Abstract
Background
Although anaemia is a common complication of advanced chronic kidney disease (CKD), knowledge of quality of care and management practices in specialist clinics varies. We examined anaemia practices at specialist nephrology clinics within the Irish health system and evaluated the opinions of practicing nephrologists.
Methods
A multicentre cross-sectional study was conducted at specialist nephrology clinics across six geographic regions in Ireland. Clinical characteristics and treatment practices were evaluated in a sample of 530 patients with CKD. An accompanying national survey questionnaire captured opinions and treatment strategies of nephrologists on anaemia management.
Results
The prevalence of anaemia [defined as haemoglobin (Hb) <12.0 g/dL] was 37.8%, which increased significantly with advancing CKD (from 21% to 63%; P < 0.01) and varied across clinical sites (from 36% to 62%; P < 0.026). Iron deficiency (ID) was present in 46% of all patients tested and 86% of them were not on treatment. More than 45% of anaemic patients were not tested for ID. Respondents differed in their selection of clinical guidelines, threshold targets for erythropoiesis-stimulating agent (ESA) and intravenous iron therapy and anaemia management algorithms were absent in 47% of the clinics. The unexpectedly low rates of ESA use (4.7%) and iron therapy (10.2%) in clinical practice were in contrast to survey responses where 63% of nephrologists indicated ESA therapy initiation when Hb was <10.0 g/dL and 46% indicated commencement of iron therapy for ferritin <150 ng/mL.
Conclusion
This study highlights substantial variability in the management of anaemia and ID at specialist nephrology clinics with low testing rates for ID, high rates of anaemia and ID and underutilization of effective treatments. Variability in the adoption and implementation of different clinical guidelines was evident.
Introduction
Chronic kidney disease (CKD) is a major public health epidemic that is associated with increased morbidity and mortality and substantially lower quality of life [1–5]. A fundamental objective in the care pathway of all CKD patients is to tackle modifiable risk factors in order to halt or slow progression to end-stage renal disease (ESRD), increase patient survival and improve overall patient physical well-being. A highly prevalent and relatively easily identifiable risk factor is anaemia associated with CKD. In the USA, for example, anaemia is twice as prevalent in CKD patients as in the general population. Moreover, the prevalence of anaemia increases as CKD progresses, affecting almost half of those with Stage 5 CKD [6]. A substantial body of evidence incriminates anaemia as a risk multiplier for major medical conditions, including coronary heart disease and stroke [7, 8]. Moreover, prospective epidemiological studies have linked anaemia with the development of left ventricular hypertrophy and left ventricular systolic dysfunction [9], along with higher rates of hospitalization, poorer quality of life and greater mortality [4, 10, 11]. Therefore, early identification and correction of anaemia is an important component of care provision for patients with advanced CKD.
Despite the increasing availability of a broad range of investigations and treatment strategies, it is striking that anaemia remains underdiagnosed and undertreated in routine clinical practice [12, 13]. Two recent studies have uncovered an unexpectedly high prevalence of anaemia among non-dialysis CKD patients and surprisingly low rates of the use of anaemia treatments [12, 13]. A study by Minutolo et al. [12] conducted in Italy estimated that 44% of CKD patients attending renal clinics were anaemic and that the proportion remained virtually unchanged after 6 months of follow-up. A further study by Cases-Amenos et al. [13] in Spain found an even higher percentage with anaemia (58.5%) and significant undertreatment of iron deficiency (ID). These studies would suggest that significant gaps remain in the management of anaemia among CKD patients attending specialist clinics and that a critical need exists for more effective implementation pathways. For example, it is unclear at the present time to what extent CKD patients are investigated for the presence of anaemia at specialist nephrology clinics. Second, it is equally uncertain whether the lack of evidence-based protocols and their implementation may in part be responsible for any observed deficiency in care provision. Third, it remains to be proven whether the treatment delivered at the coalface is concordant with the views and expectations of the supervising nephrologist.
To provide a better understanding of anaemia management, we conducted a multicentre audit of clinical practices at specialist renal clinics in Ireland. We also explored concurrently the opinions of supervising nephrologists on the use of clinical guidelines and thresholds for intervention from a survey questionnaire.
Materials and methods
Study design
We conducted a multicentre cross-sectional study of consecutive adult non-dialysis CKD patients treated at specialist nephrology clinics during the first 2 weeks of December 2012 and 2013. The 18 specialist nephrology clinics were widely distributed across six health regions in the Republic of Ireland (West, Midwest, Northwest, Midlands, East and Southeast). A standardized data collection tool was used to capture clinical information from medical case records, laboratory information systems and physician clinic letters. Data were recorded on demographic and clinical characteristics, including primary cause of kidney disease, comorbid medical conditions, prescribed medications and laboratory values recorded within the previous 3 months [or within 6 months for laboratory values for iron studies, parathyroid hormone (PTH), iron studies, lipids and haemoglobin A1c (HbA1c)]. Patients <18 years of age or on dialysis were excluded.
Anaemia was primarily defined as Hb <12.0 g/dL, although additional definitions were also used to characterize the extent of anaemia according to the following Hb thresholds: Hb <13 g/dL for men and <12 g/dL for women according to the World Health Organization (WHO) criteria, Hb <11 g/dL and Hb <10 g/dL. ID was defined as serum ferritin <100 ng/mL and/or transferrin saturation (TSAT) <20%. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [14].
The clinical study was accompanied by a survey questionnaire of adult nephrologists registered by the Irish and UK Medical Councils and practising in the North and South of Ireland. The survey questionnaire captured information on the use of clinical guidelines for CKD management in routine clinical practice, threshold values of laboratory parameters to prompt clinical intervention and the opinions of nephrologists on suggested management strategies. The study was approved by the Ethics Committee of University Hospital Limerick.
Statistical analysis
Descriptive statistics were used to describe the baseline characteristics of the study population. Categorical variables are presented as numbers and percentages, whereas continuous variables are presented as mean (SD) or median [interquartile range (IQR)]. The prevalence of anaemia according to specified Hb thresholds was computed by the stage of CKD and across health regions. Comparisons across groups were made using chi-square and Fisher’s exact tests for categorical variables. Student’s t-test and analysis of variance were used for continuous variables. Correlates of anaemia (Hb <12 g/dL) were identified in a series of separate univariate logistic regression models. A final multivariate logistic regression was constructed to identify the relative contributions of demographic, clinical and treatment factors with the presence of anaemia. Associations were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). The adequacy of the models was tested using the Hosmer and Lemeshow goodness-of-fit test and the discriminative index using the C-statistic. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of the study population
The baseline characteristics of the study population and by GFR category are shown in Table 1. The average age was 57 (SD 18) years, 56% were men and the majority were white Irish (95%). The principal causes of CKD were hypertension (27.2%), glomerulonephritis (17.7%) and diabetes (12.6%), although for a large proportion the causes were classified as unknown (13.4%). The average eGFR was 48 (SD 28) mL/min/1.73 m2 overall and just under a third (31%) had advanced CKD (Stages 4–5). Patients with more advanced CKD had significantly lower Hb and serum albumin and significantly higher phosphate and PTH hormone concentrations.
Table 1.
Baseline characteristics of the study population by stage of CKD
| n | Overall cohort | Stages 1–2a | Stage 3a | Stages 4–5a | P-value | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age (years, mean (SD) | 510 | 57.1 (18.3) | 48.1 (15.5) | 59.3 (16.8) | 65.5 (17.1) | <0.001 |
| Men (%) | 293 | 55.3 | 55.6 | 59.8 | 56.0 | 0.706 |
| Race (%) | ||||||
| White Irish | 492 | 92.8 | 91.4 | 96.6 | 96.4 | 0.049 |
| White Irish traveller | 2 | 0.4 | 0.7 | 0.0 | 0.0 | 0.055 |
| White other | 12 | 2.3 | 1.4 | 2.8 | 2.9 | 0.050 |
| Asian | 2 | 0.4 | 0.7 | 0.6 | 0.0 | 0.053 |
| Other | 9 | 1.7 | 5.7 | 0.0 | 0.7 | 0.054 |
| Cause of CKD (%) | ||||||
| Hypertension | 144 | 27.2 | 22.9 | 24.6 | 34.8 | 0.055 |
| Diabetes | 67 | 12.6 | 8.3 | 15.1 | 17.0 | 0.064 |
| Glomerulonephritis | 94 | 17.7 | 24.3 | 14.5 | 14.9 | 0.047 |
| Autosomal dominant PKD | 33 | 6.2 | 4.9 | 8.9 | 5.0 | 0.267 |
| Hereditary | 14 | 2.6 | 3.5 | 3.4 | 0.7 | 0.261 |
| Other | 172 | 32.5 | 0.0 | 0.0 | 0.0 | 0.610 |
| Not known | 71 | 13.4 | 11.1 | 10.1 | 16.3 | 0.233 |
| Kidney biopsy (%) | 79 | 14.9 | 19.4 | 14.5 | 13.5 | 0.348 |
| Comorbid conditions (%) | ||||||
| Diabetes | 111 | 20.9 | 17.4 | 24.1 | 28.3 | 0.117 |
| Hypertension | 382 | 72.1 | 73.6 | 80.1 | 84.1 | 0.116 |
| Cancer | 34 | 6.4 | 7.4 | 8.4 | 6.5 | 0.820 |
| Heart failure | 22 | 4.2 | 1.7 | 4.2 | 7.2 | 0.111 |
| Thyroid disease | 49 | 9.2 | 5.8 | 12.0 | 13.0 | 0.107 |
| Stroke/TIA | 22 | 4.2 | 3.3 | 4.2 | 6.5 | 0.490 |
| COPD | 22 | 4.2 | 3.3 | 4.2 | 5.8 | 0.609 |
| Peripheral vascular disease | 38 | 7.2 | 3.3 | 10.2 | 10.9 | 0.042 |
| Coronary heart disease | 76 | 14.3 | 9.1 | 15.8 | 23.9 | 0.004 |
| Obesity | 29 | 5.5 | 4.1 | 4.8 | 8.7 | 0.262 |
| Gout | 59 | 11.1 | 5.7 | 15.2 | 15.9 | 0.013 |
| Hypercholesterolaemia | 131 | 24.7 | 23.0 | 30.3 | 32.4 | 0.209 |
| Depression | 20 | 3.8 | 5.0 | 3.6 | 5.1 | 0.799 |
| Arthritis | 29 | 5.5 | 5.0 | 7.2 | 4.3 | 0.538 |
| Osteoporosis | 31 | 5.8 | 5.8 | 9.6 | 2.9 | 0.050 |
| Current or ex-smoker | 53 | 10.0 | 12.4 | 12.0 | 8.7 | 0.572 |
| Physical measurements, mean (SD) | ||||||
| Weight (kg) | 408 | 80.6 (17.4) | 79.8 (15.8) | 79.8 (17.5) | 82.0 (19.4) | 0.445 |
| Pulse | 301 | 75.1 (16.2) | 75.6 (13.1) | 73.6 (13.4) | 75.7 (19.2) | 0.920 |
| Systolic BP (mmHg) | 495 | 137.7 (19.6) | 131.7 (16.6) | 138.8 (19.0) | 141.5 (21.7) | <0.001 |
| Diastolic BP (mmHg) | 495 | 77.5 (13.3) | 78.7 (13.7) | 77.6 (12.9) | 76.7 (12.3) | 0.221 |
| Laboratory parameters, mean (SD) | ||||||
| eGFR (mL/min/1.73m2) | 464 | 48.5 (27.9) | 82.7 (19.4) | 43.5 (8.3) | 19.9 (6.1) | <0.001 |
| Haemoglobin (g/dL) | 461 | 12.6 (1.9) | 13.5 (1.7) | 12.7 (1.8) | 11.6 (1.7) | <0.001 |
| Ferritin (ng/L) | 163 | 251.6 (334.6) | 244.7 (401.2) | 174.4 (195.0) | 311.7 (366.0) | 0.326 |
| TSAT ratio (%) | 132 | 26.6 (13.2) | 27.4 (11.4) | 29.6 (15.9) | 25.2 (12.6) | 0.443 |
| Folate (nmol/L) | 97 | 39.6 (142.3) | 63.2 (189.5) | 10.1 (6.3) | 27.9 (113.9) | 0.230 |
| Vitamin B12 (nmol/L) | 124 | 451.5 (271.1) | 418.9 (201.0) | 480.1 (404.1) | 471.4 (189.9) | 0.386 |
| Calcium (mmol/L) | 417 | 2.4 (0.2) | 2.4 (0.2) | 2.4 (0.1) | 2.3 (0.2) | <0.001 |
| Albumin (g/L) | 364 | 41.1 (5.6) | 42.4 (5.6) | 41.6 (5.0) | 39.3 (6.2) | <0.001 |
| Phosphate (mmol/L) | 388 | 1.1 (0.3) | 1.0 (0.2) | 1.1 (0.2) | 1.3 (0.3) | <0.001 |
| PTH (pg/mL) | 166 | 131.7 (128.6) | 70.4 (42.8) | 107.5 (126.8) | 157.4 (133.7) | 0.001 |
| Total cholesterol (mmol/L) | 199 | 4.6 (1.4) | 4.9 (1.4) | 4.6 (1.0) | 4.4 (1.6) | 0.053 |
| LDL cholesterol (mmol/L) | 126 | 2.6 (1.2) | 2.8 (1.3) | 2.4 (0.7) | 2.5 (1.5) | 0.293 |
| HDL cholesterol (mmol/L) | 128 | 1.4 (0.5) | 1.4 (0.5) | 1.5 (0.5) | 1.3 (0.4) | 0.591 |
| Triglycerides (mmol/L) | 196 | 3.9 (21.2) | 1.8 (1.4) | 4.4 (23.2) | 5.5 (28.2) | 0.376 |
| HbA1c (mmol/mol) | 89 | 48.4 (23.4) | 47.5 (15.1) | 43.4 (14.3) | 49.1 (27.0) | 0.904 |
| Urine tests | ||||||
| Protein:creatinine ratio | 139 | 139.3 (246.6) | 49.0 (71.3) | 157.5 (344.8) | 210.6 (223.6) | 0.002 |
| Albumin:creatinine ratio | 53 | 101.4 (211.3) | 22.7 (28.1) | 93.4 (239.2) | 205.9 (270.6) | 0.019 |
| Medications | ||||||
| ACE-I | 121 | 22.8 | 26.4 | 25.7 | 16.3 | 0.066 |
| ARB | 96 | 18.1 | 18.8 | 20.1 | 18.4 | 0.921 |
| ACE-I and ARB | 12 | 2.3 | 2.1 | 3.9 | 0.7 | 0.164 |
| Aspirin | 181 | 34.2 | 25.0 | 34.1 | 45.4 | 0.001 |
| Iron therapy (oral or i.v.) | 54 | 10.2 | 2.1 | 8.9 | 19.1 | <0.001 |
| ESA therapy | 25 | 4.7 | 0.7 | 3.4 | 12.1 | <0.001 |
PKD, polycystic kidneys; TIA, transient ischaemic attack; COPD, chronic obstructive pulmonary disease; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker. ESA, erythropoietin stimulating agent
eGFR (estimated glomerular filtration rate per CKD-EPI equation) was available for 464 patients .
Prevalence of anaemia
The average Hb concentration in the overall cohort was 12.6 ± 1.9 g/dL. According to the WHO definition, the overall prevalence of anaemia in the entire cohort was 49% and increased significantly from 28% in CKD Stages 1–2 to 76% in CKD Stages 4–5 (P < 0.001) (Figure 1). The prevalence of anaemia, irrespective of what definition was used, increased significantly with advancing stage of CKD (P < 0.001) and this pattern corresponded to a decrease in mean Hb levels from 13.5 g/dL in Stages 1–2 to 11.6 in Stages 4–5 (P < 0.001). Using Hb < 12.0 g/dL as the target threshold for defining anaemia, the prevalence of anaemia increased significantly from 21% in CKD Stages 1–2 to 63% in CKD Stages 4–5 (P < 0.001). The prevalence of anaemia according to the WHO criteria varied significantly across health care region, from 36% in the Northwest to 62% in the Southeast (P = 0.026), although there was no statistically significant difference in mean Hb levels across regions (12.3 g/dL in Southeast and 13.1 g/dL in Northwest (P = not significant) (Figure 2 and Table 2). Similarly, serum ferritin levels and TSAT concentrations did not vary significantly across regions, as shown in Table 2.
Fig. 1.
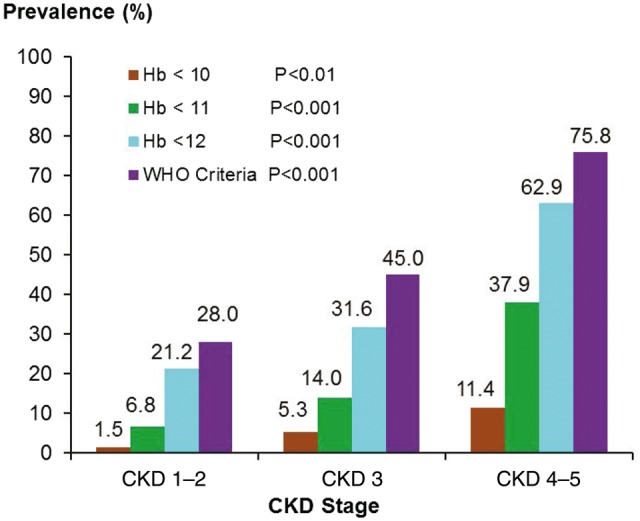
Prevalence of anaemia by CKD stage.
Fig. 2.
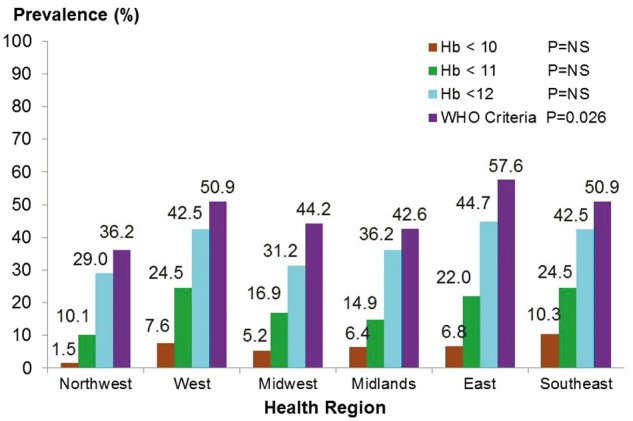
Anaemia prevalence across health care regions. NS = non-significant
Table 2.
Baseline characteristics of the study cohort by geographic region
| Overall | West | Mid-West | North-West | Midlands | East | South-East | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| n | (n = 530) | (n = 120) | (n = 103) | (n = 70) | (n = 52) | (n = 150) | (n = 35) | ||
| Demographic | |||||||||
| Age (years), mean (SD) | 510 | 57.1 (18.3) | 56.5 (19.6) | 57.2 (17.5) | 59.0 (15.6) | 63.9 (18.7) | 54.2 (18.8) | 55.4 (17.5) | 0.216 |
| Men (%) | 293 | 55.3 | 49.6 | 56.3 | 63.8 | 49.0 | 56.0 | 65.7 | 0.309 |
| Race (%) | |||||||||
| White Irish | 492 | 92.8 | 93.3 | 96.0 | 94.3 | 96.1 | 96.5 | 94.3 | 0.123 |
| White Irish traveller | 2 | 0.4 | 0.8 | 0.0 | 0.0 | 0.0 | 0.7 | 0 | 0.127 |
| White other | 12 | 2.3 | 4.2 | 1.0 | 5.7 | 3.9 | 0.0 | 0.0 | 0.125 |
| Asian | 2 | 0.4 | 0.8 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 | 0.130 |
| Other | 9 | 1.7 | 0.8 | 3.3 | 0.0 | 0.0 | 2.1 | 5.7 | 0.123 |
| Cause of CKD (%) | |||||||||
| Hypertension | 144 | 27.2 | 21.7 | 9.7 | 32.9 | 23.1 | 42.0 | 28.6 | <0.001 |
| Diabetes | 67 | 12.6 | 14.2 | 6.8 | 12.9 | 32.7 | 8.7 | 11.4 | 0.001 |
| Glomerulonephritis | 94 | 17.7 | 30.0 | 19.4 | 12.9 | 13.5 | 9.3 | 22.9 | 0.001 |
| Autosomal dominant PKD | 33 | 6.2 | 5.0 | 7.8 | 8.6 | 3.8 | 5.3 | 8.6 | 0.759 |
| Hereditary | 14 | 2.6 | 1.7 | 4.9 | 7.1 | 3.8 | 0.0 | 0.0 | 0.007 |
| Other | 172 | 32.5 | 23.3 | 27.2 | 48.6 | 42.3 | 33.3 | 28.6 | 0.006 |
| Not known | 71 | 13.4 | 14.2 | 30.1 | 7.1 | 13.5 | 6.7 | 2.9 | <0.001 |
| Kidney biopsy (%) | 79 | 14.9 | 17.5 | 25.2 | 7.1 | 13.5 | 10.7 | 11.4 | 0.011 |
| Comorbid conditions (%) | |||||||||
| Diabetes | 111 | 20.9 | 22.4 | 20.6 | 21.7 | 44.7 | 17.8 | 25.0 | 0.017 |
| Hypertension | 382 | 72.1 | 86.9 | 67.6 | 64.1 | 87.2 | 74.8 | 83.3 | 0.006 |
| Cancer | 34 | 6.4 | 1.9 | 3.9 | 8.7 | 21.3 | 7.4 | 8.3 | 0.002 |
| Heart failure | 22 | 4.2 | 4.7 | 6.9 | 8.8 | 0.0 | 0.0 | 16.7 | <0.001 |
| Thyroid disease | 49 | 9.2 | 10.3 | 8.8 | 8.7 | 21.3 | 6.7 | 16.7 | 0.107 |
| Stroke/TIA | 22 | 4.2 | 7.5 | 4.9 | 7.2 | 8.5 | 0.0 | 0.0 | 0.005 |
| COPD | 22 | 4.2 | 5.6 | 5.9 | 10.1 | 0.0 | 2.2 | 0.0 | 0.072 |
| Peripheral vascular disease | 38 | 7.2 | 3.7 | 11.8 | 8.7 | 10.6 | 5.2 | 16.7 | 0.078 |
| Coronary heart disease | 76 | 14.3 | 15.9 | 20.6 | 13.0 | 27.7 | 8.2 | 20.8 | 0.014 |
| Obesity | 29 | 5.5 | 0.0 | 5.9 | 18.8 | 8.5 | 3.7 | 4.2 | <0.001 |
| Gout | 59 | 11.1 | 16.8 | 19.6 | 4.3 | 17.0 | 5.2 | 13.0 | 0.001 |
| Hypercholesterolaemia | 131 | 24.7 | 38.1 | 14.7 | 42.9 | 42.6 | 16.3 | 17.4 | <0.001 |
| Depression | 20 | 3.8 | 6.5 | 3.9 | 1.4 | 6.4 | 3.7 | 0.0 | 0.550 |
| Arthritis | 29 | 5.5 | 4.7 | 3.9 | 15.9 | 17.0 | 0.7 | 0.0 | <0.001 |
| Osteoporosis | 31 | 5.8 | 3.7 | 7.8 | 18.8 | 10.6 | 0.7 | 0.0 | <0.001 |
| Current or ex-smoker | 53 | 10.0 | 0.0 | 19.6 | 14.5 | 21.3 | 9.6 | 0.0 | <0.001 |
| Physical measurements, mean (SD) | |||||||||
| Weight (kg) | 408 | 80.6 (17.4) | 79.5 (18.4) | 81.3 (16.0) | 80.7 (17.6) | 81.7 (19.0) | 80.2 (17.5) | 79.2 (20.0) | 0.641 |
| Pulse | 301 | 75.1 (16.2) | 71.5 (14.9) | 77.5 (17.3) | 76.5 (16.7) | 73.8 (15.5) | 76.1 (15.8) | 73.1 (14.0) | 0.743 |
| Systolic BP (mmHg) | 495 | 137.7 (19.6) | 136.4 (17.9) | 137.6 (20.1) | 139.5 (18.8) | 143.0 (23.3) | 135.0 (19.7) | 142.5 (17.2) | 0.331 |
| Diastolic BP (mmHg) | 495 | 77.5 (13.3) | 78.5 (11.3) | 77.0 (11.7) | 76.8 (11.6) | 75.3 (15.1) | 78.4 (16.4) | 77.0 (10.5) | 0.429 |
| Laboratory parameters, mean (SD) | |||||||||
| eGFRa (mL/min/1.73 m2) | 464 | 48.5 (27.9) | 43.9 (24.2) | 45.8 (29.3) | 46.7 (23.8) | 46.0 (28.8) | 52.1 (30.2) | 64.1 (28.5) | 0.118 |
| Haemoglobin (g/dL) | 461 | 12.6 (1.9) | 12.5 (2.1) | 12.7 (1.9) | 13.1 (1.7) | 12.6 (1.8) | 12.3 (1.8) | 12.3 (1.8) | 0.166 |
| Ferritin (ng/L) | 163 | 251.6 (334.6) | 272.0 (303.4) | 210.2 (223.5) | 375.5 (488.7) | 139.3 (192.0) | 179.4 (236.5) | 269.5 (187.4) | 0.730 |
| TSAT ratio (%) | 132 | 26.6 (13.2) | 23.3 (11.8) | 24.1 (7.7) | 31.1 (10.2) | 20.8 (20.3) | 27.8 (14.4) | 25.4 (18.4) | 0.327 |
| Folate (nmol/L) | 97 | 39.6 (142.3) | 72.8 (207.9) | 13.8 (8.5) | 8.8 (4.7) | 8.7 (5.1) | 44.1 (152.4) | 44.0 (NA) | 0.548 |
| Vitamin B12 (nmol/L) | 124 | 451.5 (271.1) | 445.4 (168.8) | 510 (244.1) | 603.8 (426.0) | 384.5 (154.4) | 376.2 (244.5) | 575.0 (NA) | 0.113 |
| Calcium (mmol/L) | 417 | 2.4 (0.2) | 2.3 (0.1) | 2.3 (0.2) | 2.3 (0.2) | 2.4 (0.1) | 2.4 (0.2) | 2.4 (0.2) | <0.001 |
| Albumin (g/L) | 364 | 41.1 (5.6) | 41.6 (7.1) | 36.4 (5.2) | 43.2 (4.2) | 43.5 (3.4) | 41.0 (3.3) | 41.1 (6.4) | <0.001 |
| Phosphate (mmol/L) | 388 | 1.1 (0.3) | 1.1 (0.3) | 1.3 (0.3) | 1.1 (0.3) | 1.1 (0.2) | 1.1 (0.3) | 1.2 (0.1) | <0.001 |
| PTH (pg/mL) | 166 | 131.7 (128.6) | 130.2 (95.3) | 207.8 (189.5) | 89.0 (50.4) | 108.3 (137.2) | 117.3 (106.2) | NaN (NA) | 0.004 |
| Total cholesterol (mmol/L) | 199 | 4.6 (1.4) | 4.7 (1.9) | 5.2 (1.5) | 4.3 (0.8) | 4.1 (0.9) | 4.6 (1.3) | 5.1 (0.9) | 0.110 |
| LDL cholesterol (mmol/L) | 126 | 2.6 (1.2) | 2.5 (1.6) | 2.8 (1.4) | 2.5 (0.7) | 2.3 (0.8) | 2.7 (1.3) | 2.8 (0.6) | 0.739 |
| HDL cholesterol (mmol/L) | 128 | 1.4 (0.5) | 1.4 (0.6) | 1.4 (0.6) | 1.4 (0.4) | 1.2 (0.3) | 1.0 (0.2) | 1.5 (0.6) | 0.066 |
| Triglycerides (mmol/L) | 196 | 3.9 (21.2) | 1.6 (0.8) | 1.5 (0.8) | 1.6 (0.8) | 2.1 (1.4) | 8.0 (35.1) | 1.7 (1.3) | 0.232 |
| HbA1c (mmol/mol) | 89 | 48.4 (23.4) | 46.5 (15.2) | 55.9 (25.8) | 32.0 (26.7) | 57.4 (19.1) | 53.1 (37.4) | 55.0 (14.2) | 0.770 |
| Urine tests | |||||||||
| Protein:creatinine ratio | 139 | 139.3 (246.6) | 302.6 (406.2) | 173.1 (171.7) | 67.2 (134.2) | NA (NA) | 120.8 (176.1) | 67.9 (28.8) | 0.566 |
| Albumin:creatinine ratio | 53 | 101.4 (211.3) | 170.6 (276.9) | 0.6 (NA) | 3.4 (3.0) | 51.4 (70.1) | 37.7 (47.7) | 37.4 (52.8) | 0.874 |
| Medications (%) | |||||||||
| ACE-I | 121 | 22.8 | 25.8 | 15.5 | 35.7 | 17.3 | 20.7 | 25.7 | 0.044 |
| ARB | 96 | 18.1 | 24.2 | 10.7 | 14.3 | 21.2 | 16.7 | 28.6 | 0.057 |
| ACE-I and ARB | 12 | 2.3 | 1.7 | 0.0 | 4.3 | 1.9 | 3.3 | 2.9 | 0.300 |
| Aspirin | 181 | 34.2 | 31.7 | 30.1 | 35.7 | 46.2 | 31.3 | 45.7 | 0.165 |
| Iron therapy (oral or i.v.) | 54 | 10.2 | 11.7 | 13.6 | 8.6 | 13.5 | 6.7 | 8.6 | 0.435 |
| ESA therapy | 25 | 4.7 | 5 | 2.9 | 5.7 | 7.7 | 4 | 5.7 | 0.749 |
PKD, polycystic kidneys; TIA, transient ischaemic attack; COPD, chronic obstructive pulmonary disease; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker, ESA, erythropoietin stimulating agent, NA = not available.
eGFR (estimated glomerular filtration rate per CKD-EPI equation) was available for 464 patients. NA = not available.
The univariate associations of anaemia defined as Hb <12.0 g/dL are shown in Supplementary Table S1. In the final multivariate model (Table 3), the presence of anaemia (Hb <12.0 g/dL) was associated with lower levels of kidney function [OR 1.11 (95% CI 1.04–1.18)] per 5 mL/min/1.73 m2 lower, female sex [OR 3.43 (95% CI 2.00–5.86)], younger age [OR 1.46 (95% CI 1.26–1.85) for age group 18–40 versus age 41–60 years (referent)], hyperphosphataemia [OR 1.22 (95% CI 1.09–1.37)] per 0.1 mmol/L higher and treatment with erythropoiesis-stimulating agent (ESA) or iron therapy [OR 5.82 (95% CI 1.95–17.36)]. The C-statistic for the model was 0.82.
Table 3.
Correlates of anaemia (Hb <12.0 g/dL) expressed as adjusted Odds Ratios (ORs)
| Parameter | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Age (18–40 versus 41–60 years) | 1.46 (1.26–1.85) | 0.0252 |
| Women (versus men) | 3.43 (2.01–5.86) | <0.0001 |
| Iron and/or ESA therapy (yes versus no) | 5.82 (1.95–17.36) | 0.0016 |
| Intervention for anaemia at clinic (yes versus no) | 5.52 (1.42–21.52) | 0.0138 |
| Phosphate (per 0.1 mmol/L higher) | 1.22 (1.09–1.37) | 0.0005 |
| eGFRa (per 5 mL/min lower) | 1.11 (1.04–1.18) | 0.0015 |
n = 327 in final model, C-statistic 0.818.
eGFR per CKD-EPI equation [14].
Prevalence of ID
The overall prevalence of ID among anaemic patients using WHO criteria was 30%, with the highest prevalence in patients with Stages 4–5 CKD (Table 4). Not surprisingly, the proportion of iron-deficient patients varied according to the Hb threshold; increasing from 5.2% with Hb <10 g/dL to 30.2% using WHO criteria. The mean serum ferritin and TSAT for the entire cohort were 251.6 ng/L (SD 334.6) and 26.6% (SD 13.2), respectively (Tables 1 and 2). The prevalence of ID varied across health regions, with the highest in the Midlands (50%) and the lowest in the Northwest (10%) (P = 0.01), and the pattern was similar using a lower Hb threshold of <12 g/dL (see Supplementary Table S2).
Table 4.
Prevalence [n (%)] of ID among anaemic patients by CKD stage
| CKD Stages 1–2 | CKD Stage 3 | CKD Stages 4–5 | Total | P-value | |
|---|---|---|---|---|---|
| na | (n = 41) | (n = 50) | (n = 81) | (n = 172) | |
| Hb < 10 g/dL | 1 (2.4) | 1 (2.0) | 7 (8.6) | 9 (5.2) | 0.245 |
| Hb < 11 g/dL | 5 (12.2) | 6 (12.0) | 21 (25.9) | 32 (18.6) | 0.073 |
| Hb < 12 g/dL | 8 (19.5) | 9 (18.0) | 27 (33.3) | 44 (25.6) | 0.097 |
| WHO definitionb | 10 (24.4) | 13 (26.0) | 29 (35.8) | 52 (30.2) | 0.344 |
| Totalc | 19 (46.3) | 23 (45.1) | 38 (45.2) | 80 (45.5) | 1.000 |
Number of patients with available Hb, iron studies and eGFR.
WHO criteria: Hb < 13 g/dL for men and < 12 g/dL for women.
Irrespective of anaemia status.
Screening for ID in CKD patients
Of 436 CKD patients with WHO anaemia, 113 (25.9%) were tested for ID (Table 5). The prevalence of ID screening was lowest in CKD Stages 1–2 patients at 12.8%, increasing to 50.8% in CKD Stages 4–5 patients (P < 0.001). The prevalence of ID screening did not differ across health regions.
Table 5.
Prevalence n (%) [n (%)] of ID testing among anaemic patients by CKD stage
| Total | CKD Stages 1–2 | CKD Stage 3 | CKD Stages 4–5 | P-value | |
|---|---|---|---|---|---|
| na (%) | (n = 436) | (n = 133) | (n = 171) | (n = 132) | |
| Hb < 10 g/dL | 19 (4.4) | 2 (1.5) | 6 (3.5) | 11 (8.3) | 0.023 |
| Hb < 11 g/dL | 59 (13.5) | 6 (4.5) | 13 (7.6) | 40 (30.3) | <0.001 |
| Hb < 12 g/dL | 95 (21.8) | 13 (9.8) | 22 (12.9) | 60 (45.5) | <0.001 |
| WHO definitionb | 113 (25.9) | 17 (12.8) | 29 (17.0) | 67 (50.8) | <0.001 |
| Totalc | 176 (37.9) | 41 (28.5) | 51 (28.5) | 84 (59.6) | <0.001 |
Number of patients with available Hb and eGFR.
WHO criteria: Hb <13 g/dL for men and <12 g/dL for women.
Irrespective of anaemia status; eGFR was available on a total of 464 patients.
Treatment patterns of anaemia and ID
Only 8.7% of clinic patients were receiving either iron therapy or ESA therapy and utilization rates were similar across health regions. ESA rates increased from 0.7% in CKD Stages 1–2 to 12.1% in CKD Stages 4–5 (P < 0.001) (Table 1). According to WHO-defined anaemia, 9.7% of patients were treated with ESA and utilization rates were higher at each lower Hb threshold: 12.4% for Hb <12 g/dL, 16.9% for Hb <11 g/dL and 21.4% for Hb <10 g/dL. The use of iron treatments [oral or intravenous (i.v.)] was equally low at 10.2% and increased from 2.1% in Stages 1–2 patients to 19.1% in Stages 4–5 patients (P < 0.001 (Table 1). Among iron-deficient patients, only 14.1% were receiving iron treatments (Supplementary Table S3). Among iron-replete patients (ferritin >100 ng/mL and/or TSAT >20%), only 11.1% were treated with iron therapy.
Response of nephrologists to survey questionnaire on anaemia management
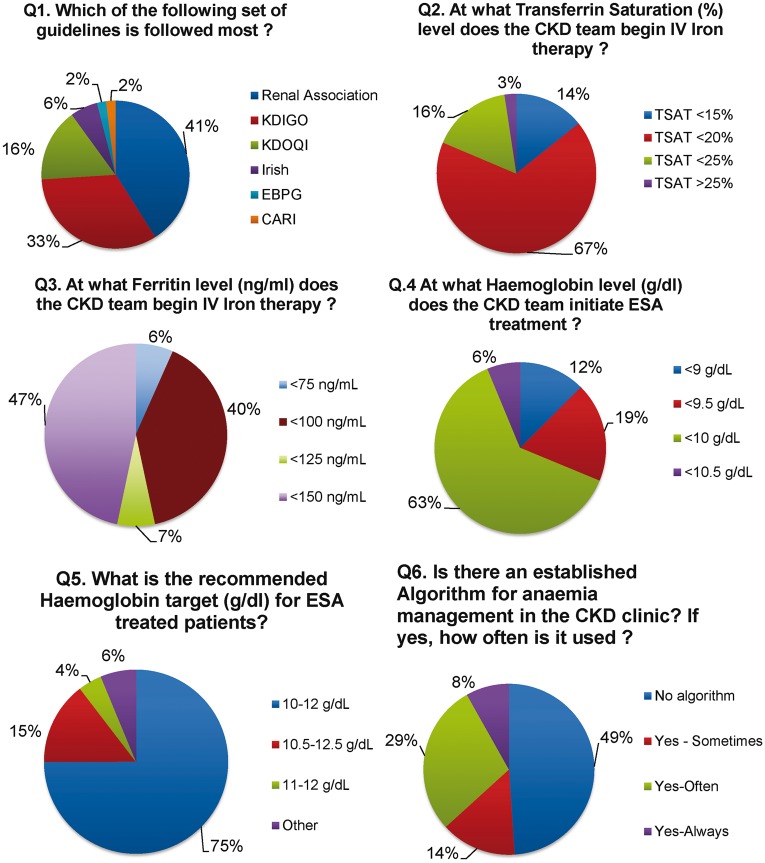
Forty-nine (83%) of 59 nephrologists responded to the survey questionnaire. There was substantial variation in the use of clinical guidelines across participating clinic sites, with the UK Renal Association guidelines being the most popular (41%) followed by the Kidney Disease: Improving Global Outcomes (KDIGO; 33%) and Kidney Disease Outcomes Quality Initiative (KDOQI; 17%) (P < 0.001) (Figure 3). With regard to ID correction, 67% and 47% of respondents identified a TSAT of <20% and a ferritin of <150 ng/mL, respectively, as target minimum thresholds for the initiation of i.v. iron therapy. The threshold haemoglobin for initiation of ESA therapy also varied, with 62.5% of nephrologists reporting a threshold limit of <10.0 g/dL, whereas 6, 19 and 13% of nephrologists reported alternative thresholds of 10.5, 9.5, and 9.0 g/dL, respectively. Most respondents (75%) identified Hb values of 10–12 g/dL as the preferred target range. Surprisingly, 47% of nephrologists reported an absence of CKD anaemia algorithms at outpatient clinics.
Fig. 3.
Opinions of Irish nephrologists on anaemia management practices from national survey questions 1-6. Renal Association, UK Renal Association Guidelines; KDIGO, Kidney Disease Improving Global Outcomes; KDOQI, Kidney Disease Outcomes Quality Initiative; Irish, Irish Chronic Kidney Disease Guidelines; CARI, Caring for Australians with Renal Impairment; EBPG, European Best Practice Guidelines.
Discussion
In this national audit of anaemia management among non-dialysis CKD patients, we observed unexpectedly high rates of anaemia and ID coupled with low screening rates and significant undertreatment at specialist nephrology clinics. The high rates of ID among clinic patients were in contrast with the relatively low utilization rates of either oral or i.v. iron therapy. Furthermore, differences in treatment patterns were observed across participating centres, suggesting differences in clinical practice. In support of this variability, our national survey found that nephrologists differed in their choice of clinical guidelines for CKD management, varied in their selection of Hb target thresholds for ESA and iron prescribing and a large proportion failed to adopt anaemia algorithms to guide clinical practice.
To the best of our knowledge, this is the first Irish study to explore practice patterns in the management of anaemia among non-dialysis CKD patients. It also sought to understand variation in real-world practice by exploring provider attitudes and behaviours through a focused national nephrologist survey. Despite tremendous advances in our understanding of CKD-associated anaemia, along with the introduction and implementation of evidence-based anaemia guidelines, we discovered high rates of anaemia and ID among patients with CKD. The prevalence of CKD-associated anaemia in our study was comparable to the reported rates from several international studies irrespective of the definition of anaemia [11–13]. While we estimated anaemia prevalence at 38.4% (using a Hb threshold <12 g/dL), a US study of 5222 adult CKD patients using the same definition reported an even higher prevalence of 47.7% [15]. Similarly, the MICENAS I study reported an even higher prevalence of 58.8% among 503 Spanish patients with Stages 3–5 CKD using the WHO criteria, and exceeded our estimate of 49.5% [13]. Using a lower Hb threshold of <11 g/dL, a study from Japan by Akizawa et al. [16] reported a prevalence of 33%, which again was substantially higher than our estimate of 19.3%. These findings would suggest that the prevalence of CKD-associated anaemia remains quite substantial despite the availability of iron and ESA replacement therapies. The disparity in prevalence estimates may be attributable to differences in the Hb thresholds used to define anaemia, differences in clinical practice relating to the use and implementation of clinical guidelines and differences in screening and treatment rates for ID.
A unique feature of our study was the inclusion of a detailed provider questionnaire that assessed the clinical strategies with regard to CKD anaemia management in the context of published clinical guidelines. The findings from this survey would suggest that variability in clinical practice patterns across sites may be due in part to differences in the adoption and interpretation of different sets of clinical guidelines [17–20]. From the survey, 41% of nephrologists reported use of the Renal Association Standards guidelines, whereas 33% reported adoption of KDIGO guidelines. It is noteworthy that these guidelines differ significantly with respect to the definition of anaemia [KDIGO adopts WHO criteria (Hb <13 g/dL for men and <12 g/dL for women)], whereas the UK Renal Association uses a lower Hb threshold of 11 g/dL] and consequently these different thresholds will influence the timing of and extent to which anaemia is screened for. We also noted considerable variability in threshold values of ID indicators that prompted initiation of i.v. iron therapy. About half the surveyed nephrologists would start i.v. iron therapy with ferritin <150 ng/mL, a threshold value that was higher than that used in our definition of ID (<100 ng/mL), and an even larger percentage (68%) would initiate i.v. iron for a TSAT <20%. Similarly for ESA initiation, the threshold values for Hb differed among nephrologists, with almost two-thirds of respondents choosing an Hb <10.0 g/dL as the threshold value for ESA initiation. Although clinical guidelines were established to improve clinical care and avoid potential harm, the inherent variations across different sets of guidelines for a specific disease may lead to greater variability in care patterns and delivery. These findings would suggest the need for greater harmonization of international clinical guidelines and clearer implementation strategies.
The combination of a multicentre audit coupled with a national nephrologist survey allowed us the opportunity to compare ‘what is being said to what is being done’ in clinical practice. This study uncovered a number of shortcomings with regard to screening practices and treatment strategies for anaemia management in CKD. For example, only 21% of those with Hb <12 g/dL had iron studies performed within the 6-month period prior to the captured clinic visit (Supplementary Table S3). Furthermore, although testing rates improved as anaemia became more severe, they remained unacceptably low, as only 68% (19 of 28) of patients with Hb <10.0 g/dL were tested. These low testing rates also extended to treatment practices. Only 8.7% of patients were on ESA or iron therapy. We also observed a high degree of inertia to anaemia therapy. Only a third of patients with Hb <10.0 g/dL were on iron (oral or i.v.) or ESA therapy. Even more striking was the fact that 85.9% of iron-deficient patients were not on iron therapy. These findings are not unique to the Irish health system. Minutolo et al. [12] reported that up to 75.7% of iron-deficient patients did not receive iron therapy and up to 36% of anaemic patients (Hb <9.5 g/dL) were not on ESA. In the MICENAS I study, only 53% of iron-deficient patients were treated with iron supplements [13]. These studies would suggest that treatment inertia is common in specialist CKD clinics. There are several reasons that may explain these trends in practice. First, residual uncertainty continues regarding the optimal timing of ESA initiation in non-dialysis CKD patients. Second, the evidence from randomized clinical trials in CKD that targeting Hb values in the normal range did not translate into clinical benefit but is in fact associated with increased risk of harm is equally important [21–24]. These factors may have dampened the level of enthusiasm for a more aggressive approach to anaemia management and prescribing of ESA agents. On the other hand, evidence has now emerged that repletion of iron stores can improve Hb levels, delay the need for ESA and improve quality of life [25–27].
Our study is not without limitations. The cross-sectional design of this study lacks a longitudinal dimension, therefore establishment of the chronology of trends of laboratory data as well as treatment interventions was not possible. A further limitation was the reliance on medical records for data extraction in a retrospective manner. Notwithstanding these limitations, this study included a detailed description of patient characteristics, including testing rates of ID and treatment interventions. Furthermore, the study was multicentre, with participation from large specialist clinics across Ireland, thus strengthening the generalizability. Moreover, the burden of anaemia was explored according to several clinically relevant and guideline-recommended Hb thresholds. Finally, and equally important, the inclusion of a national survey facilitated a better understanding of provider attitudes and allowed us to gain deeper insights into guideline interpretation and thereby shed light on potential areas of improvement.
Although anaemia is a well-recognized complication of CKD, our study found suboptimal management (both investigation and treatment) in a large proportion of CKD patients receiving specialist care. The lack of a single set of clinical guidelines coupled with low utilization rates of anaemia algorithms may be contributory factors. Low treatment rates did not correspond with the views of Irish nephrologists on treatment thresholds. This study demonstrates that there is substantial room for improvement. The adoption of electronic alert systems to improve the screening and recognition of patients at risk, the implementation of specific algorithms to manage CKD-associated anaemia at clinics and the greater utilization of anaemia nurse specialists to assist with coordination and implementation of evidence-based anaemia-based protocols are potential pathways to improve care delivery.
Supplementary Material
Acknowledgements
A.G.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and analysis. We thank all participating nephrologists from the Republic of Ireland and Northern Ireland. We are also grateful to the following individuals who assisted with data collection: Dr Liam Casserly, Dr Con Cronin, M.E., Dr Umair Sharif (University Hospital Limerick), Dr Sean Leavey, Dr Elizabeth Abernathy, Dr Louise Ryan (University Hospital Waterford), Dr Arif Mutwali, Dr Ann Marie Moran (University Hospital Letterkenny), Prof Peter Conlon, Dr Eoin Conlon, Dr Peter Conlon (Beaumont Hospital), D.N.R., Dr Dervla Connaughton (University Hospital Galway), Dr Eoin Bergin, Dr Katie Hanley, Dr Philip James, Dr Edward McMonagle, Dr Marie Connelly (Tullamore Regional Hospital).
Funding
A.G.S. was supported by grants from the Irish Heart Foundation, Midwest Kidney Disease Research and Education Foundation (MKid), Limerick and the Health Research Institute, University of Limerick. A.G.S., J.P.F., X.L. and M.E.E. were supported by grants from the Health Research Board of Ireland (HRA-2013-PHR-685 and HRA-2013-PHR-437). A.G.S. has consulted for Amgen and Vifor Pharma. D.N.R. has consulted for Amgen, Vifor Pharma and Akebia. The other authors have no relevant financial relationships to disclose.
Conflict of interest statement
None declared.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
References
- 1. Collins AJ, Foley RN, Chavers B. et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012; 59(Suppl 1): A7, e1-420 [DOI] [PubMed] [Google Scholar]
- 2. Cruz MC, Andrade C, Urrutia M. et al. Quality of life in patients with chronic kidney disease. Clinics 2011; 66: 991–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finkelstein FO, Story K, Firanek C. et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol 2009; 4: 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Voormolen N, Grootendorst DC, Urlings TA. et al. Prevalence of anemia and its impact on mortality and hospitalization rate in predialysis patients. Nephron Clin Pract 2010; 115: c133–c141 [DOI] [PubMed] [Google Scholar]
- 5. Hansen RA, Chin H, Blalock S. et al. Predialysis chronic kidney disease: evaluation of quality of life in clinic patients receiving comprehensive anemia care. Res Social Adm Pharm 2009; 5: 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stauffer ME, Fan T.. Prevalence of Anemia in Chronic Kidney Disease in the United States. PLoS One 2014; 9: e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abramson JL, Jurkovitz CT, Vaccarino V. et al. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC Study. Kidney Int 2003; 64: 610–615 [DOI] [PubMed] [Google Scholar]
- 8. Jurkovitz CT, Abramson JL, Vaccarino LV. et al. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community-based atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 2003; 14: 2919–2925 [DOI] [PubMed] [Google Scholar]
- 9. Levin A, Singer J, Thompson CR. et al. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis 1996; 27: 347–354 [DOI] [PubMed] [Google Scholar]
- 10. Collins AJ, Li S, St Peter W. et al. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol 2001; 12: 2465–2473 [DOI] [PubMed] [Google Scholar]
- 11. Mujais SK, Story K, Brouillette J. et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol 2009; 4: 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Minutolo R, Locatelli F, Gallieni M. et al. Anaemia management in non-dialysis chronic kidney disease (CKD) patients: a multicentre prospective study in renal clinics. Nephrol Dial Transplant 2013; 28: 3035–3045 [DOI] [PubMed] [Google Scholar]
- 13. Cases-Amenos A, Martinez-Castelao A, Fort-Ros J. et al. Prevalence of anaemia and its clinical management in patients with stages 3–5 chronic kidney disease not on dialysis in Catalonia: MICENAS I study. Nefrologia 2014; 34: 189–198 [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McClellan W, Aronoff SL, Bolton WK. et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin 2004; 20: 1501–1510 [DOI] [PubMed] [Google Scholar]
- 16. Akizawa T, Makino H, Matsuo S. et al. Management of anemia in chronic kidney disease patients: baseline findings from Chronic Kidney Disease Japan Cohort Study. Clin Exp Nephrol 2011; 15: 248–257 [DOI] [PubMed] [Google Scholar]
- 17. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 18. Kliger AS, Foley RN, Goldfarb DS. et al. KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am J Kidney Dis 2013; 62: 849–859 [DOI] [PubMed] [Google Scholar]
- 19. Locatelli F, Bárány P, Covic A. et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant 2013; 28: 1346–1359 [DOI] [PubMed] [Google Scholar]
- 20. Hsu CY, McCulloch CE, Curhan GC.. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 2002; 13: 504–510 [DOI] [PubMed] [Google Scholar]
- 21. Astor BC, Muntner P, Levin A. et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2002; 162: 1401–1408 [DOI] [PubMed] [Google Scholar]
- 22. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 23. Pfeffer MA, Burdmann EA, Chen CY. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 24. Drueke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 25. Macdougall IC, Bock AH, Carrera F. et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 2014; 29: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anker SD, Comin Colet J, Filippatos G. et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009. 17; 361: 2436–2448 [DOI] [PubMed] [Google Scholar]
- 27. Filippatos G, Farmakis D, Colet JC. et al. Intravenous ferric carboxymaltose in iron-deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR-HF trial. Eur J Heart Fail 2013; 15: 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.