Abstract
Background:
Passive leg raising (PLR) represents a “self-volume expansion (VE)” that could predict fluid responsiveness, but the influence of systolic cardiac function on PLR has seldom been reported. This study aimed to investigate whether systolic cardiac function, estimated by the global ejection fraction (GEF) from transpulmonary-thermodilution, could influence the diagnostic value of PLR.
Methods:
This prospective, observational study was carried out in the surgical Intensive Care Unit of the First Affiliated Hospital of Sun Yat-sen University from December 2013 to July 2015. Seventy-eight mechanically ventilated patients considered for VE were prospectively included and divided into a low-GEF (<20%) and a near-normal-GEF (≥20%) group. Within each group, baseline hemodynamics, after PLR and after VE (250 ml 5% albumin over 30 min), were recorded. PLR-induced hemodynamic changes (PLR-Δ) were calculated. Fluid responders were defined by a 15% increase of stroke volume (SV) after VE.
Results:
Twenty-five out of 38 patients were responders in the GEF <20% group, compared to 26 out of 40 patients in the GEF ≥20% group. The thresholds of PLR-ΔSV and PLR-Δ cardiac output (PLR-ΔCO) for predicting fluid responsiveness were higher in the GEF ≥20% group than in the GEF <20% group (ΔSV: 12% vs. 8%; ΔCO: 7% vs. 6%), with increased sensitivity (ΔSV: 92% vs. 92%; ΔCO: 81% vs. 80%) and specificity (ΔSV: 86% vs. 70%; ΔCO: 86% vs. 77%), respectively. PLR-Δ heart rate could predict fluid responsiveness in the GEF ≥20% group with a threshold value of −5% (sensitivity 65%, specificity 93%) but could not in the GEF <20% group. The pressure index changes were poor predictors.
Conclusions:
In the critically ill patients on mechanical ventilation, the diagnostic value of PLR for predicting fluid responsiveness depends on cardiac systolic function. Thus, cardiac systolic function must be considered when using PLR.
Trial Registration:
Chinese Clinical Trial Register, ChiCTR-OCH-13004027; http://www.chictr.org.cn/showproj.aspx?proj=5540.
Keywords: Fluid Responsiveness, Passive Leg Raising, Systolic Cardiac Function, Volume Expansion
INTRODUCTION
Hypovolemia is a very frequent clinical situation in the Intensive Care Unit (ICU), for which the rapid fluid infusion is applied as treatment. Therefore, it is essential to have reliable tools to predict the efficacy of volume expansion (VE) and ultimately distinguish patients who may benefit from VE from those who are unlikely to respond. Recently, many studies have focused on the prediction of fluid responsiveness. Static hemodynamic indices have been of little value in predicting fluid responsiveness.[1,2] In contrast, dynamic indices, based on analysis of preload dependence, have been validated as factors that can help predict fluid responsiveness.[1,3,4,5] Passive leg raising (PLR) is a reversible maneuver that mimics rapid VE by shifting venous blood from the lower limbs[6] toward the intrathoracic compartment.[7,8] Thus, PLR increases the cardiac preload and, by definition, increases the stroke volume (SV) if the heart is preload dependent.[9,10,11] Recent studies demonstrated that PLR-induced changes in SV (PLR-ΔSV) and cardiac output (PLR-ΔCO) are reliable predictive indices of fluid responsiveness.[12,13,14,15,16,17] The fluid responsiveness describes the change of SV or cardiac output which varies with the preload basing on a Frank–Starling curve. However, patients with different cardiac functions show different Frank–Starling curves, especially for the patients with normal or low cardiac function. Therefore, it is not clear whether this will affect the prediction of fluid responsiveness or not, and the predictive value of PLR in patients with normal or low cardiac function has not been clearly established.
The aim of this study was to test whether systolic cardiac function can influence the diagnostic value of PLR in predicting fluid responsiveness.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (No. 2013-12-03). Informed written consent was obtained from all patients prior to their enrollment in this study.
Patients
This prospective, observational study (Chinese Clinical Trial Register: ChiCTR-OCH-13004027) was carried out in the surgical ICU of the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) from December 2013 to July 2015. The ventilated patients with presumed hypovolemia who received fluid expansion at the discretion of the attending physician were consecutively included. This decision was based on the presence of at least one clinical sign of inadequate tissue perfusion and the absence of contraindications for fluid infusion. Clinical signs of inadequate tissue perfusion were defined as follows: clinical signs of acute circulatory failure (systolic arterial pressure [SAP] <90 mmHg [or a decrease of <40 mmHg in previously hypertensive patients]; urine output of <0.5 ml·kg−1·h−1 for at least 1 h; tachycardia [heart rate >100/min]; mottled skin), oliguria (diuresis below 20 ml/h or 0.5 ml·kg−1·h−1), acute kidney failure, and/or clinical and laboratory signs of extracellular dehydration.[16,17] Exclusion criteria included clinical signs of hemorrhage, inability to defer fluid challenge for several minutes, arrhythmia, moderate or severe valvular regurgitation, a contraindication to PLR, preterminal illness with a life expectancy of less than 24 h, or known anaphylactic reactions to albumin.
Study design
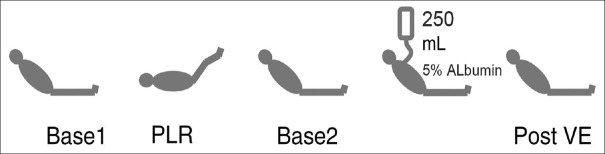
Figure 1 illustrates the design of the study. Hemodynamics (heart rate [HR], SAP, mean arterial pressure [MAP], pulse pressure [PP], CO, and SV) were recorded at each step of the protocol. Baseline 1 indicates that patients were in a semi-recumbent position, with the trunk elevated 30–45° relative to the lower limbs. PLR indicates that patients were in a supine position with the lower limbs elevated 30–45° relative to the trunk. Each hemodynamic measurement was recorded within the first 5 min. PLR-induced changes (PLR-Δ) are expressed in percentages as follows: 100 × (PLR value − baseline 1 value)/baseline 1 value. Baseline 2 indicates that the lower limbs and trunk were returned to baseline 1 position for at least 5 min. After hemodynamic measurements, VE was performed within 30 min by infusing 250 ml 5% albumin. Post-VE indicates that after VE, patients remained in the baseline 2 position. VE-induced changes (VE-Δ) are expressed in percentages as follows: 100 × (post-VE value − baseline 2 value)/baseline 2 value. Patients were considered responders to VE if VE-ΔSV increased by 15%. The ventilation parameters and vasoactive drugs were maintained during the study.
Figure 1.
Study design. Base: Baseline; PLR: Passive leg raising; VE: Volume expansion.
Measurements
The following characteristics were recorded: age, gender, Acute Physiology and Chronic Health Evaluation II score, Sequential Organ Failure Assessment score, the use of vasoactive, sedative, and analgesic drugs, indication for ICU stay, and medical history.
We used the PiCCO system (Pulsion Medical System, Munich, Germany)[18,19] for hemodynamic monitoring. This device allows continuous measurement of the arterial pressure (SAP, diastolic arterial pressure [DAP], and MAP) and HR. PP was calculated as the SAP minus the DAP. The system uses a transpulmonary-thermodilution method for measurements of volume status (global end-diastolic volume [GEDV]). Additionally, this system also can provide information on cardiac function, including global ejection fraction (GEF),[20,21] cardiac function index (CFI),[20,21,22] and left ventricular contractility index (dp/dt max). These indicators can accurately reflect systolic cardiac function. A central venous catheter was inserted into the internal jugular vein or the subclavian vein, and a PiCCO arterial catheter was inserted into the femoral artery. Then, a series of three 15-ml ice-cold saline boluses at a temperature of <8°C were injected into the central vein, and the associated dilution curves and various hemodynamic parameters were obtained.[18] All patients received volume-controlled mechanical ventilation with positive end-expiratory pressure. Drainage of blood was <50 ml/h in all patients, and no patient underwent repeated surgery for bleeding within 12 h postsurgery.
Statistical analysis
The patients to be analyzed were divided into low-GEF (<20%) and near-normal-GEF (≥20%) groups. This cutoff of 20% reflects an approximate cutoff of 40% of the ejection fraction of the left ventricle, corresponding to the low limit of normal.[20,21,23] The Kolmogorov–Smirnov test was used to check the normality of the data distribution. All continuous variables were normally distributed and expressed as mean ± standard deviation (SD). Intergroup comparisons of continuous and categorical variables were performed with Student's t-test and the Chi-square test, respectively.
In each group, patients were classified as responders and nonresponders. Absolute values at baseline and during PLR and VE were analyzed. Comparisons before and after PLR, before and after VE, and between baseline 1 and baseline 2 were performed using a paired-sample Student's t-test. The comparison between responder and nonresponder values was performed using an independent-sample Student's t-test. The receiver-operating characteristic (ROC) curves were compared using the Hanley–McNeil test.[24] Cutoff values for ΔSV, ΔCO, ΔPP, ΔSAP, ΔMAP, and ΔHR were chosen to correspond to the best respective Youden's index[25] calculated as follows: Youden's index = sensitivity + specificity − 1. Threshold indicator values such as sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were calculated for each hemodynamic indicator tested. P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 19.0 software (SPSS, Chicago, IL, USA) for all tests except the Hanley–McNeil test.
RESULTS
Patients' characteristics
A total of 78 ventilated patients with presumed hypovolemia and considered for VE were included in the study. Of these, 38 patients were assigned to the low-GEF group (<20%) and 40 patients were assigned to the near-normal-GEF group (≥20%). Table 1 summarizes the clinical characteristics, ventilation settings, and hemodynamics of the two patient groups. There were no significant differences between the two groups in terms of clinical characteristics. Cardiac function parameters were significantly higher in the near-normal-GEF group than in the low-GEF group. The ventilation settings, baseline volume status, and hemodynamics were similar between the two groups, with the exception of SV, CO, SAP, and PP, which were significantly higher in the near-normal-GEF group. Additionally, CVP was significantly lower in the near-normal-GEF group than in the low-GEF group.
Table 1.
Descriptive characteristics of the mechanically ventilated patients considered for volume expansion
| Characteristics | GEF <20% (n = 38) | GEF ≥20% (n = 40) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 64 ± 6 | 58 ± 14 | 2.019* | 0.051 |
| Male/female | 27/11 | 32/8 | 0.847† | 0.357 |
| APACHE II score | 17 ± 6 | 16 ± 6 | 0.818* | 0.418 |
| SOFA score | 8 ± 5 | 6 ± 5 | 1.432* | 0.159 |
| Sedation and analgesics use/not use | 29/9 | 26/14 | 1.200† | 0.273 |
| Vasoactive drug use/not use | 31/7 | 34/6 | 0.164† | 0.685 |
| Surgical/nonsurgical admission | 30/8 | 27/13 | 1.298† | 0.255 |
| Indication for ICU stay, n (%) | ||||
| Sepsis | 35 (92) | 34 (85) | 0.964† | 0.326 |
| Pancreatitis | 1 (3) | 1 (2.5) | 0.001† | 0.971 |
| Trauma | 0 (0) | 2 (5) | 1.950† | 0.163 |
| SIRS without infection | 2 (5) | 3 (7.5) | 0.163† | 0.687 |
| Medical history, n (%) | ||||
| Hypertension | 11 (29) | 6 (15) | 2.224† | 0.136 |
| Diabetes mellitus | 5 (13) | 4 (10) | 0.190† | 0.663 |
| COPD | 8 (21) | 6 (15) | 0.485† | 0.486 |
| Ventilation | ||||
| Tidal volume (ml/kg) | 7.8 ± 1.3 | 7.6 ± 1.9 | 0.383* | 0.525 |
| Plateau pressure (cmH2O) | 22.1 ± 3.3 | 20.8 ± 2.6 | 1.577* | 0.122 |
| PEEP (cmH2O) | 6.7 ± 2.6 | 6.0 ± 1.7 | 1.125* | 0.267 |
| Intra-abdominal pressure (cmH2O) | 12.2 ± 3.9 | 11.9 ± 3.4 | 0.288* | 0.774 |
| Cardiac function | ||||
| GEF (%) | 12 ± 2 | 26 ± 3 | −16.246* | 0.000 |
| dp/dt max (mmHg/s) | 946 ± 390 | 1346 ± 357 | −3.624* | 0.001 |
| CFI (L/min) | 3.6 ± 0.8 | 7.4 ± 0.4 | −19.363* | 0.000 |
| CPO (W) | 0.31 ± 0.13 | 0.48 ± 0.15 | −4.363* | 0.000 |
| Hemodynamics | ||||
| GEDV (ml) | 737 ± 190 | 695 ± 103 | 0.912* | 0.369 |
| CVP (mmHg) | 7 ± 1 | 5 ± 2 | 2.382* | 0.023 |
| Lac (mmol/L) | 3.5 ± 2.8 | 3.3 ± 2.4 | 0.271* | 0.788 |
| HR (beats/min) | 110 ± 11 | 110 ± 16 | 0.128* | 0.899 |
| SAP (mmHg) | 98 ± 10 | 117 ± 14 | −5.201* | 0.000 |
| DAP (mmHg) | 57 ± 8 | 53 ± 12 | 1.485* | 0.145 |
| MAP (mmHg) | 71 ± 8 | 74 ± 10 | −1.157* | 0.254 |
| PP (mmHg) | 41 ± 12 | 64 ± 16 | −5.654* | 0.000 |
| SV (ml) | 40 ± 15 | 61 ± 20 | −4.002* | 0.000 |
| CO (L/min) | 4.2 ± 1.4 | 6.6 ± 1.9 | −4.761* | 0.000 |
Values were shown as mean ± SD, n, or n (%). *t values; †χ2 values. GEF: Global ejection fraction; APACHE II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; ICU: Intensive Unit Care; SIRS: Systemic inflammatory response syndrome; COPD: Chronic obstructive pulmonary disease; CHD: Coronary heart disease; PEEP: Positive end-expiratory pressure; CFI: Cardiac function index; CPO: Cardiac power output; GEDV: Global end-diastolic volume; CVP: Central venous pressure; Lac: Lactic acid; HR: Heart rate; SAP: Systolic arterial pressure; DAP: Diastolic arterial pressure; MAP: Mean arterial pressure; PP: Radial pulse pressure; SV: Stroke volume; CO: Cardiac output; SD: Standard deviation.
Hemodynamic changes during passive leg raising and volume expansion
In the low-GEF (<20%) group, 25 patients were considered responders, with an increase in SV of 15% or more after VE. In the near-normal-GEF (≥20%) group, 26 patients were considered responders. The hemodynamic parameters at each step of the protocol (baseline 1, during PLR, baseline 2, and after VE) are shown in Table 2. Within each group, hemodynamic parameters were identical at baseline 1 and baseline 2. There were no significant differences between responders and nonresponders at baseline, with the exception of higher SAP/MAP values in nonresponders of the low-GEF group and higher CO/SV and lower PP in nonresponders of the near-normal-GEF group. After PLR and VE, the hemodynamic parameters showed significant changes in responders but not in nonresponders [Table 2]. Values of PLR-Δ are shown in Supplementary Table 1. All index changes in the near-normal-GEF group were significantly higher in responders than in nonresponders, except for PLR-ΔPP, while in the low-GEF group, only PLR-ΔSV and PLR-ΔCO were significantly higher in responders.
Table 2.
Hemodynamic parameters at each step of the protocol in responders and nonresponders in each group
| Parameters | GEF <20% (n = 38) | GEF ≥20% (n = 40) | ||
|---|---|---|---|---|
| Responders (n = 25) | Nonresponders (n = 13) | Responders (n = 26) | Nonresponders (n = 14) | |
| SV (ml) | ||||
| Base 1 | 38 ± 17 | 47 ± 10 | 51 ± 12* | 79 ± 21 |
| PLR | 43 ± 19† | 50 ± 10† | 60 ± 14*,† | 83 ± 20† |
| Base 2 | 38 ± 17 | 48 ± 11 | 51 ± 12* | 79 ± 20 |
| Post-VE | 46 ± 19‡ | 50 ± 10 | 63 ± 14*,‡ | 82 ± 20‡ |
| HR (beats/min) | ||||
| Base 1 | 112 ± 11 | 101 ± 7 | 115 ± 15 | 102 ± 16 |
| PLR | 111 ± 12* | 97 ± 9 | 111 ± 13† | 101 ± 13 |
| Base 2 | 114 ± 12* | 101 ± 6 | 115 ± 15* | 102 ± 15 |
| Post-VE | 109 ± 11* | 98 ± 6 | 105 ± 14‡ | 101 ± 15 |
| SAP (mmHg) | ||||
| Base 1 | 95 ± 10* | 106 ± 9 | 119 ± 13 | 111 ± 13 |
| PLR | 108 ± 9† | 113 ± 12 | 134 ± 23*,† | 112 ± 13 |
| Base 2 | 96 ± 9* | 107 ± 9 | 120 ± 13 | 111 ± 11 |
| Post-VE | 114 ± 9‡ | 111 ± 11 | 136 ± 20*,‡ | 121 ± 10‡ |
| MAP (mmHg) | ||||
| Base 1 | 69 ± 8* | 76 ± 6 | 73 ± 10 | 76 ± 9 |
| PLR | 79 ± 10 | 81 ± 9 | 86 ± 12† | 77 ± 8 |
| Base 2 | 69 ± 8 | 76 ± 6 | 73 ± 11 | 76 ± 7 |
| Post-VE | 83 ± 11 | 80 ± 7 | 88 ± 11‡ | 84 ± 9‡ |
| PP (mmHg) | ||||
| Base 1 | 39 ± 13 | 45 ± 10 | 68 ± 15* | 54 ± 15 |
| PLR | 42 ± 12 | 48 ± 11 | 73 ± 23† | 55 ± 16 |
| Base 2 | 39 ± 11 | 45 ± 10 | 70 ± 14* | 54 ± 15 |
| Post-VE | 47 ± 11‡ | 47 ± 11 | 74 ± 20*,‡ | 57 ± 14 |
| CO (L/min) | ||||
| Base 1 | 4.1 ± 1.6 | 4.7 ± 0.8 | 5.9 ± 1.5* | 7.9 ± 2.1 |
| PLR | 4.5 ± 1.8† | 4.8 ± 0.8 | 6.6 ± 1.6*,† | 8.3 ± 2.0† |
| Base 2 | 4.1 ± 1.6 | 4.8 ± 1.0 | 5.9 ± 1.5* | 8.0 ± 2.1 |
| Post-VE | 4.8 ± 1.8‡ | 4.8 ± 0.9 | 6.6 ± 1.5*,‡ | 8.3 ± 2.0‡ |
Values were shown as mean ± SD. *P<0.05 versus nonresponders; †P<0.05 versus baseline 1; ‡P<0.05 versus baseline 2. GEF: Global ejection fraction; CO: Cardiac output; HR: Heart rate; SAP: Systolic arterial pressure; MAP: Mean arterial pressure; PP: Radial pulse pressure; SV: Stroke volume; PLR: Passive leg raising; VE: Volume expansion; SD: Standard deviation.
Supplementary Table 1.
Hemodynamic changes induced by PLR and VE in responders and nonresponders in each group
| Items | GEF <20% (n = 38) | GEF ≥20% (n = 40) | ||||||
|---|---|---|---|---|---|---|---|---|
| Responders (n = 25) | Nonresponders (n = 13) | t | P | Responders (n = 26) | Nonresponders (n = 14) | t | P | |
| PLR-ΔSV | 14.7 ± 5.7% | 6.4 ± 5.3% | 4.304 | 0.000 | 18.2 ± 5.8% | 7.2 ± 5.9% | 5.651 | 0.000 |
| PLR-ΔCO | 11.2 ± 7.5% | 3.4 ± 2.3% | 3.454 | 0.001 | 12.3 ± 6.7% | 3.9 ± 3.4% | 4.359 | 0.000 |
| PLR-ΔHR | −0.9 ± 3.7% | −1.2 ± 4.9% | 0.219 | 0.828 | −6.0 ± 4.2% | −1.6 ± 3.0% | −3.341 | 0.002 |
| PLR-ΔSAP | 11.2 ± 7.8% | 7.9 ± 6.5% | 1.308 | 0.199 | 11.6 ± 8.8% | 5.0 ± 2.7% | 2.737 | 0.009 |
| PLR-ΔMAP | 10.5 ± 7.7% | 6.3 ± 2.9% | 1.848 | 0.073 | 13.9 ± 11.3% | 5.2 ± 3.1% | 2.801 | 0.008 |
| PLR-ΔPP | 10.4 ± 8.1% | 5.9 ± 4.8% | 0.744 | 0.454 | 8.0 ± 6.8% | 4.3 ± 3.4% | 0.863 | 0.073 |
| VE-ΔSV | 20.8 ± 5.5% | 5.0 ± 3.7% | 8.347 | 0.000 | 22.5 ± 5.4% | 4.8 ± 4.1% | 11.558 | 0.000 |
GEF: Global ejection fraction; PLR: Passive leg raising; PLR-ΔSV: PLR-induced change in stroke volume; PLR-ΔCO: PLR-induced change in cardiac output; PLR-ΔHR: PLR-induced change in heart rate; PLR-ΔSAP: PLR-induced change in systolic arterial pressure; PLR-ΔMAP: PLR-induced change in mean arterial pressure; PLR-ΔPP: PLR-induced change in pulse pressure; VE: Volume expansion; VE-ΔSV: VE-induced change in stroke volume.
Correlations and receiver-operating characteristic curves
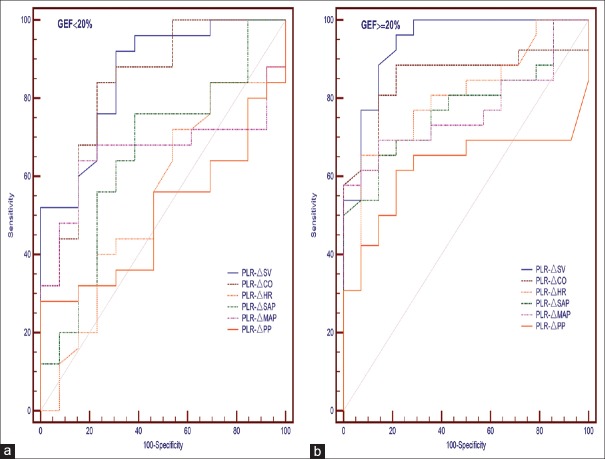
The correlation between PLR-Δ and VE-ΔSV is shown in Table 3. Regardless of GEF, PLR-ΔSV and PLR-ΔCO were positively correlated with VE-ΔSV. In addition, PLR-ΔHR was negatively correlated with VE-ΔSV in the near-normal-GEF (≥20%) group. None of the other variables were correlated with VE-ΔSV in either group. The areas under the ROC curves (AUC) for all index changes in the near-normal-GEF group were greater than the corresponding changes in the low-GEF group. The highest AUC values were for PLR-ΔSV (0.860 ± 0.059) and PLR-ΔCO (0.840 ± 0.063) in the low-GEF group and for PLR-ΔSV (0.942 ± 0.038) and PLR-ΔCO (0.859 ± 0.063) in the near-normal-GEF group [Table 3 and Figure 2].
Table 3.
Diagnostic accuracy of index changes induced by PLR for predicting fluid responsiveness
| Items | GEF <20% (n = 38) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | AUC | P | Threshold (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||||
| ΔSV | 0.539 | 0.000 | 0.860 ± 0.059 | 0.000 | 8 | 92 | 70 | 85 | 82 | |||
| ΔCO | 0.494 | 0.002 | 0.840 ± 0.063 | 0.001 | 6 | 80 | 77 | 87 | 67 | |||
| ΔHR | −0.096 | 0.568 | 0.457 ± 0.100 | 0.667 | −1 | 52 | 54 | 70 | 39 | |||
| ΔSAP | 0.217 | 0.192 | 0.628 ± 0.091 | 0.113 | 6 | 76 | 62 | 79 | 57 | |||
| ΔMAP | 0.273 | 0.098 | 0.662 ± 0.091 | 0.106 | 7 | 64 | 77 | 84 | 53 | |||
| ΔPP | 0.205 | 0.216 | 0.502 ± 0.100 | 0.998 | 6 | 56 | 54 | 70 | 39 | |||
| Items | GEF ≥20% (n = 40) | |||||||||||
| r | P | AUC | P | Threshold (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||||
| ΔSV | 0.698 | 0.000 | 0.942 ± 0.038 | 0.000 | 12 | 92 | 86 | 92 | 84 | |||
| ΔCO | 0.712 | 0.000 | 0.859 ± 0.063 | 0.000 | 7 | 81 | 86 | 91 | 71 | |||
| ΔHR | −0.533 | 0.000 | 0.799 ± 0.071 | 0.002 | −5 | 65 | 93 | 94 | 60 | |||
| ΔSAP | 0.196 | 0.225 | 0.776 ± 0.073 | 0.004 | 5 | 69 | 78 | 86 | 58 | |||
| ΔMAP | 0.249 | 0.121 | 0.769 ± 0.075 | 0.005 | 7 | 69 | 72 | 82 | 57 | |||
| ΔPP | 0.220 | 0.212 | 0.624 ± 0.090 | 0.202 | 5 | 62 | 79 | 84 | 52 | |||
GEF: Global ejection fraction; PLR: Passive leg raising; PLR-ΔSV: PLR-induced change in stroke volume; PLR-ΔCO: PLR-induced change in cardiac output; PLR-ΔHR: PLR-induced change in heart rate; PLR-ΔSAP: PLR-induced change in systolic arterial pressure; PLR-ΔMAP: PLR-induced change in mean arterial pressure; PLR-ΔPP: PLR-induced change in pulse pressure; PPV: Positive predictive value; NPV: Negative predictive value; r: Correlation coefficient between PLR-Δ and VE-ΔSV; AUC: Area under the receiver operation characteristics curve.
Figure 2.
ROC curves comparing the ability of PLR-induced changes to discriminate responders from nonresponders regarding volume expansion in the GEF <20% group (a) and GEF ≥20% group (b). PLR: Passive leg raising; ROC: Receiver-operating characteristic; GEF: Global ejection fraction.
Diagnostic performance of fluid responsiveness
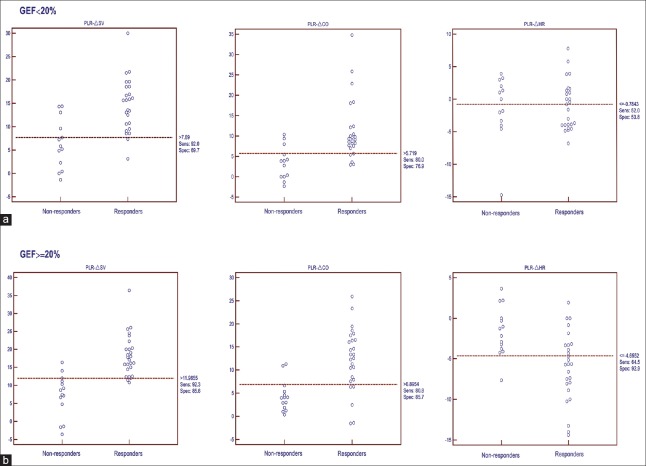
In practical terms, the optimum threshold values and associated sensitivities and specificities for distinguishing fluid responders from nonresponders are presented in Table 3. In total, the index changes in the GEF ≥20% group showed better predicting ability than those in the GEF <20% group. The thresholds of PLR-ΔSV and PLR-ΔCO for predicting fluid responsiveness were higher in the GEF ≥20% group than in the GEF <20% group (ΔSV: 12% vs. 8%; ΔCO: 7% vs. 6%), with increased sensitivity (ΔSV: 92% vs. 92%; ΔCO: 81% vs. 80%) and specificity (ΔSV: 86% vs. 70%; ΔCO: 86% vs. 77%), respectively. Regarding PLR-ΔHR, this value could predict fluid responsiveness in the GEF ≥20% group, with a threshold value of −5% (sensitivity 65%, specificity 93%), but could not do so in the GEF <20% group. The other pressure index changes, ΔSAP, ΔMAP, and ΔPP, showed poor ability to predict fluid responsiveness [Table 3 and Figure 3].
Figure 3.
PLR-induced changes in stroke volume (ΔSV), cardiac output (ΔCO) and heart rate (ΔHR) in responders and nonresponders in the GEF <20% group (a, n=38) and the GEF ≥20% group (b, n=40). PLR: Passive leg raising; GEF: Global ejection fraction.
DISCUSSION
Our study suggests that the diagnostic value of PLR depends on the systolic cardiac function in ventilated patients in the ICU. PLR-ΔSV and PLR-ΔCO enabled accurate bedside prediction of fluid responsiveness regardless of whether cardiac function of the patients is normal (GEF ≥20%) or lower (GEF <20%), but the threshold, sensitivity, and specificity were lower in the GEF <20% group. In addition, PLR-ΔHR could predict fluid responsiveness in the GEF ≥20% group but not in the GEF <20% group, while pressure index changes were poorly able to predict fluid responsiveness.
Hypovolemia is a very frequent clinical situation in the ICU and is primarily treated with VE. Unfortunately, only 40–70% of critically ill patients with acute circulatory failure display a significant increase in SV or CO in response to VE.[1] In patients with septic shock, fluid infusion is usually recommended[26] but may be harmful particularly in patients with acute respiratory distress syndrome.[27,28] PLR as a simple, economic, noninvasive, and reversible self-VE can help ICU staff avoid fluid infusion in patients who could be harmed by fluid overload.[29,30,31] Recently, Monnet et al.[32] performed a systematic review and meta-analysis including 21 studies and concluded that PLR very reliably predicted volume responsiveness. However, none of these studies evaluated the systolic cardiac function of patients, so it remained unknown whether systolic cardiac function could influence the diagnostic accuracy of PLR. Therefore, we designed this study to explore the accuracy of PLR for detecting fluid responsiveness in patients with low or normal cardiac function.
Echocardiography is the gold standard for left ventricular ejection fraction (LVEF) estimation. However, the measurement of LVEF in ICU patients is commonly performed through bedside transthoracic echocardiography (TTE), and the quality of TTE is influenced by many factors such as mechanical ventilation. Thus, by providing cardiac function indices, the PiCCO system could provide an interesting alternative to echocardiography in the assessment of LVEF. Four previous studies validated GEF as an indicator of LVEF in critically ill ICU patients. Combes et al.[20] demonstrated in thirty patients that GEF was correlated with LVEF assessed by transesophageal echocardiography (r = 0.82; P < 0.0001). A similar correlation was described in 2009 by Jabot et al.[21] from F/LVEF measurements (r = 0.67; P < 0.0001) involving 39 patients where LVEF was obtained by TTE. Meybohm et al.[33] and Perny et al.[34] also suggested that GEF was significantly correlated with LVEF. Trof et al.[23] studied the effect of systolic cardiac function on cardiac filling volumes versus pressures for predicting fluid responsiveness by dividing patients into a low-GEF group (<20%) and a near-normal-GEF group (≥20%). However, the authors studied the effect of systolic function on static hemodynamic indices but not on dynamic indices. In our study, we also divided patients into a low-GEF group (<20%) and a near-normal-GEF group (≥20%). In fact, the GEF value was 12 ± 2% in the GEF <20% group, which was significantly lower than that (26 ± 3%) in the GEF ≥20% group. The other indices of SV, CO, CFI, CPO, and dp/dt max were also significantly lower in the GEF <20% group, which demonstrated the significant difference in systolic cardiac function between the two groups.
In our study, the threshold value and diagnostic accuracy of PLR to predict fluid responsiveness were dependent on systolic cardiac function. Both PLR-ΔSV and PLR-ΔCO enabled accurate bedside prediction of fluid responsiveness regardless of cardiac function, but the threshold, sensitivity, specificity, and AUC were all higher in the near-normal-GEF group (≥20%) than in the low-GEF group (<20%). According to the Frank–Starling curve, two zones can be distinguished: (a) a slope where minimum preload changes give rise to a marked increase in SV (preload dependency zone) and (b) a flat or level segment where the SV hardly varies with changes in preload (preload-independent zone). Therefore, the relationship between the changes in SV depends on the baseline of preload and morphology or the gradient of the curve, which are determined by the contractile capacity of the heart. Thus, different patients show different Frank–Starling curves because of varied cardiac function, indicating different responses to the same preload increase. In our study, the baseline GEDV was 737 ± 190 ml in GEF <20% group versus 695 ± 103 ml in GEF ≥20% group which means that there were no significant differences between the two groups (P = 0.369) in terms of baseline of preload. The patients were divided into a near-normal-GEF group and a low-GEF group, showing different Frank–Starling curves between the two groups. Our results also demonstrated that the threshold in the near-normal-GEF group was higher (ΔSV: 12% vs. 8%; ΔCO: 7% vs. 6%) than that in the low-GEF group. Interestingly, the threshold values of PLR-ΔCO were less than those of PLR-ΔSV regardless of cardiac function, which brought our attention to PLR-ΔHR. We found that PLR-ΔHR in the near-normal-GEF group and the low-GEF group were different. The decrease in HR (−6.0 ± 4.2%; P = 0.002) was statistically significant in responders but not in nonresponders of the GEF ≥20% group, and HR was not found to be altered in responders or nonresponders of the GEF <20% group. In addition, in the GEF ≥20% group, PLR-ΔHR was negatively correlated with VE-ΔSV (r = −0.533, P = 0.000). The threshold value of PLR-ΔHR for predicting fluid responsiveness was −5% using ROC analysis with acceptable sensitivity (65%) and high specificity (93%). However, in the GEF <20% group, PLR-ΔHR could not predict fluid responsiveness. This finding may be because (a) the parasympathetic component of baroreceptor regulation of HR was impaired in patients with cardiac dysfunction[35] or (b) the overstimulation of sympathetic nerves was inconsistent with the inhibition of the vagal nerve in patients with left ventricle dysfunction, which leads to uncoordinated control of autonomic nervous system and sinoatrial node, resulting in a disorder of HR regulation.[36] Préau et al.[17] also concluded that PLR-ΔHR was statistically significant (−2.0 ± 4.0%; P < 0.05) in responders, but they included few patients with low LVEF and did not distinguish between groups of patients. These authors stated that the observed decrease in HR was very small and thus had no impact. The use of pressure index changes, such as PLR-ΔPP, ΔSAP, and ΔMAP, as preload responsiveness markers is based on the hypothesis that they depend on SV. During each systole, the left ventricle ejects a variable amount of blood through the systemic arterial circulation generating PP, SAP, and MAP waves along the arterial tree. However, all these pressure indices are influenced by complex properties of the systemic arterial tree, such as arterial compliance, wave propagation, and wave reflection. Monnet et al.[32] concluded that, when PLR effects are assessed by changes in PP, the specificity of the PLR test remains acceptable but its sensitivity is poor; Préau et al.[17] also found that the accuracy of ΔSAP and ΔMAP for predicting fluid responsiveness is lower. In our study, all these pressure indices were poorly able to predict fluid responsiveness, which is consistent with previous studies.
Our study has some limitations. First, we used GEF <20% monitored by PiCCO to distinguish patients with cardiac dysfunction. The accuracy of GEF is lower than LVEF measurements by TTE or TEE, especially in patients with valvular regurgitation. However, previous studies have demonstrated a significant correlation between GEF and LVEF. Second, we defined fluid responsiveness as an increase in SV ≥15% with VE; this cutoff value was chosen in reference to previous studies.[32] Although this cutoff seems clinically relevant, the predictive value of PLR may be altered if another cutoff value were chosen. In addition, the VE for definition of fluid responsiveness might be influenced by cardiac function. For patients with cardiac dysfunction, the influence would never be eliminated only if the VE was performed when the cardiac function has been improved to the normal level. However, it is very difficult to titrate cardiac function with inotropic agents. Third, nearly one-third of patients were not given analgesic and sedative drugs during PLR, which may cause vital signs to fluctuate due to sympathetic arousal. However, PLR is a less sympathetic stimulation for these patients who were more compatible and tolerated endotracheal intubation. In addition, there was no statistically significant difference in the proportion of patients without analgesic and sedative drugs between the two groups, which further reduced the possibility of experimental and result errors caused by sympathetic arousal. Finally, the study population was small but similar to previous PLR studies, so a large-scale study must be conducted to confirm these findings.
In conclusion, our study demonstrated that the diagnostic value of PLR depends on cardiac systolic function in ventilated patients in the ICU. PLR-ΔSV and PLR-ΔCO enabled accurate bedside prediction of volume responsiveness regardless of whether cardiac function of the patients is normal or lower, but the threshold, sensitivity, and specificity were lower in the GEF <20% group. In addition, PLR-ΔHR could predict fluid responsiveness in the GEF ≥20% group but not in the GEF <20% group, while pressure index changes were poorly able to predict fluid responsiveness. These findings suggest that, when using PLR for predicting fluid responsiveness, cardiac systolic function must be considered a factor influencing the diagnostic accuracy of PLR.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This work was supported by the grants from the Fundamental Research Funds for the Central Universities (No. 15ykpy14) and Sun Yat-sen University Clinical Research 5010 Program (No. 2007015).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: A critical analysis of the evidence. Chest. 2002;121:2000–8. doi: 10.1378/chest.121.6.2000. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 2.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–8. doi: 10.1097/01.CCM.0000249851.94101.4F. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 3.Slama M, Masson H, Teboul JL, Arnout ML, Susic D, Frohlich E, et al. Respiratory variations of aortic VTI: A new index of hypovolemia and fluid responsiveness. Am J Physiol Heart Circ Physiol. 2002;283:H1729–33. doi: 10.1152/ajpheart.00308.2002. doi: 10.1152/ajpheart.00308.2002. [DOI] [PubMed] [Google Scholar]
- 4.Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL, et al. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119:867–73. doi: 10.1378/chest.119.3.867. doi: 10.1378/chest.119.3.867. [DOI] [PubMed] [Google Scholar]
- 5.Monnet X, Bleibtreu A, Ferré A, Dres M, Gharbi R, Richard C, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012;40:152–7. doi: 10.1097/CCM.0b013e31822f08d7. doi: 10.1097/CCM.0b013e31822f08d7. [DOI] [PubMed] [Google Scholar]
- 6.Rutlen DL, Wackers FJ, Zaret BL. Radionuclide assessment of peripheral intravascular capacity: A technique to measure intravascular volume changes in the capacitance circulation in man. Circulation. 1981;64:146–52. doi: 10.1161/01.cir.64.1.146. doi: 10.1161/01.CIR.64.1.146. [DOI] [PubMed] [Google Scholar]
- 7.Schrijen FV, Henriquez A, Candina R, Polu JM. Pulmonary blood volume and haemodynamic changes with legs raised in chronic lung disease patients. Cardiovasc Res. 1991;25:895–900. doi: 10.1093/cvr/25.11.895. doi: 10.1093/cvr/25.11.895. [DOI] [PubMed] [Google Scholar]
- 8.Hofer CK, Zalunardo MP, Klaghofer R, Spahr T, Pasch T, Zollinger A, et al. Changes in intrathoracic blood volume associated with pneumoperitoneum and positioning. Acta Anaesthesiol Scand. 2002;46:303–8. doi: 10.1034/j.1399-6576.2002.t01-1-460313.x. doi: 10.1034/j.1399-6576.2002.t01-1-460313.x. [DOI] [PubMed] [Google Scholar]
- 9.Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol. 1914;48:357–79. doi: 10.1113/jphysiol.1914.sp001669. doi: 10.1113/jphysiol.1914.sp001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, et al. Linearity of the frank-starling relationship in the intact heart: The concept of preload recruitable stroke work. Circulation. 1985;71:994–1009. doi: 10.1161/01.cir.71.5.994. doi: 10.1161/01.CIR.71.5.994. [DOI] [PubMed] [Google Scholar]
- 11.He HW, Liu DW. Passive leg raising in intensive care medicine. Chin Med J (Engl) 2016;129:1755–8. doi: 10.4103/0366-6999.185866. doi: 10.4103/0366-6999.185866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G, et al. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest. 2002;121:1245–52. doi: 10.1378/chest.121.4.1245. doi: 10.1378/chest.121.4.1245. [DOI] [PubMed] [Google Scholar]
- 13.Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL, et al. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–32. doi: 10.1007/s00134-007-0646-7. doi: 10.1007/s00134-007-0646-7. [DOI] [PubMed] [Google Scholar]
- 14.Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M, et al. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med. 2007;33:1133–8. doi: 10.1007/s00134-007-0642-y. doi: 10.1007/s00134-007-0642-y. [DOI] [PubMed] [Google Scholar]
- 15.Caille V, Jabot J, Belliard G, Charron C, Jardin F, Vieillard-Baron A, et al. Hemodynamic effects of passive leg raising: An echocardiographic study in patients with shock. Intensive Care Med. 2008;34:1239–45. doi: 10.1007/s00134-008-1067-y. doi: 10.1007/s00134-008-1067-y. [DOI] [PubMed] [Google Scholar]
- 16.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–7. doi: 10.1097/01.CCM.0000215453.11735.06. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 17.Préau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38:819–25. doi: 10.1097/CCM.0b013e3181c8fe7a. doi: 10.1097/CCM.0b013e3181c8fe7a. [DOI] [PubMed] [Google Scholar]
- 18.Godje O, Peyerl M, Seebauer T, Dewald O, Reichart B. Reproducibility of double indicator dilution measurements of intrathoracic blood volume compartments, extravascular lung water, and liver function. Chest. 1998;113:1070–7. doi: 10.1378/chest.113.4.1070. doi: 10.1378/chest.113.4.1070. [DOI] [PubMed] [Google Scholar]
- 19.Gödje O, Peyerl M, Seebauer T, Lamm P, Mair H, Reichart B, et al. Central venous pressure, pulmonary capillary wedge pressure and intrathoracic blood volumes as preload indicators in cardiac surgery patients. Eur J Cardiothorac Surg. 1998;13:533–9. doi: 10.1016/s1010-7940(98)00063-3. doi: 10.1016/S1010-7940(98)00063-3. [DOI] [PubMed] [Google Scholar]
- 20.Combes A, Berneau JB, Luyt CE, Trouillet JL. Estimation of left ventricular systolic function by single transpulmonary thermodilution. Intensive Care Med. 2004;30:1377–83. doi: 10.1007/s00134-004-2289-2. doi: 10.1007/s00134-004-2289-2. [DOI] [PubMed] [Google Scholar]
- 21.Jabot J, Monnet X, Bouchra L, Chemla D, Richard C, Teboul JL, et al. Cardiac function index provided by transpulmonary thermodilution behaves as an indicator of left ventricular systolic function. Crit Care Med. 2009;37:2913–8. doi: 10.1097/ccm.0b013e3181b01fd9. doi: 10.1097/CCM.0b013e3181b01fd9. [DOI] [PubMed] [Google Scholar]
- 22.Ritter S, Rudiger A, Maggiorini M. Transpulmonary thermodilution-derived cardiac function index identifies cardiac dysfunction in acute heart failure and septic patients: An observational study. Crit Care. 2009;13:R133. doi: 10.1186/cc7994. doi: 10.1186/cc7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trof RJ, Danad I, Reilingh MW, Breukers RM, Groeneveld AB. Cardiac filling volumes versus pressures for predicting fluid responsiveness after cardiovascular surgery: The role of systolic cardiac function. Crit Care. 2011;15:R73. doi: 10.1186/cc10062. doi: 10.1186/cc10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 25.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. doi: 10.1002/1097-0142(1950)3::1%3C32:AID-CNCR2820030106%3E3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock: A retrospective pilot study. Chest. 2000;117:1749–54. doi: 10.1378/chest.117.6.1749. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- 28.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 29.Teboul JL Groupe D'experts de la SRLF. SRLF experts recommendations: Indicators of volume resuscitation during circulatory failure. Ann Fr Anesth Reanim. 2005;24:568–76. doi: 10.1016/j.annfar.2005.04.003. doi: 10.1016/j.annfar.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, Manthous C, et al. Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007;33:575–90. doi: 10.1007/s00134-007-0531-4. doi: 10.1007/s00134-007-0531-4. [DOI] [PubMed] [Google Scholar]
- 31.Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: Importance of the postural change. Intensive Care Med. 2009;35:85–90. doi: 10.1007/s00134-008-1293-3. doi: 10.1007/s00134-008-1293-3. [DOI] [PubMed] [Google Scholar]
- 32.Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 2016;42:1935–47. doi: 10.1007/s00134-015-4134-1. doi: 10.1007/s00134-015-4134-1. [DOI] [PubMed] [Google Scholar]
- 33.Meybohm P, Gruenewald M, Renner J, Maracke M, Rossee S, Höcker J, et al. Assessment of left ventricular systolic function during acute myocardial ischemia: A comparison of transpulmonary thermodilution and transesophageal echocardiography. Minerva Anestesiol. 2011;77:132–41. [PubMed] [Google Scholar]
- 34.Perny J, Kimmoun A, Perez P, Levy B. Evaluation of cardiac function index as measured by transpulmonary thermodilution as an indicator of left ventricular ejection fraction in cardiogenic shock. Biomed Res Int. 2014;2014:598029. doi: 10.1155/2014/598029. doi: 10.1155/2014/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–83. doi: 10.1056/NEJM197110142851602. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein RE, Beiser GD, Stampfer M, Epstein SE. Impairment of autonomically mediated heart rate control in patients with cardiac dysfunction. Circ Res. 1975;36:571–8. doi: 10.1161/01.res.36.5.571. doi: 10.1161/01.RES.36.5.571. [DOI] [PubMed] [Google Scholar]