Abstract
The glutamate hypothesis proposes that NMDA receptor hypofunction underlies cognitive and perhaps other schizophrenic symptoms. The present study used the odor span task to assess the effects of NMDA antagonists on remembering multiple stimuli in rodents. This task uses an incrementing non-matching-to-sample procedure in which responses to a new olfactory stimulus are reinforced on each trial, whereas responses to previously presented stimuli are not. NMDA antagonists have been associated with memory impairments in a variety of animal models, however, there are inconsistencies across different NMDA antagonists and tasks used. The current study compared the acute effects of phencyclidine, ketamine, and the novel NMDA antagonist methoxetamine on responding in the odor span task and a simple discrimination control task. Phencyclidine and methoxetamine impaired odor span accuracy at doses that did not impair simple discrimination in most rats, however the effects of ketamine were less selective. Within-session analyses indicated that the effects of phencyclidine and methoxetamine depended on the number of stimuli to remember, i.e., impairment only occurred when the memory load was relatively high. These effects of phencyclidine and methoxetamine were consistent with the hypothesis that NMDA antagonists may interfere with working memory, but the basis for less selective results with ketamine are unclear.
Keywords: NMDA antagonist, working memory, odor span task, ketamine, phencyclidine, methoxetamine
The initial impetus for the NMDA hypofunction hypothesis of schizophrenia was the observation that NMDA receptor antagonists like phencyclidine and ketamine produced many of the symptoms that characterize the disorder in humans, including the full spectrum of positive, negative and cognitive symptomatology (Merritt, McGuire & Egerton, 2013; Moghaddam & Javitt, 2012). There has been particular interest in the translational value of the hypothesis with respect to cognitive impairment (Gilmour et al., 2012). For example, the effects of NMDA antagonists have been assessed in a variety of animal models of working memory. However, results of these studies have provided mixed support for the hypothesis, and suggest that whether impairment of working memory by NMDA antagonist is observed depends on the nature of the task, task parameters, and the particular compound employed (Bannerman, Rawlins, & Good, 2006; Dix et al., 2010; Gastambide et al., 2013; Gilmour et al., 2009; Smith et al., 2011). One of the more promising tasks is the Odor Span Task (OST), which was nominated as a benchmark procedure to model working memory capacity in rodents by the CNTRICS group –Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia—(Dudchenko, Talpos, Young & Baxter, 2013).
The OST was originally developed as a way to study the neurobiology of working memory for multiple stimuli by Dudchenko, Wood & Eichenbaum (2000). The procedure uses an incrementing non-matching to sample task in which rats are presented with odors in a large arena; each odor serves as a stimulus indicating reinforcer availability the first time it is presented, but not during following presentations. For example, in the first trial one odor is presented (a cup with scented sand or covered with a scented lid) in the arena and digging in the sand or lid removal produces a food reinforcer. In the second trial, two stimuli are presented– the odor from the first trial along with a novel odor. Now the odor from Trial 1 serves as the sample stimulus, as such, a response to the novel odor results in reinforcement. With each subsequent trial, a new odor is presented along with comparison stimuli from any of the previous trials and selection of the new odor is always reinforced. Thus, the number of sample stimuli to remember increases across the session. The number of correct responses until the first error (span) is one measure purported to reflect memory capacity in the OST and percent correct plotted as a function of the number of odors to remember is another (Dudchenko et al., 2000; Galizio, Deal, Hawkey & April, 2013).
There is a growing literature using the OST to analyze drug effects that is beginning to identify the neurobiological and pharmacological determinants of performance in this task. OST performance is not affected by hippocampal lesions (Dudchenko et al., 2000) but is impaired by inactivation of the medial prefrontal cortex (Davies, Molder, Greba & Howland, 2013). Consistent with the view that OST performance might be detect cognitive deficits in animal models of schizophrenia, Murray, Davies, Molder & Howland (2017) observed span deficits in the offspring of rats after maternal immune activation during pregnancy. Guided by the NMDA hypofunction hypothesis of cognitive impairment in schizophrenia, much of the early work on the behavioral pharmacology of the OST focused on NMDA antagonists. As that hypothesis would predict, several studies have shown that these compounds impair OST performances in rats. For example, the non-competitive NMDA antagonist MK-801 impaired span length and overall OST accuracy at doses that had no effect on a simple discrimination task that controlled for drug effects on sensori-motor function, motivation and reference memory (Galizio, et al., 2013; MacQueen, Bullard & Galizio, 2011). The competitive NMDA antagonist CPP produced effects that were similarly selective to OST performance (Davies, Greba & Howland, 2013; MacQueen, Dalrymple, Drobes & Diamond, 2016). Importantly, the effects of MK-801 were shown to depend on the number of stimuli to remember. That is, effects were minimal when the memory load was low, but impairment of accuracy was accelerated as the number of odors to remember increased during the session (Galizio et al., 2013; MacQueen, et al., 2011).
Other amnestic drugs have been studied in the OST, but have produced less clear evidence of working memory impairment. Rushforth, Allison, Wonnacott, and Shoaib (2010) found that scopolamine decreased span length in rats at doses that did not increase latency to respond. However, in a replication of that study that included the discrimination control condition, scopolamine effects occurred only at doses that also impaired control performances (Galizio et al., 2013). Benzodiazepines have produced selective impairments on OST performances but the effects were quite different from those produced by NMDA antagonists. For example, the effects of chlordiazepoxide were relatively weak and limited to span length (Galizio et al., 2013). In contrast, flunitrazepam impaired OST accuracy at doses that spared control performances, but these effects did not depend on the number of stimuli to remember (Galizio, Mathews, Mason, Panoz-Brown, Prichard & Soto, 2017). Rather, the effects of flunitrazepam appeared to involve a breakdown of the overall non-matching task. Thus, at this point only NMDA antagonists have been shown to impair working memory in the OST.
Although most of the current literature implicates NMDA receptor activity as a key modulator of OST performance, a recent study found contrary results. Galizio et al. (2016) found that the effects of the NMDA antagonist ketamine affected OST performances only at doses that also impaired simple discrimination in 5/6 subjects. In most rats, effective doses of ketamine produced a cessation of responding on both OST and simple discrimination tasks. The discrepancy between findings of selective effects of MK-801 and CPP compared with the non-selective effects of ketamine is puzzling, particularly as both MK-801 and ketamine share a common mechanism as open channel blockers (Lodge & Mercier, 2015). Ketamine is less potent and is a lower affinity channel blocker than MK-801 (Lodge & Mercier, 2015; Monaghan, Irvine, Costa, Fang & Jane, 2012) which might account for the differences in their effects on OST performance. However, a number of studies have also observed differences between the behavioral effects of various uncompetitive NMDA antagonists in a variety of tasks (Dix et al., 2010; Gastambide et al., 2013, Gilmour, et al., 2009; Smith et al., 2011) and it seems important to determine the effects of different compounds in the OST.
Thus, one purpose of the present study was to extend the analysis of NMDA antagonists on OST performance to two additional compounds: phencyclidine (PCP) and methoxetamine (MXE). PCP is a potent channel blocker that produces schizophrenic-like symptoms in humans and also was one of the key compounds that originally led to the NMDA hypofunction hypothesis of schizophrenia (Merritt et al., 2013). MXE is a designer drug that has recently emerged as an abuse problem in the United States and Europe in recent years (Zanda, Fadda, Chiamulera, Fratta, & Fattore, 2016). Although MXE research is in early stages, it does appear to act as an uncompetitive NMDA channel blocker (Roth et al., 2013) and substitutes for ketamine in drug self-administration and drug discrimination procedures (Mutti et al., 2016; Chiamulera, Armani, Mutti, & Fattore, 2016). No research has yet assessed the effects of MXE using working memory tasks.
A second purpose of the study was to replicate the effects of ketamine in the OST using a more refined variation of the task. In the original version of the OST, the number of comparison stimuli in the arena incremented along with the number of stimuli to remember (Dudchenko et al., 2000). As this procedure results in a potential confound, our laboratory adjusted the task to hold the number of comparison stimuli constant at five while continuing to increase the memory load (Galizio et al., 2013; 2016; MacQueen et al., 2011). However, the presence of even five different comparison stimuli in the arena might reduce the sensitivity of the task to working memory effects by increasing the discrimination difficulty and the need to inhibit responding. Perhaps potential amnestic effects of ketamine in the Galizio et al., (2016) study were masked by other actions that led to disrupted performance due to task complexity. In order to test this hypothesis, the present version of the OST used a two-choice procedure in which each trial (after the first) presents one new odor (reinforced–S+) and one previously presented negative comparison (S−). MacQueen et al. (2016) used a two-choice OST and found it sensitive to the effects of CPP; our hypothesis was that a two-choice OST would show higher sensitivity to amnestic ketamine effects than the five-choice procedure.
Methods
Subjects
Sixteen male Sprague-Dawley rats between 90 and 150 days old at the onset of the study served as subjects. Rats were housed individually in Plexiglas cages measuring 34.5 cm L × 18 cm W × 24.5 cm H and were maintained on a 12:12 reverse dark/light schedule with artificial lighting initiated at 7 P.M. and turned off at 7 A.M. Subjects were allowed free access to water in the home cage, but food (Purina Lab Diet 5001 Rodent Diet–PMI Nutrition International, INC Brentwood, MO) was restricted to maintain 85% of free feeding weight. Animal care as well as all aspects of the procedures were approved by the UNC Wilmington IACUC.
Apparatus
A circular open-field arena (94 cm in diameter) surrounded by 32 cm high metal baffling was used for this experiment. The floor of the arena included 18 holes, spaced 13.3 cm apart in two circular arrangements in which 60 ml plastic cups were placed (see Figure 1). Each arena was located in a small testing room with a video camera positioned in the ceiling for digital recording of each session. White noise (70 dB) was presented for the duration of the session.
Figure 1.
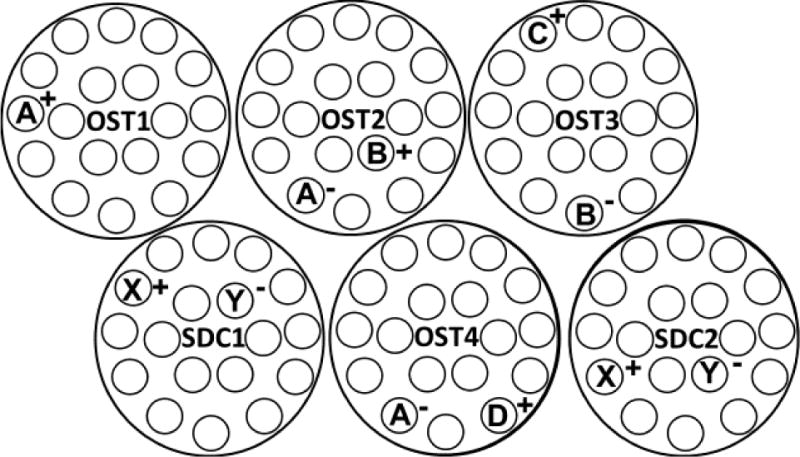
Examples of OST and SDC trials. Each large circle represents an individual trial; OST1-OST4 represent OST trials 1-4 while SDC1 and SDC2 represent interspersed SDC trials. The small circles represent 18 possible stimulus locations in the apparatus. A letter represents a specific odor in a location; letters A, B, C, D, X, & Y indicate different odor stimuli (e.g. A= apple, B= banana). A plus sign next to a letter (e.g. A+) indicates that selection of that stimulus (A) would produce a sucrose pellet. A minus (e.g. B−) indicates that no pellet is available for selection of that odor (B). Notice that possible negative stimuli increase as OST trials increment: 1 possible negative (A) for OST2, 2 (A, B) for OST3, 3 (A, B, C) for OST4, and so on. During all SDC trials, selection of X (X+) always produced a sucrose pellet, while a selection of Y (Y−) did not.
Stimuli
Plastic cups half full of sand were covered with plastic opaque scented lids. The lids were scented with various odorants. These lids were odorized via storage in plastic containers containing aromatic oils and spices as odorants (acai, allspice, anise, apple, banana, bay, black walnut, bubblegum, caramel, caraway, carob, celery, cherry, chocolate, cinnamon, clove, coconut, coriander, cumin, dill, fennel, fenugreek, garlic, ginger, honey, lilac, maple, marjoram, marshmallow, mustard, nutmeg, onion, oregano, pecan, pine forest, pumpkin, root beer, rosemary, sage, savory, spinach, sumac, thyme, watermelon) purchased from Nature’s Garden, McCormick, Sigma Aldrich, and Great American Spice Co. Lids were suspended above oils or spices during storage, rather than directly in the odorants, so that the concentration of odorant was relatively consistent across lids and were stored at least 3 weeks prior to initial use. Containers of spices and oils were refreshed every three to four weeks. To control for scent marking on lids within session, each lid was replaced with one of the same odor every time the scent was presented during a session.
Procedure
OST Training
Sessions were conducted 5 days per week (Monday–Friday). Subjects were first habituated to the apparatus, and hand shaped to remove a lid from a baited stimulus cup. Rats were then trained on an incrementing non-match-to-sample procedure. In this procedure, a novel odorant was presented during each trial along with odorants from previous trials. Removal of the novel scented lid resulted in food reinforcement, while removal of any previously presented lid did not. Initially, the number of cups in the arena incremented on each trial, and errors (removal of a previously presented scented lid) resulted in a reset—that is, on the following trial only one scented lid was present. After an average of 11 days, the baseline OST schedule was implemented.
Following initial OST training, rats began training on the 2-choice OST baseline procedure. Two features of baseline OST training were different from that of initial training. First, errors no longer resulted in resetting the stimulus arrangement to a single stimulus. Second, following Trial 2 the number of comparison stimuli in the arena was held constant at two (See Fig. 1): a new baited odor each trial (+) and an unbaited odor (−) which was randomly selected from any of the previously presented odors (with the constraint that an odor was never presented more than three times in a row). Figure 1 illustrates four typical OST trials (OST1-OST4). During the first trial (OST1) only one cup is presented, covered by a lid scented with odor A. Upon removal of lid A (A+), a sucrose pellet, which had been covered by the lid, is available as food reinforcement (signified by the + symbol). In the second trial (OST2), two odors are presented: scented lids A and B; as odor A has been previously presented, there is no sucrose pellet available under lid A (signified by the – symbol). Odor B is novel in this trial, thus removal of lid B will provide access to a sucrose pellet. Trial 3 (OST3) contains odors B and C, and as B has been previously presented, removal of lid B will not produce a pellet. These three trials each use the previous positive (+) stimulus as the negative (−) stimulus in the successive trial, however, any previously presented odor can serve as a negative stimulus. This is illustrated in trial 4 (OST4), where odor A is presented again as a negative stimulus, with the novel odor D. Figure 1 also contains an example of two simple discrimination control (SDC) trials, SDC1 & SDC2, which were added once subjects met a mastery criterion: two consecutive sessions with accuracy at 72% or greater.
Combined OST/SDC Baseline
SDC trials (See SDC1 & SDC2; Fig. 1) consisted of a pair of scented lids. Unlike the OST, in SDC trials the same odor (X+) was consistently baited across all SDC trials and sessions, while the alternative odor (Y−) was never baited. This consistency is demonstrated in figure 1: X is presented with the + symbol and Y with the – symbol in both SDC1 & SDC2. Although the pair of odors (X & Y) differed between subjects, each pair remained constant for each subject (see Table 1) once SDC trials were introduced, and these odors were never presented as choices during OST trials. As task demands were similar between the OST and SDC task, save for the increase in negative comparison stimuli (number of stimuli to remember) in the OST, the SDC served to control for drug effects on reference memory as well as non-mnemonic drug effects.
Table 1.
Training, Performance Under Baseline and Control Conditions and SDC Odorants
| Number of Sessions | Percent Correct | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rat | OST | OST/SDC | Total | Baseline | No Bait | Fresh Cup | Drug | SDC odors (+,−) | |
| O7 | 7 | 65 | 72 | 82.33% | 93.00% | 94.00% | MXE | Pumpkin, Honey | |
| M22 | 10 | 27 | 37 | 96.89% | 88.98% | – | MXE | Marshmallow, Pine Forest | |
| P3 | 11 | 86 | 97 | 81.76% | 84.00% | 83.33% | MXE | Watermelon, Black Walnut | |
| P18 | 13 | 24 | 37 | 78.00% | 81.00% | 67.00% | MXE | Maple, Acai | |
| P24 | 7 | 36 | 43 | 82.00% | 86.00% | 89.00% | MXE | Caramel, Apple | |
| P26 | 20 | 33 | 53 | 79.81% | 87.00% | 77.00% | MXE | Banana, Root beer | |
| M10 | 10 | 54 | 64 | 92.00% | 100.00% | 87.50% | PCP | Lilac, Honeydew | |
| N11 | 7 | 81 | 88 | 87.38% | 89.00% | 86.00% | PCP | Pecan, Pine Forest | |
| N33 | 7 | 30 | 37 | 85.20% | 90.00% | 100.00% | PCP | Marshmallow, Pine Forest | |
| P25 | 9 | 30 | 39 | 87.73% | 88.00% | 90.00% | PCP | Pumpkin, Honey | |
| P17 | 15 | 31 | 46 | 79.14% | 74.00% | 78.00% | PCP, KET | Pecan, Pine Forest | |
| M5 | 10 | 33 | 43 | 88.33% | 90.00% | – | PCP, KET | Chocolate, Bubblegum | |
| K10 | 11 | 56 | 67 | 86.15% | 74.35% | – | KET | Black Walnut, Champagne | |
| L7 | 11 | 24 | 35 | 93.00% | 91.67% | – | KET | Coconut, Lavender | |
| L24 | 13 | 28 | 41 | 89.00% | 89.28% | – | KET | Rum, Maple | |
| N36 | 16 | 27 | 43 | 92.00% | 94.74% | 81.58% | KET | Apple, Banana | |
Initially, six SDC trials were presented following 25 OST trials. After rats responded with 100% accuracy to the 6 SDC trials while maintaining at least 72% accuracy in the OST during one session, the SDC trials were pseudo-randomly interspersed throughout each session during the following sessions (As shown in Fig. 1). SDC trials were spread across the session so that the varying effects of drugs on performance over time could be captured. Although interspersing the SDC trials among OST trials was expected to initially cause some disruption of the odor span task, after a few sessions subjects readily learned to discriminate between odors signaling the SDC task versus the OST and respond accordingly.
Two additional control procedures were applied when the OST and SDC were combined. First, a “no-bait” control procedure was added in six pseudo-randomly chosen OST trials per session, one session per week. In these trials, neither stimulus cup contained a reinforcer, but a sucrose pellet was placed in the S+ cup immediately following a correct response. This control procedure allowed for verification that the odor of the lid, rather than the sucrose pellet beneath, controlled responding. Second, one session per week included a fresh cup control procedure. This procedure consisted of four pseudo-randomly chosen OST trials in which the S- cup was replaced with a cup that had not been used previously in the session. This control procedure allowed the experimenters to examine whether trace odors from the stimulus cup, rather than the scented lid, affected OST accuracy. Table 1 details mean percent correct across baseline, no bait, and fresh cup trials for each rat. Several subjects (M22, M5, K10, L7, & L24) did not receive the fresh cup procedure as it was implemented later in the experiment. Accurate responding was maintained during fresh cup and non-baited sessions. This indicates that neither the scent of the sucrose pellet nor the scent of the cup was likely responsible for accurate responding in the OST.
The combined OST and SDC tasks constituted the drug baseline. After meeting a stability criterion for OST accuracy, which required that the mean difference in accuracy between the most recent five sessions and the five preceding sessions was less than 10% of the grand mean of those ten sessions, subjects began to receive injections. The number of training sessions required ranged from 37 to 97 with an average of 54.7 sessions (Table 1). Following this extensive training, rats maintained stable and high levels of accuracy on both the OST and SDC task.
Drugs
All drugs were dissolved in .09% saline solution and delivered in a volume of 1ml/kg. PCP HCl (a generous gift from the NIDA drug supply program, RTP) was administered in doses of 1.0, 3.0, 5.6, and 10.0 mg/kg and Ketamine HCl (Sigma) in doses of 1.0, 3.0, 5.6, 10.0, 18.0, 30.0, and 40.0 mg/kg. MXE HCl (Debora Labs, Copenhagen) was administered in doses of 1.0, 3.0, 10.0, 18.0, 30.0 and 40.0 mg/kg. Because the provenance of MXE was uncertain, the structure was verified using 1- and 2- dimensional Nuclear Magnetic Resonance (NMR) spectroscopy, High Resolution Mass Spectrometry (HRMS), and comparison with the data in the current literature. NMR and LC/MS found no significant organic impurity.
Drug Protocol
Intraperitoneal injections were administered 15 minutes prior to session start three days per week. Saline was delivered Thursdays as an injection control, while active drug was administered on Tuesdays and Fridays. Experimenters remained blind to the dose administered. No injections were given Mondays or Wednesdays, which served as a continuation of the baseline. Two or three determinations of a dose were given for each rat; each dose in a set of determinations was administered prior to the second round of doses. Some subjects received more than one drug, either in this study or in an experiment not described here; for these animals a two-week washout period intervened before the next drug study began.
Data Analysis
Accuracy was determined by the first response in each trial for both OST and SDC trials. If a subject failed to respond within 2 minutes, the trial was scored as an omission; omitted trials were not included in the percent correct calculation. Omitted trials were repeated with presentation of only the positive stimulus, and sessions were terminated following either 3 consecutive or 6 total omissions. Consecutive correct measures included span and longest run. Span was defined by number of correct responses made until the first error in the session, excluding Trial 1 as there was no comparison stimulus. Longest run was determined as the greatest number of correct consecutive responses in the session. This measure provided more information than span, as a rat could make an error on Trial 2 resulting in a span of 1. However, the rat could then respond correctly on trials 3–20, resulting in a longest run of 17. Latencies for each trial were measured in seconds from the placement of the rat in the arena until a response was made.
Video recordings of ten randomly selected sessions were scored by a blind observer to determine inter-rater reliability (IRR). IRR was calculated by comparing scores of the observer with scores of the experimenter from the chosen session. The two raters agreed on 98% of the chosen trials, which indicated a high reliability of the operational definition of the lid removal response.
Results
Mean effects of PCP on the main dependent variables are displayed in Figure 2. The top panel shows percent correct as a function of dose for the OST (closed circles) and SDC task (open circles). Under control conditions (saline), accuracy for the OST was around 90%, while accuracy for the SDC task was near 100%. PCP produced dose-dependent impairment in accuracy on both tasks. At the 3.0 mg/kg dose, OST accuracy was slightly decreased and then more clearly impaired at the 5.6 mg/kg dose. In contrast, at both of these doses, accuracy on the SDC task was relatively unaffected, remaining above 90%. Thus, the effects of the 5.6 mg/kg dose were selective to the OST. Percent omissions is indicated by the bars on the right axis of the graph, representing trials in which a failure to respond to either stimulus within two minutes occurred. At the 5.6 mg/kg dose occasional omissions were observed, whereas at the 10.0 mg/kg dose behavior was grossly affected, resulting in over 70% omissions. Statistical analysis used a within-subject ANOVA with dose (4 levels—the 10 mg dose was omitted from the analysis due to the high number of omissions) and task (OST vs. SDC) as the main variables. The effects of PCP were verified by a significant dose x task interaction: F (3, 15)=6.95, p<.05, with main effects of dose [F(3,15)=5.16, p<.05] and task [F(3,15)=23.50, p<.05]. Fisher’s LSD tests revealed that the 5.6 mg/kg dose significantly reduced OST accuracy below saline levels (p<.05) but that SDC accuracy was unaffected (p>.05). Thus, PCP produced effects that were selective to OST performances at this dose. Figure 2 (middle panel) also shows that under control conditions, mean spans (closed circles) ranged from 7−11 odors, while longest runs (open circles) were higher at 12−15 consecutively correct responses. Span and longest run were each statistically analyzed using one-way within-subject ANOVAs. Both span [F (4, 20)= 5.92, p<.05] and longest run [F (4, 20)= 21.70, p<.05] were dose-dependently decreased by PCP administration. Fisher’s LSD tests revealed span was significantly lower at the 10.0mg/kg dose compared to saline (p<.05) and longest run was significantly below saline levels at the 5.6 and 10.0 mg/kg dose (p<.05). Latencies to respond to (Fig. 2, bottom panel) increased at the 5.6 mg/kg dose [F (3, 15)=7.01, p<.05]. The selective effects of PCP were also evident in an individual subject analysis (Figure 3). In these graphs, mean percent correct for the OST (closed circles) and SDC task (open circles) for each rat is plotted as a function of PCP dose. Five of the six rats, (M5, N11, N33, P17 and P25) showed selective effects of PCP at the 5.6 mg/kg dose with decreased OST accuracy but minimal effects on SDC performances. Rat M10 (top right panel) was the only subject in which no selective effects were evident.
Figure 2.
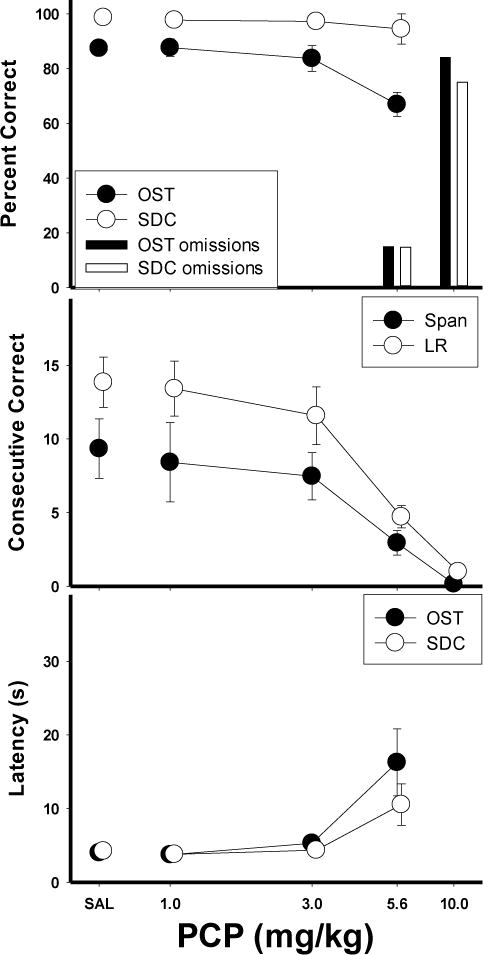
Means for percent correct, consecutive correct, and latency for the PCP group. Top panel shows the effects of PCP on percent correct (circles) and omissions (bars) for the OST (closed circles, dark bars) and SDC task (open circles, light bars). Middle panel shows span (closed circles) and longest run (open circles). Bottom panel shows latency to first response for OST (closed circles) and SDC task (open circles). Vertical lines indicate SEM.
Figure 3.
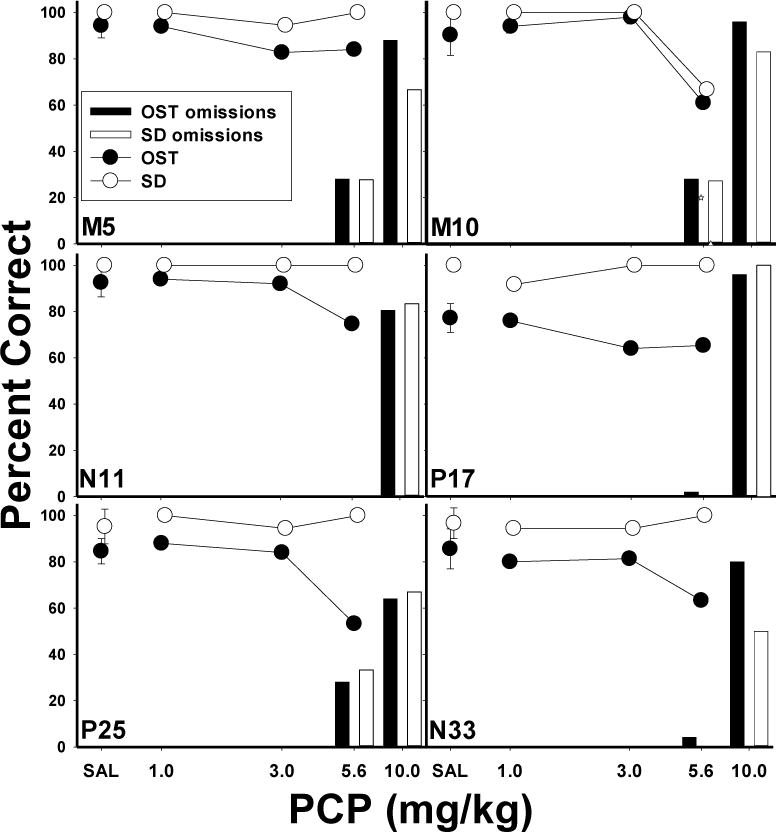
Individual subject graphs show the effects of PCP on percent correct (circles) and omissions (bars) for the OST (closed circles, dark bars) and SDC task (open circles, light bars). Vertical lines plotted for saline means indicate SEM.
The effects of MXE on the main dependent variables are shown in Figure 4. Under the saline control condition, accuracies were around 85% for the OST and 95% for the SDC task. MXE dose-dependently impaired accuracy on both tasks, however this did not occur until the 18.0 and 30.0 mg/kg doses. The data were analyzed statistically by a within-subject ANOVA with dose (6 levels) and task (OST vs. SDC) as the main variables. A main effect of dose was found [F (5, 25)= 5.93, p<.05]. There was not a significant main effect for task [F (5, 25)= 3.27, p>.05], nor a significant interaction [F (5, 25)= 0.4, p>.05] Fisher’s LSD tests revealed a significant overall difference between saline and the 30.0 mg/kg dose. Percent omissions (bars on right side) also increased to around 40% at the 18.0 and 30.0 mg/kg doses. The high doses disrupted responding in both tasks, thus the effect appears non-selective. Consistent with the decreases in overall accuracy, mean spans (closed circles) and longest runs (open circles) were also reduced at the higher doses of MXE (Fig. 4, middle panel). Both span [F (5, 25)= 3.30, p<.05] and longest run [F (5, 25)= 11.30, p<.05] were significantly decreased by MXE administration dose-dependently with Fisher’s LSD tests revealing that both measures were significantly below saline levels only at 30.0 mg/kg (p<.05). Latencies to respond to both tasks (Fig. 4, bottom panel) were somewhat elevated at the higher doses [F (5, 25)= 3.68, p<.05]. The group data did not appear to show a selective effect of MXE, as responding on both tasks was affected similarly, and impairment only occurred at doses in which high rates of omissions were observed.
Figure 4.
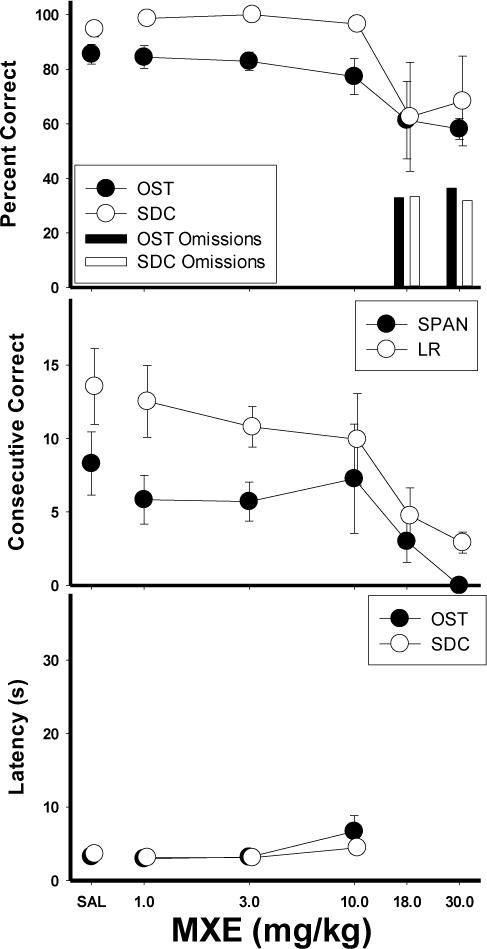
Means for percent correct, consecutive correct, and latency for the MXE group. Top panel shows the effects of MXE on percent correct (circles) and omissions (bars) for the OST (closed circles, dark bars) and SDC task (open circles, light bars). Middle panel shows span (closed circles) and longest run (open circles). Bottom panel shows latency to first response for OST (closed circles) and SDC task (open circles). Vertical lines indicate SEM.
However, the individual subject data for the rats administered MXE (Fig. 5) provide a somewhat more complex picture of the effects of MXE on responding. Three subjects showed a clear selective effect of MXE on OST percent correct: O7, P3, and M22 (right three panels). Rat O7 (top left panel) was unaffected by the lower doses of MXE, but at the 18.0 mg/kg dose, accuracy declined on the OST while remaining at 100% for the SDC task. For P3, OST accuracy decreased across the 10.0, 18.0 and 30.0 mg/kg doses, while accuracy on the SDC task was unaffected. Subject M22’s percent correct was decreased under both the 18.0 and 30.0 mg/kg doses, while responding on the SDC task remained at 100% accuracy. Two additional subjects (P24 and P26) also showed selective effects of lesser magnitude. In the case of P24, a small decrease in OST, but not SDC, accuracy was observed at the 10 mg/kg dose. P26 showed a consistent drop in OST accuracy at the 10 and 18 mg/kg doses, but SDC was also slightly impaired at these doses. Finally, Rat P18 (top left panel) showed no effects of MXE until the 18.0 and 30.0 mg/kg doses which resulted in complete omissions.
Figure 5.
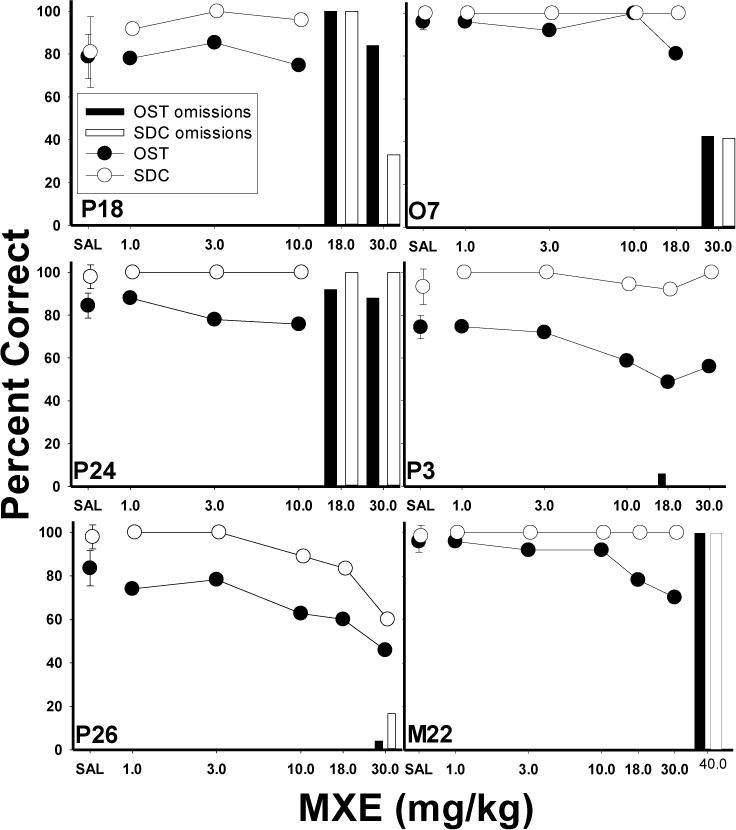
Individual subject graphs show the effects of MXE on percent correct (circles) and omissions (bars) for the OST (closed circles, dark bars) and SDC task (open circles, light bars). Vertical lines plotted for saline means indicate SEM.
In sum, MXE disrupted accuracies on the OST and SDC and reduced spans and longest runs. Although the group data analysis failed to reveal evidence of selective effects on the OST, the individual subject data for the MXE group provided some evidence of selectively in five of the six rats. The doses at which responding was disrupted were variable across the six subjects in the MXE study. This differential sensitivity to MXE effects may explain why the mean data appeared to be non-selective whereas individual rats in fact did show selective effects at some doses.
The top panel of Figure 6 shows the effects of ketamine (KET) on percent correct. KET reduced accuracy at the 30.0 mg/kg dose, [F (5, 25)= 6.34, p<.05] which also increased omissions. The 30.0 mg/kg dose disrupted responding in both tasks, thus the effect appears non-selective. Mean spans [F (5, 25)= 6.34, p<.05] and longest runs [F (5, 25)= 5.36, p<.05] were also reduced at the 30 mg/kg dose (Fig. 6, middle panel). No significant effects were obtained for response latencies (Fig. 6, bottom panel). In sum, the group data did not show a selective effect of KET, as accuracy on both tasks was maintained across doses until the 30 mg/kg dose, at which high rates of omissions were observed in both OST and SDC tasks.
Figure 6.
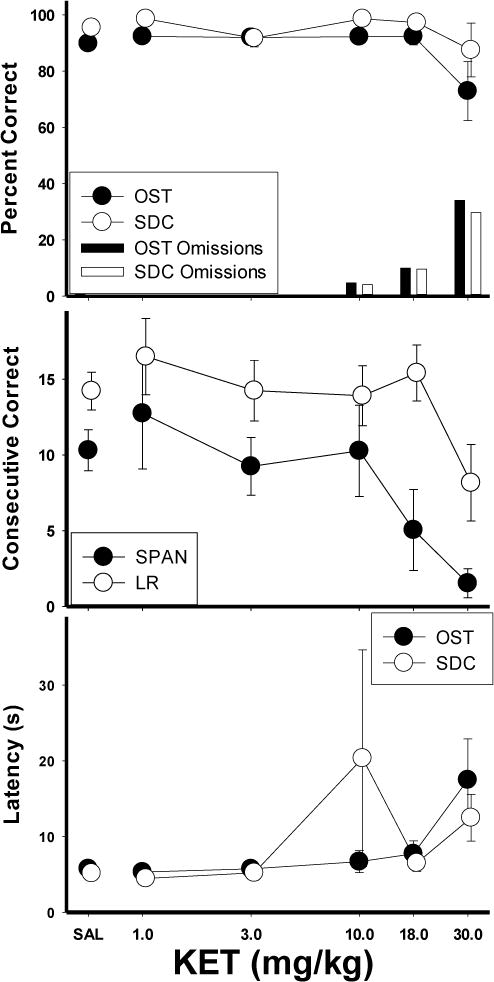
Means for percent correct, consecutive correct, and latency for the KET group. Top panel shows the effects of KET on percent correct (circles) and omissions (bars) for the OST (closed circles, dark bars) and SDC task (open circles, light bars). Middle panel shows span (closed circles) and longest run (open circles). Bottom panel shows latency to first response for OST (closed circles) and SDC task (open circles). Vertical lines indicate SEM.
Individual subject data for the KET group (Fig. 7) generally support the mean data. All subjects maintained SDC accuracy similar to saline levels, and most did not show declines in OST accuracy below saline levels at any KET dose. Four animals (M5, L24, K10, L7) were nearly unaffected by ketamine until doses were reached which caused substantial omissions. However, one subject (P17) did show a clearly selective effect with a reliable drop in OST, but not SDC, accuracy at the 30.0 mg/kg dose. Overall, effects of KET were generally limited to doses that disrupted overall responding with the selective effects in P17 standing as the exception.
Figure 7.
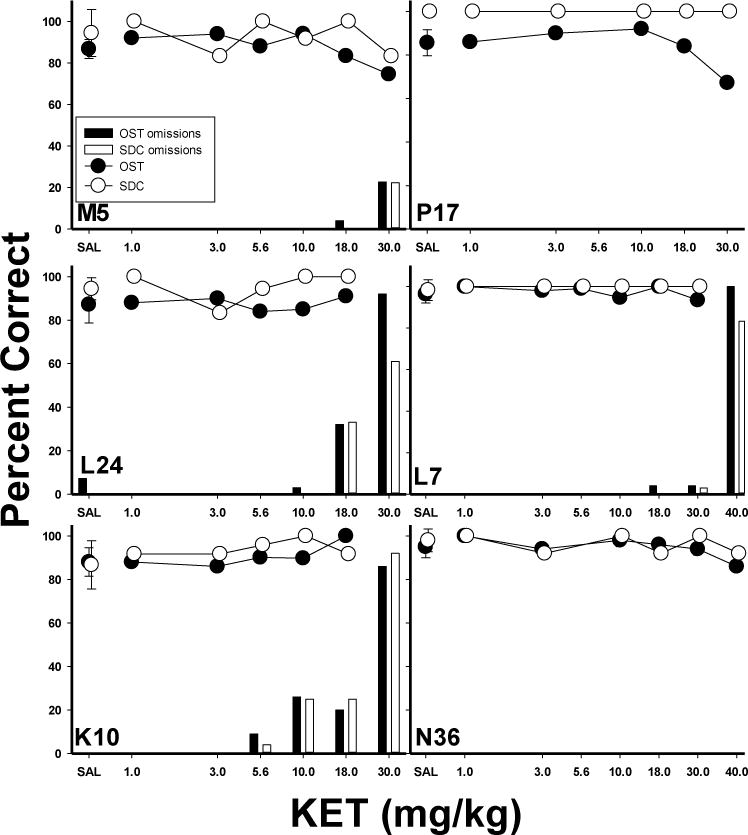
Individual subject graphs show the effects of KET on percent correct (circles) and omissions (bars) for the OST (closed circles, dark bars) and SDC task (open circles, light bars). Vertical lines plotted for saline means indicate SEM.
Although PCP selectively impaired accuracy on the OST, a within-session analysis was used to determine whether the effects of PCP depended on the number of odors to remember. Figure 8 shows such an analysis comparing the selective dose (5.6 mg/kg) of PCP (open triangles) to the saline control (closed triangles) across the OST (3 bins of 8 trials–left half of x-axis) and the SDC trials (three bins of two trials–right half of x-axis) for the five animals that showed selective effects. Note that accuracy decreased only slightly across the session under saline conditions. Indeed, during the final eight trials with 17–24 odors to remember, OST accuracy remained above 80% after saline. However, there was a sharp drop in OST accuracy as the number of stimuli to remember increased under PCP. Accuracy was only slightly lower than control in the first bin (1–8 odors), but dropped to about 60% correct when the memory load was 9–24 stimuli. ANOVA with drug (2 levels: Saline vs. PCP) and bin (3 levels: 1 vs. 2 vs. 3) as the main variables confirmed these conclusions with a significant dose X bin interaction [F (1,4)=7.10, p<.05]. Note that accuracy on SDC trials remained high throughout the session with or without PCP.
Figure 8.
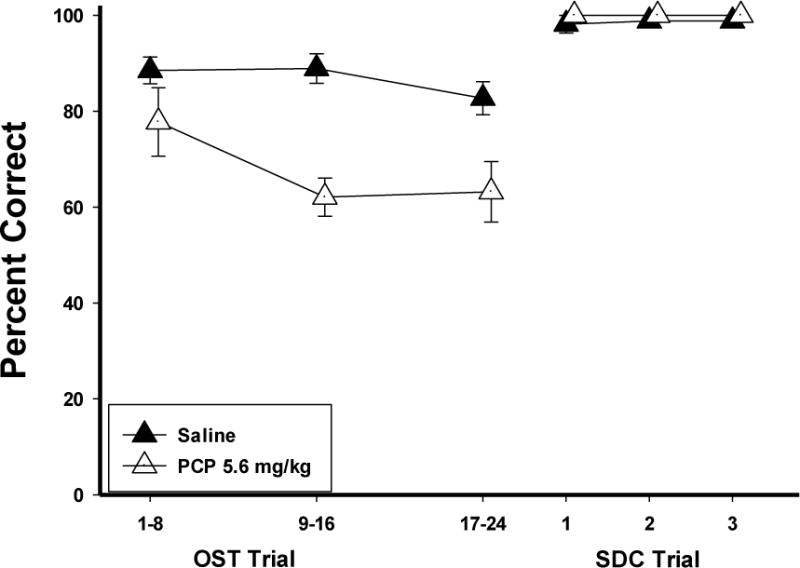
Within session graphs show the effects of 5.6 mg/kg PCP (open triangles) and Saline (closed triangles) on OST and SDC percent correct across the session in bins of 8 for the OST and 2 for the SDC. Vertical lines indicate SEM. Note that N = 5—P5 not included in this figure.
Within-session analysis for MXE was complicated by the individual differences in sensitivity. Due to this variability, MXE within-session data (Fig. 9) were analyzed using a best dose analysis which compared the means of the most selective dose for the five rats that showed selective effects (open triangles) with saline (closed triangles) across the session. The doses used were 10.0 (P24 & P26), 18.0 (O7), and 30.0 mg/kg (M22 & P3). The results were similar to PCP in that there was very little decline in accuracy across the session under saline conditions, and MXE-induced impairment of accuracy was most evident when the memory load was fairly high (9–24 stimuli). Factorial ANOVA revealed a significant interaction of drug and bins of trials [F (1,4)= 8.73, p<.05). Accuracy on the SDC trials remained relatively high across the session.
Figure 9.
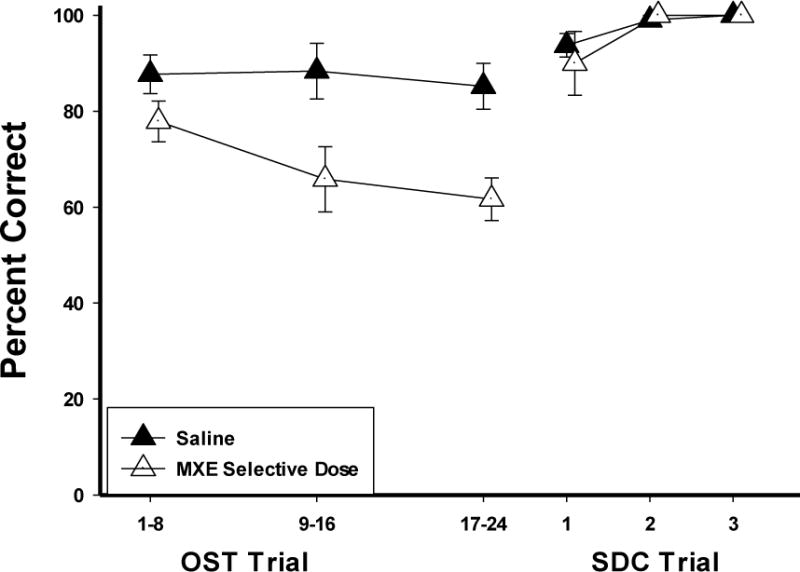
Group within session graphs show the effects of the selective dose of MXE for each rat (open triangles) and Saline (closed triangles) on OST percent correct across the session (bins of 8 trials) and the 6 SDC trials (bins of 2). Vertical lines indicate SEM. Note that N = 5—P18 not included in this figure.
Discussion
All three NMDA antagonists reduced span length, longest run, and accuracy in a dose-dependent fashion, but the three compounds differed in the extent to which the effects were selective to the OST. At high doses, all three drugs disrupted SDC as well as OST responding with frequent response omissions. At moderate doses, both PCP and MXE produced effects that were selective to the OST and were consistent with an interpretation in terms of impaired within-session or working memory. KET effects were much less selective to the OST, and appear to have been based on non-amnestic disruption of performance in most rats tested.
PCP produced the most selective effects as OST accuracy and longest run were significantly decreased (and decreases in span length approached significance) at the 5.6 mg/kg dose which had no effect on SDC accuracy. Three of the subjects did show an increase in omissions at this dose and response latency also increased, suggesting that some general task disruption was occurring. That said, the observed change in OST accuracy approached a 15% decline while SDC performance remained nearly perfect at the 5.6 mg/kg dose suggesting that the PCP effects were selective to the OST at this dose. This observation was corroborated by the within-session analysis of PCP effects, which revealed that when memory load was 1–8 stimuli, 5.6 mg/kg PCP had little effect on accuracy compared to saline. However, as the number of stimuli to remember increased from 9–23 stimuli, accuracy declined sharply under 5.6 mg/kg PCP, whereas only a slight decline was observed in the saline conditions. In short, the effects of PCP depended on the number of stimuli to remember.
Overall, MXE effects were similar to those of PCP, but individual rats varied considerably in their sensitivity to the drug. Some subjects showed selective effects of MXE at 18 mg/kg (O7, P3) or 30 mg/kg (M22) while both OST and SDC performance was disrupted in the other three rats at these doses. A best-dose analysis used to detect within session effects produced a function resembling that for PCP with very little MXE effect when the memory load was 1–8 stimuli, but substantial impairment relative to saline as the number of stimuli to remember increased throughout the session.
Finally, ketamine effects were somewhat different than the other compounds as it produced very little effect on accuracy of either OST or SDC responding until high doses were reached that disrupted responding on both tasks and increased omissions in most subjects. One subject (P17) did show a selective effect with reduced OST but not SDC accuracy at the 30 mg/kg dose, but overall ketamine effects were clearly less selective than MXE or PCP. These findings replicate those of a previous study from our laboratory, which used a five-choice OST (Galizio et al., 2016). In that study, as in the present one, only one out of six rats showed a selective effect of ketamine on the OST. Thus, the limited selectivity of ketamine on OST responding in Galizio et al. (2016) was not related to the number of comparison stimuli—rather ketamine appears to produce relatively non-selective effects with the both the five-choice and the present two-choice versions of the OST.
The present study was the first to determine the effects of PCP and MXE using the OST. The effects of PCP on OST observed in the present study closely resembled previous results with NMDA antagonists MK-801 and CPP (Davies, et al., 2013; Galizio, et al., 2013; MacQueen et al., 2011; 2016). In those studies NMDA antagonists selectively impaired OST accuracy at doses that did not affect other aspects of performance (e.g., SDC accuracy). One qualification with respect to PCP is that although the 5.6 mg/kg dose reliably produced selective impairment of OST accuracy, this dose also resulted in some global impairment (increased omissions and response latency) in several animals. Still, an interpretation of PCP disruption of OST accuracy in terms of amnestic effects seems appropriate. Interpretation of the MXE results is more complex. If the group mean data alone were considered, the conclusion would be the MXE did not produce selective effects; only when individual subject dose-response functions were analyzed was evidence of selective actions observed. Group statistical analysis using a best-dose procedure did show selectivity and supported the observation that MXE effects depended on the number of stimuli to remember. One caveat here is that best-dose analyses are potentially biased in the direction of showing positive effects (Soto, Dallery, Ator, & Katz, 2013). Very little research has been conducted with MXE and further studies will be needed to determine the extent to which MXE selectively impairs within-session/working memory.
The present study provided mixed support for the hypothesis that NMDA hypofunction is associated with reduced memory capacity. On the one hand, the selective effects and memory-load dependent effects of PCP and MXE obtained in the present study were consistent. These findings, combined with those of previous studies that studied MK-801 and CPP on OST performances, provide strong support for the hypothesis. However, the relatively non-selective effects of ketamine were inconsistent. Why would ketamine result in such different effects than other NMDA antagonists? As noted previously, other researchers have observed differences between various NMDA antagonists in a variety of behavioral tasks, and it is unclear whether these may reflect pharmacokinetic or pharmacodynamic differences across compounds (Dix et al., 2010; Gastambide et al., 2009; 2013, Smith et al., 2011). Using a somewhat different procedure—the self-ordered spatial search task—to assess memory capacity, Taffe, Davis, Gutierrez & Gold (2002) found that ketamine did produce selective impairments in rhesus monkeys that depended on the number of stimuli to remember. Thus, it is possible that a species difference in sensitivity to ketamine may be involved. It has also been noted that binding site affinity varies across NMDA antagonists. Ranking the uncompetitive antagonists in terms of affinity, MK-801 > PCP > MXE > S-(+)-KET (Wong et al., 1986; Halberstadt, Slepak, Hyun, Buell, & Powell, 2016). This hierarchy might account for the findings of ketamine’s limited selectively on OST performance. Perhaps some affinity threshold is required to produce selective effects on working memory in the OST. Studies with additional compounds (e.g., lower affinity compounds such as memantine) are needed to test such a hypothesis.
Finally, it should be added that although results from the OST are generally interpreted in terms of within-session or working memory, the translational significance of such interpretations remain to be determined. Certainly numerous studies, including the present one, have shown functional differences between the within-session remembering involved in the OST relative to reference memory tasks like the SDC. However, the relationship between the within-session memory of the OST and human working memory tasks remains puzzling. Consider that human memory span is highly limited (Gathercole, 2009). In contrast, very little decline in accuracy was observed in the present study even as the memory load reached 16–24 odors. Indeed, the upper limit on rat’s capacity to remember odors has not been reached with previous OST studies showing above chance memory for 70–100 different odors (April, Bruce, & Galizio, 2013; Bratch et al., 2016). It has been suggested that performance in the OST is best conceptualized as a form of short-term episodic-like memory (Branch et al., 2014; Panoz-Brown, Corbin, Dalecki et al, 2016) and if this hypothesis is confirmed, it would have implications for the interpretation of the effects of NMDA antagonists as well.
Public Significance Statement.
The glutamate hypothesis of schizophrenia posits that NMDA receptor hypofunction may underlie some symptoms (particularly cognitive symptoms) of the disorder. The present study provided some support for this hypothesis in that we found that two NMDA receptor antagonists, phencyclidine and methoxetamine, selectively reduced memory capacity (ketamine effects were less selective) as measured by the odor span task. These results support the idea of the NMDA receptor as a potential target for new treatments for schizophrenia.
Acknowledgments
This research was supported in part by NIDA grant DA029252, Mark Galizio, PI. Only financial support was provided by NIDA.
The authors thank Katrina Gobenciong, Angela Goolsby, Chloe Myers, Danielle Panoz-Brown, and Ashley Prichard who assisted in data collection and analysis.
Footnotes
Some of the data reported in this article were presented at the Annual Meeting of the Society for Neuroscience, Chicago, IL October, 2015.
All authors contributed in significant ways to the paper and all have read and approved the ms.
None of the authors report any conflict of interest relevant to the present research.
References
- Bannerman DM, Rawlins JNP, Good MA. The drugs don’t work—or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A- containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology. 2006;188:552–566. doi: 10.1007/s00213-006-0403-6. [DOI] [PubMed] [Google Scholar]
- Branch CL, Galizio M, Bruce K. What-where-when memory in the rodent odor span task. Learning and Motivation. 2014;47:18–29. doi: 10.1016/j.lmot.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratch A, Kann S, Cain JA, Wu JE, Rivera-Reyes N, Dalecki S, Arman D, Dunn A, Cooper S, Corbin H, Doyle AR, Pizzo MJ, Smith AE, Crystal JD. Working memory systems in the rat. Current Biology. 2016;26:351–355. doi: 10.1016/j.cub.2015.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Armani F, Mutti A, Fattore L. The ketamine analogue MXE generalizes to ketamine discriminative stimulus in rats. Behavioural Pharmacology. 2016;27:204–210. doi: 10.1097/FBP.0000000000000221. [DOI] [PubMed] [Google Scholar]
- Davies DA, Greba Q, Howland JG. GluN2B-containing NMDA receptors and AMPA receptors in medial prefrontal cortex are necessary for odor span in rats. Frontiers in behavioral neuroscience. 2013;7:1–8. doi: 10.3389/fnbeh.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DA, Molder JJ, Greba Q, Howland JG. Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learning & Memory. 2013;20:665–669. doi: 10.1101/lm.032243.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix S, Gilmour G, Potts S, Smith JW, Tricklebank M. A within-subject cognitive battery in the rat: differential effects of NMDA receptor antagonists. Psychopharmacology. 2010;212:227–242. doi: 10.1007/s00213-010-1945-1. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: a review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neuroscience & Biobehavioral Reviews. 2013;37:2111–2124. doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. Journal of Neuroscience. 2000;20:2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, April B, Deal M, Hawkey A, Panoz-Brown D, Prichard A, Bruce K. Behavioral pharmacology of the odor span task: effects of flunitrazepam, ketamine, methamphetamine and methylphenidate. Journal of the Experimental Analysis of Behavior. 2016;106:173–194. doi: 10.1002/jeab.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, Deal M, Hawkey A, April B. Working memory in the odor span task: effects of chlordiazepoxide, dizocilpine (MK801), morphine, and scopolamine. Psychopharmacology. 2013;225:397–406. doi: 10.1007/s00213-012-2825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, Mathews M, Mason M, Panoz-Brown D, Prichard A, Soto P. Amnestic drugs in the odor span task: effects of flunitrazepam, zolpidem and scopolamine. Neurobiology of learning and memory. 2017;145:67–74. doi: 10.1016/j.nlm.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastambide F, Mitchell SN, Robbins TW, Tricklebank MD, Gilmour G. Temporally distinct cognitive effects following acute administration of ketamine and phencyclidine in the rat. European Neuropsychopharmacology. 2013;23:1414–1422. doi: 10.1016/j.euroneuro.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Working memory. In: Byrne JH, editor. Concise Learning and Memory: Editor’s Selection. Amsterdam: Elsevier Press; 2009. pp. 149–168. [Google Scholar]
- Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, Tricklebank M. NMDA receptors, cognition and schizophrenia–testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. 2012;62:1401–1412. doi: 10.1016/j.neuropharm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Pioli EY, Dix S, et al. Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: implications for “NMDA antagonist modeling” of schizophrenia. Psychopharmacology. 2009;205:203–216. doi: 10.1007/s00213-009-1530-7. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Slepak N, Hyun J, Buell MR, Powell SB. The novel ketamine analog methoxetamine produces dissociative-like behavioral effects in rodents. Psychopharmacology. 2016;233:1215–1225. doi: 10.1007/s00213-016-4203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D, Mercier MS. Ketamine and phencyclidine: the good, the bad and the unexpected. British journal of pharmacology. 2015;172:4254–4276. doi: 10.1111/bph.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen DA, Bullard L, Galizio M. Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiology of learning and memory. 2011;95:57–63. doi: 10.1016/j.nlm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen DA, Dalrymple SR, Drobes DJ, Diamond DM. Influence of pharmacological manipulations of NMDA and cholinergic receptors on working versus reference memory in a dual component odor span task. Learning & Memory. 2016;23:270–277. doi: 10.1101/lm.041251.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt K, McGuire P, Egerton A. Relationship between glutamate dysfunction and symptoms and cognitive function in psychosis. Frontiers in Psychiatry. 2013;4:1–8. doi: 10.3389/fpsyt.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Irvine MW, Costa BM, Fang G, Jane DE. Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators. Neurochemistry International. 2012;61:581–592. doi: 10.1016/j.neuint.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BG, Davies DA, Molder JJ, Howland JG. Maternal immune activation during pregnancy in rats impairs working memory capacity of the offspring. Neurobiology of Learning and Memory. 2017;141:150–156. doi: 10.1016/j.nlm.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Mutti A, Aroni S, Fadda P, Padovani L, Mancini L, Collu R, Chiamulera C. The ketamine-like compound MXE substitutes for ketamine in the self-administration paradigm and enhances mesolimbic dopaminergic transmission. Psychopharmacology. 2016;233:2241–2251. doi: 10.1007/s00213-016-4275-0. [DOI] [PubMed] [Google Scholar]
- Panoz-Brown D, Corbin HE, Dalecki SJ, et al. Rats can remember items in context using episodic memory. Current Biology. 2016;26:1–6. doi: 10.1016/j.cub.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Gibbons S, Arunotayanun W, Huang XP, Setola V, Treble R, Iversen L. The ketamine analog MXE and 3-and 4-methoxy analogs of PCP are high affinity and selective ligands for the glutamate NMDA receptor. PloS one. 2013;8:e59334. doi: 10.1371/journal.pone.0059334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacut S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neuroscience Letters. 2010;47:114–118. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, Tricklebank M. A comparison of the effects of ketamine and PCP with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology. 2011;217:255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- Soto PL, Dallery J, Ator NA, Katz BR. A critical examination of best dose analysis for determining cognitive-enhancing potential of drugs: studies with rhesus monkeys and computer simulations. Psychopharmacology. 2013;228:611–622. doi: 10.1007/s00213-013-3070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Davis SA, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug and Alcohol Dependence. 2002;68:175–187. doi: 10.1016/s0376-8716(02)00194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proceedings of the National Academy of Sciences. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanda MT, Fadda P, Chiamulera C, Fratta W, Fattore L. Methoxetamine, a novel psychoactive substance with serious adverse pharmacological effects: a review of case reports and preclinical findings. Behavioural Pharmacology. 2016;27:489–496. doi: 10.1097/FBP.0000000000000241. [DOI] [PubMed] [Google Scholar]


