Abstract
Background:
Bilateral sudden sensorineural hearing loss (BSSHL) is rare and assumed to be a different clinical entity compared to unilateral SSHL (USSHL). This study examined the differences between the idiopathic BSSHL and USSHL.
Methods:
Forty-six sequential BSSHL patients (Se-BSSHL) and 68 simultaneous BSSHL (Si-BSSHL) were consecutively admitted between June 2008 and December 2015. Two sets of patients served as control groups: (1) USSHL patients with healthy contralateral ear and (2) USSHL patients with contralateral preexisting hearing loss (USSHLwCHL). We retrospectively analyzed differences among four cohorts using analysis of variance, Kruskal-Wallis test, Welch's t-test, and Chi-square test as appropriate before and after propensity score matching (PSM) based on age, gender, and body mass index (BMI).
Results:
The prevalence of idiopathic BSSHL was 8.6% (114/1329) among the total SSHL patients. In the total cohort, USSHL patients tended to be younger, female, and tended to have lower BMI, renal parameters, and total cholesterol in addition to higher high-density lipoprotein compared to the other three groups. Most routine blood indicators, some coagulation markers, and immunoglobulin M (H = 13.4, P = 0.004) were significantly different among the study groups. After PSM, the major significant differences were found in audiometric characteristics. Si-BSSHL and Se-BSSHL patients demonstrated similar hearing thresholds as USSHL but were significantly better than the USSHLwCHL patients across most frequencies before and after treatment (H = 30.0, P < 0.001 for initial hearing and H = 12.0, P = 0.007 for final hearing). Moreover, the BSSHL patients showed different hearing loss distribution patterns (more descending type, χ2 = 33.8, P = 0.001) with less hearing gain (H = 17.5, P < 0.001) compared to the USSHL patients.
Conclusions:
Idiopathic BSSHL is a relatively rare subtype of SSHL with a higher rate of descending audiogram type and inferior hearing outcome rather than being classified as a completely different disease entity compared to USSHL.
Keywords: Bilateral Hearing Loss, Propensity Score, Sudden Hearing Loss, Unilateral Hearing Loss
INTRODUCTION
Sudden sensorineural hearing loss (SSHL) is defined as an abrupt decline of >30 dB HL in at least three contiguous frequencies over a period of up to 3 days, with an estimated incidence ranging from 5 to 160 per 100,000 persons annually.[1,2] The majority of SSHL are unilaterally affected, among which 85–90% are of idiopathic etiology. In contrast, bilateral SSHL (BSSHL) accounts for 0.4–4.9% of all SSHL patients.[3,4,5,6] It has mostly been reported as a clinical symptom of systemic disease in previous studies. Some authors have further compared the differences between unilateral SSHL (USSHL) and BSSHL patients with respect to clinical manifestations, audiometric characteristics, and laboratory results. Accordingly, they arrived at similar conclusions in which BSSHL can be seen as an ominous sign for a more severe or even malignant underlying disorder and also a complete different entity compared to USSHL.[7,8,9,10,11] Nevertheless, these findings were probably not convincing due to several limitations: (1) the population sizes of BSSHL patients were too small in the originally published studies, the maximum of which is no more than 26;[3,4,5,6,9,11,12] (2) the BSSHL patients without identifiable causes were always confounded by those with known origin in the study groups; and (3) few BSSHL studies were designed to control for possible confounding covariates such as age and gender. Therefore, the present study reviewed medical information from more than 100 BSSHL patients and aimed to clarify the genuine differences between idiopathic BSSHL and USSHL, thus facilitating clinical consultation and uncovering underlying mechanisms.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the institute (No. S2017-024-01). The board granted a waiver of written informed consent for this retrospective study because the patient records were deidentified.
Study population
We performed a retrospective study of consecutive inpatient cases with idiopathic BSSHL who were treated between July 2008 and December 2015 in a large tertiary referral center. The inclusion criteria consisted of several parameters: (1) SSHL >30 dB affecting >3 consecutive frequencies of pure-tone thresholds within 72 h;[1] (2) both ears were affected either concurrently (the second ear being affected within 3 days of the first ear) or sequentially (the second ear being affected >3 days after the first ear); or (3) no identifiable causes. As a result, patients were excluded if they had one of the following diseases/conditions: (1) hereditary deafness; (2) trauma (immediately preceding sudden deafness); (3) Meniere's disease; (4) autoimmune diseases; (5) connective tissue disease; (6) syphilis; (7) parotitis; (8) noise overexposure history; (9) and/or drugs and/or other toxic effects that could be considered as causes of the sudden deafness. We also excluded SSHL patients with recurrence in the ipsilateral ear. Computed tomographic scanning was used to reveal craniofacial or temporal bone malformations, while magnetic resonance imaging helped to rule out retrocochlear pathology such as vestibular schwannoma, stroke, and/or demyelinating disease. Two subsets of patients meeting the above requirements for idiopathic USSHL, who were admitted during the study period, served as two control groups: the idiopathic USSHL patients with healthy contralateral ear (USSHL) and the idiopathic USSHL patients with preexisting contralateral hearing loss (USSHLwCHL).
Data availability
The dataset is available to all interested researchers upon request to the corresponding author. This dataset is currently not available in public data deposition due to the nature of military hospital guidelines.
Blood samples
Complete blood counts, routine chemistry, and coagulation profiles were tested for all participants on the first morning after admission. We also obtained thyroid function data and some immune response markers from a small subgroup of patients. Few patients underwent detection of antinuclear antibody (ANA) titer, thus disabling the comparison in this indicator across groups with previous studies.[3,9,11]
Auditory evaluation
The hearing level was calculated by the average air-conduction hearing thresholds across the affected frequencies. In no response cases, calculations were made by adding 5 dB to the maximum level of sound generated by the audiometer.[13] The initial hearing level was determined by the first audiometric evaluation before study entry, while the final hearing level was tested 2–4 weeks after treatment. The hearing outcomes were determined based on Siegel's criteria.[14,15] The patterns of hearing loss have been categorized into five audiogram configurations: (1) ascending (the average threshold between 0.25 and 0.50 kHz was 20 dB higher than the mean threshold of 4–8 kHz); (2) descending (the average threshold between 4 and 8 kHz was 20 dB higher than the mean threshold of 0.25–0.50 kHz); (3) flat (similar thresholds observed across the entire frequency range and the average hearing threshold not exceeding 80 dB HL); (4) profound (similar thresholds observed across the entire frequency range and the average hearing threshold over 80 dB HL); and (5) irregular type (any audiograms unqualified to be categorized into the above four types).
Treatments
All hospitalized patients were treated with similar protocols as previously described.[16] Administered medications did not differ across groups.
Statistical analysis
Data distributions were evaluated for normality by P-P (probability) plots and Q-Q (quantile) plots. Levene's test was used to assess the homogeneity of variance for a variable calculated for four groups. For continuous variables with normal distribution, we evaluated the differences in patient characteristics using analysis of variance in cases of homoscedasticity and Welch's t-test when Levene's test showed statistical significance (P < 0.05), while Kruskal-Wallis test was used in nonnormally distributed variables. On the other hand, the Chi-square test was conducted for categorical variables. Next, patients in the four groups were matched based on propensity score analyses that were computed by a logistic regression model with covariates of age, sex, and body mass index (BMI). The nearest neighbor matching without replacement on the estimated PS was utilized for matching. Of the original population, both USSHL and USSHLwCHL patients were matched 1:1 to the combined cohort of sequential (Se)-BSSHL and simultaneous (Si)-BSSHL patients with a caliper of 0.3; thereby, 112 patients were included in each group. One patient in the Se-BSSHL group and one in the Si-BSSHL group could not be matched and were thus excluded. The comparisons were then performed for the propensity score-matched patients using the same methods as those in the primary analysis. All P values were two tailed, and differences were determined to be significant when P < 0.05. Unless otherwise stated, continuous variables with normal distribution were presented as means ± standard deviation (SD) or standard error (SE), while skewed distributed variables were expressed as median (interquartile range). All statistical analyses were estimated using the statistical software package SPSS 22.0 (IBM Corp, Armonk, NY, USA).
RESULTS
Of the 1329 SSHL patients, 46 (3.5%) were Se-BSSHL, while 68 (5.1%) were Si-BSSHL, indicating the prevalence of BSSHL was 8.6% (114/1329) among the total number of SSHL patients. Besides, seven patients who reported experiencing Si-BSSHL with prompt self-recovery in one ear were excluded due to lack of audiometric evidence of bilateral hearing loss. In Si-BSSHL group, only two patients showed a 2-day interval between attacks in two ears, while the rest had deafness onset at exactly the same time. In Se-BSSHL group, the observed median gap time between attacks in the two ears was 808 days (interquartile range, 81.5 and 2543.5 days). On the other hand, of the 1215 patients with USSHL, 675 exhibiting prior CHL (but no history of sudden deafness) were categorized as the USSHLwCHL group. The remaining 540 patients with healthy contralateral ears were defined as the USSHL group.
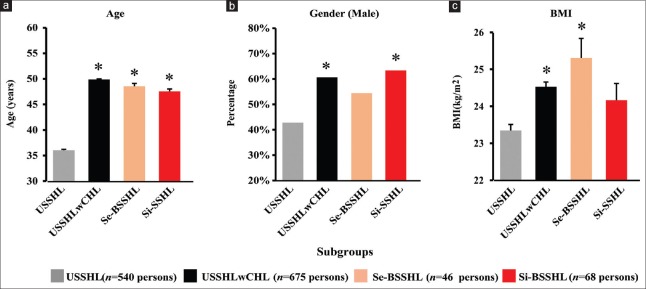
As shown in Figure 1 and Table 1, the mean age of USSHL patients (36.0 ± 14.7 years, 95% confidence interval [CI]: 34.8–37.3 years) was significantly younger than that of the other three groups (USSHLwCHL group: 49.9 ± 14.7 years, 95% CI: 48.8–51.0 years, least significant difference (LSD) t = 16.1, P < 0.001; Se-BSSHL group: 48.6 ± 16.1 years, 95% CI: 43.8–53.3 years, LSD t = 5.5, P < 0.001; and Si-BSSHL group: 47.7 ± 17.6 years, 95% CI: 43.4–52.0 years, LSD t = 6.0, P < 0.001). Male USSHL patients (231/540; 42.8%) were significantly lower than the number of male patients in the USSHLwCHL (χ2 = 38.2, P < 0.001) and Si-BSSHL (χ2 = 11.7, P = 0.001) groups. Regarding BMI, USSHL patients (23.3 ± 3.9 kg/m2, 95% CI: 23.0–23.7 kg/m2) showed a significantly lower level in comparison to the USSHLwCHL cases (24.5 ± 3.5 kg/m2, 95% CI: 24.3–24.8 kg/m2, LSD t = 5.6, P < 0.001) and to the Se-BSSHL group (25.3 ± 3.6 kg/m2, 95% CI:24.2–26.4 kg/m2, LSD t = 3.5, P = 0.001). Due to these differences in demographics, we used propensity score matching (PSM) to eliminate confounding effects due to age, gender, and BMI.
Figure 1.
Patients suffering from USSHL (gray bar) had younger age, less male, and lower BMI level compared with the other subgroups before propensity score matching. (a) Mean ± SE age of the four subgroups. (b) Male percentages of the four subgroups. (c) Mean ± SE BMI in the four subgroups (*P < 0.05 vs. USSHL). USSHL: Unilateral sudden sensorineural hearing loss with normal hearing in the contralateral ear; USSHLwCHL: Unilateral sudden sensorineural hearing loss with preexisting contralateral hearing loss; Se-BSSHL: Sequential bilateral sudden sensorineural hearing loss; Si-BSSHL: Simultaneous bilateral sudden sensorineural hearing loss; SE: Standard error of the mean; BMI: Body mass index. Note: The numerical data on which Figure 1 is based are listed in Table 1.
Table 1.
Clinical characteristics of the BSSHL and USSHL before and after PSM (n = 1329)
| Variables | Total cohort | ||||||
|---|---|---|---|---|---|---|---|
| USSHL (n = 540) | USSHLwCHL (n = 675) | Se-BSSHL (n =46) | Si-BSSHL (n = 68) | Statistics | P¶ | ||
| Age of onset (years) | 36.0 ± 14.7 | 49.9 ± 14.7 | 48.6 ± 16.1 | 47.7 ± 17.6 | 88.9‡ | <0.001 | |
| Male | 231 (42.8) | 409 (60.6) | 25 (54.3) | 44 (64.7) | 42.0§ | <0.001 | |
| BMI (kg/m2) | 23.3 ± 3.9 | 24.5 ± 3.5 | 25.3 ± 3.6 | 24.2 ± 3.9 | 12.2‡ | <0.001 | |
| Time elapse (days)*,† | 14.0 (6.0–30.0) | 13.0 (5.0–28.0) | 8.5 (3.2–30.0) | 13.0 (5.0–25.2) | 5.2|| | 0.155 | |
| Dyslipidemia | 178 (33.0) | 311 (46.1) | 1 (2.2) | 5 (7.4) | 74.4§ | <0.001 | |
| Hypertension | 74 (13.7) | 166 (24.6) | 8 (17.4) | 21 (30.9) | 30.0§ | <0.001 | |
| Diabetes | 56 (10.4) | 134 (19.9) | 2 (4.3) | 12 (20.6) | 26.3§ | <0.001 | |
| Tinnitus | 492 (91.1) | 604 (89.5) | 34 (73.9) | 57 (83.8) | 15.4§ | 0.001 | |
| Vertigo | 330 (61.1) | 366 (54.2) | 24 (52.2) | 38 (55.9) | 6.3§ | 0.097 | |
| Ear fullness | 243 (45.0) | 300 (44.4) | 11 (23.9) | 30 (44.1) | 7.8§ | 0.050 | |
| Initial hearing (dB)* | 75.0 (58.3–95.0) | 78.6 (60.8–98.3) | 66.5 (48.9–88.5) | 65.0 (46.7–88.9) | 21.4|| | <0.001 | |
| Final hearing (dB)* | 58.1 (35.0–73.7) | 61.9 (41.4–77.5) | 55.4 (37.7–73.6) | 57.7 (34.2–78.6) | 11.9|| | 0.008 | |
| Hearing gain (dB)* | 17.1 (5.0–32.5) | 15.0 (4.2–30.7) | 11.2 (2.6–21.9) | 6.7 (0.5–15.0) | 39.5|| | <0.001 | |
| Viral infection | 27 (5.0) | 41 (6.1) | 4 (8.7) | 8 (11.8) | 5.5§ | 0.136 | |
| HL as the first symptom | 364 (67) | 482 (71.4) | 36 (78.3) | 51 (75.0) | 4.7§ | 0.198 | |
| Audiogram type* | 44.5§ | <0.001 | |||||
| Flat | 126 (23.3) | 159 (23.6) | 7 (15.2) | 38 (27.9) | |||
| Descending | 98 (18.1) | 129 (19.1) | 17 (37.0) | 43 (31.6) | |||
| Profound | 229 (42.4) | 316 (46.8) | 18 (39.1) | 41 (30.1) | |||
| Ascending | 61 (11.3) | 35 (5.2) | 3 (6.5) | 6 (4.4) | |||
| Irregular | 26 (4.8) | 36 (5.3) | 1 (2.2) | 8 (5.9) | |||
| Blood biochemical indicators | |||||||
| UA (mmol/L) | 271.1 (225.3–332.1) | 300.4 (246.0–360.6) | 305.9 (246.3–364.0) | 287.1 (237.2–340.9) | 29.4|| | <0.001 | |
| Urea (mmol/L) | 4.6 (3.8–5.4) | 5.2 (4.3–6.1) | 4.7 (4.1–6.0) | 5.0 (4.2–5.9) | 54.2|| | <0.001 | |
| Cr (µmol/L) | 61.3 (52.7–72.6) | 67.0 (56.3–77.7) | 66.0 (55.7–77.3) | 68.4 (54.4–77.8) | 29.2|| | <0.001 | |
| CK (U/L) | 52.2 (38.8–75.7) | 62.9 (44.7–88.3) | 52.0 (37.4–86.8) | 63.8 (44.0–87.3) | 28.0|| | <0.001 | |
| Glucose (mmol/L) | 4.8 (4.5–5.5) | 5.2 (4.6–6.3) | 5.0 (4.7–5.9) | 5.1 (4.7–5.9) | 42.7|| | <0.001 | |
| GSP (µmol/L) | 164.4 (148.9–169.9) | 166.8 (150.9–181.7) | 165.9 (146.2–176.2) | 165.9 (154.9–175.8) | 24.3|| | <0.001 | |
| Homocysteine (µmol/L) | 9.1 (7.8–14.3) | 11.4 (8.5–14.4) | 10.9 (8.5–14.4) | 12.3 (8.5–14.4) | 22.7|| | <0.001 | |
| Lipid profile | |||||||
| TC (mmol/L) | 4.7 (4.0–5.1) | 4.9 (4.2–5.5) | 4.9 (4.2–5.3) | 4.9 (4.2–5.6) | 20.2|| | <0.001 | |
| TG (mol/L) | 1.1 (0.8–1.6) | 1.4 (0.9–1.8) | 1.4 (1.1–1.9) | 1.5 (0.9–1.9) | 30.6|| | <0.001 | |
| HDL (mmol/L) | 1.4 (1.2–1.6) | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) | 1.3 (1.1–1.6) | 21.7|| | <0.001 | |
| LDL (mmol/L) | 2.8 (2.2–3.1) | 3.0 (2.5–3.4) | 3.0 (2.5–3.4) | 2.9 (2.3–3.4) | 19.6|| | <0.001 | |
| Apo A1 (g/L) | 1.39 (1.23–1.51) | 1.35 (1.21–1.52) | 1.32 (1.19–1.47) | 1.36 (1.24–1.57) | 5.7|| | 0.125 | |
| Apo B (g/L) | 0.86 ± 0.21 | 0.93 ± 0.20 | 0.95 ± 0.19 | 0.93 ± 0.24 | 14.7‡ | <0.001 | |
| Blood routine | |||||||
| WBC (×103/µl) | 7.0 (5.6–8.9) | 6.9 (5.6–8.4) | 6.9 (5.7–8.6) | 6.7 (5.5–8.4) | 0.9|| | 0.836 | |
| Neutrophil (×103/µl) | 4.1 (2.9–6.1) | 4.1 (3.1–5.9) | 3.6 (2.9–5.7) | 4.1 (3.1–5.3) | 0.7|| | 0.870 | |
| Lymphocyte (×103/µl) | 2.0 (1.5–2.6) | 2.0 (1.5–2.5) | 2.1 (1.6–2.4) | 2.0 (1.6–2.4) | 1.8|| | 0.610 | |
| RBC (×106/µl) | 4.54 ± 0.48 | 4.62 ± 0.51 | 4.54 ± 0.48 | 4.66 ± 0.48 | 2.8‡ | 0.039 | |
| Hemoglobin (g/L) | 136.7 ± 16.2 | 140.9 ± 16.3 | 137.4 ± 18.1 | 141.6 ± 15.7 | 7.2‡ | <0.001 | |
| Platelet (×103/µl) | 231.5 ± 55.2 | 220.4 ± 58.2 | 229.1 ± 49.6 | 222.1 ± 56.5 | 4.0‡ | 0.007 | |
| Hematocrit | 0.41 ± 0.04 | 0.42 ± 0.04 | 0.41 ± 0.05 | 0.42 ± 0.04 | 7.3‡ | <0.001 | |
| MCV (fl) | 90.0 (87.0–92.8) | 90.7 (87.9–93.3) | 89.8 (87.1–93.3) | 90.3 (88.0–92.8) | 11.1|| | 0.011 | |
| MCH (pg) | 30.5 (29.4–31.3) | 30.7 (29.8–31.6) | 30.2 (29.5–31.6) | 30.7 (29.6–31.4) | 16.3|| | <0.001 | |
| Blood coagulation markers | |||||||
| Fibrinogen (g/L) | 2.5 (2.0–2.9) | 2.6 (2.1–3.2) | 2.7 (2.3–3.5) | 2.6 (2.1–3.0) | 17.6|| | <0.001 | |
| PT (s) | 13.1 (12.6–13.7) | 12.8 (12.3–13.5) | 12.9 (12.6–13.6) | 12.8 (12.5–13.2) | 24.0|| | <0.001 | |
| INR | 1.02 (0.96–1.08) | 1.01 (0.95–1.07) | 1.02 (0.96–1.09) | 1.00 (0.95–1.04) | 5.3|| | 0.153 | |
| APTT (s) | 35.4 (34.1–37.4) | 35.0 (33.4–36.5) | 35.4 (35.0–36.4) | 35.0 (33.3–36.0) | 11.2|| | 0.011 | |
| Age of onset (years) | 43.9 ± 14.6 | 48.2 ± 15.1 | 47.8 ± 15.4 | 47.2 ± 17.2 | 1.6‡ | 0.186 | |
| Male | 59 (52.7) | 63 (56.2) | 24 (53.3) | 43 (64.2) | 2.4§ | 0.485 | |
| BMI (kg/m2) | 24.0 ± 3.8 | 24.9 ± 3.4 | 25.2 ± 3.6 | 24.3 ± 3.9 | 1.9‡ | 0.133 | |
| Time elapse (days)*,† | 14.0 (5.8–25.2) | 13.0 (6.0–23.8) | 9.0 (4.0–30.0) | 13.5 (5.0–25.8) | 0.7|| | 0.865 | |
| Dyslipidemia | 47 (42.0) | 59 (52.7) | 1 (2.2) | 5 (7.5) | 62.4§ | <0.001 | |
| Hypertension | 24 (21.4) | 27 (24.1) | 7 (15.6) | 21 (31.3) | 4.2§ | 0.245 | |
| Diabetes | 17 (15.2) | 23 (20.5) | 2 (4.4) | 12 (17.9) | 6.7§ | 0.094 | |
| Tinnitus | 102 (91.1) | 97 (86.6) | 34 (75.6) | 56 (83.6) | 6.8§ | 0.077 | |
| Vertigo | 64 (57.1) | 60 (53.6) | 24 (53.3) | 38 (56.7) | 0.4§ | 0.937 | |
| Ear fullness | 51 (45.5) | 45 (40.2) | 11 (24.4) | 29 (43.3) | 6.2§ | 0.102 | |
| Initial hearing (dB)* | 75.4 (55.0–88.2) | 83.9 (65.5–102.9) | 67.1 (48.6–88.6) | 65.4 (46.7–89.6) | 30.0|| | <0.001 | |
| Final hearing (dB)* | 48.9 (30.9–67.8) | 64.3 (46.4–81.2) | 55.7 (40.7–73.6) | 58.2 (33.9–78.6) | 12.0|| | 0.007 | |
| Hearing gain (dB)* | 20.0 (5.0–35.0) | 12.3 (4.3–31.5) | 10.7 (2.5–22.1) | 7.0 (0.7–15.0) | 17.5|| | <0.001 | |
| Viral infection | 4 (3.6) | 5 (4.5) | 4 (8.9) | 8 (11.9) | 6.2§ | 0.101 | |
| HL as the first symptom | 79 (70.5) | 82 (73.2) | 35 (77.8) | 50 (74.6) | 1.0§ | 0.812 | |
| Audiogram type* | 33.8§ | 0.001 | |||||
| Flat | 33 (29.5) | 25 (22.3) | 6 (13.3) | 38 (28.4) | |||
| Descending | 19 (17.0) | 20 (17.9) | 17 (37.8) | 41 (30.6) | |||
| Profound | 44 (39.3) | 61 (54.5) | 18 (40.0) | 41 (30.6) | |||
| Ascending | 13 (11.6) | 3 (2.7) | 3 (6.7) | 6 (4.5) | |||
| Irregular | 3 (2.7) | 3 (2.7) | 1 (2.2) | 8 (6.0) | |||
| Blood biochemical indicators | |||||||
| UA (mmol/L) | 282.8 (225.3–334.8) | 297.5 (246.5–356.1) | 305.6 (246.0–355.5) | 286.5 (236.8–339.5) | 3.5|| | 0.321 | |
| Urea (mmol/L) | 4.8 (3.9–5.6) | 5.1 (4.2–6.1) | 4.8 (4.0–6.1) | 5.0 (4.2–5.9) | 3.1|| | 0.372 | |
| Cr (µmol/L) | 63.0 (55.8–75.0) | 66.0 (55.1–75.8) | 65.7 (55.5–75.3) | 68.1 (54.3–76.9) | 1.1|| | 0.785 | |
| CK (U/L) | 62.5 (42.5–82.2) | 57.6 (44.3–85.9) | 51.9 (37.0–86.1) | 63.6 (43.7–85.2) | 1.4|| | 0.708 | |
| Glucose (mmol/L) | 5.0 (4.5–6.1) | 5.1 (4.6–6.0) | 5.0 (4.7–6.0) | 5.1 (4.7–5.9) | 0.3|| | 0.968 | |
| GSP (µmol/L) | 165.5 (150.5–169.9) | 165.9 (149.8–182.0) | 165.9 (145.9–172.3) | 165.9 (154.9–176.4) | 1.9|| | 0.596 | |
| Homocysteine (µmol/L) | 9.9 (8.3–13.4) | 11.0 (8.4–14.2) | 10.9 (8.5–14.4) | 12.3 (8.5–14.4) | 3.0|| | 0.392 | |
| Lipid profile | |||||||
| TC (mmol/L) | 4.6 (4.1–5.4) | 4.9 (4.3–5.5) | 4.9 (4.2–5.3) | 4.9 (4.2–5.5) | 1.9|| | 0.597 | |
| TG (mol/L) | 1.3 (0.8–1.8) | 1.4 (1.0–1.9) | 1.4 (1.1–1.8) | 1.4 (0.9–1.9) | 2.9|| | 0.404 | |
| HDL (mmol/L) | 1.29 (1.03–1.55) | 1.26 (1.08–1.54) | 1.26 (1.07–1.48) | 1.26 (1.08–1.60) | 0.5|| | 0.913 | |
| LDL (mmol/L) | 2.8 (2.3–3.3) | 2.9 (2.4–3.3) | 3.1 (2.6–3.4) | 2.8 (2.3–3.4) | 2.1|| | 0.559 | |
| Apo A1 (g/L) | 1.32 (1.18–1.50) | 1.35 (1.23–1.50) | 1.32 (1.19–1.45) | 1.37 (1.24–1.58) | 5.1|| | 0.161 | |
| Apo B (g/L) | 0.89 ± 0.22 | 0.93 ± 0.21 | 0.95 ± 0.20 | 0.93 ± 0.24 | 1.1‡ | 0.333 | |
| Blood routine | |||||||
| WBC (×103/µl) | 6.9 (5.2–8.3) | 6.8 (5.5–8.4) | 6.9 (5.6–8.5) | 6.7 (5.5–8.4) | 1.0|| | 0.963 | |
| Neutrophil (×103/µl) | 3.9 (2.6–6.0) | 3.9 (3.0–5.5) | 3.6 (2.9–5.7) | 4.1 (3.1–5.4) | 0.9|| | 0.863 | |
| Lymphocyte (×103/µl) | 2.0 (1.4–2.5) | 1.9 (1.5–2.6) | 2.1 (1.5–2.3) | 2.0 (1.6–2.4) | 1.0|| | 0.957 | |
| RBC (×106/µl) | 4.58 ± 0.51 | 4.56 ± 0.48 | 4.54 ± 0.48 | 4.66 ± 0.48 | 0.7‡ | 0.548 | |
| Hemoglobin (g/L) | 138.6 ± 17.2 | 137.6 ± 17.2 | 137.3 ± 18.3 | 141.6 ± 15.8 | 0.9‡ | 0.444 | |
| Platelet (×103/µl) | 216.2 ± 46.8 | 217.7 ± 59.5 | 231.2 ± 48.1 | 222.4 ± 56.9 | 1.0‡ | 0.410 | |
| Hematocrit | 0.41 ± 0.05 | 0.41 ± 0.05 | 0.41 ± 0.05 | 0.42 ± 0.04 | 0.9‡ | 0.424 | |
| MCV (fl) | 90.4 (87.8–92.9) | 90.0 (87.1–93.2) | 89.8 (87.0–93.2) | 90.3 (88.0–92.7) | 1.0|| | 0.990 | |
| MCH (pg) | 30.7 (29.9–31.5) | 30.6 (29.4–31.4) | 30.1 (29.5–31.6) | 30.7 (29.6–31.5) | 0.8|| | 0.815 | |
| Blood coagulation markers | |||||||
| Fibrinogen (g/L) | 2.6 (2.1–3.0) | 2.6 (2.1–3.1) | 2.7 (2.3–3.5) | 2.6 (2.1–3.0) | 0.8|| | 0.796 | |
| PT (s) | 13.0 (12.5–13.6) | 12.8 (12.3–13.3) | 12.9 (12.6–13.7) | 12.8 (12.5–13.2) | 0.1|| | 0.132 | |
| INR | 1.01 (0.95–1.06) | 1.00 (0.95–1.06) | 1.02 (0.96–1.08) | 1.00 (0.95–1.04) | 0.4|| | 0.387 | |
| APTT (s) | 35.0 (34.0–36.7) | 35.0 (33.0–36.4) | 35.4 (35.0–36.2) | 35.0 (33.3–36.0) | 0.8|| | 0.825 | |
Continuous variables were presented as mean ± SD for normal distribution, or medians (interquartile range) for nonnormal distribution. Categorical variables were presented as n (%). *These factors were analyzed by number of ears, while the rest were analyzed by number of persons; †Time elapse between study entry and symptom onset; ‡F value; §χ2 value; ||H value; ¶Overall comparisons across four groups. BSSHL: Bilateral sudden sensorineural hearing loss; USSHL: Unilateral sudden sensorineural hearing loss with normal hearing in the contralateral ear; USSHLwCHL: Unilateral sudden sensorineural hearing loss with preexisting contralateral hearing loss; Se-BSSHL: Sequential bilateral sudden sensorineural hearing loss; Si-BSSHL: Simultaneous bilateral sudden sensorineural hearing loss; BMI: Body mass index; HL: Hearing loss; UA: Uric acid; Cr: Creatine; CK: Creatine kinase; GSP: Glycosylated serum protein; TC: Total cholesterol; TG: Triglyceride; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; WBC: White blood cell counts; MCV: Mean corpuscular volume; MCH: Mean corpuscular; PT: Prothrombin time; INR: International normalized ratio; APTT: Activated partial thromboplastin time; RBC: Red blood cell; SD: Standard deviation; PSM: Propensity score matching.
Clinical, serological, and audiometric characteristics before and after PSM are reported in [Table 1]. Of the 1329 patients who were available to be analyzed before PSM, Se-BSSHL and Si-BSSHL patients tended to be male and older, have higher BMI, and demonstrate significantly higher low-density lipoprotein cholesterol, total cholesterol, triglyceride, Apo B, serum glucose, uric acid, homocysteine, and hemoglobin levels than the USSHL cases but exhibited similar levels as those seen in the USSHLwCHL group. The median initial hearing thresholds were higher in the two USSHL subgroups (75 dB in USSHL and 78.6 dB in USSHLwCHL), as compared to their bilateral counterparts with 66.5 dB in Se-BSSHL and 65.0 dB in Si-BSSHL (H = 21.4, P < 0.001).
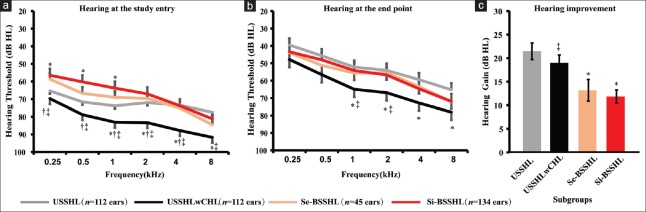
After PSM, 112 USSHL, 112 USSHLwCHL, 45 Se-BSSHL, and 67 Si-BSSHL cases were generated from the original cohort. The final sample showed a higher proportion of patients with dyslipidemia in the USSHL and USSHLwCHL groups than that in the two BSSHL groups (χ2 = 62.4, P < 0.001; USSHL versus Se-BSSHL, χ2 = 23.9, P < 0.001; USSHL versus Si-BSSHL, χ2 = 24.2, P < 0.001; USSHLwCHL versus Se-BSSHL, χ2 = 33.6, P < 0.001; and USSHLwCHL versus Si-BSSHL, χ2 = 37.3, P < 0.001). However, lipid profiles were similar across all groups. The hearing thresholds of USSHL patients were significantly lower than that of USSHLwCHL patients for most frequencies before and after treatment but were close to the level of the two BSSHL groups (H = 30.0, P < 0.001 for initial hearing and H = 12.0, P = 0.007 for final hearing, respectively) [Figure 2a and 2b]. In spite of this, the two USSHL cohorts experienced greater hearing improvement compared to the two BSSHL groups (H = 17.5, P < 0.001) [Figure 2c]. It should be noted that in comparison with the unilateral counterpart, the BSSHL patients had significantly more descending-type audiograms (χ2 = 33.8, P = 0.001) which might also result in an inferior outcome. With regard to serum markers available in a small part of participants, no statistical significance was observed across four subgroups [Table 2].
Figure 2.
Mean ± SE audiometric hearing threshold at study entry (a) and end point (b) and the hearing gain (c) among four cohorts after PSM. (*P < 0.05 vs. USSHL; †P < 0.05 versus Se-BSSHL; ‡P < 0.05 versus Si-BSSHL). USSHL: Unilateral sudden sensorineural hearing loss with normal hearing in the contralateral ear; USSHLwCHL: Unilateral sudden sensorineural hearing loss with preexisting contralateral hearing loss; Se-BSSHL: Sequential bilateral sudden sensorineural hearing loss; Si-BSSHL: Simultaneous bilateral sudden sensorineural hearing loss; SE: Standard error of the mean.
Table 2.
The immune response marker and thyroid function of bilateral and unilateral sudden sensorineural hearing loss before and after propensity score matching (PSM)
| Variables* | Before PSM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| USSHL | n | USSHLwCHL | n | Se-BSSHL | n | Si-BSSHL | n | Statistics | P|| | |
| Immunology Markers | ||||||||||
| IgA (g/L) | 2.0 (1.6–2.5) | 114 | 2.1 (1.7–2.6) | 137 | 2.0 (1.7–2.9) | 17 | 1.8 (1.5–2.2) | 29 | 4.4‡ | 0.220 |
| IgE (IU/ml) | 49.5 (20.2–129.8) | 110 | 33.5 (18.4–90.0) | 132 | 39.2 (22.0–75.0) | 17 | 47.4 (17.6–96.5) | 29 | 3.4‡ | 0.337 |
| IgG (g/L) | 10.6 (9.5–12.0) | 114 | 11.1 (8.9–12.6) | 137 | 10.7 (9.6–11.5) | 17 | 9.9 (8.7–11.8) | 29 | 2.7‡ | 0.442 |
| IgM (g/L) | 1.2 (0.9–1.6) | 114 | 0.9 (0.7–1.3) | 137 | 1.0 (0.7–1.4) | 17 | 0.9 (0.6-1.3) | 29 | 13.4‡ | 0.004 |
| IGK (g/L) | 2.7 ± 0.5 | 110 | 2.7 ± 0.7 | 131 | 2.5 ± 0.6 | 17 | 2.4 ± 0.5 | 29 | 1.5† | 0.219 |
| IGL (g/L) | 1.4 (1.2–1.6) | 110 | 1.4 (1.2–1.7) | 131 | 1.5 (1.3–1.8) | 17 | 1.3 (1.1–1.5) | 29 | 6.1‡ | 0.109 |
| Thyroid Function | ||||||||||
| TSH (mU/L) | 1.6 (0.9–2.7) | 282 | 1.9 (1–2.8) | 293 | 1.7 (0.5–2.7) | 27 | 1.7 (0.8–3.1) | 36 | 5.6‡ | 0.136 |
| T4 (nmol/L) | 99.7 ± 21.4 | 282 | 101.1 ± 20.6 | 293 | 101.0 ± 15.8 | 27 | 102.4 ± 20.5 | 36 | 0.3† | 0.803 |
| T3 (nmol/L) | 1.4 ± 0.4 | 282 | 1.5 ± 0.3 | 293 | 1.4 ± 0.3 | 27 | 1.5 ± 0.3 | 36 | 0.2† | 0.923 |
| FT3 (pmol/L) | 4.6 ± 0.9 | 282 | 4.5 ± 0.7 | 293 | 4.5 ± 0.7 | 27 | 4.5 ± 0.6 | 36 | 0.8§ | 0.488 |
| FT4 (pmol/L) | 15.1 (13.6–16.9) | 282 | 15.0 (13.4–16.8) | 293 | 15.3 (13.6–17.3) | 27 | 14.7 (12.7–15.8) | 36 | 2.0‡ | 0.571 |
| Variables* | After PSM | |||||||||
| USSHL | n | USSHLwCHL | n | Se-BSSHL | n | Si-BSSHL | n | Statistics | P|| | |
| Immunology Markers | ||||||||||
| IgA (g/L) | 2.2 (1.7–3.2) | 22 | 2.3 (1.7–3.0) | 24 | 2.0 (1.7–2.9) | 17 | 1.8 (1.4–2.2) | 28 | 5.7‡ | 0.125 |
| IgE (IU/ml) | 48.3 (19.5–78.0) | 22 | 38.2 (18.9–87.7) | 23 | 39.2 (22.0–75.0) | 17 | 41.2 (17.5–96.8) | 28 | 0.3‡ | 0.962 |
| IgG (g/L) | 10.8 (9.6–13.0) | 22 | 10.2 (9.0–11.9) | 24 | 10.7 (9.6–11.5) | 17 | 9.8 (8.7–11.7) | 28 | 2.4‡ | 0.487 |
| IgM (g/L) | 1.0 (0.7–1.4) | 22 | 1.2 (0.9–1.5) | 24 | 1.0 (0.7–1.4) | 17 | 0.9 (0.6–1.2) | 28 | 3.9‡ | 0.268 |
| IGK (g/L) | 2.8 ± 0.7 | 22 | 2.6 ± 0.7 | 23 | 2.5 ± 0.6 | 17 | 2.4 ± 0.5 | 28 | 5.5† | 0.083 |
| IGL (g/L) | 1.4 (1.2–1.7) | 22 | 1.4 (1.3–1.7) | 23 | 1.5 (1.3–1.8) | 17 | 1.3 (1.1–1.5,) | 28 | 7.3‡ | 0.062 |
| Thyroid Function | ||||||||||
| TSH (mU/L) | 1.6 (1.0–2.8,69) | 69 | 1.9 (1.0–2.9) | 53 | 1.6 (0.6–2.4) | 26 | 1.4 (0.8–3.1) | 35 | 2.5‡ | 0.529 |
| T4 (nmol/L) | 98.0 ± 21.9 | 69 | 103.5 ± 20.5 | 53 | 101.8 ± 15.6 | 26 | 102.4 ± 20.8 | 35 | 0.8† | 0.495 |
| T3 (nmol/L) | 1.5 ± 0.3 | 69 | 1.5 ± 0.4 | 53 | 1.4 ± 0.3 | 26 | 1.5 ± 0.3 | 35 | 0.3† | 0.806 |
| FT3 (pmol/L) | 4.7 ± 0.8 | 69 | 4.7 ± 1.0 | 53 | 4.5 ± 0.7 | 26 | 4.5 ± 0.6 | 35 | 1.3§ | 0.286 |
| FT4 (pmol/L) | 14.6 (13.7–16.4) | 69 | 14.8 (13.5–17.1) | 53 | 15.4 (13.7–17.3) | 26 | 14.8 (12.8–15.8) | 35 | 0.8‡ | 0.710 |
Continuous variables were presented as mean ± standard deviation for normal distribution, or medians (interquartile range) for non-normal distribution. *All the factors were analyzed by number of persons; †F value; ‡H value; §Welch's test statistics; ||Overall comparisons across four groups; USSHL: Unilateral sudden sensorineural hearing loss with normal hearing in the contralateral ear; USSHLwCHL: Unilateral sudden sensorineural hearing loss with preexisting contralateral hearing loss; Se-BSSHL: Sequential bilateral sudden sensorineural hearing loss; Si-BSSHL: Simultaneous bilateral sudden sensorineural hearing loss; Ig: Immunoglobulin; IGK: Ig Kappa light chain; IGL: Ig Lambda light chain; TSH: Thyroid stimulating hormone; T4: Thyroxine; T3: Triiodothyronine; FT3: Free triiodothyronine; FT4: Free thyroxine.
DISCUSSION
Few studies have addressed the comparison between BSSHL and USSHL. Although it was still controversial regarding the genuine differences between the two cohorts, these authors arrived at the consensus that BSSHL can predict severe systemic disorders[7,8] and is a distinct disease entity in contrast to USSHL.[9,10,11] Unfortunately, this conclusion is questionable since most of the previous studies did not exclude BSSHL patients with known etiology, and they did not adjust for probable confounding effects of differences in demographics.
To our knowledge, the present study is a rare study to make a comparison between idiopathic BSSHL and USSHL with adjustments for age, gender, and BMI differences. We conducted a PSM analysis to balance demographic characteristics that could act as confounders across groups, thus reducing the biases in the estimation process.[17] In the unmatched cohorts, Se-BSSHL and Si-BSSHL patients seemed to show higher levels of vascular risk factors such as low-density lipoprotein, total cholesterol, triglycerides, Apo B, uric acid, urea, creatine, serum glucose, glycosylated serum proteins, and homocysteine than those seen in the USSHL cases but exhibited similar levels in USSHLwCHL patients. Moreover, significant differences were also found across groups regarding coagulation markers such as fibrinogen and prothrombin time. The same was true for some routine blood indicators, including red blood cell counts, hemoglobin, platelets, hematocrit, and other blood-related factors. Nevertheless, after PSM, the significant differences found among unmatched cohorts in the lipid profiles, biochemistry indicators, coagulation parameters, and the accompanying symptoms totally disappeared. Therefore, it is justified to speculate that the significant differences found in the previous studies concerning lipid profiles, comorbid diabetes mellitus, and cardiovascular diseases between BSSHL and USSHL groups may be attributable to the confounding effects of age differences.[3,6]
The most popular hypotheses accounting for the etiology of BSSHL include viral infection, vascular disturbances, and autoimmunity. Yanagita and Murahashi[4] assumed viral infection as the primary cause of BSSHL. Fetterman et al.[3] demonstrated an increase in the prevalence of viral infections and cardiovascular diseases in addition to a high rate of positive ANA in BSSHL as compared to USSHL cases. In contrast, some authors found a different or even opposite trend regarding the prevalence of cardiovascular disease and the preceding viral infection.[8,12] In spite of this discrepancy, most of the authors described above underlined the association between autoimmunity and BSSHL. In the present study, we demonstrated a similar phenomenon concerning higher rates of the preceding viral infections in the two subgroups of BSSHL. With regard to the immune response markers, however, the result was contradictory to the previous studies.[3,9] Although we failed to compare the ANA titer across groups due to the very limited available data, we found lower levels of the immunoglobulin (Ig) A, IgM, IgG, the Ig kappa light chain, and the Ig lambda light chain in the patients with Si-BSSHL compared to the other groups, although the differences were not statistically significant. In addition, most patients in the present BSSHL cohorts were men (64.7% in Si-BSSHL and 54.3% in Se-BSSHL). This failed to meet the typical feature of female preponderance for immune-mediated hearing loss. Thus far, the findings tend to support the concept that viral infections rather than autoimmunity are the etiological cause of BSSHL.
In this study, the inclusion criteria of SSHL were based on American clinical practice[1] instead of Chinese guidelines[18] in order to facilitate direct comparison with more studies in this area. As has been shown, the audiometric criterion was set to 30-dB hearing loss across three consecutive frequencies according to the American guidelines, while it is 20-dB hearing loss at two contiguous frequencies in the Chinese counterpart. The lower threshold for inclusion in the Chinese guidelines may partly explain the relatively higher prevalence of BSSHL previously described in domestic studies.[19,20] However, after adopting the American criteria, the authors still demonstrated a higher prevalence of BSSHL (8.6% in all SSHL patients). We even excluded seven patients who initially suffered from Si-BSSHL and reported spontaneous recovery before audiometric testing. Considering this situation, the incidence appears to be underestimated. In addition, the data also suggest that 3.5% of idiopathic USSHL patients might be affected by another attack of sudden deafness in the contralateral ear later in their lifetime, which is also defined as Se-BSSHL. This helps to answer one of the typical clinical questions from SSHL patients: “Is the other ear going to be involved some day?”
The major differences between BSSHL and USSHL lay in audiometric profiling, including hearing outcome, hearing levels at study entry, and the endpoints. To our surprise, the worst hearing threshold was found in patients with USSHLwCHL instead of BSSHL irrespective of treatment. Nonetheless, BSSHL patients demonstrated significantly less hearing gain than the two subsets of USSHL. This was consistent with most of the previous studies in which better hearing improvements in USSHL than those in the BSSHL counterparts were shown.[4,6,9,11,12]
There were a number of drawbacks in the present study, which are as follows: (1) we adopted single baseline blood sampling instead of serial sampling; the latter might potentially yield a more reliable and better analytical time point; (2) the inherent defects of a retrospective and single-center study; (3) the study sample included exclusively hospitalized SSHL patients; thus the selection of patients was limited and the findings might not be extrapolated to the general population; (4) although many variables have been taken into consideration, we cannot rule out the possibility that unmeasured factors might contribute to the differences across groups. Therefore, well-designed, prospective, multilateral trials conducted on the general population are warranted to confirm the future findings. Moreover, further exploration of genotypes might provide the most convincing evidence for uncovering genuine differences between BSSHL and USSHL.
In conclusion, idiopathic BSSHL has a male preponderance and tends to occur in advanced age, accounting for 8.6% of all of the SSHL patients. Idiopathic USSHL presents the odds (3.5%) for experiencing a second sudden deafness attack in the other ear later during an individual's lifetime, which is also defined as Se-BSSHL. After adjusting for differences in demographics, the data support the finding that BSSHL is a relatively rare subtype of SSHL with more descending audiograms, inferior hearing outcomes, less chances of comorbid dyslipidemia, higher rates of preceding viral infections, and a lower level of immune markers, rather than a completely different disease entity compared to USSHL.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (No. 81530032 and No. 81500794), the National Key Basic Research Program of China (No. 2014CB943001), the China Postdoctoral Science Foundation (2017M613326), and the New Researcher Foundation of the PLA General Hospital (No. 14KMZ04).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: Sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146:S1–35. doi: 10.1177/0194599812436449. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber BE, Agrup C, Haskard DO, Luxon LM. Sudden sensorineural hearing loss. Lancet. 2010;375:1203–11. doi: 10.1016/S0140-6736(09)62071-7. doi: 10.1016/S0140-6736(09)62071-7. [DOI] [PubMed] [Google Scholar]
- 3.Fetterman BL, Luxford WM, Saunders JE. Sudden bilateral sensorineural hearing loss. Laryngoscope. 1996;106:1347–50. doi: 10.1097/00005537-199611000-00008. doi: 10.1097/00005537-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Yanagita N, Murahashi K. Bilateral simultaneous sudden deafness. Arch Otorhinolaryngol. 1987;244:7–10. doi: 10.1007/BF00453482. doi: 10.1007/BF00453482. [DOI] [PubMed] [Google Scholar]
- 5.Kirikae I, Ishii T, Shidara T, Nosue M. On types of hearing loss in sudden deafness. Jibiinkoka. 1963;35:437–41. [PubMed] [Google Scholar]
- 6.Oh JH, Park K, Lee SJ, Shin YR, Choung YH. Bilateral versus unilateral sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2007;136:87–91. doi: 10.1016/j.otohns.2006.05.015. doi: 10.1016/j.otohns.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Chen YH, Young YH. Bilateral simultaneous sudden sensorineural hearing loss. J Neurol Sci. 2016;362:139–43. doi: 10.1016/j.jns.2016.01.029. doi: 10.1016/j.jns.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Galicia-López A, Anda-Garay JC, García de la Peña M. Bilateral sudden sensorineural hearing loss in a patient with microangiopathic antiphospholipid syndrome. Reumatol Clin. 2016;12:175–7. doi: 10.1016/j.reuma.2015.05.011. doi: 10.1016/j.reuma.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Xenellis J, Nikolopoulos TP, Stavroulaki P, Marangoudakis P, Androulakis M, Tsangaroulakis M, et al. Simultaneous and sequential bilateral sudden sensorineural hearing loss: Are they different from unilateral sudden sensorineural hearing loss? ORL J Otorhinolaryngol Relat Spec. 2007;69:306–10. doi: 10.1159/000107435. doi: 10.1159/000107435. [DOI] [PubMed] [Google Scholar]
- 10.Sara SA, Teh BM, Friedland P. Bilateral sudden sensorineural hearing loss: Review. J Laryngol Otol. 2014;128(Suppl 1):S8–15. doi: 10.1017/S002221511300306X. doi: 10.1017/S002221511300306X. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang L, Zhang J, Zhang X, Zhang W, Chen X, et al. The clinical analysis of bilateral successive sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2016;273:3679–84. doi: 10.1007/s00405-016-4028-z. doi: 10.1007/s00405-016-4028-z. [DOI] [PubMed] [Google Scholar]
- 12.Akil F, Yollu U, Yilmaz M, Yener HM, Mamanov M, Inci E, et al. Simultaneous idiopathic bilateral sudden hearing loss – Characteristics and response to treatment. Braz J Otorhinolaryngol. 2017:pii: S1808-8694(17)30003-4. doi: 10.1016/j.bjorl.2016.12.003. doi: 10.1016/j.bjorl.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakata T, Esaki Y, Yamano T, Sueta N, Nakagawa T. A comparison between the feeling of ear fullness and tinnitus in acute sensorineural hearing loss. Int J Audiol. 2008;47:134–40. doi: 10.1080/14992020701760547. doi: 10.1080/14992020701760547. [DOI] [PubMed] [Google Scholar]
- 14.Siegel LG. The treatment of idiopathic sudden sensorineural hearing loss. Otolaryngol Clin North Am. 1975;8:467–73. [PubMed] [Google Scholar]
- 15.Seo YJ, Jeong JH, Choi JY, Moon IS. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio: Novel markers for diagnosis and prognosis in patients with idiopathic sudden sensorineural hearing loss. Dis Markers. 2014;2014:702807. doi: 10.1155/2014/702807. doi: 10.1155/2014/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Zhang XY, Wang QJ, Wang DY. Efficacy of methylprednisolone sodium succinate for injection (postotic injection) on the auditory threshold and speech recognition rate of sudden deafness patients. Int J Clin Exp Med. 2015;8:14110–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Cook RD, Weisberg S. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 18.Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guideline of diagnosis and treatment of sudden deafness (2015) (in Chinese) Chin J Otorhinolaryngol Head Neck Surg. 2015;50:443–7. doi: 10.3760/cma.j.issn.1673.0860.2015.06.002. [PubMed] [Google Scholar]
- 19.Ai W, Tong B, Liu Y, Duan M. Analysis of clinical characteristics and treatment outcome of bilateral and unilateral sudden sensorineural hearing loss (in Chinese) J Clin Otorhinolaryngol Head Neck Surg. 2009;23:307–10. [PubMed] [Google Scholar]
- 20.Ni M, Li D, Peng W, Peng Y, Ren J. Bilateral versus unilateral sudden sensorineural hearing loss (in Chinese) J Clin Otorhinolaryngol Head Neck Surg. 2010;24:74–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset is available to all interested researchers upon request to the corresponding author. This dataset is currently not available in public data deposition due to the nature of military hospital guidelines.