Abstract
The discovery of osteoinductivity of calcium phosphate (Ca-P) ceramics has set an enduring paradigm of conferring biological regenerative activity to materials with carefully designed structural characteristics. The unique phase composition and porous structural features of osteoinductive Ca-P ceramics allow it to interact with signaling molecules and extracellular matrices in the host system, creating a local environment conducive to new bone formation. Mounting evidence now indicate that the osteoinductive activity of Ca-P ceramics is linked to their physicochemical and three-dimensional structural properties. Inspired by this conceptual breakthrough, many laboratories have shown that other materials can be also enticed to join the rank of tissue-inducing biomaterials, and besides the bones, other tissues such as cartilage, nerves and blood vessels were also regenerated with the assistance of biomaterials. Here, we give a brief historical recount about the discovery of the osteoinductivity of Ca-P ceramics, summarize the underlying material factors and biological characteristics, and discuss the mechanism of osteoinduction concerning protein adsorption, and the interaction with different types of cells, and the involvement of the vascular and immune systems.
Keywords: calcium phosphate ceramics, porous structure, osteoinduction, tissue regeneration
Introduction
Advances in material and life sciences have brought modern medicine to the brink of innovative abilities to repair and replace damaged tissues or organs. From simply repairing the physical shape to regenerating a living tissue or organ, from large-scale surgery wounds to minimally invasive repair, the traditional non-living biomaterials are facing challenges. A promising frontier in this area priority-developing frontier is to take advantage of the body’s natural ability to regenerate, especially to endow biomaterials with the biological function to induce tissue or organ regeneration. To achieve this aim, growth factors and cultured cells were incorporated into the biomaterials to realize the biological function of inducing tissue regeneration. However, the high cost of the growth factors, the time-consuming process of cell extraction and in vitro expansion, the immunological rejection, as well as the difficulty in transportation and storage of the living cells or growth factors, hindered the development of tissue regeneration. Therefore, materials with the ability to induce tissue formation possessed high potential for tissue regeneration.
Calcium phosphate (Ca-P) ceramics are extensively regarded as excellent bone grafts due to their good biocompatibility, osteoconductivity [1, 2]. However, it was not demonstrated that Ca-P ceramics could induce bone formation until the end of 1980s. In 1988, Heughebaert [3] reported the bone-like substance induced by hydroxyapatite (HA) ceramics in soft tissue of hamsters. In 1991, the initial histological evidences of the osteoinduction of Ca-P ceramics were shown by Ripamonti [4] and our group [5]. These studies indicated that it is possible to endow biomaterials with osteoinductive ability by optimizing the material characteristics rather than by adding living cells or growth factors to induce tissue regeneration.
The discovery of materials osteoinduction has highlighted the potential to explore new generation of biomaterials; therefore, a large number of publications have extensively studied the osteoinductivity of Ca-P and other materials, and a preliminary theory of this kind of osteoinduction has been established. More promisingly, a new concept of tissue-inducing biomaterials has been brought up based on the osteoinduction of materials. In this review, we will summarize the osteoinduction of Ca-P on the point of material factors affecting osteoinduction, biological characteristics and processes related to osteoinduction, as well as the mechanism of osteoinduction initiated by materials. And finally, a perspective view for the development of tissue-inducing materials will be given.
The material characteristics of osteoinductive materials
Of all materials that are currently used in clinics for bone implants and grafts, Ca-P ceramics holds the greatest promise to be developed into true bone-replacing material owing to the similarity of its chemical composition to bone minerals as well as its biocompatibility, osteoconductivity and osteoinductivity [5–9]. A wealth of studies has now firmly linked the osteoinductivity of Ca-P ceramics to myriad material factors that can be optimized in the fabrication process [10–12]. Generally, a three-dimensional (3D) porous structure with bone-like apatite surface layer and certain structural characteristics intrinsic to Ca-P ceramics is crucial to new bone induction [7, 10–15]. In the ensuing paragraphs, we will discuss in detail the effects of various material characteristics of Ca-P materials on osteoinductivity.
Phase composition and solubility
A number of studies about material factors related to osteoinductivity of Ca-P ceramics have demonstrated that the phase composition is one of the most important factors in inducing bone formation [16, 17]. Many groups used ‘chemical compositions’ to describe this concept, but we prefer ‘phase composition’ instead because Ca-P biomaterials with same chemical composition may assume different crystal phases such as α- and β-tricalcium phosphate (TCP). Classified by phase composition, the widely referred Ca-P materials include dicalcium phosphate dihydrate (DCPD), dicalcium phosphate anhydrous (DCPA), HA, biphasic calcium phosphate (BCP, both HA/α-TCP and HA/β-TCP), tricalcium phosphate (α- and β-TCP) and octacalcium phosphate (OCP) [7–9, 15–17]. Table 1 shows the Ca/P ratio and aqueous solubility (Ksp) of several Ca-P materials [18–20].
Table 1.
Ca/P ratios and the aqueous solubility of several Ca-P phases (25 °C) [18–20]
| Ca-P phase | Formula | Ca/P ratio | Solubility (Ksp) |
|---|---|---|---|
| DCPA | CaHPO4 | 1 | 1.87 × 10−7 |
| DCPD | CaHPO4·H2O | 1 | 2.59 × 10−7 |
| HA | Ca10(PO4)6(OH)2 | 1.67 | 6.62 × 10−126 |
| α-TCP | Ca3(PO4)2 | 1.5 | 8.46 × 10−32 |
| β-TCP | Ca3(PO4)2 | 1.5 | 2.07 × 10−33 |
| OCP | Ca8H2(PO4)6·5H2O | 1.33 | 1.01 × 10−94 |
Among the Ca-P materials, HA, β-TCP and BCP (HA/β-TCP) have been studied extensively for their osteoinducing activity [7–9, 16]. HA is the most stable and least soluble one (Ksp≈6.62 × 10−126), and was occasionally found to achieve osteoinductivity due to its low dissolution rate. β-TCP possesses a Ca/P ratio of 1.5 and Ksp value of 2.07 × 10−33 at 25 °C, which is more soluble than HA. Although β-TCP has a higher dissolution rate, it is difficult to retain a temporary mechanical support for the desired duration of time [15]. Therefore, a composite mixture consisting of poorly soluble HA and highly soluble β-TCP with different β-TCP/HA ratios was regarded to be able to achieve an optimum surface solubility. Based on the reported results, the trend of osteoinductivity can be ordered as BCP > β-TCP > HA ≫ α-TCP [7–9, 15, 17, 21]. The number of reports about osteoinduction of BCP is the highest, then HA and β-TCP, while few reports referred the osteoinduction in α-TCP [17]. In the early studies, most reported Ca-P osteoinduction were found in BCP although the actual ratio of HA and TCP was vague. In our recent study [8], we compared the osteoinductivity of BCP ceramics with various mass ratios of β-TCP/HA, and found that BCP consisting of 30% HA and 70% β-TCP promoted higher expression of bone morphogenetic protein-2 (BMP-2) and showed stronger osteoinductivity in mice than BCP (70% HA and 30% β-TCP), pure β-TCP and HA.
As materials with different phase compositions have different solubility, phase composition is partly attributed to the varied solubility of the Ca-P ceramics. From the above phase composition and solubility data, as well as the comparison of osteoinductivity, it seems that a higher solubility of Ca-P ceramics would result in higher osteoinductivity, such as that in BCP and HA ceramics. However, it does not always happen in this way. For example, β-TCP ceramic has a relatively high solubility than BCP ceramic, while its osteoinductivity is not superior to BCP ceramics, according to several studies [7–9, 17]. Furthermore, the osteoinductivity was occasionally observed in some non-ceramic Ca-P materials such as Ca-P cements [22–24]. This obviously weaker osteoinductivity was partly due to the lack of porous structure and the unstable interface although they might be more soluble than Ca-P ceramics.
Based on the comparison of Ca-P with different phase compositions, a general conclusion can be inferred that with similar porous structure, BCP ceramic with higher solubility is much more osteoinductive than HA ceramic with lower solubility.
Ca2+ and − ionic releasing
Due to the fact that the osteoinductive phenomenon is usually observed in Ca-P ceramics and the varied osteoinductivity with Ca-P phase composition and solubility, calcium (Ca2+) and phosphate ions (−) are suspected to be of great importance for material osteoinduction. Another clue comes from the fact that during in vivo bone resorption, osteoclasts are likely to release Ca2+ and −, derived from bone matrix. This causes a local increase in the ion concentration to supersaturating levels, which has a significant impact on the proliferation and differentiation of osteoblasts, as well as on the subsequent bone formation process [25].
In fact, Ca2+ gradients are observed in many extracellular microenvironments and regarded as potent chemical signals for cell migration and directed growth [25, 26]. Additionally, Ca2+ is an important homing signal, which brings together different types of cells required for the initiation of bone remodeling [25, 27]. For instance, high Ca2+ concentrations are showed to stimulate pre-osteoblastic chemotaxis to the site of bone resorption, and their maturation into cells that produce new bone [25, 28]. On the other hand, the release of extracellular Ca2+ also plays an important role in controlling the proliferation and differentiation of osteoblasts near the bone resorption site [25, 29]. Similarly, − also appears to play an important role in osteoinduction. Moreover, − is believed to play a critical role in physiological bone matrix mineralization [25, 30].
In addition, the ionic environment might initiate bone induction by affecting protein adsorption. For example, in one of our works, we found that the solubility of Ca-P ceramics (β-TCP > BCP > HA, list in Table 1) also influenced protein adsorption by affecting the equilibrium ion concentration near the material surface [31]. We also found higher adsorption of fibrinogen, insulin and type I collagen (COL-I) on BCP than that on HA surfaces [31, 32]. Here, we speculated that the release of Ca2+ and − from more soluble β-TCP phase in BCP caused a local increase in ion concentration, thus resulting in more Ca-P precipitation, hence promoted protein adsorption, which in turn promoted better osteogenesis. Furthermore, these studies may also explain why BCP ceramics, with a mixture of highly soluble and poorly soluble phases, perform better than either HA or TCP in promoting osteoblastic differentiation.
Macro- and micro-porous structure
Apart from the effects of phase composition and Ca2+/− ionic environment, the pore structures have been shown to play an important role in the osteoinductivity of Ca-P ceramics. Almost all the reported osteoinductive materials have an interconnected porous structure. Our experiments have confirmed that porous Ca-P ceramics can induce bone formation while dense Ca-P ceramics cannot [10, 33]. The basic function of pores inside the scaffold is to accommodate ingrown cells, while the role of the pore interconnected channel is to allow body fluid, blood vessels, and cells to develop toward the center of the scaffold, as well as the adequate exchange of oxygen and nutrition [7, 9, 10, 13, 33, 34]. In the case of osteoinductive materials, blood vessels also have the additional function of bringing along cells with the capacity to differentiate into osteoblasts [34].
The porous structural parameters of scaffolds include porosity, pore size, pore shape and pore connectivity [7, 9]. High porosity is believed to create significantly positive effects in terms of enhancing osteogenesis, but the positive effect does not necessarily take place because the low strength arising from an overly high porosity often causes untimely collapse of the implanted scaffold [9]. Therefore, for bioactive ceramic scaffolds, a porosity ranging from 40% to 80% is available for bone defect repair, depending on the implanted sites. In addition, the number of open pores is also directly related to bone formation. The interconnected pores and passing pores look like inner tunnels allowing mass transport, cell adhesion and bone ingrowths [7, 9, 35, 36]. Habibovic et al. observed that after implanting bulk cement of DCPA that contained channels, bone was mainly formed in the interior of the peripheral channels, close to their openings, after remaining for 12 weeks in the muscle of goats [24]. For a given porosity ranging from 40% to 80%, an optimal pore diameter is generally within the range of 200–500 μm, and the pore-interconnected channel size is in the range of 100–200 μm [7, 9].
Besides that, micro-pores (pore diameter < 10 μm) play a crucial role in promoting osteogenesis. The micro-pores on the walls of macro-pores are not only beneficial to penetrating bodily fluids, but also produce rough surfaces on the walls, which are favorable for cell attachment and the expression of osteogenic phenotype [7, 9, 10, 16, 17, 33]. The internal pores yet confine the flow of bodily fluid, thus keeping the concentration of dissolved Ca2+ and − ion in the pores and decreasing the shear stresses exerted on the cells and proteins attached on macro-pore surfaces [33]. In one of our works, we found that HA and BCP particles with high porosity and/or more pores >20 nm in size adsorbed more fibrinogen and insulin than particles with low porosities [31]. We further suggested that the distribution of micro-pores on the walls of macro-pores might play a positive role in favoring the adsorption of proteins with low molecular weights (e.g. β1 transforming growth factor, TGF-β1) [37].
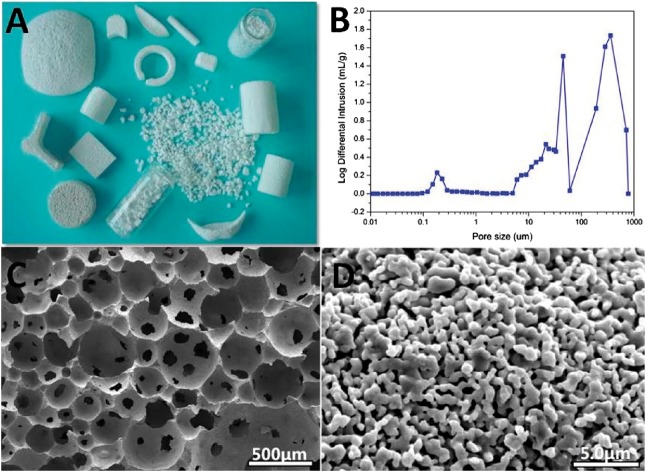
In our works, gas-foaming method (hydrogen peroxide as gas-foaming agents) was used to fabricate Ca-P ceramics and osteoinductive Ca-P ceramics were successfully prepared [5, 7–9, 31, 33, 34, 37]. In this foaming module, abundant micro-pores besides interconnecting macro-pores (100 μm) can be formed (shown in Fig. 1). Although the pore size and morphology cannot be controlled rigorously in this process, the porosity can be well controlled at the range of 40–80%, as well as the macro-pores’ diameter at 100–500 μm, with abundant micro-pores (<10 μm).
Figure 1.
Pore structure of Ca-P ceramic fabricated by the gas-foaming method. (A) Porous Ca-P ceramic scaffolds, (B) pore size distribution and (C and D) macro- and micro-pore structure
Topographical features
Among a variety of material characteristics, the material surface topography seems particularly important to their osteoinductivity, and many reports have explored the effect of material surface topography on the behavior of cell adhesion and differentiation [38–44]. In the study of Dalby et al. [45, 46], they fabricated different kinds of surface nanotopographies, and found that surface topography caused significant changes in mesenchymal stem or stromal cells (MSCs) response. A kind of nanodisplaced topography significantly increased osteospecific differentiation; further study concluded that both symmetric and random nanopit arrays did not induce osteogenic differentiation, while the disordered nanopit pattern induced osteogenic differentiation. This is a typical example of surface topography affecting cell function, which means the material surface topography modulating bone or other tissue formation.
In one of our works, we fabricated several types of surface topography on the HA disc-shaped pellets with various pore sizes and different pore morphologies [47]. The osteoblastic cells’ response on them showed that the macro-pore structure on HA surface favored cell proliferation, while the micro-pore structure up-regulated the early osteoblastic differentiation process. This surface pore structure can be regarded as surface topography, hence the varied cell response represented the effect of topography on cell biofunction and even osteogenetic potential. In another work, we fabricated HA ceramic with an orderly micro-patterned surface varied in groove width [48]. The results showed that the cell response also changed with the micro-patterns, proving the effect of surface topography on cell function.
These studies revealed the possibility of surface topography on cell behavior, even cell osteogenic differentiation. In the Ca-P ceramics, the surface topography might also be reflected in surface microstructure variation, resulting from different crystal sizes by different sintering temperatures.
Bone-like apatite formation
Many studies suggested that the bone-like apatite forming on the surface of Ca-P ceramics played important roles in bone induction [5, 7, 9, 10, 49]. The bone-like apatite is composed of calcium hydroxyl carbonate apatite and is equivalent in composition and structure to the major inorganic phase of natural bones [50–52]. Thus, bone-like apatite forming on an implanted biomaterial has become a direct criterion for evaluating the biomaterial’s bioactivity, although bioactivity is generally defined to be the ability to form chemical bonds between the implanted biomaterials and host tissues [1, 51, 53, 54]. Various studies have demonstrated that porous Ca-P ceramics with the bone-like layer, or those Ca-P ceramics that could induce the bone-like apatite formation in vivo, showed strong osteoinductivity [49, 55]. The formation of a bone-like apatite layer on Ca-P ceramics includes the nucleation, growth and crystallization of Ca-P. For Ca-P precipitation, the nucleation mainly depends on the concentrations of Ca2+ and − ions, pH, temperature and surface features of the solid onto which Ca-P will be deposited [54, 56–59].
It has been found that in the aqueous system, almost all of the Ca-P ceramics can form a second nuclear apatite layer by dissolution–reprecipitation in vitro. The order of the ability of bone-like apatite formation corresponds to the order of osteoinduction of Ca-P ceramics [49]. The study on the relationship of surface micro/nanostructure and biological function of osteoinductivity in the porous BCP ceramics revealed that varied microstructure of the bone-like apatite also affected the material osteoinductivity [49]. And the formation of the bone-like layer with some special characteristics is necessary for osteoinduction of Ca-P ceramics [49].
Nanoscale and nanostructure
It is well known that the optimal approach of fabricating artificial bone grafts is biomimicry. Bone apatite is composed of nanosized carbonated Ca-P crystals. However, conventional Ca-P ceramics, despite mimicking in part the bony composition and porous structure, have large size of Ca-P grains at the micro-scale, which is likely to debase the biological properties of the porous Ca-P ceramics [7, 9, 60]. In the view of biomimicry, a nanoscale crystal size might improve the biological properties of a Ca-P ceramic. Many in vitro studies did prove this hypothesis. Bone forming cells tend to interact with the nanoscale surface of biomaterials, which can promote adhesion, proliferation and differentiation [7, 9, 61–66].
In one of our works, we successfully fabricated the porous Ca-P nanoceramics using modified co-precipitation synthesis and microwave sintering [7, 66]. These Ca-P nanoceramics absorbed more bone-related proteins and showed selective adsorption to some specific proteins, including bone growth factors. Regarding the effect of different surface microstructures, the BCP ceramic surface with feature sizes <100 nm promoted higher protein adsorption than that with feature sizes >100 nm. In addition, because of the high specific surface area, the nanolevel surface topography, the high surface defects and the interconnecting macro-pores with abundant micro-pores, the Ca-P nanoceramics can effectively initiate and regulate a cascade of cellular activities, thereby resulting in higher invivo osteoconductivity and osteoinductivity than the conventional Ca-P ceramics.
In summary, Ca-P ceramics can be endowed with osteoinductivity by optimizing the material characteristics, including phase composition, ionic environment, macro- and micro-pore structure, topographical feature, bone-like apatite and nanostructure. These material properties can influence the process of osteoinduction directly or indirectly (shown in Fig. 2). Although the mechanism of osteoinductivity is still not fully understood, the main material characteristics of Ca-P ceramics relevant to their osteoinductivity are explored. Based on these results, the osteoinductivity of the Ca-P ceramics can be further enhanced by optimizing the physical–chemical properties of materials, and they are also useful for us to gain a deeper and wider understanding of the mechanism for material osteoinductivity.
Figure 2.
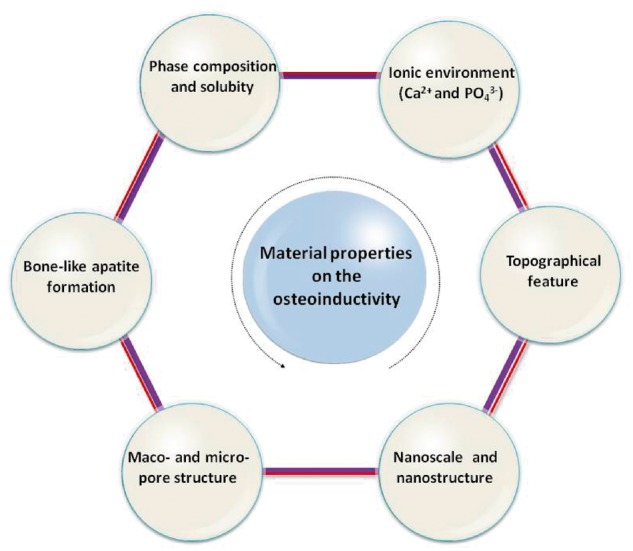
The Material characteristics related to osteoinduction of Ca-P ceramics
Biological characterization of material osteoinduction
The first report of ‘osteoinduction’ came from the works of Urist et al. [67], in which bone formation was observed by implantation of decalcified bone matrix (DBM) or BMPs in muscle of mice, rats, guinea pigs and rabbits. After that, the concept of ‘osteoinduction’ was used for the description of ‘ectopic bone formation’ of biomaterials. In Urist’s experiment, osteoinduction by DBM or BMPs in non-osseous sites were biologically characterized by histological observation. Similar to this phenomenon, some biomaterials such as Ca-P ceramics [68], porous titanium [69] and poly-HEMA [70], were reported to induce ectopic bone formation after implantation in some non-osseous sites, hence demonstrating ‘material osteoinduction’. Normally, in vivo implantation of biomaterials in non-osseous sites of animals, followed by a thorough histological analysis and depiction the bone formation, were used to assess the osteoinduction of biomaterials. With the development of bioscience and the extending study of material osteoinduction, more and more characterizing methods and models could be used for exploring material osteoinduction.
Direct proofs of osteoinduction: histological morphology analysis
Early in 1990s, Zhang, Yamasaki and Ripamonti reported, respectively, the heterotopic bone formation in porous Ca-P ceramics after implantation in dogs and baboons [5, 6, 71]. Later, many following reports gave evidence of Ca-P osteoinduction [10, 14, 15, 36, 68, 72, 73]. In this period, most of the evidence comes from histological staining which revealed morphological evidence of bone tissue. In some cases, TEM or SEM photos were also shown to describe the histological morphology of induced bone or cells [74–76].
Continuous histological observation revealed the process of osteoinduction [10, 13, 72]. Firstly, the fibrous connective tissue and capillaries invaded into the internal pore regions of the Ca-P ceramics. Then the mesenchymal cells arranged around the capillary, ALP-positive cells aggregated at the interface between tissue and biomaterials. After that, osteoblasts were identified. Then, osteoid and bone marrow cavity was formed gradually, and new bone grew from the boundary to the center of the pores. Finally, mature bone with bone marrow cavities formed, and Harvesian system developed in the pores in some cases.
So far, non-osseous or ectopic implantation and histological observations have been well-known standard methods for testifying the osteoinductive ability of biomaterials. By histological analysis, the osteoinduction of different materials, as well as that in different animal species, was extensively studied [13, 68, 75]. In earlier studies, this material osteoinduction was mainly observed in large animals, such as baboons [4], goats [21] and dogs [77]. However, recent works revealed the histological evidence of osteoinduction by Ca-P ceramics in rabbits and mice [73–75, 78].
Cellular and molecular characterization of material osteoinduction
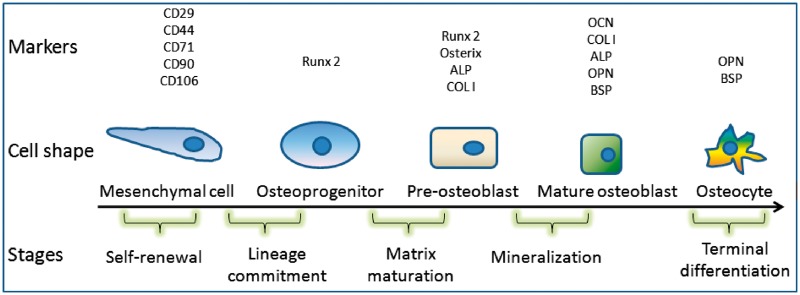
In natural osteogenesis, a series of cellular and molecular events are involved. These events include the osteoblastic differentiation from stem cells to preosteoblasts, osteoblasts and osteocytes, as well as the expression of related bone special markers (genes and proteins) as shown in Fig. 3. As to the new bone formation induced by biomaterials, these cells, genes and proteins should also be presented as evidence, and therefore, characterization of osteogenetic cells and related gene or protein markers has drawn more and more attention in the study of material osteoinduction.
Figure 3.
Osteoblastic differentiation of MSCs and markers for different stages
Based on the importance of osteoblastic differentiation of cell in the osteogenesis process, a series of works explored the correlation between material osteoinduction and cell differentiation induced by materials. Although the predication of osteoinduction by in vitro culture remains unstable [76], it provides a simplified predictive system for us to understand the mechanism of osteoinduction. These cell models mainly include MSCs and osteoblasts.
Mesenchymal stem cells
Stem cells, in particular adult MSCs, with multi-potential differentiation capacity, are currently recognized as a promising cell source for tissue engineering applications and cell-based therapies. The differentiation of MSCs along osteoblastic linage is undoubtedly evidence of osteogenesis. Therefore, identification of MSCs in material osteoinduction indicates the source of potential cells to differentiate into osteoblast, and the differentiation potential influenced by materials might give evidence of material osteoinduction and help to illuminate the possible mechanism.
In searching for MSCs in the osteoinduction of Ca-P, some studies proved the existence of multifunctional cells which might act as MSCs for the osteogenesis inside the materials in vivo [72, 79]. We found some multifunctional cells from the samples of Ca-P implanted in muscle [72, 80], which were going through osteoblastic differentiation, indicating the presence of MSCs in the implants and the induction of osteogenesis by Ca-P.
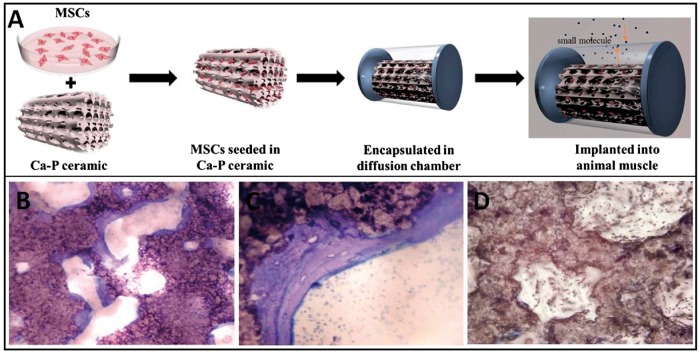
Many researchers explored in vitro co-culture of MSCs and Ca-P materials, including HA, BCP and TCP, and presented evidence of osteoblastic differentiation of MSCs [81–84]. The consistence between osteoblastic differentiation and osteoinduction was found. In one of our studies, MSCs from rat’s bone marrow (rBMSCs) were co-cultured with BCP in a diffusion chamber and implanted in dog’s back muscle. Half a year later, bone-like tissue was observed (Fig. 4). In this model, the rBMSCs cells were separated from the host tissue by the diffusion chamber, hence the results proved the possibility of materials inducing MSCs’ differentiation along osteoblastic linage and thus forming new bone. In another report, Yuan et al. [82] described an in vitro model in which human bone marrow stromal cells (hBMSCs) were co-cultured with four types of Ca-P ceramics, and a trend of gene expression was correlated with the amount of bone induced by the materials implanted intramuscularly in sheep. This indicates that it is possible to predict the osteoinductivity of biomaterials by co-culture of MSCs and biomaterials.
Figure 4.
MSCs Isolated from rats’ bone marrow, cultured with Ca-P in a diffusion chamber, then implanted in dogs’ muscle for 6 months (A). Histology observation found that bone tissue was formed in the pores of BCP (B and C), while not in HA (D)
Osteoblasts
Osteoblasts are specialized, differentiated products of MSCs. Their function is the synthesis of collagen, and several additional specialized proteins, such as osteocalcin (OCN) and osteopontin (OPN), which compose the organic matrix of bone. Besides MSCs, osteoblasts are also widely used to study the influence of Ca-P materials on cell response, such as their attachment, proliferation and secreting function. Many cell lines, including MC3T3-E1, MG63, SaOS-2, ROS17/28 or primary isolated osteoblasts, all belong to osteoblasts, and they have been used as classic cell models for detection of osteoblastic differentiation stimulated by biomaterials [85–89]. Generally, osteoblasts tend to attach more and spread better on osteoinductive Ca-P, and their secretion of ALP, as well as some osteoblastic differentiation markers, are also promoted by these materials. For example, according to the microarray analysis, the phenotype of mouse MC3T3-E1 osteoblasts was significantly altered by Ca-P with varied phase composition, especially the increased ALP activity and mRNA expression of ALP, COL-I and OPN [80]. Many reports showed that Ca-P ceramics with different phase compositions changed the mRNA expression of ALP, COL-I or OCN in diverse kinds of osteoblasts [87–89].
Expressions of bone-related genes and proteins
As mentioned earlier, during the natural osteogenesis process, a lot of genes and proteins, especially bone-related genes, will be activated. These genes and proteins, therefore, have been considered as markers of osteogenesis and have provided significant evidences for material osteoinduction. These markers include BMP-2, BMP-4, BMP-7, Runx2/Cbfa1, Osterix, COL-I, OPN, OCN, BSP and ALP (Fig. 3).
BMPs are potent osteoinductive factors that have been evaluated extensively for both preclinical and clinical use. They can regulate the function and differentiation of cells involved in bone formation and bone healing [90]. The original discovery of BMP-2 as osteoinductive factors of DBM prompted researchers to study BMPs’ expression levels in ectopic bone formation induced by Ca-P [89, 91].
In vivo, gene expressions of BMP-4 were detected as early as the third day after the implantation of BCPs [92]. Eyckmans [91] found that the mRNA expression of BMP-2, -4, -6 and -7 in human periosteum-derived cells (hPDCs) increased over time, during bone formation process induced by Ca-P granules.
In an in vivo experiment, the immunohistochemical staining showed the highest expression of BMP-2 and OCN in BCP, corresponding to the stronger osteoinductive ability presented in this BCP group [8]. In the in vitro co-culture with MSCs, more osteoinductive Ca-P ceramics induced higher expression of BMP-2 and BMP-4 [83, 93].
Besides BMPs, researchers paid attention to lots of other bone specific genes and proteins which were also related to osteoinduction of biomaterials. For example, the mRNA expressions of COL-I, ALP was detected from the 3rd day to 24th week upon the implantation of BCP ceramics in the dorsal muscle of dogs [92]. The protein secretion of osteonectin (ON), OPN and OCN were also detected by immunohistochemical staining after intramuscular implantation of HA, BCP or TCP ceramics in rats [80].
All these bone-related markers are used to mark or predict the trend of osteogenesis by materials. For example, expression of COL-I and OCN genes in human bone marrow MSC (BMSCs) was higher on Ca-P surfaces than on tissue culture plastic [81]. Another report also showed that more osteoinductive materials contributed to a relative higher expression of many genes involved in bone matrix formation [94].
Altogether, these reports offered cellular and molecular evidence for Ca-P-stimulated ectopic bone formation or material osteoinduction. Moreover, based on these data and models, we can further design specific experiments in the future to get a more comprehensive understanding of this osteoinduction process, which is to unveil the cellular and molecular mechanism for osteoinduction of Ca-P.
Mechanism of osteoinduction
Cells and signals related to bone building and repair
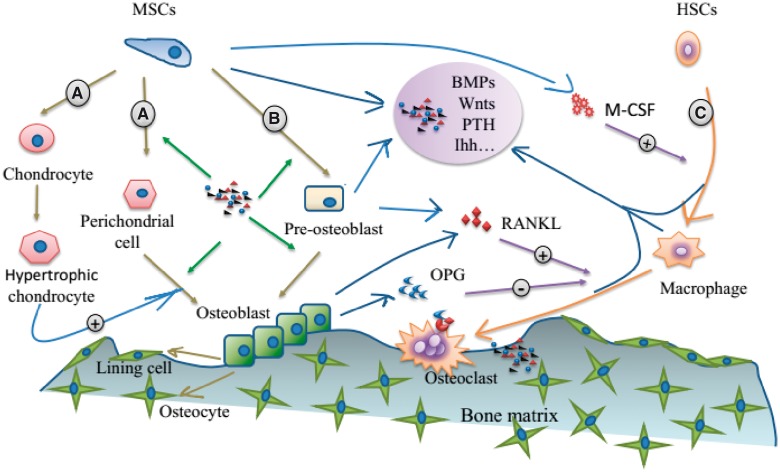
As the ectopic bone formation induced by materials resembles the natural bone both in morphology and function, it is reasonable to search for cues from the cellular and molecular aspect involved in the building and repair of natural bone, for exploring the mechanism of material osteoinduction [10, 13, 72, 92]. The cells and signals related to bone building and repair are shown in Fig. 5.
Figure 5.
Cells and signals related to bone building and repair: (A) the osteoblastic differentiation in endochondral ossification, (B) the osteoblastic differentiation in intramembranous ossification and (C) osteoclast differentiation of monocytes in hematopoietic stem cells
The building of bone during vertebrate embryogenesis involves two linages of cells: osteoclast and osteoblast lineage cells. The balance between the production and function of osteoblasts and osteoclasts is crucial for bone homeostasis [95].
As the chief bone-making cells, osteoblasts can differentiate from MSCs through two processes: endochondral or intramembranous ossification (marked by A and B in Fig. 5). The two processes both start with the aggregation and condensation of MSCs. Then, with adequate vascularization, intramembranous ossification happens as MSCs directly differentiate into osteoblasts. By contrast, during endochondral ossification, MSCs differentiate into perichondrial cells and chondrocytes, the latter then undergo hypertrophy, which then triggers the differentiation of perichondrial cells to osteoblasts [95]. Later, accompanied with the invasion of blood vessels, the chondrocytes produced cartilage would gradually be replaced by bone [96]. Thus, it seems that no matter through which process, the formation of bone is closely related to angiogenesis. Accordingly, every step along osteogenic differentiation from MSCs in either way is regulated by various molecules, including morphogens, hormones, growth factors, cytokines, matrix proteins, transcription factors and their co-regulatory proteins. These molecules work as signaling pathways, such as BMP signaling [97], Hedgehog signaling [98], Notch signaling [99], Wnt signaling [100] and FGF signaling [101]. These signaling pathways may interact with each other in orchestrating bone formation or bone repair [102, 103].
For the osteoclast differentiation (marked C in Fig. 5), i.e. osteoclastogenesis, it includes the transformation of mononuclear hematopoietic stem cells (HSCs) to bone marrow-derived macrophages, and the further forming of osteoclasts. These transformations are mainly mediated by macrophage colony-stimulating factor (MCSF), receptor activator of NF-κB (RANK), receptor activator of NF-kB ligand (RANKL), with osteoprotegerin (OPG) as a natural decoy receptor for RANKL [104].
These elements are further regulated by various kinds of biological factors from diverse types of cells in interrelated ways. For instance, the tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), secreted by macrophages in response to innate inflammation, can activate T-cell to express soluble RANKL [105] and up-regulate the RANK on the surface of osteoclast precursors, thus inducing osteoclastogenesis [106–109]. Some cytokines secreted by endothelial cells, such as IL-4 and IL-13, can stimulate stromal cells to express OPG to antagonize osteoclast differentiation [110].
Thus, altogether, the osteoblast differentiation and osteoclastogenesis are regulated by various cytokines of different cells, which make a complicated crosstalk between these cells from different systems, which at least involve the bone, vascular system and immune system [109]. Therefore, in regard to ectopic bone formation induced by Ca-P, studying these cells and related processes might unveil the mechanism in cellular and molecular level.
Many studies have revealed the biological process of Ca-P-induced bone formation, as mentioned earlier. These studies have not only offered enormous insight into these cells and events but also raised questions for further understanding. As various kinds of cells and events were observed before the emerging of osteoblast, hereby, the first question arose for unlocking the mechanism is that: what kind of cells is the precursor for the osteoblast? Then, as the osteoinductivity of Ca-P is intrinsic, which means their inherent properties could somehow interact with cells and other biological factors in vivo to induce the ectopic bone formation, thus the second critical question for unveiling the mechanism is: how do the materials trigger the accumulation and subsequent osteogenic differentiation of those precursors?
Cell origin of osteoblasts
For the first question, as it has been described before, the presentation of MSCs and their differentiation into osteoblasts have been demonstrated in the Ca-P-induced osteoinduction, which undoubtedly indicate that these MSCs are precursors of the osteoblasts. However, the following question remains: where do these MSCs come from to rest in Ca-P materials within the ectopic site?
Previously, it was observed that lots of fibrous connective tissue and capillaries invaded into the internal pores of Ca-P ceramics, where the novel bone formation begins [13]. Furthermore, it was found that some of the polymorphic cells that aggregated in association with capillaries in the vicinity of the ceramic surface could be positively stained for the ALP [72]. These indicate that the capillaries and the connective tissue that grow into the internal pores of Ca-P ceramics may provide the MSCs for further differentiation [111]. Similarly, Ripamonti [79] observed that, before and during bone formation, laminin staining (for vascular endothelial cells) was localized around capillaries in the vicinity of the ceramic, as well as around individual cells that seemed to migrate out of the vascular compartment. In all, osteoblastic differentiation from MSCs seems to be first observed near capillaries, as well as the internal surface of ceramics. Therefore, pericytes or endothelial cells associated with the capillaries were proposed to be the origin of those cells that aggregate and undergo osteoblastic differentiation in the vicinity of ceramics. This was further supported by the fact that ectopic bone formation induced by Ca-P biomaterials occurs almost via the intramembranous ossification [16].
Endothelial cells compose the inner lining of blood vessels and lymphatic vessels [112]. They are similar to epithelial cells, which can undergo epithelial–mesenchymal transition (EMT) to participate in physiological processes such as wound healing. This means endothelial cells can also transform into mesenchymal cells via a process known as endothelial–mesenchymal transition (EndMT) [113]. Specifically, a recent study has found that in a pathological heterotopic ossification, the vascular endothelial cells differentiated into chondrocytes and osteoblasts through an intermediate of MSCs which were generated by EndMT; and this differentiation was mediated by local inflammatory signals and/or other changes in the tissue microenvironment [114]. This work makes it reasonable that in the Ca-P material induced heterotopic bone formation, the endothelial cells may contribute to the origin of osteoblast by undergoing EndMT. Interestingly, a stem cell population with myoblastic and endothelial characteristics was found within the striated muscular tissue to support the continuous cell differentiation and replacement [115, 116]. This population of stem cells was defined as myoendothelial stem cells, and these myoendothelial stem cells were proposed as the cells that can differentiate into osteoblasts when in contact with osteoinductive biomaterials by Ripamonti [117].
With regard to pericytes, they are ubiquitous subendothelial cells which reside in vessels ranging in size from the microvasculature to the aorta [116]. Basically, pericytes serve as perivascular progenitor cells, which play an important role during capillogenesis, as they invest budding endothelial sprouts and control the proliferation and differentiation of endothelial cells [118]. Recently, it was found that pericytes are mesenchymal cells that have been described to have stem-like properties and differentiate into other cell types [119, 120]. However, others suggested pericytes may arise from EndMT [112, 121, 122]. Specifically, pericytes could express ALP [123, 124], and it has been suggested that they are a supplementary source of osteoblasts in periosteal osteogenesis [125]. Hence, pericytes were suspected to contribute to the process of bone formation at the surface of an osteoinductive material [16].
As a matter of fact, MSCs could originate from several other resources, such as bone marrow. In one of our group’s earlier works, we have observed that MSCs originating from bone marrow presented in the pores of Ca-P by immunohistochemical staining [126]. This was further confirmed by a recent study, which demonstrated the homing of BMSCs to non-osseous sites for ectopic bone formation, induced by osteoinductive Ca-P [127].
Fibroblasts, another kind of MSCs, may also contribute to the origin of osteoblasts, since they are present in the connective tissue that grows into the pores of ceramics. Furthermore, our in vitro study, which investigated the osteoinductive effect of Ca-P on C2C12 fibroblasts [128], supported this theory.
Signals and their origin for initiating the osteoblastic differentiation in osteoinduction of Ca-P
It is generally accepted that MSCs are the origin of OB in the ectopic bone formation induced by materials. However, what triggers the MSCs’ accumulation on the material surface and the subsequent osteogenic differentiation remains unclear.
Adsorption or concentration of proteins that initiate the osteoinduction
Many studies have demonstrated that Ca-P showed a high affinity for proteins by providing higher amount of surface with high surface energy, as well as by providing Ca2+ positively charged binding site for negatively charged proteins. Thus, protein adsorption would be the first event upon the implantation of Ca-P. And since several physical or chemical features, such as topographical features (e.g. roughness, micro-porosity) of Ca-P strongly affect the amount and specificity of adsorbed proteins, just as they have been shown to affect the osteoinduction of Ca-P, it is reasonable to consider this process may be relevant to the osteoinduction, or even be the very reason of osteoinduction by Ca-P.
Adsorption of BMPs on Ca-P
In the exploration for the mechanism of osteoinduction by Ca-P biomaterials, it has long been suspected that endogenous BMPs might be the very biological reason [13], due to the early works of Urist [129].
This hypothesis was supported by our in vitro study, which showed that Ca-P ceramics have a strong ability to absorb bovine BMPs. Further supporting evidence was obtained from the following in vivo studies, in which the immunohistochemical BMPs staining before bone formation in the ceramics was detected after 30 days upon implantation in pigs, in addition to enhanced bone formation in Ca-P ceramics by bovine BMPs [11]. Moreover, it was found that inhibition of BMPs by a monoclonal antibody resulted in reduced osteoinductivity of the Ca-P [130]. Therefore, the importance of BMPs in osteoinduction of Ca-P is undeniable. In addition, this was shared by Ripamonti et al. [131], as they also detected BMP-3 and BMP-7 on the interface of tissue-HA substrate, where bone was observed after implantation in the muscle of primates.
Later, since the deposited apatite layer after implantation facilitated the adhesion of proteins such as BMPs, De Groot [132] proposed that the dissolution and deposition of a biological apatite layer on Ca-P surface leads to the co-precipitation of BMPs, to the sufficient concentration to trigger the osteoinduction.
Although the theory concerning the adsorbed or co-precipitated BMPs contribute to the osteoinductivity of Ca-P is reasonable, there are still apparent differences between osteoinduction by BMPs and that by Ca-P biomaterials. For instance, Ca-P-induced ectopic bone formation usually occurs via intramembranous ossification [21, 133, 134], while BMPs induced ectopic bone formation is mostly through endochondral ossification [135].
Other proteins adsorbed on Ca-P
Besides BMPs, other proteins are also absorbed by Ca-P. Among them, cell-adhesive proteins, such as fibronectin and vitronectin, can bind with specific integrin on the cytomembrane to generate anchors for cells, as well as initiate the cell–ECM interactions, which have been shown to influence different kinds of cell behavior, such as adhesion, proliferation and even osteogenic differentiation [136, 137]. This specificity of binding and the related modulation of cell behavior were dominated by the different types of integrins, and their association with the cellular signaling network to initiate downstream signaling cascades, such as the focal adhesion kinase (FAK), protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) pathways [138].
In this aspect, Matsuura et al. [139] suggested that the RGD domains of fibronectin and vitronectin on Ca-P surfaces play major roles in the spreading of osteoblasts, thus contributing to the osteoconductivity of the surfaces. Later, Kilpadi proved that hydroxylapatite binds more cell adhesive proteins such as fibronectin and vitronectin from the serum than do two commonly used hard-tissue materials, namely pure titanium and stainless steel. In turn, this increased protein adsorption would lead to better binding of integrins α5β1 and α5β3, as well as osteoblast precursor cells [140].
In Marino’s [141] study, which cultured human adipose-derived stem cells on TCP surfaces in the absence of supplements, the presence of phosphorylated-FAK and evidence of differentiation were observed. Subsequently, in the study of Salasznyk et al. [142], human BMSCs on COL-I and vitronectin coated surfaces showed osteoblastic differentiation via the phosphorylation of FAK, which resulted in the activation of Runx2. Therefore, it is plausible that the Ca-P adsorbed cell-adhesive proteins, such as vitronectin, may modulate osteoblastic differentiation through the subsequent phosphorylation of FAK and activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway. Recently, it was found that α2β1 integrin and MAPK/ERK signaling pathways were involved in the regulation of osteoconduction for TCP by the BMP-2 autocrine loop [143].
Moreover, based on the overlap between the integrins and BMP-2 receptors [144], an integrin-BMP/Smad signaling pathway was suggested to promote bone regeneration for nanofibrous HA/chitosan scaffolds, in which the HA accounts for cell adhesion or spreading by influencing integrin-mediated signaling cascades and the BMP pathway in BMSCs [145].
Osteoinduction due to interaction between MSCs and Ca-P
Since several types of cells would co-exist with the Ca-P in the in vivo environment after implantation, new theory for the osteoinductive mechanism was brought up, which implied it was the materials triggered secretion of factors in these cells, rather than accumulation of factors from body fluid, that lead to bone formation. This theory can be divided into two parts according to the cells (MSCs or HSC-derived cells) that were directly affected by Ca-P. For this part, we discuss the MSCs which are directly stimulated by Ca-P to undergo osteoblastic differentiation through their own secreted factors or other changes, as well as how the Ca-P triggered this effect.
Ca-P-stimulated signaling molecules in MSCs
In searching for the critical molecules that initiate the osteoblastic differentiation of stem cells stimulated by Ca-P materials, once again BMPs drew the attention of many researchers because of their potent osteoinductive capacity. Previously, though immunohistochemical stains of BMPs have been observed [126, 131], it was still not sure where these BMPs came from, or whether they were adsorbed or secreted, and if so, by what kind of cells. Later, in a clinically relevant model of the osteoinduction process, which required the existence of Ca-P and BMP/Wnt signaling, the activation of the BMP/Smad signaling pathway in hPDCs was evidenced by immunohistochemistry stains for p-Smad 1/5/8 positive cells [91]. Recently, to avoid other cells and their cytokines within the in vivo environment, we conducted an in vitro experiment by simply culturing the BMSCs on HA and BCP ceramics in the absence of additional osteogenic factors [93]. These two ceramics were shown to be osteoinductive by a previous in vivo study [77], with BCP showing higher osteoinductivity. By comparing the osteoblastic differentiation status and gene expression of related molecules in BMP/Smads signaling, we suggested that Ca-P ceramics may initiate the osteoblastic differentiation of MSCs by stimulating the autocrine of BMP-2 in MSCs, as well as by up-regulating other relevant molecules in the BMP/Smads pathway [93]. Since BMP-2 could induce expression of other BMPs by an autocrine pathway [90, 146], we further suspected that this material-stimulated BMP-2 up-regulation may result in a cascade of amplification for BMP signaling. This in vitro study was in line with our in vivo study [8], as in which BMP-2 secretion was proved to be stimulated by osteoinductive Ca-P, and the highest was correlated with the best ectopic bone formation. Barradas’s [83] recent in vitro study, which compared HA and β-TCP ceramics, showed a similar result with regards to BMP-2 expression. Thus, the osteoinductive Ca-P stimulated BMP-2 autocrine loop in MSCs may be the very factor which initiates the osteoblast differentiation. The involvement of BMP-2 was further supported by an in vivo inhibitory study, which demonstrated that the blocking of calcium channel and osteoclastogenesis resulted in down-regulation of BMP-2 and limited bone formation [147].
Besides the BMP autocrine signaling loop, other signaling pathways that are involved in bone development or repair might also be related to the osteoinduction process triggered by Ca-P. Through in vitro co-culture of MSCs with TCP or HA ceramics, Barradas et al. [83] found MSCs cultured on β-TCP showed more pronounced attachment and spreading than that on HA. Subsequently, using DNA microarray analysis, they also found that the MSCs were induced to express G-protein coupled receptor (GPCR) 5 A and regulator of G-protein signaling 2 by β-TCP [83]. Therefore, they proposed that these genes, which were related to the protein kinase A and GPCR signaling pathways, might serve as the prediction of the earliest response of MSC to bone-inducing ceramics [83]. Recently, a study demonstrated that secretion of IL-1α in BMSCs was stimulated by the nanohydroxyapatite (nHAp) within some kind of composite scaffolds, and it was through an autocrine/paracrine way [148]. Moreover, the activity of IL-1α could be mediated by the producing of IL1R2 when BMSCs interacted with nHAp [148].
The adenosine signaling pathway is also involved. For instance, Shih [149] proved that by the release of phosphate, which participated in the synthesis of ATP in mitochondria of cells, Ca-P-bearing matrices could induce stem cells to differentiate along osteoblastic linage via the adenosine signaling.
Using in vivo study, Eyckmans [150] mapped the Ca-P-activated gene network by ectopic implantation of constructs consisting of hPDCs, with HA/collagen scaffolds (Ca-P-rich matrix, CPRM), or decalcified scaffolds (Ca-P-depleted matrix, CPDM). The result revealed that both constructs stimulated a similar gene expression cascade upon subcutaneous implantation, yet the gene expression dynamics was slower in CPDM scaffolds than in osteoinductive CPRM scaffolds [150]. The variation in gene expression dynamics was supposed to be related to the hub genes’ differential activation, as well as the molecular signaling pathways associated with bone development (TGF-β, β-catenin, BMP, EGF and ERK signaling), calcium signaling (CREB) and inflammation (TNF-α, NF-κB and IL-6) [150].
How does Ca-P stimulate MSC to secrete these signaling molecules?
Though the expression of BMP-2 or other signaling molecules in MSCs stimulated by Ca-P were confirmed, it was still unknown how this happens. Just like Klar [147] has correlated BMP-2 expression and bone formation with calcium ions, this signaling also originates from the physical and chemical or structural nature of Ca-P. The Ca2+, − ions and concavity or geometry are suspected as essential factors of Ca-P to achieve osteoinduction according to their related signal pathways in MSCs.
Calcium ions (Ca2+ ions)
The Ca2+ ion is the most universal carrier of biological signals, which modulates cell life from its origin at fertilization to its end in the apoptotic process [151]. Firstly, it was suggested that extracellular Ca2+ gradients could work as a chemotactic signaling for homing of bone marrow progenitor cells or pre-osteoblast to the site of bone resorption [27, 28, 152, 153]. Then, higher concentration of Ca2+ could also stimulate these progenitor cells’ maturation into cells that produce new bone [28, 153]. Thus, it is reasonable to suspect that the Ca2+ ions, released from the dynamic dissolution of Ca-P materials, may recruit MSCs chemotactically, and stimulate their osteogenic differentiation in a concentration-dependent way.
Previously, Jung et al. studied the effects of HA released Ca2+ on osteoblastic differentiation of MC3T3-E1 cells. They suggested that the released Ca2+ may enter cells through calcium channels and calcium sensing receptors (CaSR), and activate the CaMK2a/CAM pathway [154]. This may finally modulate the osteoblastic differentiation via the CREB and/or the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway [155]. Later, Barradas et al. demonstrated that a high extracellular concentration of Ca2+ enhanced proliferation and morphological changes in hMSCs, accompanied with up-regulation of BMP-2 expression. However, the interference with a series of proteins involved in Ca2+ sensing showed that it was not the CaSR, but rather type L voltage-gated calcium channels were involved in mediating the signaling pathway between extracellular Ca2+ and BMP-2 expression. Furthermore, their microarray analysis indicated that MEK1/2 activity was essential for the effect of calcium, thus they came to propose that the internalization of Ca, probably by ion channels, would activate PKC, EK1/2, ERK1/2 pathways in sequence, and at last enter the nucleus to up-regulate the BMP-2 expression via Fos expression and activator protein 1 (AP-1) formation [156]. This study is in line with an in vivo study, which showed that blocking of Ca ions channel would result in down-regulation of BMP-2 expression and limited bone formation [147].
There is also another theory about the osteoinductive mechanism of Ca-P that involves Ca2+. Syed-Picard [157] demonstrated that amorphous calcium phosphate alters cellular functions and 3D spatial tissue differentiation patterns by increasing local calcium concentration, which in turn modulated the connexin 43 mediated gap junctions between cells.
Phosphate ions (− ions)
For the released phosphate, previous work by Beck [158, 159] showed that a higher concentration of phosphate can induce mineralization-associated genes, such as matrix glaprotein (MGP) and OPN, in MC3T3-E1 cells through the activation of ERK1/2- and PKC-dependent pathways, rather than the p38-dependent pathway. Nevertheless, the study of Khoshniat et al. [160] showed that calcium is required for phosphate-dependent ERK1/2 phosphorylation to regulate MGP/OPN expression, since this effect may originate from extracellular-related effects of Ca-P that are dependent on the integrity of lipid rafts. Recently, Shih [149] proved that released phosphate from Ca-P-bearing matrices could enter the cells via a phosphate transporter called solute carrier family 20 member1 (SLC20α1). Then, the internalized phosphate participated in the synthesis of ATP, which would promote the osteoblastic differentiation of hMSCs as an autocrine/paracrine signaling molecule. Moreover, the osteoblastic differentiation would not happen if the SLC20α1 was perturbed, due to the decreased intra-mitochondrial phosphate and ATP synthesis [149]. Therefore, it seems that − released by Ca-P may work in diverse means which involve different signal pathways.
Particularly, Chai et al. [161] showed both ions, administrated individually or in combination with calcium, consistently up-regulated BMP-2 expression for human periosteum-derived cells in a dose-dependent manner. Barrada’s recent work and our in vitro study, which both used Ca-P ceramics instead of ions, showed the similar result with regards to BMP-2 expression [83, 93]. Thus, both ions released by Ca-P may be involved in triggering the BMP-2 autocrine signaling loop, even though they may work through different pathways.
Altogether, these studies showed that either ion can effectively promote the osteoblastic differentiation of progenitor cells. Alternatively, their combination may be more osteoinductive, for this combination may form other elements, such as the Ca-P precipitates [160], which may enhance the osteogenesis by other means that individual ions could not achieve.
Physical or structural characteristics
Apart from the adsorption of related proteins (integrin or growth factors) and the chemical influence of Ca2+ and − ions, the Ca-P could also directly influences cells through its physical or structural characteristics, such as the grain size, surface structure or geometry, which may also contribute to the osteoinduction of Ca-P.
Regarding molecular mechanism, it was reported that these physical or structural factors worked mainly in a mechanically sensitive way through those molecules which participated in cytoskeletal tension generation, including actin, myosin II, Rho, ROCK and the Rho modulator [162]. A recent study showed that geometric features increase actomyosin contractility promote osteogenesis; its microarray analysis and pathway inhibition studies suggested that contractile cells promoted osteogenesis by enhancing c-Jun N-terminal kinase and ERK1/2 activation in conjunction with elevated wingless-type (Wnt) signaling [163].
Besides integrin and associated cytoskeletal filaments, cells may also utilize other cell surface molecules to sense or transduce mechanical stress, such as cadherins and selectins that form intercellular junctional complexes or the stress-sensitive ion channels on the cell surface [164–167]. The concavity of porous ceramics may generate shear stress forces of the in vivo fluid, which work through mechanosensitive ion channels to direct MSCs differentiation [168, 169]. Furthermore, the concavity would work as a protective environment to accumulate the released Ca2+ and − ions, thus recruit and stimulate the differentiation of MSCs [29, 170, 171].
Osteoinduction due to interaction between Ca-P and other cells
Except MSCs, there are other cells participating in the host response to materials, and these cells may be more important for osteoinduction than MSCs. What’s more, the interaction between these cells via their secreted cytokines factors may be even more important.
Interaction between Ca-P and inflammation-associated cells
Actually, the specific inflammatory response of tissues to the osteoinductive ceramics is an important factor that renders a material osteoinductivity [16, 134, 172, 173]. As described previously, macrophages/monocytes are involved in the inflammation process, as well as in the bone remodeling or repair process. This, along with the appearance of these cells in the ectopic bone formation induced by Ca-P, makes a link between the inflammatory response and osteoinduction process of Ca-P. Certainly, the osteoclasts and their resorption activity would also be involved since they are derived from these macrophages/monocytes.
Interaction between Ca-P and macrophages/monocyte
The inflammation process started from the wound by injury. However, with Ca-P existed by the implantation, the consistently available Ca2+ ions would cause the circulating monocytes to fuse into macrophages. Then the macrophages, under certain stimulation, may secrete cytokines that could induce the osteoblast differentiation of available MSC cells. For instance, De Bruijn et al. [174] showed that macrophages in response to micro-rough surfaced HA, which were osteoinductive, particularly produced more prostaglandin E2 (PGE2) than that of smooth HA, and this PGE2 was shown to be chemotactic for hMSCs and could stimulate their osteoblastic differentiation.
Recently, in the study of Chen, it was found that macrophage phenotype switched to M2 extreme in response to β-TCP extracts, and this was related to the activation of CaSR; BMP-2 was also significantly up-regulated by the β-TCP stimulation, which indicates that macrophages may participate in the β-TCP-stimulated osteogenesis. Moreover, when macrophage-conditioned β-TCP extracts were applied to BMSCs, the osteogenic differentiation of BMSCs was significantly enhanced, indicating the important role of macrophages in biomaterial-induced osteogenesis [175]. Not only the ions, but also the microparticles from the degraded Ca-P, can influence the behavior of those monocytes or macrophages, in addition to factors such as TNF-α, IL-6 and IL-10 [134, 176, 177]. Actually, harnessing the power of macrophages/monocytes to get enhanced bone tissue engineering has been put forward by Dong et al. [178]. They suggested that several osteotropic factors (such as IL-1 b and IL-4) could be produced by macrophages/monocytes, and that these cytokines played constructive roles in bone development and repair. The significant role of macrophages/monocytes in osteoinduction could also be illustrated by the implantation of respective scaffolds in nude mouse, which demonstrated that BCP associated with total bone marrow, which contained MSCs and macrophages/monocytes, consistently improved osteoinduction than BCP associated with pure human stromal cells [179].
Interaction between Ca-P and osteoclasts
The continuous presence of Ca2+ released by the Ca-P, as well as the presence of incompletely dissolved Ca-P, could promote the macrophages to undergo further transformation into osteoclasts, i.e. osteoclastogenesis [180, 181]. Once osteoclasts attached, the Ca-P ceramic is resorbed subjacent to the ruffled border of the cell by acid hydrolysis. The resorption by osteoclasts results in concavity, either as lacunae, grooves and/or pits, as the osteoclasts trek across the surface of the Ca-P, which in turn works as micro-topographic cues to affect the MSCs’ differentiation [169]. For the macro-porous Ca-P-based constructs, which primarily/only possessed a macro-topographical geometric surface, this resorption by osteoclasts to create concavities was proved to be essential for them to achieve ‘intrinsic’ osteoinductivity [117]. This process induced the micro- and macro-topographical geometric surface patterning alterations, as Ripamonti [117] proposed based on their study which utilized Zoledronate Zometa to inhibit this effect of osteoclasts.
Additionally, osteoclastogenesis and the osteoclastic priming of the macro-porous surfaces may also result in nanotopography, which have been proved to be capable of controlling stem cell differentiation [45, 182]. Moreover, Ca2+ and − ions released by the resorption activity of osteoclasts also contribute to the osteogenic differentiation and subsequent bone formation [183], as Ca2+ and − ions accumulated in the concavity would reach a higher concentration.
For the micro-porous Ca-P, which already possessed micro or even nanoscale features before implantation, the osteoclastogenesis and the osteoclast resorption was not irrelevant or less important than their osteoinduction. Recently, it was showed that the physical–chemical nature of the micro-porous Ca-P, especially the surface architecture, could affect the osteoclastogenesis [184] and osteoclast resorption [185], which in turn would influence the osteoinduction of this Ca-P. In the latter study, the TCPs, which exhibited osteoinductivity with submicron-scale surface structure, induced osteoclastogenesis and ectopic bone formation in a process that was blocked by monocyte/macrophage depletion, in comparison with TCPb, which were not osteoinductive with micron-scale surface architecture [185]. By utilizing the same pair of materials, the authors also demonstrated that the osteoclast resorption of Ca-P was also controlled by tuning of surface architecture, with only the submicro-structured TCPs showed to be resorbed by osteoclast. Particularly, they showed that the foreign body giant cells could not resorb either TCP material, suggesting that osteoclast specific machinery is necessary to resorb TCP [185].
Thus, it could be seen that the specific inflammatory response to Ca-P, which involved the macrophages/monocyte or osteoclasts, would help to create or enhance several factors which could mediate the ectopic bone formation, including soluble cytokines (BMP-2, PGE-2, TNF-α, IL-6, IL-10), insoluble topographic features (concavities) and the accompanied higher concentration of Ca2+ and − ions, or cytokines.
Additionally, besides macrophages/monocytes, other kind of cells, such as T cells and B cells in the immune system, may also play a role in this osteoinduction process. In the Nse-BMP4 mice, the lack of mature B and T lymphocytes resulted in smaller spreading and overall amount of heterotopic ossification, which suggested that the adaptive immune system plays a role in spreading of heterotopic ossification [186]. Recently, developments in osteoimmunology have revealed the vital role of immune cells in regulating bone dynamics. Chen et al. [187–189] have suggested that neglecting the importance of the immune response may explain the inconsistencies between in vitro and in vivo conditions, and they proposed osteoimmunomodulation in recognition of the importance of the immune response during biomaterial-mediated osteogenesis.
Interaction between Ca-P and cells associated with angiogenesis
As described in the cell origin section of this review, the ectopic bone formation by Ca-P always needs the preformation of blood vessels, which hints the importance of angiogenesis to the osteogenesis induced by Ca-P. Actually, the cells responsible for the angiogenesis are endothelial cells and pericytes, which may also be able to transition into osteoblasts under the mediation of inflammation, as described previously. Moreover, these cells can express cytokines such as BMP-2 and BMP-7 [190], and their expression has been shown to be markedly up-regulated in response to inflammatory stresses [191, 192]. In fact, BMPs are cable of not only regulating osteogenesis but also angiogenesis [193]. To add a further level of complexity, BMPs are capable of regulating EndMT [114], which would provide MSCs for further differentiation. Thus, the role of angiogenesis in the osteoinduction of Ca-P may come through several ways. One is through their secreted cytokines, such as BMPs, to recruit and induce MSCs to undergo osteoblastic differentiation. A second way is by providing more endothelial cells and pericytes to transform into MSCs, which finally differentiate into osteoblasts under the induction of materials or cytokines. The final way is to serve as a conduit for other osteoprogenitor cells, such as BMSCs. These aspects are only in accordance with how the angiogenesis contribute to the initiation of osteoinduction. As for the whole osteoinduction process, it should not be forgotten that the new blood vessels are essential for providing oxygen and nutrients to support the growing bone.
Furthermore, recent studies have found that the angiogenesis in ectopic bone formation of Ca-P is also influenced by materials. Our recent in vivo study showed that the more resolvable TCP resulted in more blood vessels in the ectopic site than did other HA or BCP ceramics, which indicate the effect of Ca2+ ions in angiogenesis [34]. Actually, Ca2+ mediated signaling is important in angiogenesis [194, 195], and extracellular Ca was suggested to be a key factor responsible for the angiogenic responses of bone marrow progenitor cells [196]. Other factors, such as concavity, have also been suggested to trigger rapid angiogenesis through the Ca2+ ions retained within it. Further, the angiogenesis could work with mechanical distortion to trigger the invasion of MSCs (myoblastic/myoendothelial and/or endothelial, pericytic) to rest in the concavity [170]. Hereby, within this protective microenvironment, MSCs may response to the accumulated ions or cytokines, or to topographic features to undergo differentiation [169].
Together, as one of the pre-requisites for osteoinduction of Ca-P [134], the angiogenesis showed to work with Ca-P to promote osteogenesis. However, even with sufficient angiogenesis, osteoinduction of Ca-P will not definitely happen. This may be due to the role of inflammation in mediating the angiogenesis and the EndMT, as well as the secretion of osteogenic factors from the endothelial cells. Thus, as the inflammation also interacted with Ca-P, the proper combination of Ca-P-triggered inflammation with angiogenesis might be the reason for osteoinduction.
Summary for the mechanism of material osteoinduction
In summary, the mechanism for osteoinduction of Ca-P may be very complicated, owing to the involvement of various kinds of cells, molecules, as well as biological progresses with crosslinked interactions. As we have depicted the existing theories concerning the adsorption of proteins, the interaction between Ca-P materials and different types of cells, and none of them have been isolated as the specific reason for the osteoinduction of Ca-P, we therefore propose a hybrid hypothesis for the osteoinduction mechanism of Ca-P, which involves not only the bone system, but also the vascular and immune systems (shown in Fig. 6).
Figure 6.
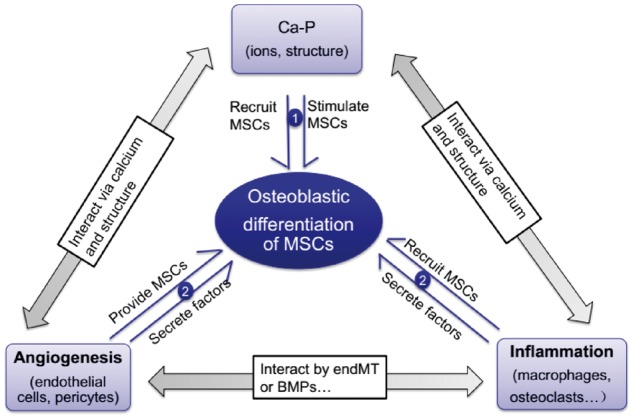
Hypothesis for the osteoinductive mechanism of Ca-P: ① represents the direct effect of Ca-P (as the changed form) to stimulate the osteoblastic differentiation of MSCs and ② represents Ca-P interacting with the inflammatory cells to trigger the osteoblastic differentiation of MSCs. This may involve the angiogenesis, which could interact with both the Ca-P and inflammation
Based on the previous studies and theories, it can be seen that micro-porous Ca-P may work through soluble factors (such as Ca2+ and − ions), and/or insoluble factors (such as their micro or even nanoscale topographic features) to interact with various kinds of proteins and cells, as well as mediate their interaction. Therefore, a summarized hypothesis for the osteoinduction mechanism of micro-porous Ca-P is formed: firstly, the injury caused invading of the inflammatory factors and inflammatory cells. Secondly, the micro-porous Ca-P work through soluble factors (such as Ca2+ and − ions), and/or insoluble factors (such as their micro or even nanoscale topographic features), or even by interacting with these inflammatory factors and cells, which results in adsorption or concentration of related proteins (osteogenic growth factors, cell adhesive proteins and inflammatory factors), as well as the recruitment of various kinds of progenitor cells (monocytes, MSC, endothelial cells, pericytes). This process may be accompanied by the dynamical dissolution/precipitation of Ca-P, and co-precipitation of proteins to form the bone apatite-like layer on the Ca-P surface. Then, there are two pathways that may result in the osteoinduction: the first is that Ca-P (as the changed form) directly stimulates the osteoblastic differentiation of MSCs (marked 1 in Fig. 6). The second is by Ca-P interacting with the inflammatory cells (monocytes, macrophages, osteoclast), which result in more osteoinductive surface features and higher concentration of Ca2+ and − ions, as well as osteogenic cytokines. Finally, these factors trigger the MSCs’ differentiation (marked 2 in Fig. 6). The latter pathway may involve the angiogenesis, which could interact with both the Ca-P and inflammatory response to contribute to osteoinduction by providing more osteogenic factors and more MSCs (marked 2 in Fig. 6).
Perspectives: from osteoinductive materials to tissue-inducing materials
Since the discovery of Ca-P osteoinduction, extensive studies have been performed to deepen our understanding of this fantastic phenomenon, as well as its possible mechanism. The key inspiration from this is that we can endow the materials with the biofunction of inducing tissue regeneration. The realization of this idea would thoroughly change the concept of biomaterials. Although there are still many unclear secrets hiding behind the material osteoinduction, it guides us to explore revolutionary change in material design. Based on this concept of material osteoinduction, extensive attempts to design tissue-inducing materials have been performed [42, 163, 197–202]. These frontier studies include the design of material characteristics to change cell fate, such as the osteogenesis or chondrogenesis differentiation, and the phenotype and multipotency maintenance of MSCs [46]. In some pioneer studies, chondrogenesis, neuranagenesis and angiogenesis are reported [34, 197–202].
In summary, current biomaterials science and engineering is undergoing revolutionary change. Tissue-inducing biomaterials are becoming an important direction and frontier for biomaterials science and industry.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81190131) and the National Basic Research Program of China (‘973’ Program No. 2011CB606201).
Conflict of interest statement. None declared.
References
- 1. Hench LL, Polak JM.. Third-generation biomedical materials. Science 2002;295:1014–7. [DOI] [PubMed] [Google Scholar]
- 2. Langer R, Vacanti JP.. Tissue engineering. Science 1993;260:920–6. [DOI] [PubMed] [Google Scholar]
- 3. Heughebaert M, LeGeros R, Gineste M. et al. Physicochemical characterization of deposits associated with HA ceramics implanted in nonosseous sites. J Biomed Mater Res 1988;22:257–68. [DOI] [PubMed] [Google Scholar]
- 4. RIPAMONTI U. Bone induction in nonhuman primates: An experimental study on the baboon. Clin Orthop Relat Res 1991;269:284–94. [PubMed] [Google Scholar]
- 5. Zhang X, Zou P, Wu C. et al. A Study of Porous Block HA Ceramics and Its Osteogenesis. Bioceramics and The Human Body. Amsterdam: Elsevier, 1991,408–15.
- 6. Li X, Guo B, Xiao Y. et al. Influences of the steam sterilization on the properties of calcium phosphate porous bioceramics. J Mater Sci Mater Med 2016;27:5–14. [DOI] [PubMed] [Google Scholar]
- 7. Hong Y, Fan H, Li B. et al. Fabrication, biological effects, and medical applications of calcium phosphate nanoceramics. Mater Sci Eng R Rep 2010;70:225–42. [Google Scholar]
- 8. Wang J, Chen Y, Zhu X. et al. Effect of phase composition on protein adsorption and osteoinduction of porous calcium phosphate ceramics in mice. J Biomed Mater Res A 2014;102:4234–43. [DOI] [PubMed] [Google Scholar]
- 9. Wang Z, Tang Z, Qing F. et al. Applications of calcium phosphate nanoparticles in porous hard tissue engineering scaffolds. Nano 2012;7:1230004. [Google Scholar]
- 10. Yuan H, Yang Z, Li Y. et al. Osteoinduction by calcium phosphate biomaterials. J Mater Sci Mater Med 1998;9:723–6. [DOI] [PubMed] [Google Scholar]
- 11. Yuan H, Zou P, Yang Z. et al. Bone morphogenetic protein and ceramic-induced osteogenesis. J Mater Sci Mater Med 1998;9:717–21. [DOI] [PubMed] [Google Scholar]
- 12. Denry I, Kuhn LT.. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent Mater 2016;32:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Z, Yuan H, Tong W. et al. Osteogenesis in extraskeletally implanted porous calcium phosphate ceramics: Variability among different kinds of animals. Biomaterials 1996;17:2131–7. [DOI] [PubMed] [Google Scholar]
- 14. Yuan H, Yang Z, de Bruijn JD. et al. Material-dependent bone induction by calcium phosphate ceramics: A 2.5-year study in dog. Biomaterials 2001;22:2617–23. [DOI] [PubMed] [Google Scholar]
- 15. Yuan H, De Bruijn J, Li Y. et al. Bone formation induced by calcium phosphate ceramics in soft tissue of dogs: A comparative study between porous α-TCP and β-TCP. J Mater Sci Mater Med 2001;12:7–13. [DOI] [PubMed] [Google Scholar]
- 16. Barradas AMC, Yuan HP, van Blitterswijk CA. et al. Osteoinductive biomaterials: Current knowledge of properties, experimental models and biological mechanisms. Eur Cells Mater 2011;21:407–29. [DOI] [PubMed] [Google Scholar]
- 17. Samavedi S, Whittington AR, Goldstein AS.. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater 2013;9:8037–45. [DOI] [PubMed] [Google Scholar]
- 18. Elliott JC. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates. Studies in Inorganic Chemistry 1994; 18. [Google Scholar]
- 19. Yamamuro T, Hench LL, Wilson J.. CRC Handbook of Bioactive Ceramics: Vol. 2, Calcium Phosphate and Hydroxylapatite Ceramics. CRC Press, 1990. [Google Scholar]
- 20. Aoki H. Science and Medical Applications of Hydroxyapatite. JAAS 1991. [Google Scholar]
- 21. Yuan H, Van Den Doel M, Li S. et al. A comparison of the osteoinductive potential of two calcium phosphate ceramics implanted intramuscularly in goats. J Mater Sci Mater Med 2002;13:1271–5. [DOI] [PubMed] [Google Scholar]
- 22. Yuan H, Li Y, De Bruijn J. et al. Tissue responses of calcium phosphate cement: A study in dogs. Biomaterials 2000;21:1283–90. [DOI] [PubMed] [Google Scholar]
- 23. Chen L, Yan Q, Wu T. et al. Antibacterial and osteoinductive properties of icariin-metronidazole/calcium phosphate cement sustained release system. J Biomater Tissue Eng 2017;7:437–47. [Google Scholar]
- 24. Habibovic P, Gbureck U, Doillon CJ. et al. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials 2008;29:944–53. [DOI] [PubMed] [Google Scholar]
- 25. Chai YC, Carlier A, Bolander J. et al. Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater 2012;8:3876–87. [DOI] [PubMed] [Google Scholar]
- 26. Doosti BA, Lobovkina T.. Ca2+ gradient induces membrane bending and formation of nanotubes in giant lipid vesicles. Biophys J 2016;110:584a.26840724 [Google Scholar]
- 27. Breitwieser GE. Extracellular calcium as an integrator of tissue function. Int J Biochem Cell Biol 2008;40:1467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dvorak MM, Riccardi D.. Ca2+ as an extracellular signal in bone. Cell Calcium 2004;35:249–55. [DOI] [PubMed] [Google Scholar]
- 29. Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem 2006;97:56–70. [DOI] [PubMed] [Google Scholar]
- 30. Murshed M, Harmey D, Millán JL. et al. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev 2005;19:1093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu X, Zhang H, Fan H. et al. Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater 2010;6:1536–41. [DOI] [PubMed] [Google Scholar]
- 32. Zhu X, Fan H, Li D. et al. Protein adsorption and zeta potentials of a biphasic calcium phosphate ceramic under various conditions. J Biomed Mater Res B Appl Biomater 2007;82:65–73. [DOI] [PubMed] [Google Scholar]
- 33. Zhang X, Zhou J, Chen W. et al. A calcium phosphate, bioceramics with osteoinduction. The 4th World Biomaterials Congress, Berlin, Germany1992,24–8.
- 34. Chen Y, Wang J, Zhu XD. et al. Enhanced effect of β-tricalcium phosphate phase on neovascularization of porous calcium phosphate ceramics: In vitro and in vivo evidence. Acta Biomater 2015;11:435–48. [DOI] [PubMed] [Google Scholar]
- 35. Yamasaki H, Sakai H.. Osteogenic response to porous hydroxyapatite ceramics under the skin of dogs. Biomaterials 1992;13:308–12. [DOI] [PubMed] [Google Scholar]
- 36. Klein C, de Groot K, Chen W. et al. Osseous substance formation induced in porous calcium phosphate ceramics in soft tissues. Biomaterials 1994;15:31–4. [DOI] [PubMed] [Google Scholar]
- 37. Zhu X, Fan H, Xiao Y. et al. Effect of surface structure on protein adsorption to biphasic calcium-phosphate ceramics in vitro and in vivo. Acta Biomater 2009;5:1311–8. [DOI] [PubMed] [Google Scholar]
- 38. Dunn G, Heath J.. A new hypothesis of contact guidance in tissue cells. Exp Cell Res 1976;101:1–14. [DOI] [PubMed] [Google Scholar]
- 39. Rovensky YA, Slavnaja I, Vasiliev JM.. Behaviour of fibroblast-like cells on grooved surfaces. Exp Cell Res 1971;65:193–201. [DOI] [PubMed] [Google Scholar]
- 40. Deligianni DD, Katsala ND, Koutsoukos PG. et al. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000;22:87–96. [DOI] [PubMed] [Google Scholar]
- 41. Rosa AL, Beloti MM, van Noort R.. Osteoblastic differentiation of cultured rat bone marrow cells on hydroxyapatite with different surface topography. Dent Mater 2003;19:768–72. [DOI] [PubMed] [Google Scholar]
- 42. Yim EK, Pang SW, Leong KW.. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res 2007;313:1820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chaubey A, Ross KJ, Leadbetter RM. et al. Surface patterning: Tool to modulate stem cell differentiation in an adipose system. J Biomed Mater Res B Appl Biomater 2008;84:70–8. [DOI] [PubMed] [Google Scholar]
- 44.L CR, M SF, L NT et al. Titanium with nanotopography induces osteoblast differentiation by regulating endogenous bone morphogenetic protein expression and signaling pathway. J Cell Biochem 2016;117:1718.. [DOI] [PubMed] [Google Scholar]
- 45. Dalby MJ, Gadegaard N, Tare R. et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 2007;6:997–1003. [DOI] [PubMed] [Google Scholar]
- 46. McMurray RJ, Gadegaard N, Tsimbouri PM. et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater 2011;10:637–44. [DOI] [PubMed] [Google Scholar]
- 47. Chen XN, Zhu XD, Fan HS. et al. Rat bone marrow cell responses on the surface of hydroxyapatite with different topography. Key Eng Mat Trans Tech Publ 2008;361:1107–10. [Google Scholar]
- 48. Wang Z, Xiao ZW, Fan HS. et al. Fabrication of micro-grooved patterns on hydroxyapatite ceramics and observation of earlier response of osteoblasts to the patterns. Wuji Cailiao Xuebao (J Inorg Mater) 2013;28:51–7. [Google Scholar]
- 49. Fan H, Ikoma T, Tanaka J. et al. Surface structural biomimetics and the osteoinduction of calcium phosphate biomaterials. J Nanosci Nanotechnol 2007;7:808–13. [DOI] [PubMed] [Google Scholar]
- 50. Rehman I, Hench L, Bonfield W. et al. Analysis of surface layers on bioactive glasses. Biomaterials 1994;15:865–70. [DOI] [PubMed] [Google Scholar]
- 51. Nuss KM, von Rechenberg B.. Biocompatibility issues with modern implants in bone – a review for clinical orthopedics. Open Orthop J 2008;2:66.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park S-Y, Bae D-J, Kim M-J. et al. Extracellular low pH modulates phosphatidylserine-dependent phagocytosis in macrophages by increasing stabilin-1 expression. J Biol Chem 2012;287:11261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clark A, Hench L, Paschall H.. The influence of surface chemistry on implant interface histology: A theoretical basis for implant materials selection. J Biomed Mater Res 1976;10:161–74. [DOI] [PubMed] [Google Scholar]
- 54. Lu X, Leng Y.. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005;26:1097–108. [DOI] [PubMed] [Google Scholar]
- 55. Fan HS, Ikoma T, Bao C. et al. Surface characteristics and osteoinductivity of biphasic calcium phosphate ceramics with different sintering temperature. Key Eng Mater2006;309–311:1299–302. [Google Scholar]
- 56. Ohtsuki C, Kokubo T, Yamamuro T.. Mechanism of apatite formation on CaO SiO 2 P 2 O 5 glasses in a simulated body fluid. J Non-Crystal Solids 1992;143:84–92. [Google Scholar]
- 57. Lu HH, Pollack SR, Ducheyne P.. Temporal zeta potential variations of 45 S 5 bioactive glass immersed in an electrolyte solution. J Biomed Mater Res 2000;51:80–7. [DOI] [PubMed] [Google Scholar]
- 58. Zhu P, Masuda Y, Koumoto K.. The effect of surface charge on hydroxyapatite nucleation. Biomaterials 2004;25:3915–21. [DOI] [PubMed] [Google Scholar]
- 59. Zhang D, Hupa M, Hupa L.. In situ pH within particle beds of bioactive glasses. Acta Biomater 2008;4:1498–505. [DOI] [PubMed] [Google Scholar]
- 60. Tan Y, Wang G, Fan H. et al. Expression of core binding factor 1 and osteoblastic markers in C2C12 cells induced by calcium phosphate ceramics in vitro. J Biomed Mater Res A 2007;82:152–9. [DOI] [PubMed] [Google Scholar]
- 61. Sarikaya M. Biomimetics: Materials fabrication through biology. Proc Natl Acad Sci 1999;96:14183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hartgerink JD, Beniash E, Stupp SI.. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001;294:1684–8. [DOI] [PubMed] [Google Scholar]
- 63. Seeman NC, Belcher AM.. Emulating biology: Building nanostructures from the bottom up. Proc Natl Acad Sci 2002;99(Suppl 2):6451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cölfen H, Mann S.. Higher‐order organization by mesoscale self‐assembly and transformation of hybrid nanostructures. Angew Chem Int Ed 2003;42:2350–65. [DOI] [PubMed] [Google Scholar]
- 65. Xu A-W, Ma Y, Cölfen H.. Biomimetic mineralization. J Mat Chem 2007;17:415–49. [Google Scholar]
- 66. Li B, Chen X, Guo B. et al. Fabrication and cellular biocompatibility of porous carbonated biphasic calcium phosphate ceramics with a nanostructure. Acta Biomater 2009;5:134–43. [DOI] [PubMed] [Google Scholar]
- 67. Urist MR. Bone: Formation by autoinduction. Science 1965;150:893–9. [DOI] [PubMed] [Google Scholar]
- 68. Yang RN, Ye F, Cheng LJ. et al. Osteoinduction by Ca-P biomaterials implanted into the muscles of mice. J Zhejiang Univ Sci B 2011;12:582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fujibayashi S, Neo M, Kim HM. et al. Osteoinduction of porous bioactive titanium metal. Biomaterials 2004;25:443–50. [DOI] [PubMed] [Google Scholar]
- 70. Winter GD, Simpson BJ.. Heterotopic bone formed in a synthetic sponge in the skin of young pigs. Nature 1969;223:88–90. [DOI] [PubMed] [Google Scholar]
- 71. Ripamonti U. Inductive bone matrix and porous hydroxyapatite composites in rodents and nonhuman primates. Handb Bioactive Ceramics 1990;2:245–53. [Google Scholar]
- 72. Yang ZJ, Yuan H, Zou P. et al. Osteogenic responses to extraskeletally implanted synthetic porous calcium phosphate ceramics: An early stage histomorphological study in dogs. J Mater Sci Mater Med 1997;8:697–701. [DOI] [PubMed] [Google Scholar]
- 73. Cheng L, Shi Y, Ye F. et al. Osteoinduction of calcium phosphate biomaterials in small animals. Mater Sci Eng C 2013;33:1254–60. [DOI] [PubMed] [Google Scholar]
- 74. Sautier JM, Nefussi JR, Forest N.. Ultrastructural-study of bone-formation on synthetic hydroxyapatite in osteoblast cultures. Cells Mater 1991;1:209–17. [Google Scholar]
- 75. Qu SX, Leng Y, Guo X. et al. Histological and ultrastructural analysis of heterotopic osteogenesis in porous calcium phosphate ceramics. J Mater Sci Lett 2002;21:153–5. [Google Scholar]
- 76. Annaz B, Hing KA, Kayser M. et al. Porosity variation in hydroxyapatite and osteoblast morphology: A scanning electron microscopy study. J Microsc Oxf 2004;215:100–10. [DOI] [PubMed] [Google Scholar]
- 77. Yuan H, Van Blitterswijk C, De Groot K. et al. A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods. J Biomed Mater Res A 2006;78:139–47. [DOI] [PubMed] [Google Scholar]
- 78. Tang Z, Tan Y, Ni Y. et al. Comparison of ectopic bone formation process induced by four calcium phosphate ceramics in mice. Mater Sci Eng C Mater Biol Appl 2016;70:1000–10. [DOI] [PubMed] [Google Scholar]
- 79. Ripamonti U, Van den Heever B, Van Wyk J.. Expression of the osteogenic phenotype in porous hydroxyapatite implanted extraskeletally in baboons. Matrix 1993;13:491–502. [DOI] [PubMed] [Google Scholar]
- 80. Zhang LL, Hanagata N, Maeda M. et al. Porous hydroxyapatite and biphasic calcium phosphate ceramics promote ectopic osteoblast differentiation from mesenchymal stem cells. Sci Technol Adv Mater 2009;10:025003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Muller P, Bulnheim U, Diener A. et al. Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J Cell Mol Med 2008;12:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yuan H, Fernandes H, Habibovic P. et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci 2010;107:13614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Barradas AM, Monticone V, Hulsman M. et al. Molecular mechanisms of biomaterial-driven osteogenic differentiation in human mesenchymal stromal cells. Integr Biol (Camb) 2013;5:920–31. [DOI] [PubMed] [Google Scholar]
- 84. Lin L, Chow KL, Leng Y.. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A 2009;89:326–35. [DOI] [PubMed] [Google Scholar]
- 85. Ong JL, Cavin R. Osteoblast cell response to hydroxyapatite of different heat treatments. Proceedings of the 1997 16th Southern Biomedical Engineering Conference, Broadwater Beach Resort and Hotel, Biloxi, Mississippi1997,183–6.
- 86. Wang C, Duan Y, Markovic B. et al. Phenotypic expression of bone-related genes in osteoblasts grown on calcium phosphate ceramics with different phase compositions. Biomaterials 2004;25:2507–14. [DOI] [PubMed] [Google Scholar]
- 87. Huang L, Zhou B, Wu H. et al. Effect of apatite formation of biphasic calcium phosphate ceramic (BCP) on osteoblastogenesis using simulated body fluid (SBF) with or without bovine serum albumin (BSA). Mater Sci Eng C 2017;70:955–61. [DOI] [PubMed] [Google Scholar]
- 88. Specchia N, Pagnotta A, Cappella M. et al. Effect of hydroxyapatite porosity on growth and differentiation of human osteoblast-like cells. J Mater Sci 2002;37:577–84. [Google Scholar]
- 89. Sibilla P, Sereni A, Aguiari G. et al. Effects of a hydroxyapatite-based biomaterial on gene expression in osteoblast-like cells. J Dent Res 2006;85:354–8. [DOI] [PubMed] [Google Scholar]
- 90. Chen D, Zhao M, Mundy GR.. Bone morphogenetic proteins. Growth Factors 2004;22:233–41. [DOI] [PubMed] [Google Scholar]
- 91. Eyckmans J, Roberts SJ, Schrooten J. et al. A clinically relevant model of osteoinduction: A process requiring calcium phosphate and BMP/Wnt signalling. J Cell Mol Med 2010;14:1845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sun LY, Wu L, Bao CY. et al. Gene expressions of Collagen type I, ALP and BMP-4 in osteo-inductive BCP implants show similar pattern to that of natural healing bones. Mater Sci Eng C Mater Biol Appl 2009;29:1829–34. [Google Scholar]
- 93. Tang ZR, Wang Z, Qing FZ. et al. Bone morphogenetic protein Smads signaling in mesenchymal stem cells affected by osteoinductive calcium phosphate ceramics. J Biomed Mater Res A 2015;103:1001–10. [DOI] [PubMed] [Google Scholar]
- 94. Xu S, Qiu Z, Wu J. et al. Osteogenic differentiation gene expression profiling of hMSCs on hydroxyapatite and mineralized collagen. Tissue Eng A 2015;22:170. [DOI] [PubMed] [Google Scholar]
- 95. Long F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 2012;13:27–38. [DOI] [PubMed] [Google Scholar]
- 96. Long F, Ornitz DM.. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol 2013;5:a008334.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen G, Deng C, Li YP.. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 2012;8:272–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. St-Jacques B, Hammerschmidt M, McMahon AP.. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 1999;13:2072–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hilton MJ, Tu X, Wu X. et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med 2008;14:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bolander J, Chai YC, Geris L. et al. Early BMP, Wnt and Ca2+/PKC pathway activation predicts the bone forming capacity of periosteal cells in combination with calcium phosphates. Biomaterials 2016;86:106–18. [DOI] [PubMed] [Google Scholar]
- 101. Ornitz DM, Marie PJ.. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev 2002;16:1446–65. [DOI] [PubMed] [Google Scholar]
- 102. Lin GL, Hankenson KD.. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem 2011;112:3491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lu D, Qu J, Lei J. et al. LPS-stimulated inflammation inhibits BMP-9-induced osteoblastic differentiation through crosstalk between BMP/MAPK and Smad signaling: LPS stimulation inhibits BMP-9-induced osteoblastic differentiation. Exp Cell Res 2016;341:54–60. [DOI] [PubMed] [Google Scholar]
- 104. Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res 2013;92:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Glass GE, Chan JK, Freidin A. et al. TNF-α promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci 2011;108:1585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Takayanagi H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 2007;7:292–304. [DOI] [PubMed] [Google Scholar]
- 107. Sima C, Glogauer M.. Macrophage subsets and osteoimmunology: Tuning of the immunological recognition and effector systems that maintain alveolar bone. Periodontol 2000 2013;63:80–101. [DOI] [PubMed] [Google Scholar]
- 108. Steeve KT, Marc P, Sandrine T. et al. IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev 2004;15:49–60. [DOI] [PubMed] [Google Scholar]
- 109. Theoleyre S, Wittrant Y, Tat SK. et al. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev 2004;15:457–75. [DOI] [PubMed] [Google Scholar]
- 110. Stein NC, Kreutzmann C, Zimmermann SP. et al. Interleukin‐4 and interleukin‐13 stimulate the osteoclast inhibitor osteoprotegerin by human endothelial cells through the STAT6 pathway. J Bone Miner Res 2008;23:750–8. [DOI] [PubMed] [Google Scholar]
- 111. Zhang X, Yuan H, De Groot K. Calcium phosphate biomaterials with intrinsic osteoinductivity. The 6th World Biomaterials Congress, Kamuela, Hawaii, USA,2000. p.1–13.
- 112. Potenta S, Zeisberg E, Kalluri R.. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer 2008;99:1375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Medici D, Kalluri R.. Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin Cancer Biol 2012;22:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Medici D, Olsen BR.. The role of endothelial-mesenchymal transition in heterotopic ossification. J Bone Miner Res 2012;27:1619–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zheng B, Cao B, Crisan M. et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol 2007;25:1025–34. [DOI] [PubMed] [Google Scholar]
- 116. Kovacic JC, Boehm M.. Resident vascular progenitor cells: An emerging role for non-terminally differentiated vessel-resident cells in vascular biology. Stem Cell Res 2009;2:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ripamonti U, Klar RM, Renton LF. et al. Synergistic induction of bone formation by hOP-1, hTGF-beta3 and inhibition by zoledronate in macroporous coral-derived hydroxyapatites. Biomaterials 2010;31:6400–10. [DOI] [PubMed] [Google Scholar]
- 118. Betsholtz C, Lindblom P, Gerhardt H.. Role of Pericytes in Vascular Morphogenesis. EXS 2005;94:115–25. [DOI] [PubMed] [Google Scholar]
- 119. Crisan M, Yap S, Casteilla L. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–13. [DOI] [PubMed] [Google Scholar]
- 120. Brachvogel B, Moch H, Pausch F. et al. Perivascular cells expressing annexin A5 define a novel mesenchymal stem cell-like population with the capacity to differentiate into multiple mesenchymal lineages. Development 2005;132:2657–68. [DOI] [PubMed] [Google Scholar]
- 121. Moonen J-RA, Krenning G, Brinker MG. et al. Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovasc Res 2010;86:506–15. [DOI] [PubMed] [Google Scholar]
- 122. Armulik A, Abramsson A, Betsholtz C.. Endothelial/pericyte interactions. Circ Res 2005;97:512–23. [DOI] [PubMed] [Google Scholar]
- 123. Péault B, Rudnicki M, Torrente Y. et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther 2007;15:867–77. [DOI] [PubMed] [Google Scholar]
- 124. Dellavalle A, Sampaolesi M, Tonlorenzi R. et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 2007;9:255–67. [DOI] [PubMed] [Google Scholar]
- 125. DIAZ-FLORES L, Gutierrez R, Lopez-Alonso A. et al. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res 1992;275:280–6. [PubMed] [Google Scholar]
- 126. Zhang X. Osteoinduction and Ostogenic Genes Expression Regulated by Ca-P Bioceramics. Biomaterials in Asia: In Commemoration of the 1st Asian Biomaterials Congress, Tsukuba, Japan, 6–8 December 2007 World Scientific, 2008: 24–34.
- 127. Song G, Habibovic P, Bao C. et al. The homing of bone marrow MSCs to non-osseous sites for ectopic bone formation induced by osteoinductive calcium phosphate. Biomaterials 2013;34:2167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tan Y, Wang G, Fan H. et al. Quantitative analysis of core binding factor 1 (Cbfa1) and osteocalcin in C2C12 cells induced by Ca/P ceramics in vitro. Key Eng Mater 2007;330–332:1067–70. [Google Scholar]
- 129. Urist MR, Strates BS.. Bone morphogenetic protein. J Dent Res 1971;50:1392–406. [DOI] [PubMed] [Google Scholar]
- 130. Rao H, Lu Z, Liu W. et al. The adsorption of bone-related proteins on calcium phosphate ceramic particles with different phase composition and its adsorption kinetics. Surf Interface Anal 2016;48:1048–55. [Google Scholar]
- 131. Ripamonti U, Crooks J, Kirkbride A.. Sintered porous hydroxyapatites with intrinsic osteoinductive activity: Geometric induction of bone formation. S Afr J Sci 1999;95:335. [Google Scholar]
- 132. De Groot J. Carriers that concentrate native bone morphogenetic protein in vivo. Tissue Eng 1998;4:337–41. [DOI] [PubMed] [Google Scholar]
- 133. Ripamonti U. The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. J Bone Joint Surg Am 1991;73:692–703. [PubMed] [Google Scholar]
- 134. Le Nihouannen D, Daculsi G, Saffarzadeh A. et al. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone 2005;36:1086–93. [DOI] [PubMed] [Google Scholar]
- 135. Reddi A. Cell biology and biochemistry of endochondral bone development. Collagen Relat Res 1981;1:209–26. [DOI] [PubMed] [Google Scholar]
- 136. Giancotti FG, Ruoslahti E.. Integrin signaling. Science 1999;285:1028–33. [DOI] [PubMed] [Google Scholar]
- 137. Chen X, Wang J, Chen Y. et al. Roles of calcium phosphate-mediated integrin expression and MAPK signaling pathways in the osteoblastic differentiation of mesenchymal stem cells. J Mater Chem B 2016;4:2280–9. [DOI] [PubMed] [Google Scholar]
- 138. Shekaran A, Garcia AJ.. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J Biomed Mater Res A 2011;96:261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Matsuura T, Hosokawa R, Okamoto K. et al. Diverse mechanisms of osteoblast spreading on hydroxyapatite and titanium. Biomaterials 2000;21:1121–7. [DOI] [PubMed] [Google Scholar]
- 140. Kilpadi KL, Chang PL, Bellis SL.. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J Biomed Mater Res 2001;57:258–67. [DOI] [PubMed] [Google Scholar]
- 141. Marino G, Rosso F, Cafiero G. et al. β-Tricalcium phosphate 3D scaffold promote alone osteogenic differentiation of human adipose stem cells: In vitro study. J Mater Sci Mater Med 2010;21:353–63. [DOI] [PubMed] [Google Scholar]
- 142. Salasznyk RM, Klees RF, Williams WA. et al. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res 2007;313:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lu Z, Zreiqat H.. The osteoconductivity of biomaterials is regulated by bone morphogenetic protein 2 autocrine loop involving α2β1 integrin and mitogen-activated protein kinase/extracellular related kinase signaling pathways. Tissue Eng A 2010;16:3075–84. [DOI] [PubMed] [Google Scholar]
- 144. Lai CF, Cheng SL.. αvβ Integrins play an essential role in BMP‐2 induction of osteoblast differentiation. J Bone Miner Res 2005;20:330–40. [DOI] [PubMed] [Google Scholar]
- 145. Liu H, Peng H, Wu Y. et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013;34:4404–17. [DOI] [PubMed] [Google Scholar]
- 146. Deschaseaux F, Sensébé L, Heymann D.. Mechanisms of bone repair and regeneration. Trends Mol Med 2009;15:417–29. [DOI] [PubMed] [Google Scholar]
- 147. Klar RM, Duarte R, Dix-Peek T. et al. Calcium ions and osteoclastogenesis initiate the induction of bone formation by coral-derived macroporous constructs. J Cell Mol Med 2013;17:1444–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liu H, Xu GW, Wang YF. et al. Composite scaffolds of nano-hydroxyapatite and silk fibroin enhance mesenchymal stem cell-based bone regeneration via the interleukin 1 alpha autocrine/paracrine signaling loop. Biomaterials 2015;49:103–12. [DOI] [PubMed] [Google Scholar]
- 149. Shih Y-RV, Hwang Y, Phadke A. et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc Natl Acad Sci 2014;111:990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Eyckmans J, Roberts SJ, Bolander J. et al. Mapping calcium phosphate activated gene networks as a strategy for targeted osteoinduction of human progenitors. Biomaterials 2013;34:4612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Krebs J, Michalak M.. Calcium: A Matter of Life or Death. Ann Dermatol Venereol 2007;136:125–32. [Google Scholar]
- 152. Aguirre A, González A, Planell J. et al. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem Biophys Res Commun 2010;393:156–61. [DOI] [PubMed] [Google Scholar]
- 153. Gonzalez-Vazquez A, Planell JA, Engel E.. Extracellular calcium and CaSR drive osteoinduction in mesenchymal stromal cells. Acta Biomater 2014;10:2824–33. [DOI] [PubMed] [Google Scholar]
- 154. Jung G-Y, Park Y-J, Han J-S.. Effects of HA released calcium ion on osteoblast differentiation. J Mater Sci Mater Med 2010;21:1649–54. [DOI] [PubMed] [Google Scholar]
- 155. Zayzafoon M, Fulzele K, McDonald JM.. Calmodulin and calmodulin-dependent kinase IIα regulate osteoblast differentiation by controlling c-fos expression. J Biol Chem 2005;280:7049–59. [DOI] [PubMed] [Google Scholar]
- 156. Barradas AM, Fernandes HA, Groen N. et al. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012;33:3205–15. [DOI] [PubMed] [Google Scholar]
- 157. Syed-Picard FN, Jayaraman T, Lam RS. et al. Osteoinductivity of calcium phosphate mediated by connexin 43. Biomaterials 2013;34:3763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Beck GR, Zerler B, Moran E.. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci 2000;97:8352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Beck GR, Knecht N.. Osteopontin regulation by inorganic phosphate is ERK1/2-, protein kinase C-, and proteasome-dependent. J Biol Chem 2003;278:41921–9. [DOI] [PubMed] [Google Scholar]
- 160. Khoshniat S, Bourgine A, Julien M. et al. Phosphate-dependent stimulation of MGP and OPN expression in osteoblasts via the ERK1/2 pathway is modulated by calcium. Bone 2011;48:894–902. [DOI] [PubMed] [Google Scholar]
- 161. Chai YC, Roberts SJ, Schrooten J. et al. Probing the osteoinductive effect of calcium phosphate by using an in vitro biomimetic model. Tissue Eng A 2011;17:1083–97. [DOI] [PubMed] [Google Scholar]
- 162. Mammoto T, Ingber DE.. Mechanical control of tissue and organ development. Development 2010;137:1407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kilian KA, Bugarija B, Lahn BT. et al. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A 2010;107:4872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ingber DE. Cellular mechanotransduction: Putting all the pieces together again. Faseb J 2006;20:811–27. [DOI] [PubMed] [Google Scholar]
- 165. Ko KS, Arora PD, McCulloch CA.. Cadherins mediate intercellular mechanical signaling in fibroblasts by activation of stretch-sensitive calcium-permeable channels. J Biol Chem 2001;276:35967–77. [DOI] [PubMed] [Google Scholar]
- 166. Maroto R, Raso A, Wood TG. et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 2005;7:179–85. [DOI] [PubMed] [Google Scholar]
- 167. Sukharev S, Corey DP.. Mechanosensitive channels: Multiplicity of families and gating paradigms. Sci Signal 2004;2004:re4-re4.. [DOI] [PubMed] [Google Scholar]
- 168. Tanaka SM, Sun HB, Roeder RK. et al. Osteoblast responses one hour after load-induced fluid flow in a three-dimensional porous matrix. Calcif Tissue Int 2005;76:261–71. [DOI] [PubMed] [Google Scholar]
- 169. Ripamonti U, Roden L, Rentona L. et al. The influence of geometry on bone: Formation by autoinduction. Sci Afr 2012. www.scienceinafrica.com/old/2012/The%20influence%20of%20geometry%20on%20bone%20%20SIA.%205.5.12.pdf. [Google Scholar]
- 170. Ripamonti U, Roden LC, Ferretti C. et al. Biomimetic matrices self-initiating the induction of bone formation. J Craniofac Surg 2011;22:1859–70. [DOI] [PubMed] [Google Scholar]
- 171. Ohgushi H, Dohi Y, Tamai S. et al. Osteogenic differentiation of marrow stromal stem cells in porous hydroxyapatite ceramics. J Biomed Mater Res 1993;27:1401–7. [DOI] [PubMed] [Google Scholar]
- 172. Fellah BH, Josselin N, Chappard D. et al. Inflammatory reaction in rats muscle after implantation of biphasic calcium phosphate micro particles. J Mater Sci Mater Med 2007;18:287–94. [DOI] [PubMed] [Google Scholar]
- 173. Jing W, Dan L, Bo G. et al. Role of biphasic calcium phosphate ceramic-mediated secretion of signaling molecules by macrophages in migration and osteoblastic differentiation of MSCs. Acta Biomater 2017;51:447–60. [DOI] [PubMed] [Google Scholar]
- 174. De Bruijn J, Shankar K, Yuan H. et al. OsteoinductionandIts Evaluation . Bioceramics and Their Clinical Applications. Cambridge: Woodhead Publishing Ltd, 2008. [Google Scholar]
- 175. Chen Z, Wu C, Gu W. et al. Osteogenic differentiation of bone marrow MSCs by beta-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials 2014;35:1507–18. [DOI] [PubMed] [Google Scholar]
- 176. Le Nihouannen D, Saffarzadeh A, Gauthier O. et al. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J Mater Sci Mater Med 2008;19:667–75. [DOI] [PubMed] [Google Scholar]
- 177. Laquerriere P, Grandjean-Laquerriere A, Jallot E. et al. Importance of hydroxyapatite particles characteristics on cytokines production by human monocytes in vitro. Biomaterials 2003;24:2739–47. [DOI] [PubMed] [Google Scholar]
- 178. Dong L, Wang C.. Harnessing the power of macrophages/monocytes for enhanced bone tissue engineering. Trends Biotechnol 2013;31:342–6. [DOI] [PubMed] [Google Scholar]
- 179. Miramond T, Corre P, Borget P. et al. Osteoinduction of biphasic calcium phosphate scaffolds in a nude mouse model. J Biomater Appl 2014;29:595–604. [DOI] [PubMed] [Google Scholar]
- 180. Kanatani M, Sugimoto T, Fukase M. et al. Effect of elevated extracellular calcium on the proliferation of osteoblastic MC3T3-E1 cells: Its direct and indirect effects via monocytes. Biochem Biophys Res Commun 1991;181:1425–30. [DOI] [PubMed] [Google Scholar]
- 181. Olszak IT, Poznansky MC, Evans RH. et al. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J Clin Investig 2000;105:1299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. McNamara LE, McMurray RJ, Biggs MJ. et al. Nanotopographical control of stem cell differentiation. J Tissue Eng 2010; 1:120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Chai YC, Roberts SJ, Desmet E. et al. Mechanisms of ectopic bone formation by human osteoprogenitor cells on CaP biomaterial carriers. Biomaterials 2012;33:3127.. [DOI] [PubMed] [Google Scholar]
- 184. Davison NL, Gamblin AL, Layrolle P. et al. Liposomal clodronate inhibition of osteoclastogenesis and osteoinduction by submicrostructured beta-tricalcium phosphate. Biomaterials 2014;35:5088–97. [DOI] [PubMed] [Google Scholar]
- 185. Davison NL, ten Harkel B, Schoenmaker T. et al. Osteoclast resorption of beta-tricalcium phosphate controlled by surface architecture. Biomaterials 2014;35:7441–51. [DOI] [PubMed] [Google Scholar]
- 186. Kan L, Liu Y, McGuire TL. et al. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells 2009;27:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chen Z, Klein T, Murray RZ. et al. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today 2016;19:304–21. [Google Scholar]
- 188. Chen Z, Yuen J, Crawford R. et al. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated β-tricalcium phosphate. Biomaterials 2015;61:126–38. [DOI] [PubMed] [Google Scholar]
- 189. Wu C, Chen Z, Wu Q. et al. Clinoenstatite coatings have high bonding strength, bioactive ion release, and osteoimmunomodulatory effects that enhance in vivo osseointegration. Biomaterials 2015;71:35–47. [DOI] [PubMed] [Google Scholar]
- 190. Collett G, Canfield A.. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res 2005;96:930–8. [DOI] [PubMed] [Google Scholar]
- 191. Sorescu GP, Song H, Tressel SL. et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 2004;95:773–9. [DOI] [PubMed] [Google Scholar]
- 192. Cola C, Almeida M, Li D. et al. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun 2004;320:424–7. [DOI] [PubMed] [Google Scholar]
- 193. de Jesus Perez VA, Alastalo T-P, Wu JC. et al. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt–β-catenin and Wnt–RhoA–Rac1 pathways. J Cell Biol 2009;184:83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Kohn EC, Alessandro R, Spoonster J. et al. Angiogenesis: Role of calcium-mediated signal transduction. Proc Natl Acad Sci 1995;92:1307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Munaron L. Intracellular calcium, endothelial cells and angiogenesis. Recent Patents Anticancer Drug Discov 2006;1:105–19. [DOI] [PubMed] [Google Scholar]
- 196. Aguirre A, Gonzalez A, Navarro M. et al. Control of microenvironmental cues with a smart biomaterial composite promotes endothelial progenitor cell angiogenesis. Eur Cells Mater 2012;24:90.. [DOI] [PubMed] [Google Scholar]
- 197. Zheng L, Sun J, Chen X. et al. In vivo cartilage engineering with collagen hydrogel and allogenous chondrocytes after diffusion chamber implantation in immunocompetent host. Tissue Eng A 2009;15:2145–53. [DOI] [PubMed] [Google Scholar]
- 198. Yuan T, Zhang L, Feng L. et al. Chondrogenic differentiation and immunological properties of mesenchymal stem cells in collagen type I hydrogel. Biotechnol Prog 2010;26:1749–58. [DOI] [PubMed] [Google Scholar]
- 199. Zhang L, Yuan T, Guo L. et al. An in vitro study of collagen hydrogel to induce the chondrogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A 2012;100:2717–25. [DOI] [PubMed] [Google Scholar]
- 200. Shi M, Zhou Y, Shao J. et al. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater 2015;21:178–89. [DOI] [PubMed] [Google Scholar]
- 201. Yang Z, Zhang A, Duan H. et al. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci 2015;112:13354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Levingstone TJ, Ramesh A, Brady RT. et al. Cell-free multi-layered collagen-based scaffolds demonstrate layer specific regeneration of functional osteochondral tissue in caprine joints. Biomaterials 2016;87:69–81. [DOI] [PubMed] [Google Scholar]