Abstract
Children are at increased risk of developing metabolic syndrome (MS) after kidney transplantation, which contributes to long-term cardiovascular (CV) morbidities and decline in allograft function. While MS in the general population occurs due to excess caloric intake and physical inactivity, additional chronic kidney disease and transplant-related factors contribute to the development of MS in transplant recipients. Despite its significant health consequences, the interplay of the individual components in CV morbidity in pediatric transplant recipients is not well understood. Additionally, the optimal methods to detect early CV dysfunction are not well defined in this unique population. The quest to establish clear guidelines for diagnosis is further complicated by genetic differences among ethnic groups that necessitate the development of race-specific criteria, particularly with regard to individuals of African descent who carry the apolipoprotein L1 variant. In children, since major CV events are rare and traditional echocardiographic measures of systolic function, such as ejection fraction, are typically well preserved, the presence of CV disease often goes undetected in the early stages. Recently, new noninvasive imaging techniques have become available that offer the opportunity for early detection. Carotid intima-media thickness and impaired myocardial strain detected by speckle tracking echocardiography or cardiac magnetic resonance are emerging as early and sensitive markers of subclinical CV dysfunction. These highly sensitive tools may offer the opportunity to elucidate subtle CV effects of MS in children after transplantation. Current knowledge and future directions are explored in this review.
Keywords: echocardiography, ethnicity, dyslipidemia, hypertension, nutrition, pediatrics
Introduction
Cardiovascular (CV) disease is the second leading cause of morbidity and mortality among children after kidney transplant [1]. The development of metabolic syndrome (MS) after transplantation (MSAT) adds to the burden of CV disease in this population. While MS in the general population develops due to excessive caloric intake and physical inactivity, additional factors inherent to primary kidney disease and Tx-related factors add to MSAT. Common immunosuppression therapies used in renal transplantation alter glucose and lipid metabolism and increase the risks of obesity, hypertension, glucose intolerance and dyslipidemia, adding to the components of MS [2]. Since obesity and MS have become increasingly common among children worldwide, the effects of this epidemic on CV risk in an already vulnerable pediatric transplant population are of particular concern.
It is known that the effects of obesity and MS on CV function begin during childhood, but the signs are likely to be subtle in the early stages. Autopsy studies have identified fatty streaks and atherosclerotic lesions attributable to obesity and MS in the arteries of children and young adults who died of accidental causes [3, 4]. Since major CV events are rare in children, abnormalities may not become apparent until they are in the late stages, thus the opportunity for early intervention is missed. Recently, new noninvasive imaging techniques have become available that may offer the opportunity for early detection. Increased carotid intima-media thickness (CIMT), impaired myocardial strain detection by speckle tracking echocardiography and impaired myocardial strain and oxygenation response to stress detected by cardiac magnetic resonance imaging (CMRI) are emerging as early and sensitive markers of subclinical CV dysfunction. These highly sensitive tools may offer the opportunity to elucidate subtle effects of MS in pediatric renal transplant recipients. Current knowledge and future directions on this topic will be explored in this review.
MSAT
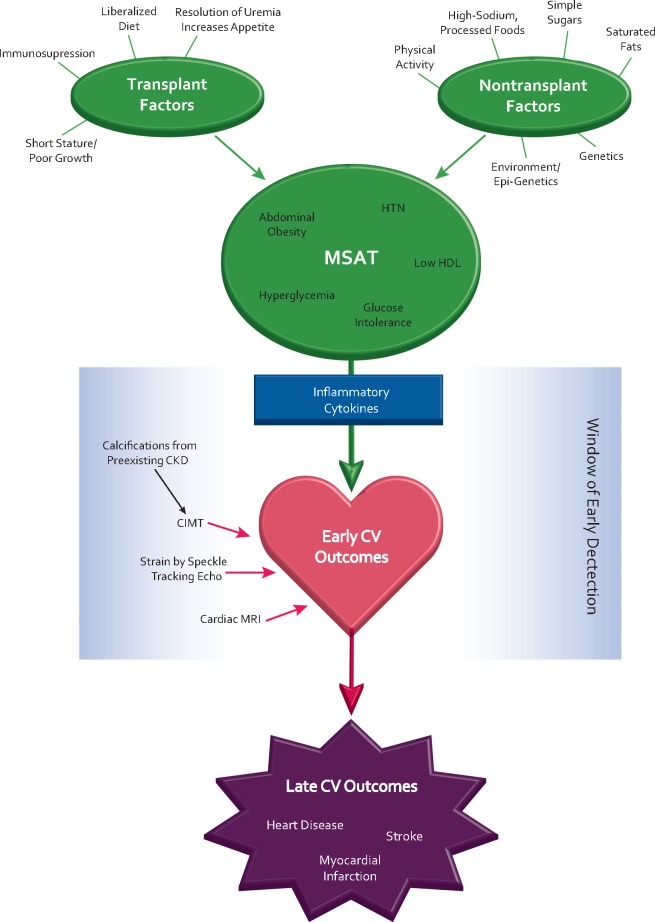
MS is the name coined for a constellation of risk factors that increase future risk of CV disease, stroke and diabetes. MS is traditionally defined as the presence of at least three of the following morbidities: abdominal obesity, impaired glucose tolerance (reflecting insulin resistance), hypertension, hypertriglyceridemia and low high-density lipoprotein (HDL) cholesterol [5]. The basic theory underlying MS is that while each individual component is an independent predictor of CV disease, the combination of multiple factors creates a synergistic CV risk profile that is greater than the sum of its parts. In the general population, studies have shown that childhood MS is a significant predictor of CV disease in adulthood [6]. The traditional paradigm of MS is altered by a myriad of factors associated with chronic kidney disease (CKD) and transplantation. In transplant recipients, typical risk factors for MS, such as excess intake of processed foods and physical inactivity, converge with transplant medication–induced effects of dyslipidemia, glucose intolerance and hypertension to create a hybridized version of MS unique to this population (see Figure 1). MSAT has been associated with more rapid decline in allograft function over time [7–9] and increased risk of atherosclerotic events [10]. As such, the exact definition of MS has not been consistent across the transplant literature (see Table 1). A retrospective cohort study of pediatric transplant recipients (n = 234) reported that the rate of MS significantly increased from 18% pretransplant to 37% at 1 year posttransplant, using body mass index (BMI) rather than abdominal adiposity to define obesity [11]. A key finding of this study was that MS was significantly associated with the presence of left ventricular hypertrophy (LVH) at 1 year posttransplant {odds ratio [OR] 2.6, [95% confidence interval (CI) 1.2–5.9]}. Another study that investigated MS in 32 children after renal transplant did assess abdominal obesity but did not require obesity to define MS. Of eight children determined to have MS in this study, only three were obese. The fact that the majority of children determined to have MS in this study were lean suggests the involvement of individual transplant-related risk factors other than obesity at play in recipients [12]. Therefore, MSAT differs from the classic model of MS, and the interplay of its individual components in pediatric transplant recipients is unclear.
Fig. 1.
Determinants, progression, detection, and early and late CV outcomes of MSAT
Table 1.
Summary of studies on MS in pediatric transplant recipients
| Author | Year | MS definition | Prevalence | Results/associations |
|---|---|---|---|---|
| Ramirez-Cortez et al. [11] | 2009 | ≥3 criteria:
|
|
|
| Wilson et al. [12] | 2010 | ≥3 criteria:
|
|
|
| Maduram et al. [8] | 2010 | ≥3 criteria:
|
|
Lower GFR in children at 1 year post-transplant (65) versus those without MS (65 versus 88 mL/min/1.73 m2) |
| Tainio et al. [7] | 2014 | ≥3 criteria:
|
|
Lower GFR at 1.5 years but no difference at ≥5 years post-transplant |
BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; OGTT, oral glucose tolerance test; TG, triglycerides; WC, waist circumference.
Individual components of MS and transplant recipients
This section will discuss the individual components of MS and how they manifest in pediatric transplant recipients.
Obesity
Childhood obesity is a significant issue affecting the general pediatric population, with recent estimates indicating that 16.9% of US children are obese [13]. A cross-sectional analysis of national data representing US children 6–17 years of age revealed that obese children are at higher risk for dyslipidemia, glucose intolerance and hypertension compared with healthy-weight children [14]. It is projected that by 2025, ∼268 million children will be overweight, including 91 million obese worldwide. These children are expected to have obesity-related comorbidities, including impaired glucose tolerance, type 2 diabetes and hypertension [15]. Causes of obesity include poor diet and physical inactivity, as well as genetic factors; genome-wide association studies (GWAS) have recently identified >90 susceptibility loci for BMI [16].
Obesity is known to increase CV risk in both children and adults. The pathology of obesity-related CV risk is related to the secretion of inflammatory cytokines, such as tumor necrosis factor alpha, from adipose tissue. The inflammatory cytokines induce a variety of unfavorable effects, including endothelial dysfunction, glucose intolerance, vasoconstriction and vascular dysfunction, all of which increase CV risk [17].
The BMI (kg/m2) percentile for age is the most common method used to diagnose overweight (85th–95th percentile) and obesity (≥95th percentile) in children [18]. However, abdominal obesity, measured by waist circumference or waist:height ratio, is more strongly associated with high metabolic and CV risk than is high BMI in the general pediatric population [19–21]. However, each of these anthropometric methods has limitations, particularly in reference to the assessment of children with CKD. Studies have shown that BMI does not accurately reflect body composition in children with CKD, due to their altered body habitus characterized by reduced lean mass and high fat mass, as revealed by dual X-ray absorptiometry [22, 23]. However, waist circumference for age percentiles are likely to underestimate abdominal obesity in children with CKD, since impaired growth and short stature are common. Therefore, more sensitive anthropometric methods are needed to accurately diagnose obesity in this population.
Obesity trends in children with CKD mirror those in the general pediatric population. In a recent analysis of 799 children who participated in the Chronic Kidney Disease in Children Study (CKiD), 15% were overweight and an additional 18% were obese [24]. In this CKiD cohort, median height and weight standard deviation scores (SDSs) were −0.55 and 0.03, respectively, and 12% had severe short stature (SDS < −1.88). The combination of short stature with preserved or above average weight compounds the risk of obesity in this population [24].
Obesity is even more common in the pediatric transplant population, and studies show that the prevalence of obesity doubles (from ∼15% to 30%) during the first year after transplantation [25]. Factors contributing to posttransplant weight gain include increased appetite and improved taste sensation with the resolution of uremia, liberalization of renal diet restrictions, as well as sedentary lifestyle and poor overall physical fitness, which are pervasive in this population [25]. Obesity in pediatric transplant recipients has been associated with decreased allograft survival and increased mortality. Families should receive intensive nutrition education and counseling to promote a heart-healthy diet and regular physical activity, with a goal of at least 60 min of active play daily, to promote maintaining healthy weight and decreasing CV risk after transplant [26].
Hypertension
Hypertension is a major cause of end organ damage and CV morbidity and mortality in the general pediatric population as well as in children with renal disease. A recent evaluation of trends in pediatric hypertension based on a large sample of National Health and Nutrition Examination Survey data indicates that the prevalence is increasing and is associated with the childhood obesity epidemic across the USA [27]. The strong link between obesity and hypertension is substantiated physiologically. The release of angiotensinogen by adipose tissue promotes an increase in blood pressure via stimulation of the renin–angiotensin–aldosterone system (RAAS) and increased sodium reabsorption in obese individuals [28]. In pediatric transplant recipients, hypertension risk is further compounded by the effects of immunosuppressive medications. Corticosteroids are known to increase sodium and water reabsorption and increase renal vascular resistance, while calcineurin inhibitors (CNIs) induce hypertension via afferent arteriolar constriction, stimulation of the RAAS and secretion of inflammatory cytokines leading to fibrosis of the allograft over time [29].
As such, the prevalence of hypertension in the pediatric transplant population is strikingly high. In a study of 74 children, 77% had hypertension prior to transplant and 82.4%, 71.7% and 61% had hypertension at 1, 5 and 10 years posttransplant, respectively [30]. Hypertensive children at 10 years posttransplant had an 8.1 times higher risk of graft loss compared with normotensive children [30]. Hypertension has been associated with increased CIMT and myocardial strain in otherwise healthy children [31, 32] and in pediatric transplant recipients [33]. As hypertension is a key component of MSAT, frequent blood pressure monitoring and aggressive treatment of hypertension are critical in mitigating CV risk in pediatric transplant recipients.
Dyslipidemia
Dyslipidemia is a strong risk factor for CV disease, and compelling evidence from autopsy and cohort studies in the general population indicates that atherosclerotic lesions silently begin to develop during early childhood [4, 34, 35]. Individuals with MS typically exhibit a highly atherogenic lipid profile characterized by hypertriglyceridemia and low HDL cholesterol, which frequently occur together in association with obesity and physical inactivity. Dyslipidemia is common in children with CKD and after kidney transplantation. In a study of 366 children with CKD, 32% had hypertriglyceridemia and 18.3% had low HDL cholesterol and hypertriglyceridemia independently predicted increased CIMT, an indicator of increased risk for CV disease [36].
After transplantation, the risk of dyslipidemia is compounded by the effects of commonly used immunosuppressive agents, including corticosteroids, CNIs and mechanistic target of rapamycin (mTOR) inhibitors. A recent registry study of 386 pediatric transplant recipients reported hypertriglyceridemia in 71% of children at 3 months posttransplant and 59% at 1 year posttransplant and hypertriglyceridemia was associated with lower glomerular filtration rate (GFR) [37]. Corticosteroids alter lipoprotein metabolism and promote dyslipidemia by stimulating hepatic synthesis of very-low-density lipoprotein (VLDL) and down-regulating LDL receptors [38]. A dose-dependent effect of CNI on increased lipid levels has also been demonstrated in adults; however, the use of tacrolimus has generally been associated with a more favorable lipid profile compared with cyclosporine [39]. In pediatric patients, the use of immunosuppressive regimens containing cyclosporine, mTOR inhibitor and steroids was associated with a 25-fold increased risk of dyslipidemia compared with a regimen of tacrolimus, mycophenolate and steroids [37]. The overall high CV morbidity in the pediatric transplant population places them at high risk for early-onset CV disease, as categorized by the National Heart, Lung, and Blood Institute (NHLBI) expert panel, warranting close monitoring of lipid levels and lifestyle and dietary interventions [40]. Obese children without kidney disease are also included in the NHLBI high CV risk category, thus compounding the projected CV risk among obese children with MSAT. The first line of management for dyslipidemia characterized by high triglycerides with low HDL should focus on weight management, including limited intake of saturated fats and simple sugars, and increased physical activity [26]. Although there are no current pharmacological treatments for lowering triglycerides in children, limited data on the beneficial effects of omega-3 fatty acids may hold promise as a future therapy [26]. In addition, adjustment of the immunosuppression regimen may be considered judiciously.
Impaired glucose tolerance
Impaired glucose tolerance can lead to vascular endothelial dysfunction, dyslipidemia, hypertension and vascular inflammation and hence promote the development of CV disease. In a study of moderately obese adults with varying degrees of insulin sensitivity, those with the highest insulin resistance were found to have the highest CV morbidity [41]. After transplantation, glucocorticoids induce peripheral insulin insensitivity while CNIs cause an acquired defect in insulin synthesis and secretion from pancreatic beta cells [42], setting the stage for impaired glucose tolerance. In children, it has been reported that 26.2% have impaired glucose tolerance and 8.1% develop new-onset diabetes after transplantation by 6 months posttransplant [43]. New-onset diabetes after transplantation is associated with abdominal obesity and MSAT and has also been linked with increased risk of CV events after transplant [42, 44–46].
The Kidney Disease Outcomes Quality Initiative pediatric guidelines recommend that patients should be counseled on a diet low in simple sugars, avoiding juices, soda and other sweetened beverages to minimize the risks of excess weight gain and hyperglycemia posttransplant [26].
Ethnic differences in MS and CV risks
Individuals of African descent generally carry a higher risk for MS, CV disease and kidney disease than other races [13, 47–49]. The reasons for these disparities are not completely understood and are likely multifactorial in nature. Genetic factors are known to play a role, due to the strong association of the apolipoprotein L1 (APOL1) gene with the risk of CKD in individuals of African ancestry. Relationships of APOL1 renal risk variants with increased risk of CV disease among those of African descent are also starting to emerge [50]. In the Women’s Health Initiative study of 749 postmenopausal African American women, those with two APOL1 alleles had a lower GFR and higher risk for incident CV disease compared with those with zero APOL1 alleles (OR 1.98; P = 8.37 × 10−3) [51]. New insights into the role of genetic factors and obesity are also being uncovered as GWASs delve into this area. Recently the first GWAS for BMI in individuals of African descent identified a novel BMI locus known as SEMA4D, which appears to promote obesity through regulation of a transcription start site, and may explain some of the increased burden of obesity among this population [52]. Beyond genetics, other variables contributing to differences in cardiometabolic risk among ethnic groups may include environmental exposures, cultural beliefs, psychosocial factors and access to healthy foods and health care.
African American adults, including young adults, have higher rates of adverse CV events and CV death compared with other ethnic groups [53, 54]. Evidence shows that African Americans have higher left ventricular (LV) mass compared with other ethnic groups [55]. Looking at subclinical markers of CV disease, studies have shown that CIMT is higher among healthy adults and children of African ancestry compared with other ethnicities [56, 57]. A recent cross-sectional study by Lefferts et al. [58] examined racial differences in CIMT and aortic stiffness measured by pulse wave velocity in healthy children. This study reported higher pulse wave velocity and CIMT in African American children compared with Caucasian children after adjustment for age, sex, blood pressure and socioeconomic status. The etiology of increased CIMT in this population is unknown. It remains to be seen whether it may be related to environmental exposures, genetic polymorphisms or other causes. One study suggests that higher CIMT among individuals of African ancestry may be related to physiological differences in the size of CV structures and lean body mass, which vary among different ethnic groups [59], while another study concluded that a blunted nocturnal cortisol increase, caused by psychosocial stress, may account for increased risk for atherosclerosis and CIMT in obese African American youth [60].
Few studies have investigated ethnic differences in myocardial strain. The Multi-Ethnic Study of Atherosclerosis, a prospective, observational cohort study of 6814 healthy adults representing four ethnic groups (Caucasian, African American, Hispanic and Chinese American), investigated racial and ethnic differences in subclinical myocardial function using CMRI. African Americans were found to have the least favorable systolic strain, even after correcting for hypertension and LV mass [55]. Another study assessed LV strain by speckle tracking echo cardiography in a tri-ethnic (African American, Hispanic and Caucasian) healthy population with normal ejection fraction (EF) and similarly found that African Americans had the greatest degree of subclinical LV systolic dysfunction detected by longitudinal strain [61].
Recent evidence suggests that the relationship and degree of synergy between individual metabolic risk factors vary greatly among different ethnic groups [62]. Little is known about race-specific factors impacting MSAT in pediatric transplant recipients. Given the high rate of metabolic derangement, CV morbidity and end-stage renal disease among those of African ancestry, future studies should investigate how the effects of transplant-related factors on cardiometabolic risk differ among pediatric transplant recipients of different ethnicities. These differences add another layer of complexity and should be further investigated in order to establish race-specific guidelines for defining MSAT and evaluating CV risk in a diverse population of pediatric transplant recipients.
Evaluation of CV disease
Traditional evaluation by standard echocardiography
The detection of overt abnormalities in standard measures of systolic LV function by echocardiography, such as EF or fractional shortening, is rare in the pediatric population [25]. Even among children with obesity and MS, EF is typically found to be normal [63]. The presence of abnormal EF or fractional shortening in a child would indicate that advanced CV disease is already present. Evaluation of LV mass by traditional echocardiography is also of limited use in detecting CV dysfunction in children with CKD, due to its lack of accuracy in young children, underestimation of LVH in overweight patients and lack of reliability in fluid overloaded patients. Recent evidence is emerging to suggest that subclinical CV abnormalities do develop early in children with CKD and may portend adverse outcomes. Thus there is a need for newer, more sensitive tools such as speckle tracking echocardiography, CMRI and CIMT for early detection of CV dysfunction in pediatric transplant recipients.
LVH
LVH has traditionally been considered important in the identification of CV risk. LVH can develop early in the course of CKD, and often persists after transplantation, particularly in association with MSAT. A retrospective study of 234 pediatric transplant recipients reported a 40% prevalence of LVH and 2.6 times greater risk of LVH in recipients with MS than in those without MS [11]. However, there is no consensus on the best way to define LVH in young children, due to the significant changes in the relationship of their height to body and heart size with rapid growth, or in the pediatric transplant population, due in part to their abnormal body composition and short stature [64]. LV mass normalized to height2.7 is a commonly used method to evaluate LVH, as it describes the relationship between heart and body size without obscuring the effects of obesity. In children ≥10 years of age, LVH is defined as >40 g/m2.7 in girls and >45 g/m2.7 in boys. However, this method is not reliable in children <10 years of age and, in addition may underestimate relative left ventricular mass (LVM) in thin children and overestimate LVM in overweight children [65–67]. To address this limitation, Khoury et al. [66] developed normal age-specific percentiles for LVM/height2.7, using LVM/height2.7 >95th percentile for age to define LVH. Subsequently, Foster et al. [68] developed new LV mass reference percentiles expressing LV mass relative to lean body mass, which is the strongest determinant of LV mass. Thus, while some studies have reported improvement in LVH after transplant, findings have been inconsistent across the pediatric literature due in part to the limitations discussed.
Novel imaging tools for early detection of CV morbidity
Carotid intima-media thickness
CIMT has emerged as a reproducible surrogate marker for early atherosclerosis [69]. Noninvasive imaging of the carotid arteries is used to demonstrate the status of the IMT of the vessel. Prospective studies have demonstrated that an increase in CIMT is associated with an increase in the relative risk for stroke and myocardial infarction in the general adult population [70, 71]. Increased CIMT has been found to be associated with MS and its components (hyperlipidemia, hypertension, obesity, type 1 diabetes) in the general pediatric population [72]. Woo et al. [73] conducted a study investigating the effect of diet and exercise on noninvasive markers of atherosclerosis in otherwise healthy obese children and found a significant improvement in CIMT and percent body fat after 1 year of intervention, suggesting that a significant change in CIMT can be detected after a 1-year period. Studies of CIMT in pediatric transplant recipients are summarized in Table 2. Two recent studies investigating CIMT in pediatric renal transplant recipients found that CIMT was significantly greater compared with healthy controls and CIMT correlated with the duration of dialysis prior to transplantation [80, 78]. Litwin et al. [76] reported that the CIMT of children with CKD or on dialysis increased by 0.7 SD over a period of 1 year, while CIMT improved by an average of 0.6 SD within 1 year in those who received a transplant. The impact of MSAT on the CIMT of children after transplant is not well known and should be investigated in future studies. In addition, the CIMT of pediatric transplant recipients of African ancestry has not been reported. Only two of the nine previous studies summarized below included a small number of African American patients and the CIMT of the African American recipients was combined with other races in the analysis.
Table 2.
Summary of studies on CIMT in pediatric transplant recipients[TQ4]
| Author | Year | Design | Population (location) | A-A (n) | Results | Associations |
|---|---|---|---|---|---|---|
| Mitsnefes et al. [25] | 2004 | Cross-sectional | 31 transplant/31 control (Cincinnati, OH, USA) | 7 | CIMT higher in transplant versus controls | SBP, number of BP meds |
| Litwin et al. [74] | 2005 | Cross-sectional | 34 transplant/55 CKD/37 dialysis/270 control (Germany, Poland) | 0 | CIMT higher in all patient groups versus control | Higher calcium × phosphorus, dialysis |
| Bilginer et al. [75] | 2007 | Cross-sectional | 24 transplant/20 control (Turkey) | 0 | CIMT higher in transplant versus controls | Calcium × phosphorus, duration of dialysis |
| Litwin et al. [76] | 2008 | Cohort, 12-month duration | 32 ESRD; 19 underwent transplant during study (Germany, Poland) | 0 | CIMT decreased over time by 0.7 SD | Phosphorus, duration of dialysis, BP |
| Krmar et al. [77] | 2008 | Cohort, mean 4.1-year duration | 31 transplant/21 control (Sweden) | 0 | CIMT stable over time in transplant, higher versus controls | No association between BP and CIMT |
| Delucchi et al. [78] | 2008 | Cross-sectional | 12 transplant/8 dialysis/20 control (Chile) | 0 | CIMT in dialysis and transplant higher versus controls | Duration of dialysis |
| Siirtola et al. [79] | 2010 | Cross-sectional | 13 transplant/26 control (Finland) | 0 | CIMT higher in transplant versus controls | GFR <60 mL/min/1.73 m2, triglycerides |
| Basiratnia et al. [80] | 2010 | Cross-sectional | 66 transplant/66 control (Iran) | 0 | CIMT higher in transplantversus controls | Calcitriol dose |
| Tawadrous et al. [81] | 2012 | Cross-sectional | 14 transplant/15 dialysis/15 control (Brooklyn, NY, USA) | 6 | CIMT higher in dialysis versus transplant and controls | None identified |
ESRD, end-stage renal disease; SBP, systolic blood pressure.
Myocardial strain by speckle tracking echocardiography
Assessment of myocardial strain by speckle tracking echocardiography utilizes new technology to analyze myocardial motion by tracking natural acoustic markers (or speckles) as they move during myocardial contraction [82]. Strain is emerging as an important, noninvasive tool for the assessment of LV systolic function. Its key advantage is the ability to detect early signs of LV dysfunction that are not evident by standard echo cardiography. In addition, strain analysis is load independent, making it well suited for evaluation of myocardial function in children with kidney disease. Recent evidence indicates that subclinical myocardial dysfunction, detected by impaired myocardial strain but not by standard echocardiography, is present in otherwise healthy children and young adults with obesity, hypertension and type 1 diabetes [63, 83–87]. Impaired strain has also been shown to be indicative of early myocardial dysfunction in septic shock in children [88], of prognostic value for myocardial recovery after myocardial infarction [89] and an accurate predictor of cardiac events and CV mortality, superior to EF [90]. Longitudinal strain was a strong independent predictor of mortality in a recent study of adult hemodialysis patients with preserved EF, further supporting the utility of speckle tracking echocardiography in identifying early subclinical CV risk [91]. Little is known about the myocardial strain of pediatric transplant recipients. To date, three studies investigating myocardial strain in children with CKD have been published in the literature. The Cardiovascular Comorbidity in Children with CKD (4C) study investigated myocardial strain in 272 children with CKD in 14 European countries compared with 61 healthy controls [92]. The study found that despite having a normal EF, the myocardial strain of children with CKD was impaired in the radial and circumferential directions. In addition, LVH was more common in children with CKD compared with controls (55% versus 7%; P = 0.001) and the LVH geometry was preferentially concentric. There were no differences noted in longitudinal strain between the CKD and control groups. The authors hypothesize that the concentric LVH may have occurred as a response to impaired circumferential function, possibly suggestive of intrinsic structural abnormalities of the heart muscle in children with CKD. Another recent cross-sectional study investigated myocardial strain in children with end-stage renal disease (19 dialysis patients and 17 transplant patients) compared with 33 healthy controls [93]. This study similarly found no differences in EF between patients and controls, but significantly increased LV wall thickness and impaired myocardial strain in the dialysis and transplant patients compared with controls. The myocardial dysfunction in the dialysis and transplant groups was characterized by impaired longitudinal strain, while circumferential and radial strain did not differ from controls. This is in contrast to the findings of circumferential dysfunction in the CKD population reported by the 4C study and may reflect different LV mechanics during different stages along the continuum of renal disease. Looking closer at this continuum, Rumman et al. [94] conducted a retrospective analysis of myocardial strain in 48 children at three different time points: CKD, dialysis and 1 year posttransplant compared with 192 healthy controls. Results of this study indicated that the EF of children was similar to controls and remained normal throughout dialysis and transplantation. Longitudinal and circumferential strain parameters were similar to controls during CKD. Longitudinal strain worsened during dialysis [β = 2.0 (95% CI 0.4–3.6)], but the association was not significant after adjustment for blood pressure and CKD. Following transplantation, longitudinal strain improved back to CKD levels.
The existing data on myocardial strain are limited, but suggest that children with CKD have subclinical myocardial dysfunction that develops during the course of CKD, worsens during dialysis and may persist after kidney transplantation. In children, the signs of myocardial dysfunction are typically not apparent by standard echocardiography, but subclinical abnormalities are detectable by speckle tracking echocardiography. The investigation of strain in the pediatric transplant population is of particular importance, as it may provide an opportunity to identify those children at highest risk and provide an opportunity for early intervention. The studies discussed above were limited primarily to Caucasian populations. Pediatric transplant recipients of African ancestry are an underrepresented group that should be included in future studies.
CMRI
CMRI is another highly sensitive imaging technology that is emerging as a new technique to provide insight into subclinical CV dysfunction in transplant recipients. Advantages of CMRI, in which heart contours are individually traced, include high reproducibility and ability to measure LVM independent of volume and geometric assumptions [95]. As this technique is in its infancy, only a few studies have investigated CMRI in transplant recipients (see Table 3). CMRI was recently shown to be a superior method for measurement of LVM in children with CKD and transplant compared with standard echocardiography [99]. In addition to LVM, CMRI has also been used to detect signs of early CV dysfunction by impaired myocardial circumferential strain in pediatric dialysis and transplant patients with normal EF [96]. Another study utilized CMRI to conduct tissue phase mapping in 20 children with CKD (before or after transplant) and found reduced regional LV wall velocities compared with controls, in the absence of LVH. Finally, blood oxygen level–dependent CMRI has been used to detect myocardial ischemia by assessing the tissue oxygenation of myocardial segments at rest and under stress by blood oxygen level–dependent signal intensity. A recent study employing this technique found that patients with CKD, dialysis and renal transplant had impaired myocardial response to stress compared with hypertensive and normal control subjects [98]. More research is needed to learn about the ways that this promising and versatile technique can be applied to the detection of subclinical CV dysfunction in the pediatric transplant population.
Table 3.
Summary of studies of CMRI in transplant recipients
| Author | Population | Technique | Results/conclusions |
|---|---|---|---|
| Malatesta-Muncher et al. [96] | Pediatric: ESRD (10 dialysis/10 transplant) versus 24 healthy controls | CMRI and CMRS | CMRI and MRS detected subclinical cardiac dysfunction, decreased energy metabolism and myocardial microcomposition in ESRD patients, despite normal EF |
| Schaefer et al. [97] | Pediatric: 15 children (2 CKD, 6 PD, 7 HD, 18 transplants) | CMRI (before and after transplant | All CMRI parameters (EF, end diastolic LV volume index, end systolic LV volume index and LVMI) improved after transplant |
| Parnham et al. [98] | Adult: 12 CKD, 11 dialysis, 10 transplant, 10 HTN controls, 10 healthy controls | BOLD CMRI | CKD, dialysis and transplant had impaired myocardial response to stress in comparison to HTN and normal controls |
| Arnold et al. [99] | Pediatric: 25 CKD (14 post-transplant) | CMRI versus standard echocardiography | Echo underestimates LVM compared to CMRI |
| CMR-LVMI but not echo-LVMI predicted future GFR decline | |||
| Gimpel et al. [100] | Pediatric: 20 CKD/transplant versus 12 healthy controls | CMRI tissue phase mapping | Reduced regional LV wall velocities in CKD and transplant, with normal LVH |
ESRD, end-stage renal disease; HD, hemodialysis; HTN, hypertension; LVMI, left ventricular mass index; MRS, magnetic resonance spectroscopy; PD, peritoneal dialysis.
Conclusion
MSAT occurs in pediatric transplant recipients when obesity and transplant-related factors converge, leading to high CV risk and adverse long-term prognosis. Improved understanding of the interplay of factors that create MSAT in this population as well as more aggressive focus on prevention and early detection are important for improving patient outcomes. Identification of subclinical CV damage, detected by methods such as CIMT, strain by speckle tracking echo cardiography and CMRI are important for early CV risk stratification in this population. Investigation of the effects of ethnic differences in MSAT and CV risk are critical to creating accurate risk prediction models for diverse pediatric transplant recipients. Further research on the pathophysiology, prevention and early detection of MSAT are important steps toward the ultimate goal of delaying or avoiding the occurrence of major CV events and prolonging the life expectancy of children after kidney transplantation.
Conflict of interest statement
None declared.
References
- 1. Dharnidharka VR, Fiorina P, Harmon WE.. Kidney transplantation in children. N Engl J Med 2014; 371: 549–558 [DOI] [PubMed] [Google Scholar]
- 2. Brady TM, Parekh RS.. Metabolic syndrome: signs and symptoms running together. Pediatr Transplant 2010; 14: 6–9 [DOI] [PubMed] [Google Scholar]
- 3. McGill HC, Jr, McMahan CA, Herderick EE, et al. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr 2000; 72(Suppl 5): 1307S–1315S [DOI] [PubMed] [Google Scholar]
- 4. Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998; 338: 1650–1656 [DOI] [PubMed] [Google Scholar]
- 5. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–2752 [DOI] [PubMed] [Google Scholar]
- 6. Morrison JA, Friedman LA, Gray-McGuire C.. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics 2007; 120: 340–345 [DOI] [PubMed] [Google Scholar]
- 7. Tainio J, Qvist E, Hölttä T, et al. Metabolic risk factors and long-term graft function after paediatric renal transplantation. Transpl Int 2014; 27: 583–592 [DOI] [PubMed] [Google Scholar]
- 8. Maduram A, John E, Hidalgo G, et al. Metabolic syndrome in pediatric renal transplant recipients: comparing early discontinuation of steroids vs. steroid group. Pediatr Transplant 2010; 14: 351–357 [DOI] [PubMed] [Google Scholar]
- 9. Ruangkanchanasetr P, Bunnag S, Vongwiwatana A, et al. Metabolic syndrome in Thai renal transplant recipients: a multicenter study. Ann Transplant 2015; 20: 500–505 [DOI] [PubMed] [Google Scholar]
- 10. Courivaud C, Kazory A, Simula-Faivre D, et al. Metabolic syndrome and atherosclerotic events in renal transplant recipients. Transplantation 2007; 83: 1577–1581 [DOI] [PubMed] [Google Scholar]
- 11. Ramirez-Cortes G, Fuentes-Velasco Y, García-Roca P, et al. Prevalence of metabolic syndrome and obesity in renal transplanted Mexican children. Pediatr Transplant 2009; 13: 579–584 [DOI] [PubMed] [Google Scholar]
- 12. Wilson AC, Greenbaum LA, Barletta GM, et al. High prevalence of the metabolic syndrome and associated left ventricular hypertrophy in pediatric renal transplant recipients. Pediatr Transplant 2010; 14: 52–60 [DOI] [PubMed] [Google Scholar]
- 13. Ogden CL, Carroll MD, Kit BK. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311: 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skinner AC, Mayer ML, et al. Health status and health care expenditures in a nationally representative sample: how do overweight and healthy-weight children compare? Pediatrics 2008; 121: e269–e277 [DOI] [PubMed] [Google Scholar]
- 15. Lobstein T, Jackson-Leach R.. Planning for the worst: estimates of obesity and comorbidities in school-age children in 2025. Pediatr Obes 2016; 11: 321–325 [DOI] [PubMed] [Google Scholar]
- 16. Welter D, MacArthur J, Morales J, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res 2014; 42(Database issue): D1001–D1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 2000; 148: 209–214 [DOI] [PubMed] [Google Scholar]
- 18. Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002; 109: 45–60 [DOI] [PubMed] [Google Scholar]
- 19. Kahn HS, Imperatore G, Cheng YJ.. A population-based comparison of BMI percentiles and waist-to-height ratio for identifying cardiovascular risk in youth. J Pediatr 2005; 146: 482–488 [DOI] [PubMed] [Google Scholar]
- 20. Maffeis C, Banzato C, Talamini G.. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J Pediatr 2008; 152: 207–213 [DOI] [PubMed] [Google Scholar]
- 21. Caprio S, Hyman LD, McCarthy S, et al. Fat distribution and cardiovascular risk factors in obese adolescent girls: importance of the intraabdominal fat depot. Am J Clin Nutr 1996; 64: 12–17 [DOI] [PubMed] [Google Scholar]
- 22. Johnson VL, Wang J, Kaskel FJ, et al. Changes in body composition of children with chronic renal failure on growth hormone. Pediatr Nephrol 2000; 14: 695–700 [DOI] [PubMed] [Google Scholar]
- 23. Rashid R, Neill E, Smith W, et al. Body composition and nutritional intake in children with chronic kidney disease. Pediatr Nephrol 2006; 21: 1730–1738 [DOI] [PubMed] [Google Scholar]
- 24. Rodig NM, McDermott KC, Schneider MF, et al. Growth in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Pediatr Nephrol 2014; 29: 1987–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson AC, Mitsnefes MM.. Cardiovascular disease in CKD in children: update on risk factors, risk assessment, and management. Am J Kidney Dis 2009; 54: 345–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis 2009; 53(3 Suppl 2): S11–S104 [DOI] [PubMed] [Google Scholar]
- 27. Rosner B, Cook NR, Daniels S, et al. Childhood blood pressure trends and risk factors for high blood pressure: the NHANES experience 1988–2008. Hypertension 2013; 62: 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of the Obesity Society and the American Society of Hypertension. J Clin Hypertens 2013; 15: 14–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alpay N, Ozkok A, Caliskan Y, et al. Influence of conversion from calcineurin inhibitors to everolimus on fibrosis, inflammation, tubular damage and vascular function in renal transplant patients. Clin Exp Nephrol 2014; 18: 961–967 [DOI] [PubMed] [Google Scholar]
- 30. Stabouli S, Printza N, Dotis J, et al. Long-term changes in blood pressure after pediatric kidney transplantation. Am J Hypertens 2016; 29: 860–865 [DOI] [PubMed] [Google Scholar]
- 31. Juhola J, Magnussen CG, Berenson GS, et al. Combined effects of child and adult elevated blood pressure on subclinical atherosclerosis: the International Childhood Cardiovascular Cohort Consortium. Circulation 2013; 128: 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lande MB, Carson NL, Roy J, et al. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension 2006; 48: 40–44 [DOI] [PubMed] [Google Scholar]
- 33. Mitsnefes MM, Kimball TR, Witt SA, et al. Abnormal carotid artery structure and function in children and adolescents with successful renal transplantation. Circulation 2004; 110: 97–101 [DOI] [PubMed] [Google Scholar]
- 34. Strong JP, Malcom GT, McMahan CA, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 1999; 281: 727–735 [DOI] [PubMed] [Google Scholar]
- 35. McMahan CA, Gidding SS, Malcom GT, et al. Pathobiological determinants of atherosclerosis in youth risk scores are associated with early and advanced atherosclerosis. Pediatrics 2006; 118: 1447–1455 [DOI] [PubMed] [Google Scholar]
- 36. Brady TM, Schneider MF, Flynn JT, et al. Carotid intima-media thickness in children with CKD: results from the CKiD study. Clin J Am Soc Nephrol 2012; 7: 1930–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Habbig S, Volland R, Krupka K, et al. Dyslipidemia after pediatric renal transplantation—the impact of immunosuppressive regimens. Pediatr Transplant 2017; 21. doi:10.1111/petr.12914 [DOI] [PubMed] [Google Scholar]
- 38. Berg AL, Nilsson-Ehle P.. ACTH lowers serum lipids in steroid-treated hyperlipemic patients with kidney disease. Kidney Int 1996; 50: 538–542 [DOI] [PubMed] [Google Scholar]
- 39. Kramer BK, Montagnino G, Del Castillo D, et al. Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation: 2 year follow-up results. Nephrol Dial Transplant 2005; 20: 968–973 [DOI] [PubMed] [Google Scholar]
- 40. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011; 128(Suppl 5): S213–S256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McLaughlin T, Abbasi F, Lamendola C, et al. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med 2007; 167: 642–648 [DOI] [PubMed] [Google Scholar]
- 42. Mora PF. New-onset diabetes after renal transplantation. J Invest Med 58: 755–763 [DOI] [PubMed] [Google Scholar]
- 43. Buyan N, Bilge I, Turkmen MA, et al. Post-transplant glucose status in 61 pediatric renal transplant recipients: preliminary results of five Turkish pediatric nephrology centers. Pediatr Transplant 2010; 14: 203–211 [DOI] [PubMed] [Google Scholar]
- 44. Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int 2006; 69: 588–595 [DOI] [PubMed] [Google Scholar]
- 45. Luan FL, Langewisch E, Ojo A.. Metabolic syndrome and new onset diabetes after transplantation in kidney transplant recipients. Clin Transplant 2010; 24: 778–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dedinska I, Laca L, Miklusica J, et al. Waist circumference as an independent risk factor for NODAT. Ann Transplant 2015; 20: 154–159 [DOI] [PubMed] [Google Scholar]
- 47. Carson AP, Howard G, Burke GL, et al. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 2011; 57: 1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bash LD, Astor BC, Coresh J.. Risk of incident ESRD: a comprehensive look at cardiovascular risk factors and 17 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2010; 55: 31–41 [DOI] [PubMed] [Google Scholar]
- 49. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–e292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McLean NO, Robinson TW, Freedman BI.. APOL1 gene kidney risk variants and cardiovascular disease: getting to the heart of the matter. Am J Kidney Dis 2017; 70: 281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ito K, Bick AG, Flannick J, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res 2014; 114: 845–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen G, Doumatey AP, Zhou J, et al. Genome-wide analysis identifies an african-specific variant in SEMA4D associated with body mass index. Obesity 2017; 25: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meadows TA, Bhatt DL, Cannon CP, et al. Ethnic differences in cardiovascular risks and mortality in atherothrombotic disease: insights from the Reduction of Atherothrombosis for Continued Health (REACH) registry. Mayo Clin Proc 2011; 86: 960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360: 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fernandes VR, Cheng S, Cheng YJ, et al. Racial and ethnic differences in subclinical myocardial function: the Multi-Ethnic Study of Atherosclerosis. Heart 2011; 97: 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mittelman SD, Gilsanz P, Mo AO, et al. Adiposity predicts carotid intima-media thickness in healthy children and adolescents. J Pediatr 2010; 156: 592–597.e2 [DOI] [PubMed] [Google Scholar]
- 57. Markus H, Kapozsta Z, Ditrich R, et al. Increased common carotid intima-media thickness in UK African Caribbeans and its relation to chronic inflammation and vascular candidate gene polymorphisms. Stroke 2001; 32: 2465–2471 [DOI] [PubMed] [Google Scholar]
- 58. Lefferts WK, Augustine JA, Spartano NL, et al. Racial differences in aortic stiffness in children. J Pediatr 2017; 180: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chowdhury SM, Henshaw MH, Friedman B, et al. Lean body mass may explain apparent racial differences in carotid intima-media thickness in obese children. J Am Soc Echocardiogr 2014; 27: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Toledo-Corral CM, Myers SJ, Li Y, et al. Blunted nocturnal cortisol rise is associated with higher carotid artery intima-media thickness (CIMT) in overweight African American and Latino youth. Psychoneuroendocrinology 2013; 38: 1658–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Russo C, Jin Z, Homma S, et al. Race-ethnic differences in subclinical left ventricular systolic dysfunction by global longitudinal strain: a community-based cohort study. Am Heart J 2015; 169: 721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cheung EL, Bell CS, Samuel JP, et al. Race and obesity in adolescent hypertension. Pediatrics 2017; 139: e20161433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koopman LP, Mertens LL.. Impact of childhood obesity on cardiac structure and function. Curr Treat Options Cardiovasc Med 2014; 16: 345. [DOI] [PubMed] [Google Scholar]
- 64. Schoenmaker NJ, van der Lee JH, Groothoff JW, et al. Low agreement between cardiologists diagnosing left ventricular hypertrophy in children with end-stage renal disease. BMC Nephrol 2013; 14: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 1992; 20: 1251–1260 [DOI] [PubMed] [Google Scholar]
- 66. Khoury PR, Mitsnefes M, Daniels SR, et al. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 2009; 22: 709–714 [DOI] [PubMed] [Google Scholar]
- 67. Foster BJ, Gao T, Mackie AS, et al. Limitations of expressing left ventricular mass relative to height and to body surface area in children. J Am Soc Echocardiogr 2013; 26: 410–418 [DOI] [PubMed] [Google Scholar]
- 68. Foster BJ, Khoury PR, Kimball TR, et al. New reference centiles for left ventricular mass relative to lean body mass in children. J Am Soc Echocardiogr 2016; 29: 441–447e2 [DOI] [PubMed] [Google Scholar]
- 69. de Groot E, Hovingh GK, Wiegman A, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation 2004; 109(23 Suppl 1): III33–III38 [DOI] [PubMed] [Google Scholar]
- 70. Bots ML, Grobbee DE.. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc Drug Ther 2002; 16: 341–351 [DOI] [PubMed] [Google Scholar]
- 71. van der Meer IM, Bots ML, Hofman A, et al. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation 2004; 109: 1089–1094 [DOI] [PubMed] [Google Scholar]
- 72. Urbina EM, Williams RV, Alpert BS, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension 2009; 54: 919–950 [DOI] [PubMed] [Google Scholar]
- 73. Woo KS, Chook P, Yu CW, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation 2004; 109: 1981–1986 [DOI] [PubMed] [Google Scholar]
- 74. Litwin M, Wuhl E, Jourdan C, et al. Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 2005; 16: 1494–1500 [DOI] [PubMed] [Google Scholar]
- 75. Bilginer Y, Ozaltin F, Basaran C, et al. Carotid intima-media thickness in children and young adults with renal transplant: internal carotid artery vs. common carotid artery. Pediatr Transplant 2007; 11: 888–894 [DOI] [PubMed] [Google Scholar]
- 76. Litwin M, Wuhl E, Jourdan C, et al. Evolution of large-vessel arteriopathy in paediatric patients with chronic kidney disease. Nephrol Dial Transplant 2008; 23: 2552–2557 [DOI] [PubMed] [Google Scholar]
- 77. Krmar RT, Balzano R, Jogestrand T, et al. Prospective analysis of carotid arterial wall structure in pediatric renal transplants with ambulatory normotension and in treated hypertensive recipients. Pediatr Transplant 2008; 12: 412–419 [DOI] [PubMed] [Google Scholar]
- 78. Delucchi A, Dinamarca H, Gainza H, et al. Carotid intima-media thickness as a cardiovascular risk marker in pediatric end-stage renal disease patients on dialysis and in renal transplantation. Transplant Proc 2008; 40: 3244–3246 [DOI] [PubMed] [Google Scholar]
- 79. Siirtola A, Kallio T, Ala-Houhala M, et al. Carotid intima-media thickness after pediatric renal or liver transplantation at high-resolution B-mode ultrasonography. Transplant Proc 2010; 42: 1695–1698 [DOI] [PubMed] [Google Scholar]
- 80. Basiratnia M, Fazel M, Lotfi M, et al. Subclinical atherosclerosis and related risk factors in renal transplant recipients. Pediatr Nephrol 25: 343–348 [DOI] [PubMed] [Google Scholar]
- 81. Tawadrous H, Kamran H, Salciccioli L, et al. Evaluation of arterial structure and function in pediatric patients with end-stage renal disease on dialysis and after renal transplantation. Pediatr Transplant 2012; 16: 480–485 [DOI] [PubMed] [Google Scholar]
- 82. Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 23: 351–369; quiz 453–455 [DOI] [PubMed] [Google Scholar]
- 83. Mehta SK, Holliday C, Hayduk L, et al. Comparison of myocardial function in children with body mass indexes ≥25 versus those <25 kg/m2. Am J Cardiol 2004; 93: 1567–1569 [DOI] [PubMed] [Google Scholar]
- 84. Lorch SM, Sharkey A.. Myocardial velocity, strain, and strain rate abnormalities in healthy obese children. J Cardiometab Syndr 2007; 2: 30–34 [DOI] [PubMed] [Google Scholar]
- 85. Labombarda F, Leport M, Morello R, et al. Longitudinal left ventricular strain impairment in type 1 diabetes children and adolescents: a 2D speckle strain imaging study. Diabetes Metab 2014; 40: 292–298 [DOI] [PubMed] [Google Scholar]
- 86. Kishi S, Teixido-Tura G, Ning H, et al. Cumulative blood pressure in early adulthood and cardiac dysfunction in middle age: the CARDIA Study. J Am Coll Cardiol 2015; 65: 2679–2687 [DOI] [PubMed] [Google Scholar]
- 87. Di Salvo G, Pacileo G, Del Giudice EM, et al. Abnormal myocardial deformation properties in obese, non-hypertensive children: an ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur Heart J 2006; 27: 2689–2695 [DOI] [PubMed] [Google Scholar]
- 88. Basu S, Frank LH, Fenton KE, et al. Two-dimensional speckle tracking imaging detects impaired myocardial performance in children with septic shock, not recognized by conventional echocardiography. Pediatr Crit Care Med 2012; 13: 259–264 [DOI] [PubMed] [Google Scholar]
- 89. Kylmala MM, Antila M, Kivisto SM, et al. Can strain rate imaging predict recovery of contraction after acute myocardial infarction? Eur J Echocardiogr 2011; 12: 364–371 [DOI] [PubMed] [Google Scholar]
- 90. Cho GY, Marwick TH, Kim HS, et al. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol 2009; 54: 618–624 [DOI] [PubMed] [Google Scholar]
- 91. Kramann R, Erpenbeck J, Schneider RK, et al. Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. J Am Soc Nephrol 2014; 25: 2351–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chinali M, Matteucci MC, Franceschini A, et al. Advanced parameters of cardiac mechanics in children with CKD: the 4C Study. Clin J Am Soc Nephrol 2015; 10: 1357–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. van Huis M, Schoenmaker NJ, Groothoff JW, et al. Impaired longitudinal deformation measured by speckle-tracking echocardiography in children with end-stage renal disease. Pediatr Nephrol 2016; 31: 1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rumman RK, Ramroop R, Chanchlani R, et al. Longitudinal assessment of myocardial function in childhood chronic kidney disease, during dialysis, and following kidney transplantation. Pediatr Nephrol 2017. doi:10.1007/s00467-017-3622-7 [DOI] [PubMed] [Google Scholar]
- 95. Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002; 90: 29–34 [DOI] [PubMed] [Google Scholar]
- 96. Malatesta-Muncher R, Wansapura J, Taylor M, et al. Early cardiac dysfunction in pediatric patients on maintenance dialysis and post kidney transplant. Pediatr Nephrol 2012; 27: 1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schaefer B, Rusai K, Toth A, et al. Cardiac magnetic resonance imaging in children with chronic kidney disease and renal transplantation. Pediatr Transplant 2012; 16: 350–356 [DOI] [PubMed] [Google Scholar]
- 98. Parnham S, Gleadle JM, Bangalore S, et al. Impaired myocardial oxygenation response to stress in patients with chronic kidney disease. J Am Heart Assoc 2015; 4: e002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Arnold R, Schwendinger D, Jung S, et al. Left ventricular mass and systolic function in children with chronic kidney disease-comparing echocardiography with cardiac magnetic resonance imaging. Pediatr Nephrol 2016; 31: 255–265 [DOI] [PubMed] [Google Scholar]
- 100. Gimpel C, Jung BA, Jung S, et al. Magnetic resonance tissue phase mapping demonstrates altered left ventricular diastolic function in children with chronic kidney disease. Pediatr Radiol 2017; 47: 169–177 [DOI] [PubMed] [Google Scholar]