | (22.0) | 39 | (21.1) | 54 | (22.7) | 0.6 |
|
| |||||||
| Presenting symptoms | |||||||
| Cough longer than 2 weeks | 269 | (63.6) | 114 | (61.6) | 155 | (65.1) | 0.5 |
| Low weight or failure to thrive | 200 | (47.3) | 86 | (46.5) | 114 | (47.9) | 0.8 |
| Fever | 87 | (20.6) | 39 | (20.5) | 49 | (20.6) | 1.0 |
| Sweats | 92 | (21.8) | 50 | (27.0) | 42 | (17.7) | 0.02 |
| Lack of appetite | 89 | (21.0) | 44 | (23.8) | 45 | (18.9) | 0.2 |
| Pulmonary TB not responding to anti-TB treatment | 162 | (38.3) | 72 | (38.9) | 90 | (37.8) | 0.8 |
| EP TB not responding to anti-TB treatment | 12 | (2.8) | 1 | (0.5) | 11 | (4.6) | 0.01 |
| Mass (e.g. adenopathy) | 22 | (5.2) | 3 | (1.6) | 19 | (8.0) | 0.004 |
| Neurological complaints | 19 | (4.5) | 5 | (2.7) | 14 | (5.9) | 0.1 |
| Non-specific complaints | 67 | (15.8) | 12 | (6.5) | 55 | (23.1) | <0.001 |
| Other Complaints | 259 | (61.2) | 111 | (60.0) | 148 | (62.2) | 0.6 |
|
| |||||||
| Mycobacteriology | |||||||
|
| |||||||
| TB culture performed | 419 | (99.1) | 184 | (99.5) | 235 | (98.7) | 0.6 |
| Positive culture | 398 | (95.0) | 173 | (94.0) | 225 | (95.7) | 0.4 |
| Smear microscopy performed | 409 | (96.7) | 176 | (95.1) | 233 | (97.9) | 0.1 |
| At least one positive smear | 258 | (63.1) | 120 | (68.2) | 138 | (59.2) | 0.06 |
|
| |||||||
| Drug susceptibility test performed | 391 | (92.4) | 172 | (93.0) | 219 | (92.0) | 0.7 |
| Resistance profile | |||||||
| XDR TBd | 38 | (9.7) | 22 | (12.8) | 16 | (7.3) | |
| PreXDR TBe | 22 | (5.6) | 13 | (7.6) | 9 | (4.1) | |
| MDR TBf | 302 | (77.2) | 131 | (76.2) | 171 | (78.1) | |
| RIF mono-resistant TBg | 4 | (1.0) | 1 | (0.6) | 3 | (1.4) | |
| INH mono-resistant TBh | 7 | (1.8) | 1 | (0.6) | 6 | (2.8) | |
| Poly-resistant TBi | 10 | (2.6) | 2 | (1.2) | 8 | (3.7) | |
| None/Other | 8 | (2.1) | 2 | (1.2) | 6 | (2.7) | 0.07 |
|
| |||||||
| Patient received DR TB treatment | 422 | (99.8) | 185 | (100) | 237 | (99.6) | >0.99 |
| Any toxicity reported | 42 | (9.9) | 17 | (9.2) | 25 | (10.5) | 0.7 |
| Recorded date of TB diagnosis and initiation of DR TB treatment | 410 | (96.9) | 181 | (97.8) | 229 | (96.2) | 0.3 |
| Treatment started before DX | 61 | (14.8) | 20 | (11.1) | 41 | (17.9) | |
| Treatment started with or after DX | 349 | (85.1) | 161 | (89.0) | 188 | (82.1) | 0.053 |
| 0–30 days after diagnosis | 210 | (60.2) | 91 | (56.5) | 119 | (63.3) | |
| >30 days and <1 year after diagnosis | 134 | (38.4) | 67 | (41.6) | 67 | (35.6) | |
| >1 year after diagnosis | 5 | (1.4) | 3 | (1.86) | 2 | (1.1) | 0.4 |
|
| |||||||
| Outcomes | |||||||
|
| |||||||
| Cure/Complete | 245 | (57.9) | 114 | (61.6) | 131 | (55.0) | |
| Died | 69 | (16.3) | 20 | (10.8) | 49 | (20.6) | |
| Failed | 3 | (0.7) | 1 | (0.5) | 2 | (0.8) | |
| Lost to Follow up | 36 | (8.5) | 22 | (11.9) | 14 | (5.9) | |
| Unknown/On Treatment/Transfer | 70 | (16.6) | 28 | (15.1) | 42 | (17.7) | 0.02 |
Abbreviations: HIV, human immunodeficiency virus; TB, tuberculosis; MDR, multidrug resistant; XDR, extensively drug resistant; LN, lymph node; MDR, multidrug resistant; RIF, rifampicin; INH, isoniazid; DX, laboratory-confirmed diagnosis.
For continuous variable (age), p value was calculated with a Wilcoxon-Mann-Whitney test. For categorical variables, p values were calculated using a Pearson’s chi square test or Fisher’s exact test where appropriate.
Weight for age adjusted Z-score >2 standard deviations below the mean.
Weight for age adjusted Z-score >3 standard deviations below the mean.
In addition to MDR, resistant to any fluoroquinolone and at least one of three second-line injectable drugs (capreomycin, kanamycin and amikacin).
In addition to MDR, resistant to any fluoroquinolone or at least one of three second-line injectable drugs (capreomycin, kanamycin and amikacin).
Resistant to both isoniazid and rifampicin.
Resistant to rifampicin only.
Resistant to isoniazid only.
Resistant to more than one first-line anti-TB drug, other than both isoniazid and rifampicin.
Among participants with data on previous TB treatment history (n=341), HIV-infected individuals were more likely to have been previously treated (58.7%) than HIV negative participants (36.4%, p<0.001). Among participants who had information about their contact history (n=249), previous contact with an MDR/XDR TB case was higher among HIV negative individuals, but this difference did not reach statistical significance (p=0.08). There was no significant difference in the number of symptoms reported by HIV status (p=0.6).
HIV-negative participants were more likely to have had a positive smear microscopy test (68.2%) compared to HIV-infected participants (59.2%), but this did not reach statistical significance (p=0.06). There were 389 (92.0%) participants that had at least one chest radiograph and 378 (97.2%) were reported as abnormal. HIV infected individuals were more likely to have extrapulmonary TB (32.9%) compared to individuals without HIV (9.1%, p<0.001). There were 391 (92.4%) participants that had at least one drug susceptibility test recorded. There was not a statistically significant difference in the distribution of resistance profiles by HIV status (p=0.07).
Of the 238 HIV-infected participants, only 133 (55.9%) had at least one documented CD4 count, of which 52 (39.1%) had at least one CD4 count below 200. There were 162 (68.1%) HIV-infected participants who had a record of Highly Active Antiretroviral Therapy (HAART) before or during the current TB episode and 142 (60.0%) that had record of Co-trimoxazole preventive therapy (CPT).
There were 245 (57.9%) participants successfully treated (cure/complete), 69 (16.3%) died, 3 (0.7%) treatment failed, 36 (8.5%) were lost to follow-up, and 70 (16.6%) were still on treatment, transferred or unknown. A significantly higher proportion of HIV negative participants were cured or completed treatment than participants who were HIV-positive (61.6% vs. 55.0%; p=0.02). A higher proportion of HIV-infected participants (20.6% vs. 10.8% of HIV negative) died while on treatment.
Rate of Poor Treatment Outcomes
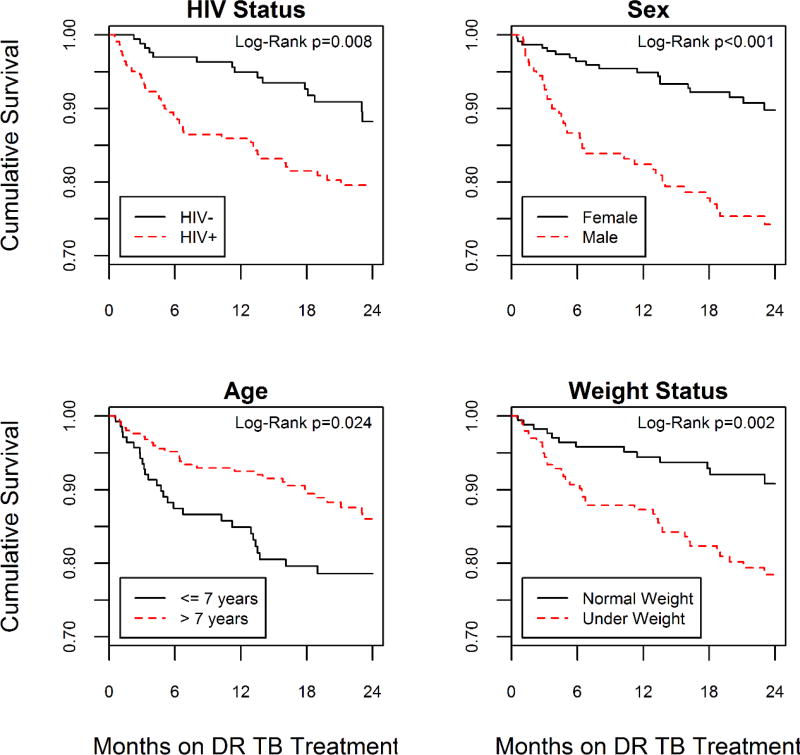
The Kaplan-Meier estimator indicated that time to death was significantly different by HIV status (p=0.008), sex (p<0.001), province (p=0.045) and weight status (p=0.002) (Table 2). Year of TB diagnosis (p=0.054) and age (p=0.09) were close to being statistically different. Over the two-year risk period, HIV-infected participants experienced an unadjusted hazard of death that was more than 2-fold higher than participants who were HIV-negative (HR=2.20, 95%CI: 1.22, 3.97) (Table 3) (Figure 1). The multivariable Cox proportional hazard model was stratified on province and year of diagnosis and contained covariates for HIV status, sex, age and weight. There were 372 observations that contributed person-time to this risk set. Of those, 50 (13.4%) participants experienced death and 322 (86.6%) were censored. Over the two-year risk period, the adjusted rate of death was over 2-fold higher among participants with HIV compared to participants who were HIV-negative (aHR=2.28, 95%CI: 1.11, 4.68). Males (aHR=2.69, 95%CI: 1.47, 4.9) and participants that were underweight (aHR=2.65, 95%CI: 1.37, 5.13) also experienced significantly higher hazards of death.
Table 2.
Bivariate association of survival time and variables of interest among children and adolescents with drug-resistant tuberculosisa in three provinces, South Africa, 2005–2008.
| Total | Died | Other treatment outcomes |
||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Effect | n | n | % | n | % | Log-rank p-value |
| HIV status | 395 | 56 | 14.2 | 339 | 85.8 | 0.008 |
| Negative | 171 | 15 | 8.8 | 156 | 91.2 | |
| Positive | 224 | 41 | 18.3 | 183 | 81.7 | |
| Sex | 394 | 56 | 14.2 | 338 | 85.8 | <0.001 |
| Female | 229 | 19 | 8.3 | 210 | 91.7 | |
| Male | 165 | 37 | 22.4 | 128 | 77.6 | |
| Age, in years | 395 | 56 | 14.2 | 339 | 85.8 | 0.09 |
| 0–1 | 43 | 6 | 14.0 | 37 | 86.1 | |
| 2–7 | 97 | 21 | 21.6 | 76 | 78.4 | |
| 8–12 | 106 | 10 | 9.4 | 96 | 90.6 | |
| 13–17 | 149 | 19 | 12.8 | 130 | 87.3 | |
| Province | 395 | 56 | 14.2 | 339 | 85.8 | 0.05 |
| Kwazulu-Natal | 230 | 33 | 14.3 | 197 | 85.7 | |
| Eastern Cape | 76 | 16 | 21.1 | 60 | 79.0 | |
| Gauteng | 89 | 7 | 7.9 | 82 | 92.1 | |
| Year of TB diagnosis | 395 | 56 | 14.2 | 339 | 85.8 | 0.05 |
| 2005 | 57 | 14 | 24.6 | 43 | 75.4 | |
| 2006 | 73 | 8 | 11.0 | 65 | 89.0 | |
| 2007 | 130 | 17 | 13.1 | 113 | 86.9 | |
| 2008 | 135 | 17 | 12.6 | 118 | 87.4 | |
| Resistance Profile | 357 | 50 | 14.0 | 307 | 86.0 | 0.4 |
| XDR TB | 37 | 9 | 24.3 | 28 | 75.7 | |
| Pre-XDR TB | 22 | 3 | 13.6 | 19 | 86.4 | |
| MDR TBb | 286 | 37 | 12.9 | 249 | 87.1 | |
| Other | 12 | 1 | 8.3 | 11 | 91.7 | |
| Weight status | 372 | 50 | 13.4 | 322 | 86.6 | 0.002 |
| Normal weight | 172 | 13 | 7.6 | 159 | 92.4 | |
| Underweightc | 92 | 13 | 14.1 | 79 | 85.9 | |
| Very underweightd | 108 | 24 | 22.2 | 84 | 77.8 | |
| Culture+ >6 months after DR Tx starte | 395 | 58 | 14.7 | 337 | 85.3 | 0.5 |
| No | 310 | 41 | 13.2 | 269 | 86.8 | |
| Yes | 85 | 17 | 20.0 | 68 | 80.0 | |
Abbreviations: HIV, human immunodeficiency virus; TB, tuberculosis; XDR, extensively-drug resistant; MDR, multidrug resistant; DR, drug resistant; Tx, treatment.
Participants who had not been on treatment for at least 15 days were excluded. Participants who had not experienced the event of interest after 2 years of follow-up were censored at 730.5 days.
Includes rifampicin mono-resistance.
Weight for age adjusted Z-score >2 standard deviations below the mean.
Weight for age adjusted Z-score >3 standard deviations below the mean.
Any positive culture results 6 months or later from start of first DR TB treatment regimen.
Table 3.
Crude and adjusted hazard ratios for death during first 2-years of MDR TB treatment in three provinces, South Africa, 2005–2008.
| Bivariate Modelsa | Multivariable Modelb (N=372) |
|||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Factor | N | HR | (95% CI) | aHR | (95% CI) | |
| HIV Status | HIV+ vs. HIV− | 395 | 2.20 | (1.22 3.97) | 2.28 | (1.11 4.68) |
| Sex | Male vs. Female | 394 | 3.01 | (1.73 5.24) | 2.69 | (1.47 4.91) |
| Age | >7 years vs. ≤7 years | 395 | 0.55 | (0.33 0.93) | 0.56 | (0.29 1.06) |
| Resistance Type | PreXDR/XDR TB vs. MDRc TB | 345 | 1.55 | (0.81 2.97) | Not included | |
| Weight Status | Underweightd vs. normal | 372 | 2.59 | (1.37 4.87) | 2.65 | (1.37 5.13) |
| Culture+ > 6mose | Yes vs. No | 395 | 1.24 | (0.70 2.18) | Not included | |
Abbreviations: MDR, multidrug resistant; TB, tuberculosis; HR, hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval; HIV, human immunodeficiency virus; XDR, extensively drug resistant; mos, months.
Bivariate Cox proportional hazard models.
Multivariable Cox proportional hazard model, stratified on province and year of diagnosis.
Includes rifampicin mono-resistance.
Weight for age adjusted Z-score >2 standard deviations below the mean.
Any positive culture results 6 months or after start of first DR TB treatment regimen.
Figure 1.
Unadjusted Kaplan-Meier survival curves of poor treatment outcome during first 2-years of MDR TB treatment in three provinces, South Africa, 2005–2008.
Discussion
This analysis of pediatric patients with DR TB describes the clinical presentation and treatment outcomes of those diagnosed in three of the eastern provinces of South Africa. These results indicate that favorable outcomes are achievable in pediatric MDR TB patients, with 57.9% of the participants in our cohort experiencing cure or treatment completion outcome. However, the rate of death was higher among children who were HIV positive, male or underweight. The treatment success rate in our cohort of children with DR TB was higher than the global treatment success rate for all MDR TB cases, but lower than other published cohorts of pediatric MDR TB (refs: WHO global TB report 2015, hicks, seddon, isaadakis).
This is, to our knowledge, the largest cohort of DR TB pediatric patients with HIV infection and TB treatment outcome described in the literature. Patients in the MDR TB hospitals were required to have culture confirmation before admission and initiation of treatment. As a result, this population of patients was more likely to have advanced disease (11). Almost every child included in this cohort had a positive sputum culture and more than half were HIV-infected.
Despite the severity of disease and high prevalence of co-infection, over half of our participants experienced favorable outcomes—treatment completion or cure. Individualized treatment regimens provided by experienced providers in a hospitalized setting has been shown to be successful in both adults and children with DR TB (28, 34). However, HIV-infection was associated with higher mortality and survival analysis indicated that HIV-infection had an impact on mortality of children with drug-resistant TB. Overall, 9.7% of our cohort had XDR TB, which is very similar to adults in South Africa (10.5% XDR TB among MDR TB cases) (35). These results indicated that the proportion of children with XDR TB or PreXDR TB was lower among those infected with HIV. This may be because pediatric patients with HIV-infection and pre-XDR or XDR TB did not live long enough to be admitted to treatment facilities that require culture confirmation, but a consistent association between HIV infection and XDR TB has not been documented. Primary drug resistance rather than acquired drug resistance is more common among children. Therefore, we think it unlikely that the pediatric patients acquired pre-XDR or XDR TB, but, rather, that these differences by HIV status could be related to differences in HIV infection and drug resistance transmission patterns in each region.
The occurrence of death in our cohort was relatively low when compared to adult patients with DR TB (36), but these findings are similar to those from a previous study of 84 children with MDR TB in South Africa that reported 11% mortality (36). This is likely, in part, related to prolonged hospitalization in facilities where providers are experienced in treating DR TB both with and without HIV infection. It is unclear why male pediatric patients were more likely to have poor outcomes. Similar to a previous study, although HIV-infection was associated with shorter survival, malnutrition (based on weight-for-age Z-score) was the most predictive of death (36).
Limitations
This study has important limitations resulting from retrospective data collection limited to available medical records and charts. In general, medical charts at the MDR TB hospitals were comprehensive, though charting practices were inconsistent and variable across sites. Some data, such as contact history and history of antiretroviral therapy (ART), were often incomplete. When key data were unavailable in medical charts, they were obtained from laboratory and programmatic surveillance systems. Many of the HIV-infected patients did not have records of CD4 data or ART use. Therefore, we were unable to analyze the HIV-infected group by severity of HIV-related disease or by HIV treatment status. In our time-to-event analysis, about 25% of our participants had incomplete follow up data (lost to follow up, still on treatment, transferred or unknown). Finally, cause of death was not documented to a level of detail that could have enabled us to understand the influence of opportunistic infections (other than TB) on survival. Because we reviewed pediatric patients treated at MDR TB facilities, our findings are not generalizable to all children and adolescents with DR TB. More data from a variety of settings are needed to better understand true morbidity and mortality of all pediatric patients with DR TB.
Conclusion
The analysis of treatment outcomes among this large cohort of DR TB pediatric patients with HIV infection demonstrates that favorable outcomes are achievable. Although these patients were treated at MDR TB facilities by experienced clinicians, expertise and availability of medications to build effective regimens are improving. Based on history of previous treatment and contact to DR TB patients, there are opportunities for earlier diagnosis and treatment to improve outcomes for pediatric patients with DR TB. Renewed focus on early treatment with medications effective against resistant TB, life-saving ART, and nutritional rehabilitation will serve to improve outcomes among the most vulnerable of those affected by TB.
Acknowledgments
The authors would like to acknowledge Michael Chen for his statistical support in the analysis and Joey Lancaster from the South Africa Medical Research Center (MRC) for her contributions to this project.
Funding: This evaluation was supported with funding provided by the US Agency for International Development and the US Centers for Disease Control and Prevention (CDC), with additional support from the South Africa National Institute for Communicable Diseases of the National Health Laboratory Service and the South African Medical Research Council (Cooperative Agreement U23-CCU021809 FOA No. PS-07-006).
Footnotes
Conflict of Interest
None declared
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.World Health Organization. Global tuberculosis report 2015. 2015 9241565055. [Google Scholar]
- 2.Laserson KF, Thorpe LE, Leimane V, Weyer K, Mitnick CD, Riekstina V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2005;9(6):640–5. Epub 2005/06/24. PubMed PMID: 15971391. [PubMed] [Google Scholar]
- 3.Jenkins HE, Tolman AW, Yuen CM, Parr JB, Keshavjee S, Perez-Velez CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet (London, England) 2014;383(9928):1572–9. doi: 10.1016/s0140-6736(14)60195-1. Epub 2014/03/29. PubMed PMID: 24671080; PubMed Central PMCID: PMCPMC4094366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaaf HS, Gie RP, Kennedy M, Beyers N, Hesseling PB, Donald PR. Evaluation of young children in contact with adult multidrug-resistant pulmonary tuberculosis: a 30-month follow-up. Pediatrics. 2002;109(5):765–71. doi: 10.1542/peds.109.5.765. Epub 2002/05/03. PubMed PMID: 11986434. [DOI] [PubMed] [Google Scholar]
- 5.Schaaf HS, Marais BJ, Hesseling AC, Gie RP, Beyers N, Donald PR. Childhood drug-resistant tuberculosis in the Western Cape Province of South Africa. Acta paediatrica (Oslo, Norway : 1992) 2006;95(5):523–8. doi: 10.1080/08035250600675741. Epub 2006/07/11. PubMed PMID: 16825130. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–43. doi: 10.1016/s0140-6736(10)60410-2. Epub 2010/05/22. PubMed PMID: 20488523. [DOI] [PubMed] [Google Scholar]
- 7.Schaaf HS, Marais BJ, Hesseling AC, Brittle W, Donald PR. Surveillance of antituberculosis drug resistance among children from the Western Cape Province of South Africa--an upward trend. American journal of public health. 2009;99(8):1486–90. doi: 10.2105/ajph.2008.143271. Epub 2009/02/07. PubMed PMID: 19197080; PubMed Central PMCID: PMCPmc2707459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zignol M, Sismanidis C, Falzon D, Glaziou P, Dara M, Floyd K. Multidrug-resistant tuberculosis in children: evidence from global surveillance. The European respiratory journal. 2013;42(3):701–7. doi: 10.1183/09031936.00175812. Epub 2012/12/12. PubMed PMID: 23222872; PubMed Central PMCID: PMCPmc3759300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon JA, Hesseling AC, Marais BJ, Jordaan A, Victor T, Schaaf HS. The evolving epidemic of drug-resistant tuberculosis among children in Cape Town, South Africa. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2012;16(7):928–33. doi: 10.5588/ijtld.11.0679. Epub 2012/05/16. PubMed PMID: 22583610. [DOI] [PubMed] [Google Scholar]
- 10.Fairlie L, Beylis NC, Reubenson G, Moore DP, Madhi SA. High prevalence of childhood multi-drug resistant tuberculosis in Johannesburg, South Africa: a cross sectional study. BMC infectious diseases. 2011;11:28. doi: 10.1186/1471-2334-11-28. Epub 2011/01/29. PubMed PMID: 21269475; PubMed Central PMCID: PMCPmc3045316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaaf HS, Garcia-Prats AJ, Hesseling AC, Seddon JA. Managing multidrug-resistant tuberculosis in children: review of recent developments. Current opinion in infectious diseases. 2014;27(3):211–9. doi: 10.1097/qco.0000000000000062. Epub 2014/04/23. PubMed PMID: 24751893. [DOI] [PubMed] [Google Scholar]
- 12.Friedland G. Tuberculosis, drug resistance, and HIV/AIDS: a triple threat. Current infectious disease reports. 2007;9(3):252–61. doi: 10.1007/s11908-007-0039-7. Epub 2007/04/14. PubMed PMID: 17430708. [DOI] [PubMed] [Google Scholar]
- 13.Chintu C. Tuberculosis and human immunodeficiency virus co-infection in children: management challenges. Paediatric respiratory reviews. 2007;8(2):142–7. doi: 10.1016/j.prrv.2007.05.003. Epub 2007/06/19. PubMed PMID: 17574158. [DOI] [PubMed] [Google Scholar]
- 14.Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. The Journal of infectious diseases. 2007;196(Suppl 1):S86–107. doi: 10.1086/518665. Epub 2007/08/30. PubMed PMID: 17624830. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi NR, Shah NS, Andrews JR, Vella V, Moll AP, Scott M, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. American journal of respiratory and critical care medicine. 2010;181(1):80–6. doi: 10.1164/rccm.200907-0989OC. Epub 2009/10/17. PubMed PMID: 19833824. [DOI] [PubMed] [Google Scholar]
- 16.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(Suppl 3):S184–94. doi: 10.1086/651490. Epub 2010/04/20. PubMed PMID: 20397947. [DOI] [PubMed] [Google Scholar]
- 17.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2004;8(5):636–47. Epub 2004/05/13. PubMed PMID: 15137548. [PubMed] [Google Scholar]
- 18.Hesseling AC, Kim S, Madhi S, Nachman S, Schaaf HS, Violari A, et al. High prevalence of drug resistance amongst HIV-exposed and -infected children in a tuberculosis prevention trial. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2012;16(2):192–5. doi: 10.5588/ijtld.10.0795. Epub 2012/01/13. PubMed PMID: 22236919; PubMed Central PMCID: PMCPmc3265022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesfin YM, Hailemariam D, Biadgilign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PloS one. 2014;9(1):e82235. doi: 10.1371/journal.pone.0082235. Epub 2014/01/15. PubMed PMID: 24416139; PubMed Central PMCID: PMCPmc3885391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seddon JA, Hesseling AC, Godfrey-Faussett P, Fielding K, Schaaf HS. Risk factors for infection and disease in child contacts of multidrug-resistant tuberculosis: a cross-sectional study. BMC infectious diseases. 2013;13:392. doi: 10.1186/1471-2334-13-392. Epub 2013/08/28. PubMed PMID: 23977834; PubMed Central PMCID: PMCPmc3765928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velasco M, Castilla V, Sanz J, Gaspar G, Condes E, Barros C, et al. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. Journal of acquired immune deficiency syndromes (1999) 2009;50(2):148–52. doi: 10.1097/QAI.0b013e31819367e7. Epub 2009/01/10. PubMed PMID: 19131895. [DOI] [PubMed] [Google Scholar]
- 22.Satti H, McLaughlin MM, Hedt-Gauthier B, Atwood SS, Omotayo DB, Ntlamelle L, et al. Outcomes of multidrug-resistant tuberculosis treatment with early initiation of antiretroviral therapy for HIV co-infected patients in Lesotho. PloS one. 2012;7(10):e46943. doi: 10.1371/journal.pone.0046943. Epub 2012/11/02. PubMed PMID: 23115633; PubMed Central PMCID: PMCPmc3480376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arentz M, Pavlinac P, Kimerling ME, Horne DJ, Falzon D, Schunemann HJ, et al. Use of anti-retroviral therapy in tuberculosis patients on second-line anti-TB regimens: a systematic review. PloS one. 2012;7(11):e47370. doi: 10.1371/journal.pone.0047370. Epub 2012/11/13. PubMed PMID: 23144818; PubMed Central PMCID: PMCPmc3489892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satti H, McLaughlin MM, Omotayo DB, Keshavjee S, Becerra MC, Mukherjee JS, et al. Outcomes of comprehensive care for children empirically treated for multidrug-resistant tuberculosis in a setting of high HIV prevalence. PloS one. 2012;7(5):e37114. doi: 10.1371/journal.pone.0037114. Epub 2012/05/26. PubMed PMID: 22629356; PubMed Central PMCID: PMCPmc3358299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas TA, Shenoi SV, Heysell SK, Eksteen FJ, Sunkari VB, Gandhi NR, et al. Extensively drug-resistant tuberculosis in children with human immunodeficiency virus in rural South Africa. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14(10):1244–51. Epub 2010/09/17. PubMed PMID: 20843414; PubMed Central PMCID: PMCPmc3030274. [PMC free article] [PubMed] [Google Scholar]
- 26.Seddon JA, Hesseling AC, Willemse M, Donald PR, Schaaf HS. Culture-confirmed multidrug-resistant tuberculosis in children: clinical features, treatment, and outcome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(2):157–66. doi: 10.1093/cid/cir772. Epub 2011/11/05. PubMed PMID: 22052896. [DOI] [PubMed] [Google Scholar]
- 27.Schaaf HS, Marais BJ. Management of multidrug-resistant tuberculosis in children: a survival guide for paediatricians. Paediatric respiratory reviews. 2011;12(1):31–8. doi: 10.1016/j.prrv.2010.09.010. Epub 2010/12/22. PubMed PMID: 21172673. [DOI] [PubMed] [Google Scholar]
- 28.Ettehad D, Schaaf HS, Seddon JA, Cooke GS, Ford N. Treatment outcomes for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. The Lancet Infectious diseases. 2012;12(6):449–56. doi: 10.1016/s1473-3099(12)70033-6. Epub 2012/03/01. PubMed PMID: 22373593. [DOI] [PubMed] [Google Scholar]
- 29.Moore BK, Anyalechi E, van der Walt M, Smith S, Erasmus L, Lancaster J, et al. Epidemiology of drug-resistant tuberculosis among children and adolescents in South Africa, 2005–2010. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(6):663–9. doi: 10.5588/ijtld.14.0879. Epub 2015/05/07. PubMed PMID: 25946356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaakidis P, Casas EC, Das M, Tseretopoulou X, Ntzani EE, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(8):969–78. doi: 10.5588/ijtld.15.0123. Epub 2015/07/15. PubMed PMID: 26162364. [DOI] [PubMed] [Google Scholar]
- 31.WHO. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children. World Health Organization; 2014. [PubMed] [Google Scholar]
- 32.World Health Organization. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: 2011 Update. Geneva: World Health Organization; 2011. WHO Guidelines Approved by the Guidelines Review Committee. [Google Scholar]
- 33.World Health Organization. Guidelines for the Programmatic Management of Drug-resistant Tuberculosis: Emergency Update. Geneva: World Health Organization; 2008. [Google Scholar]
- 34.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. The Lancet Infectious diseases. 2009;9(3):153–61. doi: 10.1016/s1473-3099(09)70041-6. Epub 2009/02/28. PubMed PMID: 19246019. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. Geneva: 2010. [Google Scholar]
- 36.Hicks RM, Padayatchi N, Shah NS, Wolf A, Werner L, Sunkari VB, et al. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2014;18(9):1074–83. doi: 10.5588/ijtld.14.0231. Epub 2014/09/06. PubMed PMID: 25189555. [DOI] [PubMed] [Google Scholar]