Abstract
Varenicline reduces drinking in people with alcohol use disorder, but little is known about the mechanisms underlying this effect. Varenicline targets α4β2 and α7 nicotinic acetylcholine receptors, which are associated with several cognitive functions such as working memory. Varenicline may improve drinking outcomes by enhancing cognitive functioning. The current manuscript reports on cognitive outcomes from a placebo-controlled, double-blind human laboratory experiment examining the effects of varenicline on drinking behavior (Verplaetse et al., 2016a). Participants were 55 adult heavy drinkers who met criteria for an alcohol use disorder. They were randomized to receive varenicline (1 mg/day, 2 mg/day) or placebo. They completed a baseline assessment of cognitive functioning (i.e., digits backwards task, continuous performance task) before starting medication. Following a medication titration period, they attended a laboratory session (post medication day 8) where they completed the cognitive assessment battery and an alcohol-primed ad libitum drinking task. Blood was collected to measure plasma varenicline levels. Varenicline produced dose-dependent improvements in working memory. Although there was no significant effect of oral varenicline dose on response time on the continuous performance task, participants with higher levels of plasma varenicline showed greater improvement of RT. Among participants receiving 2 mg/day varenicline, larger improvements in working memory were associated less drinking, although mediation analyses did not find a significant indirect effect. These findings suggest that varenicline can improve working memory above baseline levels in heavy drinkers. Varenicline may reduce rates of alcohol use by improving working memory.
Keywords: varenicline, working memory, cognitive enhancement, alcohol use disorder, laboratory study
Introduction
Varenicline (Chantix; Pfizer, New York) is a partial agonist of nicotinic acetylcholine receptors (nAChRs) that was initially developed as a smoking cessation aid (Gonzales et al., 2006). Although varenicline is thought to reduce smoking by acting on α4β2, the drug also acts on other nAChR subunits, such as α7 receptors (Mihalak, Carroll, & Luetje, 2006). Given the role of the cholinergic system in higher-order cognitive functions, such as working memory (Chan, Wong, & Sheu, 2007), it is possible that varenicline improves smoking outcomes by enhancing cognitive performance. Indeed, prior clinical trials have shown that varenicline can improve cognitive performance in people with a range of disorders, including people with schizophrenia (Shim et al., 2012) and nicotine dependence (Patterson et al., 2010). However, no study to date has examined whether varenicline can improve cognitive function in heavy drinkers or whether these improvements are associated with drinking outcomes.
Recent research has shown that varenicline can also be used to reduce rates of alcohol use. Initially, a preclinical study found that varenicline-treated rats consumed less alcohol than untreated rats in both operant response and free access paradigms (Steensland, Simms, Holgate, Richards, & Bartlett, 2007). In the human laboratory, varenicline reduced rates of alcohol consumption and craving among non-treatment seeking heavy drinkers (McKee et al., 2009; Verplaetse et al., 2016a). Subsequent clinical trials in treatment-seeking drinkers with alcohol use disorder (AUD) also have supported the notion that varenicline can reduces rates of harmful drinking (Fucito et al., 2011; Litten et al., 2013), although a similar clinical trial in people with AUD found no difference between varenicline and placebo in drinking outcomes (de Bejczy et al., 2015). Overall, this body of work supports that varenicline reduces rates of alcohol use (see Erwin & Slaton, 2014 for a comprehensive review).
Despite consistent evidence that varenicline can reduces rates of drinking, less is known about the mechanisms by which the drug exerts this effect. One possibility is that varenicline reduces rates of drinking by enhancing cognitive performance. Impairments of cognitive functioning is common among individuals with AUD (Bates, Bowden, & Barry, 2002), and improvements in cognitive functioning may mediate reductions in drinking among people with AUD in treatment (reviewed in Bates, Buckman, & Nguyen, 2013). Even among substance users without significant cognitive impairments, cognitive enhancements can still be beneficial as it allows better adherence to goal-directed behavior such as limiting drinking or resisting cravings (Houben, Wiers, & Jansen, 2011; Sofuoglu, DeVito, Waters, & Carroll, 2013). Although it is established that varenicline improves cognitive functioning in smokers (Hong et al., 2011; Patterson et al., 2009; Rhodes, Hawk, Ashare, Schlienz, & Mahoney, 2012; Smith et al., 2009), many of these studies are conducted in physically dependent smokers who are attempting to quit or are otherwise nicotine deprived. Temporary cognitive impairment is a common symptom of nicotine withdrawal (Shiffman, Paty, Gnys, Kassel, & Elash, 1995). It is therefore difficult to determine in these studies whether varenicline improves cognitive performance by reducing withdrawal symptoms in deprived nicotine users (Ashare & Schmidt, 2014) or produces a more general improvement above baseline. This is an important distinction for understanding whether cognitive enhancing effects of varenicline mediate the drug’s effect on drinking.
The current study sought to characterize the effects of varenicline on cognitive functioning in heavy drinking adults by reanalyzing data from a human laboratory study that examined the effects of varenicline (1/mg day, 2 mg/day) on alcohol use outcomes. Results examining medication effects on drinking outcomes are reported in the parent publication (Verplaetse et al., 2016a). Cognitive outcomes are not reported in the parent publication; these results are newly reported in the current study. Cognitive performance was assessed using the digit backwards task (working memory) and a continuous performance task (reaction time, sustained attention, response inhibition). We hypothesized that varenicline would produce a dose-dependent increase in cognitive performance, such that participants receiving active varenicline would show improved cognitive performance compared to those receiving placebo. Further, we predicted that varenicline-induced improvement in cognitive functioning would predict drinking outcomes, such that participants who showed the greatest improvement under varenicline would drink less when given ad libitum access to alcohol. In the parent publication for this study, we found significant variability in plasma varenicline levels, even among participants receiving identical varenicline doses. In the current analysis, we also examined the association among degree of improvement in cognitive performance and plasma varenicline levels.
Method
Participants
Volunteers were eligible to participate if they were ≥ 21 years of age and could read and speak English. Data were collected prior to the transition to the DSM5, so all participants met DSM-IV criteria for past 6-month alcohol abuse or dependence. They also endorsed recent patterns of heavy drinking (binge drinking at least once per week over the past 30 days, defined as ≥ 4/5 drinks in a single episode women/men, respectively). Exclusion criteria included illicit drug use (except for occasional cannabis use defined as use not associated with cannabis dependence), past 30-day use of psychoactive drugs, having sought treatment to reduce alcohol or tobacco use, current suicidal or homicidal ideation, pregnancy or nursing, or a medical condition contraindicating alcohol use (e.g., liver enzymes ≥ 3× normal) or varenicline administration. Volunteers who met criteria for a serious and persistent mental illness (e.g., psychotic spectrum disorder, bipolar disorder) were not invited to participate, nor were those deemed to be at high risk for suicide. Volunteers at risk to undergo alcohol withdrawal, as indicated by an elevated score on the CIWA-Ar (Sullivan, Sykora, Schneiderman, Naranjo, & Sellers, 1989), were not invited to participate.
Experimental Design
The parent study was a double-blind, placebo-controlled, parallel-group human laboratory experiment. Participants were randomized to receive varenicline (1 mg/day, 2 mg/day) or placebo. Assignment was stratified by sex and smoking status. Additional methodological details concerning the study design, procedures, and materials are reported in the primary manuscript associated with this experiment (Verplaetse et al., 2016a). A group of the participants also completed alcohol-challenge sessions between 2 and 3 weeks post-randomization (Verplaetse et al., 2016b). The parent project is registered with ClinicalTrials.gov, NCT00580645 (Study 1c).
Materials and Measures
Alcohol use
Participants’ drinking habits were assessed using the Timeline Follow-back (TLFB) procedure (Sobell & Sobell, 1992). This protocol uses a structured calendar anchored with holidays and other notable events to assist participants in recording their drinking behavior. Two measures from the TLFB are reported in the current study, including (1) the average number of drinks consumed per week, and (2) the average number of drinking days per week. Participants also completed the alcohol use disorders identification test (AUDIT) as a measure of alcohol use problem severity (Saunders, Aasland, Babor, de la Fuente, & Grant, 1993). These measures were used to assess for baseline differences between groups in drinking habits.
Digit span backwards
The digit span backwards is a subtest of the Wechsler intelligence and memory batteries (Wechsler, 1997). Participants were read a series of numbers and required to repeat the digit sequence back to the research assistant in reversed order. The first items on the task require the participant to recall two numbers and the length of the span increases until the participant misses two trials consecutively. This task requires storage of information for mental manipulation of the digits and is considered a measure of working memory capacity (Baddeley, 1992; Conklin, Curtis, Katsanis, & Iacono, 2000; Conway et al., 2005). Raw scores are reported, indicating the total number of trials where the participants correctly recalled the string of numbers. Possible scores ranged from 0 to 14.
Conners’ Continuous Performance Task (CPT)
The CPT is a computerized assessment tool that measures several aspects of cognitive performance (Conners & Staff, 2000). Participants viewed a series of letters on a computer monitor for 14 minutes. They were instructed to respond as quickly as possible to target stimuli (all letters but “X”) and the refrain from responding to the infrequent non-target stimuli (“X”). Criterion variables included reaction time to target stimuli (RT), variability of response time to go targets (RTvar) and the percentage of non-target (X) trials during which participants made a response (commission errors).
Procedures
Eligibility screening
The human investigation committee of Yale University approved this study and written informed consent was obtained. Participants underwent an extensive eligibility screening, including physical examination, electrocardiogram, urine toxicology, pregnancy test, and basic blood chemistries.
Medication
Varenicline and matching placebo were provided by Pfizer (NY), and were overencapsulated with riboflavin added to monitor compliance. Varenicline was titrated to steady-state levels over 7 days. Medication compliance was monitored with pill counts and riboflavin marker on days 5 and 8 (Del Boca et al., 1996). Plasma levels were assessed at the start of the laboratory session on day 8. Additional details about our medication dosing strategies are reported in the parent publication (Verplaetse et al., 2016a).
Laboratory session
Eight days after starting medication, each participant completed a 14-hour laboratory session conducted at the Yale Center for Clinical Investigation, New Haven, Connecticut. The laboratory procedures were like those used in our previous alcohol self-administration studies (McKee et al., 2009), which conform to NIAAA guidelines for alcohol administration (National Advisory Council on Alcohol Abuse and Alcoholism, 2005). The timing of the laboratory session was as follows: Participants arrived at the laboratory at 8:00 AM and their final dose of medication was provided at 9:00 AM. Smokers were allowed smoke at 10:00 am, 12:00 pm, and 2:00 pm on the research unit in a negative pressure room to ensure that they were not nicotine deprived. They completed the cognitive assessment battery between 11:15am and noon.
Priming dose
The alcohol priming drinks were administered from 3:00 to 3:05 pm and consisted of 1 part 80-proof liquor of the participant’s choosing and 3 parts mixer chosen from a selection of equally caloric, non-caffeinated, noncarbonated beverages. Doses were calculated to produce a blood alcohol concentration (BAC) of 0.030 g/dL based on Watson’s (1989) formula that considers participants’ body weight, height, sex, and age to estimate total body water. BAC data following the priming dose are described in the parent publication (Verplaetse et al., 2016a).
Alcohol self-administration
Participants started the first and second hour of alcohol self-administration 50 and 120 minutes after consuming the priming drink, respectively. Each ad-libitum drinking period lasted 1 hour and participants were able to drink up to 4 alcoholic beverages (maximum = 8 drinks total over both 1-hour self-administration sessions). Doses were based on Watson’s (1989) formula and were calculated such that each drink would raise BAC by 0.015 g/dL. At the beginning of the first self-administration session, participants were given a drinking tab ($24) and told that they could purchase up to 4 drinks each session. Participants kept $3 for each drink they did not consume, which incentivized their abstinence during this task and modeled a situation in which reducing drinking was a desirable outcome. They were given free access to their drinks and their drinking tab was totaled after the end of the self-administration periods.
Plasma varenicline levels
Human plasma containing VAR and the internal standard, Varenicline-15N2D2 was extracted using a solid-phase extraction method for the determination of VAR in human K2-EDTA plasma by Worldwide Clinical Trials, Austin, TX. The range of quantitation was 0.0500 to 10.0 ng/mL based on the analysis of 0.500 mL of plasma. Blood was drawn immediately at the beginning of the laboratory session on day 8.
Data Analyses
One participant was identified as an outlier due to a lack of effort on the digit backwards task (i.e., failed to recall 2 items during the first assessment and successfully recalled a string of 7 items during the second assessment; change score = 3.6 SD above mean). Data from this participant were removed. Some participants elected not to complete the computerized assessment potion of the experiment, which included the continuous performance task. The number of participants who completed the CPT included 14, 18, and 17 in the placebo, 1 mg/day, 2 mg/day varenicline conditions, respectively. Analyses of variance and chi-square analyses were used to test for group differences in demographic and baseline substance use variables.
Primary outcome variables of interest were scores on the cognitive assessments, plasma varenicline levels, and ad-libitum alcohol consumption during the free access period. Data from the cognitive tests were analyzed using 2 (time: baseline versus laboratory session) × 3 (dose: placebo, 1 mg/day, 2 mg/day) mixed-design analyses of variance (ANOVAs). If the time × dose interaction of these omnibus ANOVAs failed to reach significance, we used two a priori analyses to identify significant effects. First, we conducted a reduced-model 2 (time) × 2 (dose: placebo vs. 2 mg/day) ANOVA to determine whether the maximum dose of varenicline improved cognitive functioning compared to placebo. Models with significant time × medication effects were rerun with gender and smoking status (smoker versus nonsmoker) included as covariates Second, we used polynomial contrasts to test for a varenicline dose-dependent linear increase in cognitive performance. These polynomial contrasts were conducted using analyses of covariance (ANCOVAs) that were similar to the omnibus mixed-design ANOVAs except that baseline cognitive functioning was included as a covariate and time was not included as a factor. All analyses were first conducted with smoking status and gender included as covariates. If the inclusion of these covariates did not change the significance of either main effect (time or medication condition) or the time × medication condition interaction, then results are presented without covariates.
We also examined whether varenicline plasma levels were associated with improvements in cognitive performance. We calculated difference scores (i.e., post-medication performance – premedication performance) that were always coded such that more positive values indicated greater improvements under medication. Zero-order correlation analyses were used to test whether plasma varenicline levels were associated with pre- to post-medication improvements. For these correlations, all participants with available data were included in each analysis, regardless of their medication condition.
The second set of analyses examined whether varenicline-induced improvements in cognitive performance were associated with ad libitum drinking outcomes. These analyses were guided by the results of the dose-response analyses in that we only conducted these analyses for cognitive functions that evidenced a response to varenicline according to the analyses by oral dose or plasma varenicline levels. Bivariate correlation analyses tested for a significant association among improvements in cognitive performance and the drinking behavior. We tested these correlations with each self-administration period (range = 0–4) and total number of drinks across both periods (range = 0–8) as dependent variables. Acknowledging the potential for a non-normal distribution of ad libitum drinks consumed, we re-ran these correlations using Spearman’s rank order correlations, which are robust against normality violations (Kowalski, 1975). The correlation coefficients from these analyses were consistent with those from the bivariate analyses, so we present the bivariate correlations below. For significant correlations between cognitive enhancement and drinking outcomes, we conducted mediational analyses to test the significance of the indirect path from medication condition to alcohol self-administration via improvements in cognitive performance using the product-of-coefficients approach (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002). These analyses only tested for mediation in medication conditions where cognitive enhancement was evident. A bootstrapping procedure (5000 replications) was used to estimate the standard error of the indirect effect.
Results
Demographic, Alcohol Use, and Smoking
Participants demographic information is reported in Table 1. As seen in this table, groups did not differ in terms of demographic makeup or alcohol use and smoking variables.
Table 1.
Baseline demographic, alcohol use, and smoking variables
| Placebo (n = 15) |
1 mg/day varenicline (n = 20) |
2 mg/day varenicline (n = 20) |
|
|---|---|---|---|
|
|
|||
| Age (years) | 35.27 (9.25) | 33.35 (8.51) | 34.15 (11.59) |
| Sex (% male) | 67% | 60% | 75% |
| Race | |||
| White | 9 (60%) | 10 (50%) | 15 (75%) |
| Other | 6 (40 %) | 10 (50%) | 5 (25%) |
| Marital status | |||
| Not married | 12 (80%) | 16 (80%) | 19 (95%) |
| Married | 3 (20%) | 4 (20%) | 1 (5%) |
| Alcohol use | |||
| AUDIT | 13.67 (7.00) | 13.45 (5.01) | 10.75 (3.92) |
| Drinks/week | 36.24 (29.21) | 32.89 (13.62) | 29.32 (17.57) |
| Drinking episodes/week | 5.33 (1.69) | 4.73 (1.59) | 4.75 (1.61) |
| Smoking Status | |||
| Smokers | 10 (67%) | 13 (65%) | 12 (60%) |
| Nonsmokers | 5 (33%) | 7 (35%) | 8 (40%) |
| Smoking* | |||
| FTND | 5.00 (2.58) | 5.23 (2.09) | 4.67 (2.10) |
| Cig/Day | 14.76 (6.62) | 12.56 (4.98) | 15.95 (6.26) |
Note. AUDIT is the alcohol use disorder identification test. Drinks/week and drinking/episodes week report values from the timeline follow-back. FTND is the Fagerstrom Test of Nicotine Dependence.
Variables reported for only smokers (n =35). No ANOVA or chi-square test found any significant difference between groups.
Varenicline Effects on Cognitive Performance
Digit span backwards
Medication effects on digit backwards scores are charted in Figure 1. There was a significant main effect of time, F (1, 52) = 7.81, p = 0.007, ηp2 = 0.131, but no significant time × medication condition interaction, F (2, 52) = 2.29, p = 0.111, ηp2 = 0.081. However, the reduced-model ANOVA found a significant time × medication condition interaction, F (1, 33) = 5.84, p = 0.021, ηp2 = 0.150, confirming that participants receiving 2 mg/day varenicline showed greater improvements in digit span backwards than did those on placebo. The linear polynomial contrast was significant, p = 0.036, suggesting a dose-dependent improvement in digit span backwards.
Figure 1.
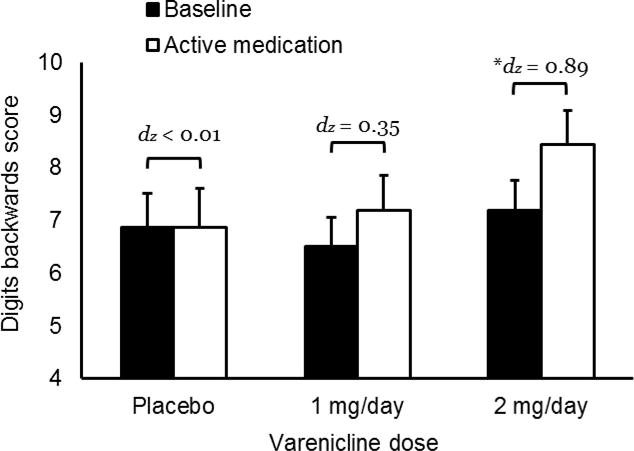
Effects of varenicline on digit backwards score. Capped bars indicated standard error. Starred bracket indicates significant difference between baseline and active medication, *p = 0.001. dz is the effect size.
Reaction time
Medication effects on RT are charted in Figure 3. There was no significant main effect of time, F (1, 46) = 1.06, p = 0.309, ηp2 = 0.023, or medication × time interaction, F (2, 46) = 1.56, p = 0.222, ηp2 = 0.063. Likewise, the reduced-model ANOVA found no significant medication × time interaction, F (1, 29) = 2.38, p = 0.133, ηp2 = 0.076. The linear polynomial contrast was not significant, p = 0.130.
Figure 3.
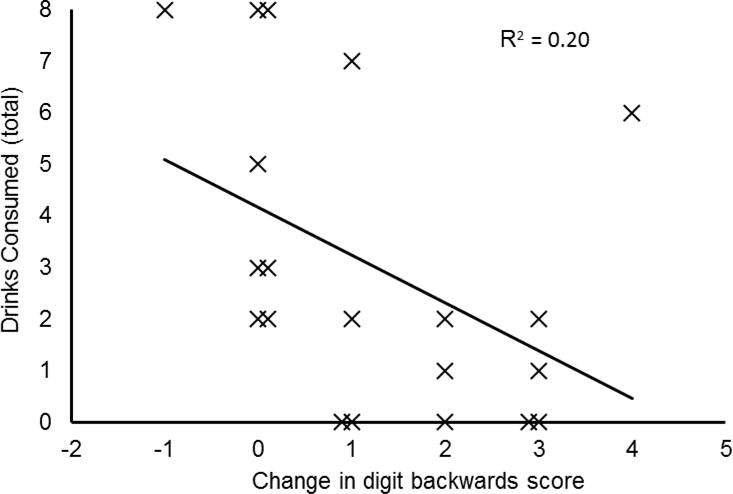
Association between medication-induced improvements on digit backwards task and number of drinks consumed during the ad libitum consumption task. Solid line shows the least squares regression line of best fit with coefficient of determination. The associations in the placebo and 1 mg/day conditions were not significant and are not shown.
Response time variability
Medication effects on RTvar are reported in a supplemental table. There was no significant main effect of time, F (1, 46) = 0.43, p = 0.516, ηp2 = 0.009, or time × medication condition interaction, F (2, 46) = 0.19, p = 0.830, ηp2 = 0.008. The reduced model ANOVA found no significant time × medication condition interaction, F (1, 29) = 1.03, p = 0.319, ηp2 = 0.79. The linear polynomial contrast was not significant, p = 0.280.
Commission errors
Medication effects on commission errors are reported in a supplemental table. There was no significant main effect of time, F (1, 46) < 0.01, p = 0.992, ηp2 < 0.001, nor was there a significant time × medication condition interaction, F (2, 46) = 1.57, p = 0.219, ηp2 = 0.064. The reduced model ANOVA found no significant time × medication condition interaction, F (1, 29) = 1.05, p = 0.315, ηp2 = 0.016. The linear polynomial contrast was not significant, p = 0.611.
Association between Plasma Varenicline and Cognitive Enhancement
Mean plasma varenicline concentration was 2.36 ng/mL (SD = 1.23) among those receiving 1 mg/day and 4.60 ng/mL (SD = 1.86) among those receiving 2 mg/day. Higher concentrations of plasma varenicline were associated with greater improvements in digit span backwards, r (53) = 0.299, p = 0.027, and faster RT on the continuous performance task, r (47) = 0.417, p = 0.003. There was no significant correlation between plasma varenicline and improvements in RT variability, r (47) = 0.095, p =−0.516, or commission errors, r (47) = −0.052, p = 0.723.
Drinking Outcomes
Correlational analyses summarizing the association between degree of cognitive enhancement and drinking outcomes are presented in Table 2. As seen in this table, among participants receiving 2 mg/day varenicline, those who showed the most improvement in digit span backwards also drank less when given ad-libitum access to alcohol. This relation was strongest when limiting the analysis to drinks consumed during the first period of free access, but the association was still significant when considering total drinks across both periods. Improvements in response time were associated with less drinking during the second period of free access, although there was no significant relation with total number of drinks across both periods. Medication dose effects on drinking behavior are described in the parent publication (Verplaetse et al., 2016). Briefly, evidence supported that 2mg varenicline reduced consumption, primarily in the first period.
Table 2.
Association between medication improvements in cognitive functioning and ad-libitum alcohol consumption
| 1 mg/day varenicline | P1 drinks | P2 drinks | Total drinks | |||
|---|---|---|---|---|---|---|
|
| ||||||
| r | p | r | p | r | p | |
|
|
|
|||||
| RT med effect | 0.11 | 0.66 | −0.13 | 0.605 | −0.02 | 0.951 |
| Digits backward med effect | 0.17 | 0.47 | 0.04 | 0.877 | 0.11 | 0.659 |
|
| ||||||
| 2 mg/day varenicline | ||||||
|
| ||||||
| RT med effect | −0.28 | 0.278 | −0.57 | 0.016* | −0.46 | 0.062 |
| Digits backward med effect | −0.62 | 0.003* | −0.24 | 0.310 | −0.45 | 0.048* |
Note. Reported values are correlation coefficients. P1 and P2 drinks are number of drinks consumed during the first and second free access period. Total drinks is the total number of drinks consumed across both periods. Med effects are degree of improvement that occurred after starting medication compared to baseline performance.
p < .05.
Mediation analyses tested the significance of the indirect effect of medication condition (placebo versus 2 mg/day varenicline) on total number of drinks via improvements in working memory. The indirect effect was not significant, b = −0.45, SE = 0.55, 95% CI = −2.05 – 0.27, p > 0.05. Full mediational analyses are presented in a supplemental table.
Discussion
The current study tested whether varenicline would enhance cognitive functioning in a group of heavy drinking adults. Results were generally consistent with our hypotheses. There was a dose-dependent increase in working memory performance observed following the medication titration period, and higher concentrations of plasma varenicline were associated with greater improvements in working memory. This finding is consistent with prior research showing that varenicline can improve working memory in smokers (e.g., Loughead et al., 2010). Evidence that varenicline speeded RT was mixed. Although there was no significant effect of oral dose on RT, participants who showed higher plasma varenicline levels showed faster RT. These findings provide additional support for our previous conclusions that plasma varenicline is a more sensitive indicator of drug effect compared to oral dose (Verplaetse et al., 2016a).
Another goal of this study was to test whether varenicline-induced cognitive enhancement was associated with drinking outcomes in a laboratory model of alcohol-primed drinking. We found that improved working memory and, to a lesser extent, faster RT were associated with less drinking following alcohol prime. This study is the first to show that cognitive enhancement under varenicline is associated with drinking behavior. Working memory is a potential treatment target for the management of substance use disorders (Bickel, Yi, Landes, Hill, & Baxter, 2011; Houben et al., 2011; Sofuoglu et al., 2013). Our findings here provide direct support for this hypothesis by demonstrating a strong association between medication-induced improvements in working memory and reduced alcohol use following alcohol prime. Interestingly, there was no significant association among medication response and drinking behavior among participants receiving 1 mg/day varenicline. Other results reported in the parent publication (Verplaetse et al., 2016a) were consistent with this finding. Although there was no significant association between varenicline plasma levels and alcohol drinking overall, a significant relationship emerged when the analysis was restricted to participants with varenicline plasma levels greater than 3 ng/mL, suggesting that a therapeutic threshold must be reached before varenicline influences behavior.
An associated study by our group was conducted in a subset of participants in the current sample to determine whether varenicline would reduce alcohol-impairment of cognitive functioning (Verplaetse et al., 2016b). The study found that participants maintained on varenicline who received a high dose of alcohol (0.08 g/dl) showed less alcohol impairment of working memory (N-back task), processing speed (digit-symbol substitution task), and motor control (pursuit-rotor task) compared to those maintained on placebo. Results of the current study extend these findings by showing that in addition to blocking alcohol impairment of these cognitive functions, varenicline also can enhance cognitive performance above baseline levels. This distinction is important and suggest that varenicline can cause general cognitive enhancement even in people who are abstinent from alcohol, which may help them maintain their abstinence.
Varenicline-induced improvement of working memory is consistent with the known pharmacological profile of the drug. Varenicline is a partial agonist at α4β2 and a full agonist at α7 nAChR (Mihalak et al., 2006). Both receptor subtypes have been implicated in working memory functioning (Chan et al., 2007; Levin, 2002), because activation of these receptors causes increased dopamine release in brain regions that are activated during working memory tasks (Livingstone et al., 2009). Loughead and colleagues (2010) used fMRI to examine varenicline-induced changes in brain regions associated with cognitive functioning in nicotine deprived smokers, finding that varenicline increased brain activity during a working memory task in associated regions. Other studies have shown that the α7 agonist ABT-126 has a similar enhancing effect on cognitive performance in patients with schizophrenia (Haig, Bain, Robieson, Baker, & Othman, 2016).
There is a strong theoretical rationale for why pharmacological enhancement of working memory should improve alcohol use outcomes. Dual-process models of substance use propose that drinkers rely on intact higher-order “executive” processes, such as working memory, to adhere to internally represented goals that require resisting more immediate and salient reinforcers (Goldstein & Volkow, 2002). Although participants in the current study were not treatment seeking, we utilized a laboratory model of motivated abstinence by monetarily incentivizing choosing not to drink. We have successfully used this strategy in the past to model motivated abstinence in medication screening studies with non-treatment seeking substance users (McKee, 2009). It was especially important to use this approach in the current study because the competing contingencies allowed us to observe how enhanced working memory might influence drinking behavior.
Despite the robust effect of varenicline on working memory functioning, our study found no evidence that inhibitory control or sustained attention improved under the drug. Other studies have administered batteries of cognitive assessments to people maintained on varenicline to identify which cognitive processes change following varenicline administration. Rhodes and colleagues (2012) found that non-abstinent smokers maintained on varenicline showed a reduction in attentional lapses but no change in inhibitory control on a stop-signal task. Our group (Ashare & McKee, 2012) previously found that varenicline speeded reaction time but did not have an effect on working memory in nicotine-deprived smokers. Smith and colleagues (2009) found that varenicline improved specific memory functions but had no effect on attention, whereas another study found that varenicline improved a wide array of cognitive functions, including RT and interference control, in smokers with schizophrenia (Shim et al., 2012). Another study in nonsmokers found that varenicline boosted memory functioning (Mocking et al., 2013). The discrepancies in findings likely occurred due to the methodological variability among the studies, including differences in their samples (e.g., smokers versus nonsmokers), testing conditions (e.g., nicotine deprived versus non-deprived) and the tasks used to assess cognitive functioning. Clearly more research is needed to draw firm conclusions about which cognitive functions are sensitive to varenicline and under what conditions these effects emerge.
Medications that are currently FDA approved to treat alcohol use disorder include naltrexone, disulfiram, and acamprosate. None of these medications are thought to reduce drinking by enhancing cognitive functioning. Indeed, neither naltrexone (Hatsukami, Mitchell, Morley, Morgan, & Levine, 1986) nor disulfiram (Peeke et al., 1979) have an effect on cognitive performance, and acamprosate may even hinder aspects of memory functioning (Schneider et al., 1999). This cognitive enhancement strategy for treating substance use disorder is important given that recent theoretical models of substance use disorder emphasize impaired cognitive control as a predisposing and maintaining factor (Goldstein & Volkow, 2011; Lyvers, 2000). Findings from the current study on varenicline may be particularly important because they identify a new mechanism by which a drug can reduce drinking behavior.
These findings have important implications for pharmacological management of heavy drinking, but findings should be interpreted considering some limitations. First, participants were non-treatment seeking heavy drinkers, and were therefore not highly motivated to reduce their alcohol consumption. It is possible that any improvements in cognitive functioning may have been more beneficial in a group of treatment-seeking participants. Such a possibility could be tested by examining working memory in the context of a clinical trial of varenicline in a group of treatment-seeking heavy drinkers. Second, the current reanalysis only included a single alcohol-use outcome. We did not test the association between medication effects on working memory and alcohol use outside of the laboratory. However, laboratory studies provide important information regarding the mechanisms by which medications can reduce substance use (McKee, 2009) and complement findings from larger clinical trials showing that varenicline can reduce alcohol use among treatment-seeking heavy drinkers outside of the laboratory (Litten et al., 2013). Third, we were not able to show statistically that improvements in working memory explained medication effects on alcohol self-administration using mediation analysis. This null finding may have resulted from Type II error because we had insufficient statistical power to detect mediation effects.
In sum, the current study found that varenicline improved working memory in a group of non-treatment seeking heavy drinkers, an effect that was associated with reduced alcohol use in a laboratory drinking paradigm. These findings are important because they identify a potential mechanism by which varenicline reduces drinking. Although 1 mg/day varenicline produced incremental improvements in working memory, evidence from the current study alongside related investigations (Verplaetse et al., 2016a) suggest that the currently recommended clinical dose (i.e., 2 mg/day) is necessary to produce optimal reductions in alcohol use. Researchers should continue to investigate the potential clinical utility of varenicline for managing alcohol use disorder.
Supplementary Material
Figure 2.
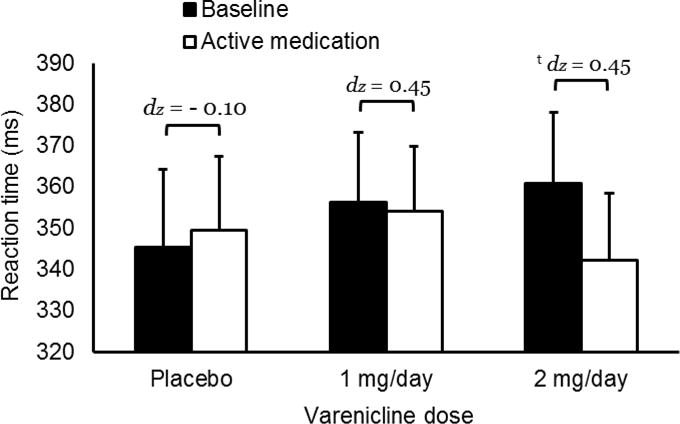
Effects of varenicline on reaction time during target trials on the continuous performance task. Capped bars indicate standard error. Bracket with t indicates that medication effect approached significance, p = 0.081. dz is effect size.
Public Health Significance.
Varenicline can reduce rates of alcohol consumption in people who drink heavily. One reason for this effect may be that varenicline improves cognitive functioning, which may afford drinkers greater control over their alcohol use. This study found that varenicline improved working memory functioning. Participants who showed the greatest improvement in working memory also drank less when given free access to alcohol. These findings support using varenicline to treat alcohol use disorder.
Footnotes
Primary outcome measures from this clinical trial were reported in the parent publication (Verplaetse et al., 2016a). These data have not been previously disseminated elsewhere.
References
- Ashare RL, McKee SA. Effects of varenicline and bupropion on cognitive processes among nicotine-deprived smokers. Experimental and Clinical Psychopharmacology. 2012;20:63–70. doi: 10.1037/a0025594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Schmidt HD. Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert Opinion on Drug Discovery. 2014;9:579–594. doi: 10.1517/17460441.2014.908180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Experimental and Clinical Psychopharmacology. 2002;10:193–212. doi: 10.1037/1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Bates ME, Buckman JF, Nguyen TT. A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychology Review. 2013;23:27–47. doi: 10.1007/s11065-013-9228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Wong PT, Sheu FS. Frontal cortical α7 and α4β2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology. 2007;52:1641–1649. doi: 10.1016/j.neuropharm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Curtis CE, Katsanis J, Iacono WG. Verbal working memory impairment in schizophrenia patients and their first-degree relatives: Evidence from the digit span task. American Journal of Psychiatry. 2000;157:275–277. doi: 10.1176/appi.ajp.157.2.275. [DOI] [PubMed] [Google Scholar]
- Conners CK, Staff M. Conners Continuous Performance Test II (CPT II V 5) North Tonawanda, NY: Multi-Health Systems; 2000. [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12:769–786. doi: 10.3758/Bf03196772. [DOI] [PubMed] [Google Scholar]
- de Bejczy A, Lof E, Walther L, Guterstam J, Hammarberg A, Asanovska G, Soderpalm B. Varenicline for treatment of alcohol dependence: A randomized, placebo-controlled trial. Alcoholism: Clinical and Experimental Research. 2015;39:2189–2199. doi: 10.1111/acer.12854. [DOI] [PubMed] [Google Scholar]
- Erwin BL, Slaton RM. Varenicline in the treatment of alcohol use disorders. Annals of Pharmacotherpy. 2014;48:1445–1455. doi: 10.1177/1060028014545806. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Varenicline Phase 3 Study Group Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Haig GM, Bain EE, Robieson WZ, Baker JD, Othman AA. A randomized trial to assess the efficacy and safety of ABT-126, a selective α7 nicotinic acetylcholine receptor agonist, in the treatment of cognitive impairment in schizophrenia. American Journal of Psychiatry. 2016;173:827–835. doi: 10.1176/appi.ajp.2015.15010093. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Mitchell JE, Morley JE, Morgan SF, Levine AS. Effect of naltrexone on mood and cognitive functioning among overweight men. Biological Psychiatry. 1986;21:293–300. doi: 10.1016/0006-3223(86)90050-8. [DOI] [PubMed] [Google Scholar]
- Hong LE, Thaker GK, McMahon RP, Summerfelt A, Rachbeisel J, Fuller RL, Nye A. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Archives of General Psychiatry. 2011;68:1195–1206. doi: 10.1001/archgenpsychiatry.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben K, Wiers RW, Jansen A. Getting a grip on drinking behavior: Training working memory to reduce alcohol abuse. Psychological Science. 2011;22:968–975. doi: 10.1177/0956797611412392. [DOI] [PubMed] [Google Scholar]
- Kowalski CJ. On the effects of non-normality on the distribution of the sample product-moment correlation coefficient. (Series C (Applied Statistics)).Journal of the Royal Statistical Society. 1975;21:1–12. doi: 10.2307/2346598. [DOI] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. Journal of Neurobiology. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Abuse, N. N. I. A. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. Journal of Addiction Medicine. 2013;7:277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, Wonnacott S. α7 and non- α7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. European Journal of Neuroscience. 2009;29:539–550. doi: 10.1111/j.1460-9568.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Lerman C. Effects of the α4β2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biological Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: a neuroscientific interpretation. Experimental and Clinical Psychopharmacology. 2000;8:225–249. doi: 10.1037/1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. doi: http://dx.doi.org/10.1037/1082-989X.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biological Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Molecular Pharmacology. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mocking RJ, Patrick Pflanz C, Pringle A, Parsons E, McTavish SF, Cowen PJ, Harmer CJ. Effects of short-term varenicline administration on emotional and cognitive processing in healthy, non-smoking adults: A randomized, double-blind, study. Neuropsychopharmacology. 2013;38:476–484. doi: 10.1038/npp.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeke SC, Prael AR, Herning RI, Rogers W, Benowitz NL, Jones RT. Effect of disulfiram on cognition, subjective response, and cortical-event-related potentials in nonalcoholic subjects. Alcoholism: Clinical and Experimental Research. 1979;3:223–229. doi: 10.1111/j.1530-0277.1979.tb05304.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JD, Hawk LW, Jr, Ashare RL, Schlienz NJ, Mahoney MC. The effects of varenicline on attention and inhibitory control among treatment-seeking smokers. Psychopharmacology (Berl) 2012;223:131–138. doi: 10.1007/s00213-012-2700-6. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schneider U, Wohlfarth K, Schulze-Bonhage A, Haacker T, Muller-Vahl KR, Zedler M, Emrich HM. Effects of acamprosate on memory in healthy young subjects. Journal of Studies on Alcohol. 1999;60:172–175. doi: 10.15288/jsa.1999.60.172. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine withdrawal in chippers and regular smokers: Subjective and cognitive effects. Health Psychology. 1995;14:301–309. doi: 10.1037/0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, Kelly DL. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37:660–668. doi: 10.1038/npp.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer JP, Davis JM, Cornwell J, Noth K, Gupta S, Lajtha A. Cognitive and antismoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophrenia Research. 2009;110:149–155. doi: 10.1016/j.schres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) British Journal of Addiction. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA. Effect of lowering the dose of varenicline on alcohol self-administration in drinkers with alcohol use disorders. Journal of Addiction Medicine. 2016a;10:166–173. doi: 10.1097/Adm.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA. Effect of varenicline combined with high-dose alcohol on craving, subjective intoxication, perceptual motor response, and executive cognitive function in adults with alcohol use disorders: Preliminary findings. Alcoholism: Clinical and Experimental Research. 2016b;40:1567–1576. doi: 10.1111/acer.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow K, Batt R, editors. Human metabolism of alcohol: Vol 1 Pharmacokinetics, mediolegal aspects, and general interest. Boca Raton, FL: CRC Press; 1989. pp. 41–58. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–III (WAIS–III) San Antonio, TX: The Psychological Corportation; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


