Abstract
Background:
Snakebites are a neglected threat to global human health with a high morbidity rate. The present study explored the efficacy of antivenom with hyperbaric oxygen (HBO) intervention on snakebites, which could provide the experimental basis for clinical adjuvant therapy.
Methods:
Male Sprague–Dawley rats (n = 96) were randomized into four groups: the poison model was established by injecting Deinagkistrodon acutus (D. acutus) venom (0.8 LD50) via the caudal vein; the antivenom group was injected immediately with specific antivenom via the caudal vein after successful establishment of the envenomation model; and the antivenom + HBO group was exposed to HBO environment for 1 h once at predetermined periods of 0 h, 4 h, 12 h, and 23 h after antivenin administration. Each HBO time point had six rats; the control group was left untreated. The rats in the experimental group were euthanized at the corresponding time points after HBO therapy, and brain tissue and blood were harvested immediately. Hematoxylin and eosin (H&E) staining was used to investigate the pathological changes in the rat brain. Immunohistochemistry (IHC), real-time polymerase chain reaction (PCR), and Western blotting were used to detect the expression of Nestin mRNA and protein in the subventricular zone (SVZ) of the brain. The levels of coagulation function (prothrombin time, activated partial thromboplastin time [APTT], and fibrinogen) and oxidation/antioxidation index (malondialdehyde [MDA] and superoxide dismutase [SOD]) were analyzed. Data were analyzed using one-way analysis of variance.
Results:
The brain tissue from rats in the poison model was observed for pathological changes using H&E staining. Tissues showed edema, decreased cell number, and disordered arrangement in the SVZ in the snake venom group. The antivenom − HBO intervention significantly alleviated these observations and was more prominent in the antivenom + HBO group. The serum levels of SOD and MDA in the snake venom group were increased and the antivenom − HBO intervention further increased the SOD levels but significantly decreased the MDA levels; however, this was enhanced within 1 h after HBO administration (MDA: F = 5.540, P = 0.008, SOD: F = 7.361, P = 0.000). Activated partial thromboplastin time (APTT) was significantly abnormal after venom administration but improved after antivenom and was even more significant in the antivenom + HBO group 5 h after envenomation (F = 25.430, P = 0.000). Only a few nestin-positive cells were observed in the envenomation model. The expression levels were significant in the antivenom and antivenom + HBO groups within 1 and 5 h after envenomation and were more significant in the antivenom + HBO group as determined by IHC, real-time PCR, and Western blotting (P < 0.05). D. acutus envenomation has neurotoxic effects in the brain of rats.
Conclusions:
Antivenin and HBO, respectively, induced a neuroprotective effect after D. acutus envenomation by attenuating brain edema, upregulating nestin expression in SVZ, and improving coagulopathy and oxidative stress. The intervention efficacy of antivenom with HBO was maximum within 5 h after envenomation and was more efficacious than antivenom alone.
Keywords: Deinagkistrodon acutus Venom, Antivenom, Hyperbaric Oxygenation, Nestin, Snakebites, Subventricular Zone
INTRODUCTION
Snakebites often occur in tropical and subtropical regions. Of the 25 million snakebite incidences reported around the world annually, about 10,000 are fatal.[1] Deinagkistrodon acutus (D. acutus) is endemic to China and common in Guizhou province. The neurovirulent component of D. acutus bite will damage brain tissues and cause coagulation disorders and vascular endothelial cell injury, accompanied with severe cerebral hemorrhage or ischemic stroke, which could be life-threatening.[2,3,4] However, studies to reverse these toxicities have seldom been performed. One potential mechanism to reduce the toxicity may be through the proliferation of neural stem cells (NSCs) in the subventricular zone (SVZ) that could differentiate toward the neurons, thereby repairing damaged nerve. Nestin is a widely used NSC marker, and its expression initiates from the formation of neurula. With the differentiation and maturity of the neurons, the expression of nestin is gradually diminished and lost. In cases of hypoxia–ischemia, trauma, and physicochemical irritation, the expression of nestin gets upregulated and may be involved in repairing damaged nerves.[5,6]
Antivenom is the preferred and world-recognized therapy to treat snakebites. It can neutralize free snake venom immediately and improve the symptoms of poisoning; however, it has limited effects on damaged local tissue.[7] Hyperbaric oxygen (HBO) has been widely applied clinically since the 1960s. It could be used as an adjuvant therapy for snakebites and improve the toxic symptoms observed in local tissues.[8] However, the relevant experimental studies and evidence-based observations are currently lacking.
In the present study, the efficacy and mechanism of antivenom + HBO intervention on snakebites was determined. We hypothesized that HBO as an auxiliary treatment for snakebites could provide the experimental basis for clinical use in snakebite patients.
METHODS
Animals and groups
Male Sprague–Dawley rats (weight of 250 ± 20 g, n = 96) were purchased from the Animal Experimental Center of the Southwest Hospital, Third Military Medical University, Chongqing, China (license number: SCXK [military] 2012-0009). The rats were housed in a temperature- and humidity-controlled animal facility with 12 h light/dark cycles. All procedures used in this study were in accordance with our institutional guidelines that complied with the international ethics and humane standards for animal use. Every effort was made to minimize the number of animals and reduce their suffering.
The rats were randomly divided into four groups: the control group (n = 24), the snake venom group (n = 24), the antivenom group (n = 24), and the antivenom + HBO group (n = 24). The rats in each group were further divided into four subgroups (n = 6) according to the time of initiation of HBO intervention (0 h, 4 h, 11 h, and 23 h after administration of antivenom).
Snake venom and antivenom
Lyophilized D. acutus venom was provided by the Qimen Snake Injury Research Institute (Huangshan, Anhui Province, China). The anti-D. acutus antivenin was purchased from Shanghai Biotechnology Co. Ltd., (Shanghai, China).
Animal models
(1) The control group: Received no treatment. (2) The snake venom group: Based on reported methods,[9,10] the LD50 of D. acutus venom in rat was 1.594 mg/kg; the D. acutus venom freeze-dried powder was solubilized in sterile normal saline at 1.594 mg/ml. The rats were anesthetized by intraperitoneal injection of ketamine (70 mg/kg) and xylazine (8 mg/kg) mixture, and 0.8 LD50 was injected via the caudal vein (the selected dose resulted in pathological manifestations of the brain tissue based on published literature[9,10] and prior experiments). It was observed that systemic pathogenic changes could be observed at doses of 0.8 times LD50 of D. acutus venom and 20% mortality within 24 h. After administration of the snake venom, rats demonstrated polypnea accompanied by moist rales and increased heart rates, but no lethality was observed, indicating a successful establishment of the model. (3) The antivenom group: After establishing the model, rats were administered with D. acutus antivenin immediately via the caudal vein (78 U/kg,[10] which was 0.8-fold of the above dose). (4) The antivenom + HBO group: At 0 h, 4 h, 11 h, and 23 h after injecting the specific antivenom, the experimental animals were placed in HBO chambers (GB-150 steel experimental animal hyperbaric chamber) and treated once for 1 h. The time points of HBO intervention were set based on the following: first, this project studied the acute pathological changes and treatment measures, and the poisoned rats usually died within 24 h after venom administration; second, abnormal laboratory indicators could be detected within 5 h in clinical snakebite patients; third, the half-life of D. acutus venom was 53.1 min; and fourth, the neuroprotection effect of HBO was observed in conditions of 0.2 MPa HBO environment for 1 h,[11] and brain pathological findings could be recorded within 2 h after venom administration.[12] Hence, the different intervention times for HBO were selected to observe the earliest changes in envenomed rats and the earliest effects of HBO. The HBO method was as follows:[13] compression was conducted at 0.12 MPa for 5 min (1.2ATA) with pure oxygen. Subsequently, the cabin pressure was increased to 0.2 MPa in 5 min (2ATA) with voltage stabilized oxygen inhalation for 40 min. At the end of HBO exposure, decompression was conducted for 10 min. The concentration of oxygen in the chamber remained above 95%.
Sample collection
At the corresponding time points after HBO therapy (1 h, 5 h, 12 h, and 24 h after antivenin injection), blood samples (2 ml) were collected via the abdominal aorta. Within 2 h, the blood was centrifuged and the supernatant was collected and stored at −80°C until analyzed. The animals in each time point were euthanized by perfusion through the left ventricle with 200-ml chilled phosphate-buffered saline (PBS 0.1 mol/L) (Chinese fir Biological Technology Co., Ltd., China), followed by 400-ml 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4). The brains were harvested and fixed for 24 h. Brain tissues were coronally sectioned into 5-μm paraffin slices for H&E and immunohistochemistry (IHC) stain. The brain tissues in the SVZ were immediately isolated and stored at −80°C for real-time polymerase chain reaction (PCR) and Western blotting.
Methods for detection of indices
Coagulation function
Six plasma samples were withdrawn from each group (500 μl per sample), and coagulation functions (prothrombin time [PT], activated partial thromboplastin time [APTT], and fibrinogen [FIB]) were analyzed using the automatic biochemistry analyzer (Progress 300, China).
Serum superoxide dismutase and malondialdehyde detection
Xanthine oxidase and thiobarbituric acid methods were used to detect superoxide dismutase (SOD) and malondialdehyde (MDA), respectively, using the SOD and MDA Kits (Jiancheng, Nanjing, China).
Hematoxylin and eosin in brain tissue
Paraffin slices were dewaxed and rehydrated, then stained with hematoxylin (Beyotime Biotechnology, China) for 3 min, washed for 5 min, destained with hydrochloric acid alcohol, washed for 5 min, and fast stained with eosin (Beyotime Biotechnology, China). They were then washed, dehydrated, cleaned, and cover-slip mounted using neutral balsam.
Detection of Nestin expression by immunohistochemistry
The immunohistochemical strept avidin-biotin complex (SABC) method was used to detect the expression of nestin protein using mouse anti-rat monoclonal primary antibody (1:200, CST, USA). PBS (pH 7.4) was used as the negative control. Primary antibody was incubated overnight at 4°C, followed by incubation with biotinylated goat-anti-mouse IgG (mouse Kit, Beijing Chinese Fir Biological Technology Co., Ltd., China) for 30 min at 37°C. HRP–streptavidin–SABC substrate was then added for 30 min at 37°C, and the color was visualized using 3-3-diaminobenzidine as the chromogen substrate. The cytoplasm contained brown or brownish yellow granules indicating positive expression of nestin. Using high-magnification microscope (×400), the percentage and the degree of positive cells in SVZ in each section of six different fields were analyzed (×400). IHC scores were assigned and higher scores indicated higher expression of nestin.[14]
Nestin mRNA expression detected by real-time polymerase chain reaction
TRIzol (Invitrogen, Massachusetts, USA) was used to extract total RNA from the brain tissue in SVZ, and the concentration and purity of RNA were analyzed using the ultramicro ultraviolet spectrophotometer (ND2000C, Thermo, Massachusetts, USA). Then, 2 μg of total RNA was reverse transcribed according to the manufacturer's instruction (TOYOBO, Osaka, Japan). cDNA was then further amplified using specific primers [synthesized by Sangon Biotech, Shanghai Co Ltd, Shanghai, China [Table 1]. The real-time PCR reaction conditions were as follows: predenaturation at 96°C for 6 min, denaturation at 96°C for 30 s, annealing at 59°C for 30 s, and extension at 72°C for 30 s. The target gene Nestin and the internal reference gene Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were amplified for 40 cycles, and the fluorescence signal was recorded at 72°C (Bio-Rad CFX96 real-time fluorescence quantitative instrument, Califonia, USA). Each sample was analyzed in triplicate, and Ct was termed as the average value of the samples. 2−ΔΔCt represented the relative expression level of Nestin mRNA.
Table 1.
Primer sequences for Nestin expression
| Gene | Primer sequence (5’-3’) | Amplified product (bp) |
|---|---|---|
| Nestin | Sense: TAC TGA AAA GTT GCT GCCA | 164 |
| Antisense: TCT GAT CTC TGC AT CTACA | ||
| GAPDH | Sense: GGC CTC CAA GGA GTA AGA AA | 203 |
| Antisense: GCC CCT CCT GTT ATT ATGG |
Nestin protein expression by Western blotting
SVZ samples were obtained from three rats from each group at each time point. The protein was extracted by homogenizing tissues in prechilled radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology, Beijing, China). The homogenate was centrifuged at 12,000 r/min at 4°C for 20 min to collect the supernatant. Total protein concentration was measured and 50 μg of protein was resolved using an 8% SDS-PAGE (Beyotime Biotechnology, Beijing, China) for the detection of specific proteins. The proteins were transferred to polyvinylidene fluoride (PVDF) membranes (0.45 μm, Millipore, Massachusetts, USA) and probed with anti-Nestin rabbit anti-rat polyclonal (1:200, Boster, Wuhan China) and anti-GAPDH mouse anti-rat monoclonal antibodies (1:1000, Beyotime Biotechnology Beijing, China) overnight at 4°C. Subsequently, goat anti-rabbit HRP and goat anti-mouse HRP (1:2500, Beijing Chinese fir Biological Technology Co., Ltd., Beijing, China) were incubated for 2 h at room temperature, and then the membrane was developed with an enhanced chemiluminescence kit by Bio-Rad system. The band intensity was measured using Image-Pro software (Media Cybernetics, Maryland, USA).
Statistical analysis
Standard test was used to identify whether the data conformed to normal distribution, and the measurement data were expressed as mean ± standard deviation (SD). Homogeneity of variance test was used to identify whether the data conformed to homogeneity of variance, and the statistical significance was determined with one-way analysis of variance (ANOVA) using SPSS17.0 (IBM Company, Chicago, USA). P < 0.05 was considered statistically significant.
RESULTS
Pathological changes in brain tissue
Cells were arranged in a regular and dense manner in the upper lateral SVZ in the control group, with no edema observed. However, the cell numbers decreased and were disorganized in the snake venom group. Brain tissues showed evident edema, cloudiness, and lesions 1 h after administration of the snake venom. After 5 h, there was a significant improvement compared to 1 h after snake envenomation and continued to recover until 24 h. In the antivenom group and the antivenom + HBO group, the cell number increased, cell arrangement was more regular, and edema was alleviated. The condition was considerably significant in the antivenom + HBO group. The brain lesion recovery in the SVZ was near normal after HBO combination therapy, especially at 1 h after envenomation [Figure 1].
Figure 1.
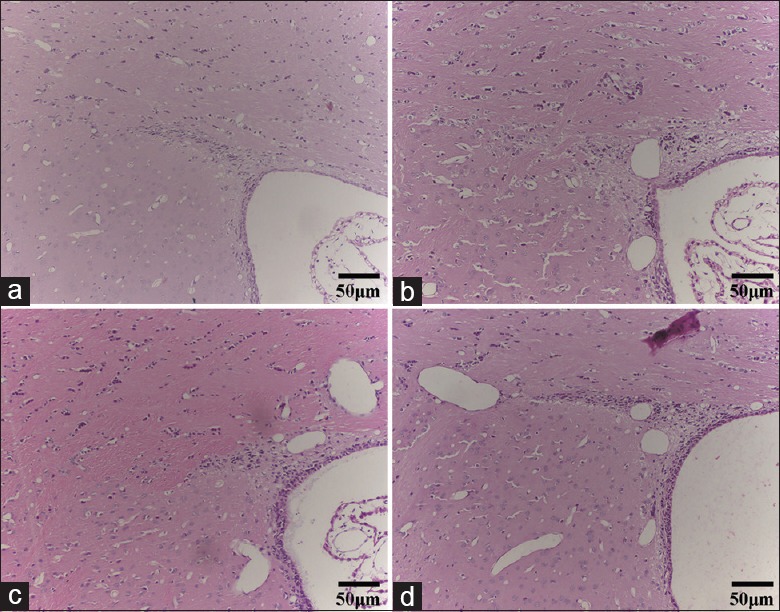
The antivenom with HBO could more markedly improve tissue edema in the SVZ than antivenom alone at 1 h after injection of the Deinagkistrodon acutus venom (H&E, bar = 50 μm). (a) The control group, (b) the snake venom group, (c) the antivenom group, and (d) the antivenom + HBO group. HBO: Hyperbaric oxygen.
Nestin protein expression in brain tissue detected by immunohistochemistry
The control group did not show positive nestin expression but was observed in the SVZ at each time point in the snake venom group as brown or brownish yellow fine granules. The positive expression was mainly observed in the cytoplasm of the NSCs and distinctly in the upper lateral SVZ. Expression was upregulated at 1 h after envenomation and decreased after 5 h. Nestin-positive cell increased at each time point after antivenom administration. Moreover, following intervention with antivenom combined HBO, the expression was substantially upregulated, and the difference was most evident at 1 h postenvenomation [Figure 2].
Figure 2.
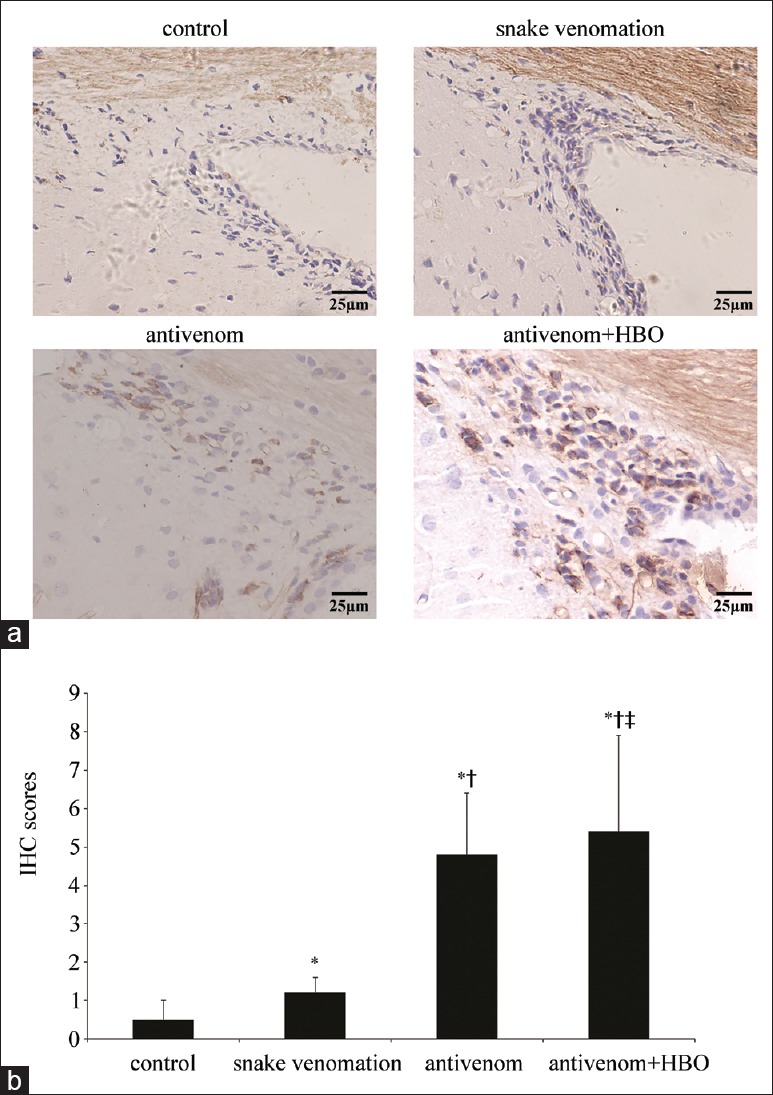
Nestin protein expression was significantly upregulated in the SVZ of antivenom + HBO group than the antivenom group at 1 h after injection of Deinagkistrodon acutus venom. (a) The nestin immunohistochemistry in the SVZ of each group at 1 h after injection of snake venom. The nestin-positive expression was mainly seen in the cytoplasm of the NSC. Significant increase in the number of nestin-positive cell was noted in snake venomation group at 1 h after injection of the snake venom. The above phenomenon was more obvious in antivenom + HBO group than the antivenom group. (b) The histogram showed the IHC scores of (a). The scores were results of the positive percentage score multiplied by the positive degree score (IHC staining, bar = 25 μm). *P < 0.05: Compared with control at the same time point; †P < 0.05: Compared with the snake venom group at the same time point; ‡P < 0.05: Compared with the antivenom group at the same time point; SVZ: Subventricular zone; HBO: Hyperbaric oxygen; NSC: Neural stem cell; IHC: Immunohistochemistry.
Nestin mRNA and protein expression changes in brain tissue
Nestin mRNA and protein expression in the brain tissue of SVZ were both significantly increased after administration of snake venom (P < 0.05). The expression peaked at 1 h after injection and gradually reduced in a time-dependent manner. After treatment with antivenom, nestin mRNA and protein expression levels were further upregulated. The difference was statistically significant at 1 h post-envenomation (F = 24.382, P = 0.000). Furthermore, nestin upregulation was further increased after HBO intervention and was most significant within 5 h postenvenomation (F = 150.781, P = 0.000) [Figure 3].
Figure 3.
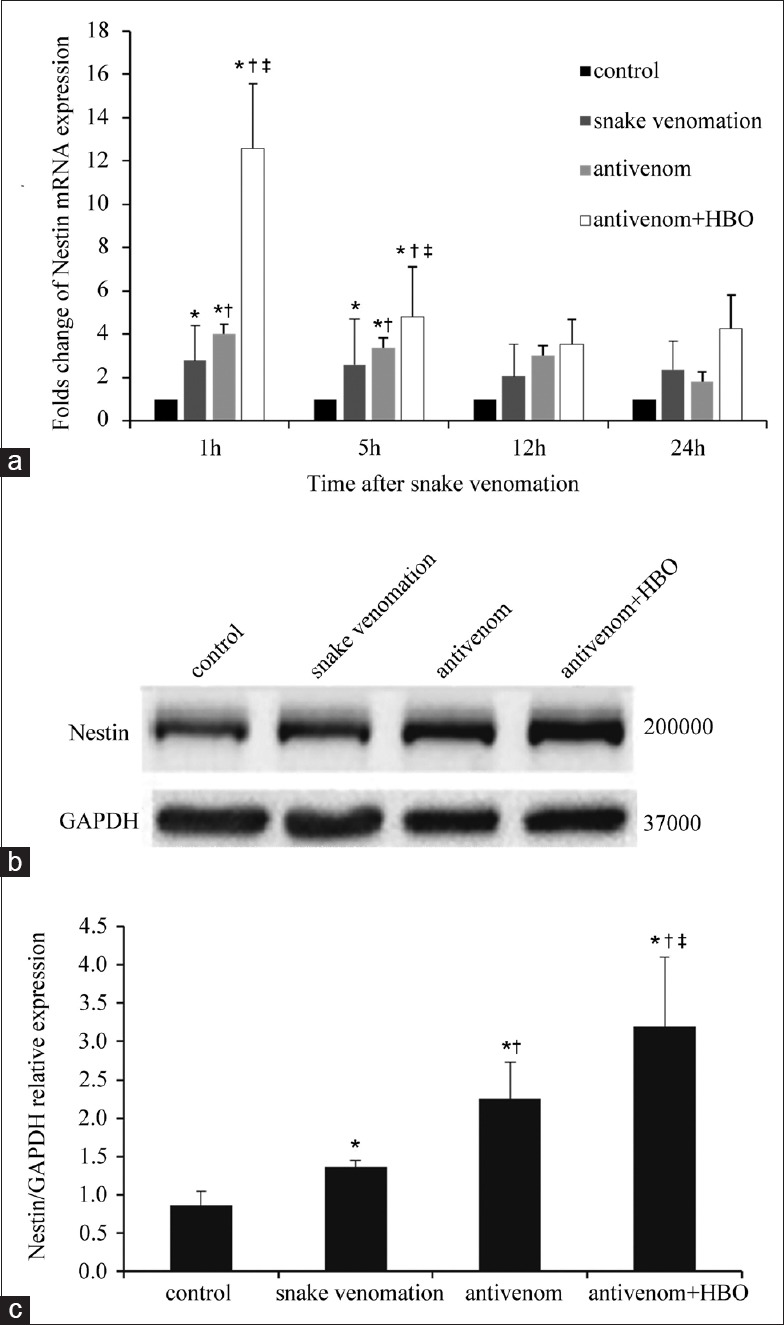
Nestin gene and protein expressions were significantly upregulated in the condition of antivenom with HBO at 1 h after injection Deinagkistrodon acutus venom. (a) The histogram was the quantified PCR of Nestin gene expression. The antivenom with HBO significantly upregulated the Nestin gene expression, especially at 1 h after injection of snake venom. (b) Changes in expression level of nestin protein by Western blotting assay. (c) Semiquantitative results from the Western blotting assay in (b). The density of nestin band was normalized against beta-actin. Values were normalized to control and expressed as the mean ± standard deviation (n = 3). *P < 0.05: Compared with control at the same time point; †P < 0.05: Compared with the snake venom group at the same time point; ‡P < 0.05: Compared with the antivenom group at the same time point; HBO: Hyperbaric oxygen; PCR: Polymerase chain reaction.
Altered coagulation function
At 1 h after envenomation, the rats in the snake venom group showed evident coagulation dysfunction. The coagulation dysfunction mildly improved after envenomation with time but was still abnormal compared with control rats (P = 0.000). After antivenom administration, coagulation dysfunction improved 1 h after envenomation, which was significantly better after HBO combined intervention (APTT: F = 1797.849, P = 0.000; PT: F = 600.760, P = 0.000; and FIB: F = 14.883, P = 0.000) and was dramatic at 5 h after envenomation (APTT: F = 25.430, P = 0.000; PT: F = 7.361, P = 0.002; and FIB: F = 2.179, P = 0.124) [Figure 4].
Figure 4.
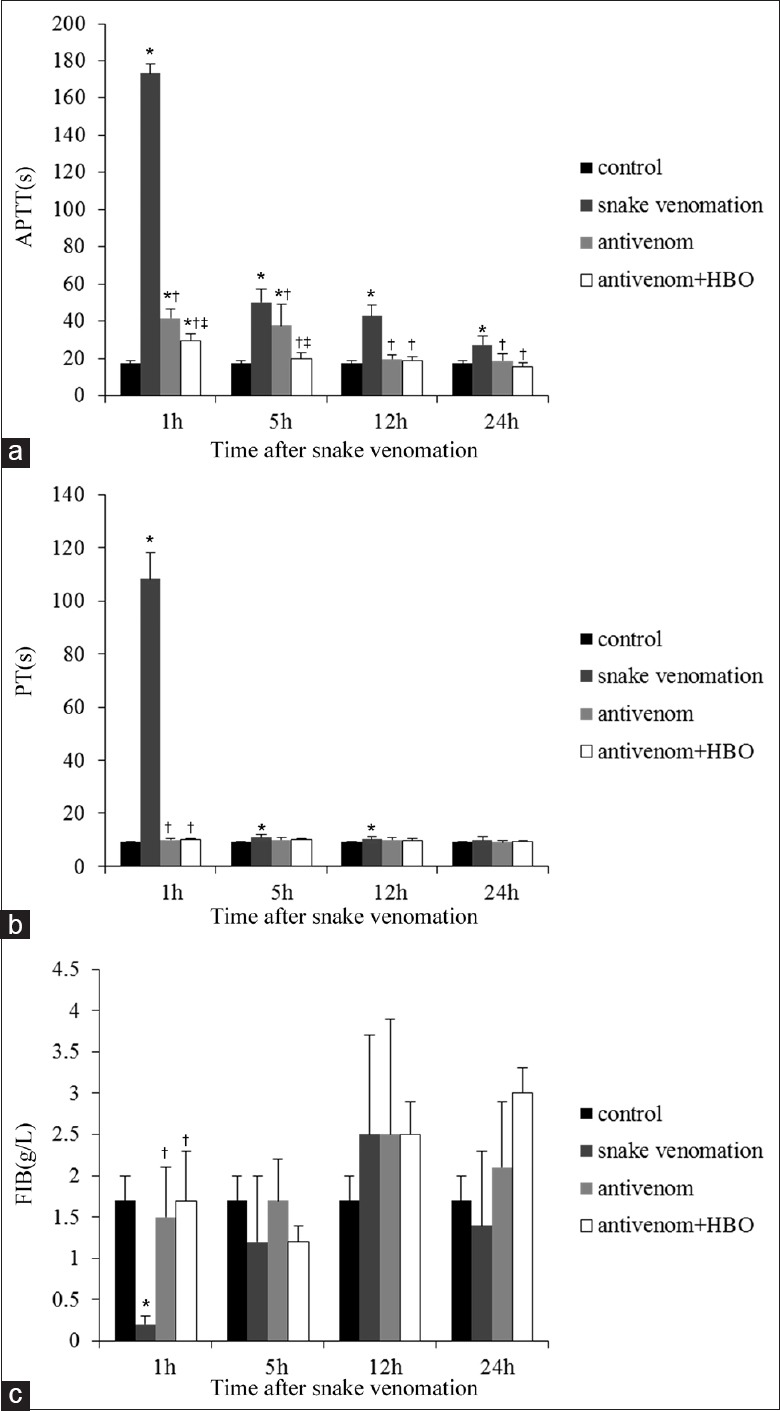
Antivenom with HBO remarkably improved the coagulation dysfunction within 5 h after injection of Deinagkistrodon acutus venom. The above figures showed that coagulation function including APTT, PT, and FIB (a-c) was significantly abnormal, especially at 1 h after snake venomation injection. Antivenom with HBO could significantly improve the coagulation dysfunction within 5 h after snakebite, and especially at 1 h. Values (mean ± standard deviation) are representative of six independent experiments (n = 6 mice/group). APTT: Activated partial thromboplastin time; PT: Prothrombin time; FIB: Fibrinogen; HBO: Hyperbaric oxygen; *P < 0.05: Compared with control group; †P < 0.05: Compared with the snake venom group; ‡P < 0.05: Compared with the antivenom group.
Serum superoxide dismutase and malondialdehyde levels
The serum SOD and MDA levels were increased after snake venom administration. Upon antivenom administration, the SOD levels increased further while MDA levels were significantly reduced. The phenotype was more dramatic after HBO combination therapy, and the differences were distinct at 1 h post-envenomation (MDA: F = 5.540, P = 0.008; SOD: F = 7.361, P = 0.000) [Figure 5].
Figure 5.
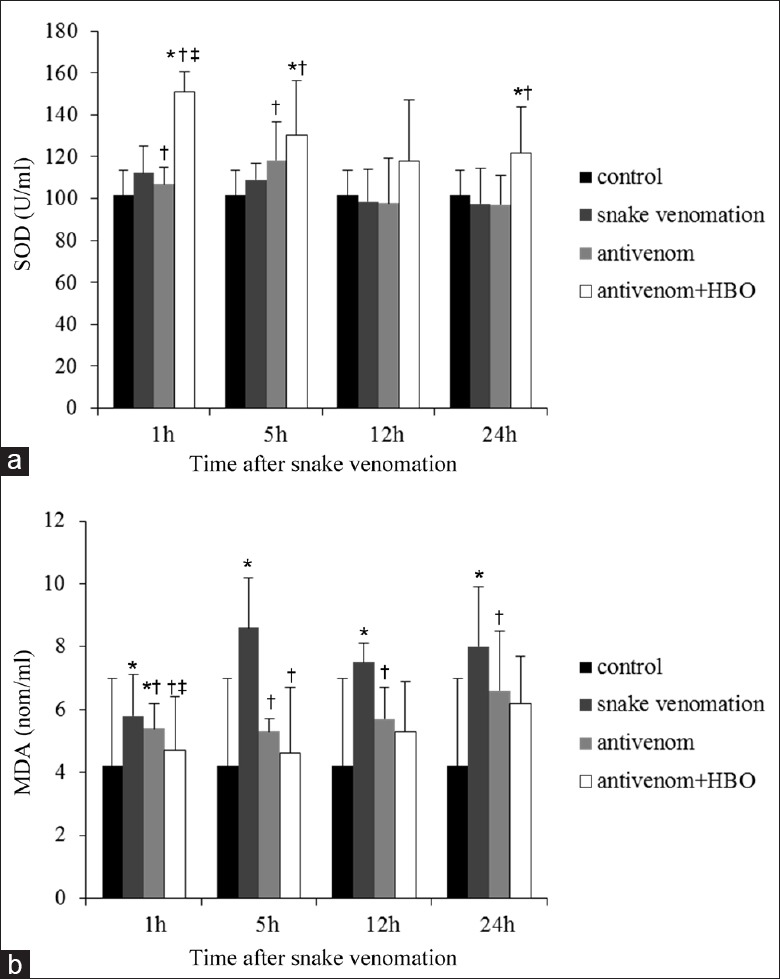
Antivenom with HBO significantly reduced serum MDA and increased serum SOD levels at 1 h after injection of the snake venom. (a) Serum SOD level lightly increased after injection of the snake venom and the phenomenon could be improved by the antivenom, which was more significant with the administration of antivenom + HBO especially within 5 h after envenomation. (b) The serum MDA level after envenomation. It showed the completely opposite tendency compared with the serum SOD levels. The antivenom with HBO administration showed more significant antioxidant effects within 5 h. Values (mean ± standard deviation) are representative of six independent experiments (n = 6 mice/group). *P < 0.05: Compared with control group; †P < 0.05: Compared with the snake venom at the same time point; ‡P < 0.05: Compared with the antivenom group; HBO: Hyperbaric oxygen; MDA: Malondialdehyde; SOD: Superoxide dismutase.
DISCUSSION
D. acutus is one of the top ten poisonous snakes in China. The local symptoms caused by these snakebites are overlooked due to the significant clinical manifestation, but brain injury was neglected due to the rare incidence. Some cases have been reported that the neurotoxic component of D. acutus venom can induce brain injury and intracerebral hemorrhage in severely poisoned patients.[2,3,4] However, neurotoxic effects of D. acutus venom in animal experiments have seldom been studied. The clinical therapy in worldwide for snakebite is based on the administration of adequate antivenom in a timely manner, assisted by traditional Chinese herbs, local debridement drainage, anti-inflammatory detoxification, hemodialysis, and symptomatic treatment. The treatments mentioned above are aimed to protect organ function, reduce edema, and attenuate ulceration and disability. However, the efficient restoration of damaged nerves induced by snakebites has seldom been studied.
Our results demonstrate that the coagulation functions of rats were dramatically abnormal after D. acutus envenomation and were the worst 1 h after envenomation. Twelve hours after envenomation, the condition gradually improved but was still abnormal compared with control rats. This phenotype was consistent with the coagulation dysfunction that has been observed in patients 6 h after snakebite. The serum MDA levels in rats gradually increased with time after envenomation, while the SOD levels declined, suggesting that the body was under oxidative stress after snake venom poisoning. The antioxidant enzymes were unable to cope with the increase in oxygen radicals, thereby resulting in a significant amount of free oxygen radicals aggravating injury to the brain. This was in line with previous studies which showed that the component of D. acutus venom, phosholipase, proteolytic enzyme, and matrix metalloproteinase can destroy target cell membranes to produce a large amount of active oxygen free radicals. The above materials damage the brain tissue.[15] In addition, the pathological changes demonstrate that after D. acutus, the edema structure in SVZ was compromised, cell numbers decreased, and there was an irregular order to cell spacing. One hour post-D. acutus envenomation, Nestin mRNA and protein expression in SVZ was clearly upregulated and demonstrated maximal expression compared to other time points. Five hours post-envenomation, Nestin expression was gradually attenuated, although it was still higher than normal levels. The upregulation of nestin in brain tissue indicated that D. acutus venom could change the microenvironment by inducing neurovirulence, dysfunctional coagulation or vascular endothelial cell injury, and oxidative stress resulting in brain injury. The above conditions further stimulated the upregulation of nestin. This suggests that upregulation of Nestin expression in brain tissue after D. acutus envenomation may be a protective response to snake toxin. This results in enhanced proliferation and differentiation activity of NSCs in brain tissue, potentially protecting the brain from injury.
HBO has achieved significant efficacy for diseases such as traumatic brain injury, carbon monoxide-induced toxic encephalopathy, and hypoxic–ischemic encephalopathy. Several clinical case reports have demonstrated that HBO adjuvant therapy for snakebite could significantly shorten the disease duration and improve the systemic poison symptoms and prognosis.[16,17] However, whether HBO could improve the repair of brain tissue after snakebite poisoning is yet to be determined. Our previous studies demonstrated that HBO could exert antioxidant effects by decreasing MDA levels, thereby inhibiting the expression of hypoxia-inducible factor-1 and a protective effect on early-stage acute organophosphorus pesticide toxicity in brain injury. The results in this study demonstrated that intervention of antivenom combined with HBO could dramatically improve the coagulation dysfunction more than that of antivenom alone after 5 h of a snakebite. This indicates that HBO could decrease the risk of bleeding by improving the coagulation, which further exerts a protective effect on brain tissue. This is in agreement with previous studies demonstrating HBO to improve coagulation disorders.[11,18] The current study also demonstrated that therapy with antivenom in combination with HBO could dramatically improve brain edema in SVZ, increase the number of cells, and promote normal cellular arrangement. Moreover, it decreased serum MDA levels and increased SOD levels, which was in agreement with our previous study.[12] This suggests that HBO could increase the antioxidant capability of the injured local tissue and alleviate brain injury by increasing oxygen supply, thereby improving hypoxia in brain tissues. Several studies have demonstrated that HBO could increase Nestin expression in SVZ and hippocampus, which is involved in the repair of injured nerves.[19,20,21] We found that combination therapy enhanced Nestin expression in SVZ when compared to antivenom alone, especially in the early period. Furthermore, HBO intervention could promote damaged nerve repair by stimulating the proliferation and differentiation of NSCs in the SVZ. A previous study reported that extending the course of HBO treatment could significantly promote NSC proliferation and differentiation to implement the neuroprotection,[22] which was confirmed in our present study. Our results suggest a new auxiliary therapy for snakebites in the clinic.
In summary, the current study demonstrated that D. acutus envenomation showed neurotoxic effects. Antivenom combined with HBO could significantly upregulate Nestin expression in SVZ and attenuate the coagulation dysfunction and oxidative stress, especially within the initial period after snakebite envenomation. This may be involved in the repair of injured brain tissue, exerting a protective effect. However, based on the current study, it is not easy to clarify the effect of delayed HBO therapy and the mechanism of the combined therapy. We need to further explore whether extending the course of HBO treatment will exert a neuroprotective effect after D. acutus envenomation and the mechanism of neuroprotection of therapy with antivenom combined with HBO.
Financial support and sponsorship
This study was supported by grants from the Science and Technology Foundation of Guizhou Province (No. SY [2013]3067) and National Natural Science Foundation of China (No. 81560217).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.White J. Snake venoms and coagulopathy. Toxicon. 2005;45:951–67. doi: 10.1016/j.toxicon.2005.02.030. doi: 10.1016/j.toxicon.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 2.He DJ, Xu ZQ, Le DY. One case report: Subarachnoid hemorrhage caused by Deinagkistrodon acutus (in Chinese) Chin J Emerg Med. 2013;22:247. doi: 10.3760/cma.j.issn.1671-0282.2013.03.05. [Google Scholar]
- 3.Cheng CL, Mao YC, Liu PY, Chiang LC, Liao SC, Yang CC. Deinagkistrodon acutus envenomation: a report of three cases. J Venom Anim Toxins Incl Trop Dis. 2017;23:20. doi: 10.1186/s40409-017-0111-1. doi: 10.1186/s40409-017-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li WD, Jiang ZG, Dong MP, Jiang XT. The neurotoxic effect of the Agkistrodon acutus (in Chinese) South Anhui Med Rep. 1989;3:153–6. [Google Scholar]
- 5.Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Del Brutto OH, Del Brutto VJ. Neurological complications of venomous snake bites: A review. Acta Neurol Scand. 2012;125:363–72. doi: 10.1111/j.1600-0404.2011.01593.x. doi: 10.1111/j.1600-0404.2011.01593.x. [DOI] [PubMed] [Google Scholar]
- 8.Mazer-Amirshahi M, Boutsikaris A, Clancy C. Elevated compartment pressures from copperhead envenomation successfully treated with antivenin. J Emerg Med. 2014;46:34–7. doi: 10.1016/j.jemermed.2013.05.025. doi: 10.1016/j.jemermed.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Li XP, Lin MQ, Xu FB, Zhong MS, Qiu YQ. Experimental study on Qingjie Decoction in the treatment of snake venom of disseminated intravascular coagulation induced by Agkistrodon acutus venom in rabbit model (in Chinese) New J Trad Chin Med. 2005;37:92–3. doi: 10.3969/j.issn.0256-7415.2005.06.053. [Google Scholar]
- 10.Yan ZR, Wang WC, Mao WL, Zhen JT, Wang HG. Study of the intervention effect of 717 Jiedu Decoction on the expression of serum PLA2 in viper bited rats (in Chinese) Chin J Trad Chin Med. 2013;28:197–9. [Google Scholar]
- 11.Yang Y, Yang JL, Xie ZH. The effect of hyperbaric oxygen on the expression of hypoxia inducible factor-l alpha in brain tissue after acute organophosphate poisoning (in Chinese) Chin J Phys Med Rehabil. 2015;37:332–5. doi: 10.3760/cma.j.issn.0254-1424.2015.05.003. [Google Scholar]
- 12.Yang YJ, Wang XL, Yu XH, Wang X, Xie M, Liu CT, et al. Hyperbaric oxygen induces endogenous neural stem cells to proliferate and differentiate in hypoxic-ischemic brain damage in neonatal rats. Undersea Hyperb Med. 2008;35:113–29. [PubMed] [Google Scholar]
- 13.Xie ZH, Chen ZP, Cao R, Yang L, Chen ZX, Liang GB. The effect of hyperbaric oxygen on the expression of hypoxia inducible factor-l alpha mRNA in renal tissue after renal ischemia-reperfusion injury (in Chinese) Chin J Phys Med Rehabil. 2010;32:182–5. doi: 10.3760/cma.j.issn.0254-1424.2010.03.006. [Google Scholar]
- 14.Solomon AS. Pterygium. Br J Ophthalmol. 2006;90:665–6. doi: 10.1136/bjo.2006.091413. doi: 10.1136/bjo.2006.091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunitha K, Hemshekhar M, Thushara RM, Santhosh MS, Sundaram MS, Kemparaju K, et al. Inflammation and oxidative stress in viper bite: An insight within and beyond. Toxicon. 2015;98:89–97. doi: 10.1016/j.toxicon.2015.02.014. doi: 10.1016/j.toxicon.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Korambayil PM, Ambookan PV, Abraham SV, Ambalakat A. A multidisciplinary approach with hyperbaric oxygen therapy improve outcome in snake bite injuries. Toxicol Int. 2015;22:104–9. doi: 10.4103/0971-6580.172287. doi: 10.4103/0971-6580.172287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rainer PP, Kaufmann P, Smolle-Juettner FM, Krejs GJ. Case report: Hyperbaric oxygen in the treatment of puff adder (Bitis arietans) bite. Undersea Hyperb Med. 2010;37:395–8. [PubMed] [Google Scholar]
- 18.Wang XL, Zhao YS, Yang YJ, Xie M, Yu XH. Therapeutic window of hyperbaric oxygen therapy for hypoxic-ischemic brain damage in newborn rats. Brain Res. 2008;1222:87–94. doi: 10.1016/j.brainres.2008.05.016. doi: 10.1016/j.brainres.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Yin X, Meng F, Wang Y, Wei W, Li A, Chai Y, et al. Effect of hyperbaric oxygen on neurological recovery of neonatal rats following hypoxic-ischemic brain damage and its underlying mechanism. Int J Clin Exp Pathol. 2013;6:66–75. [PMC free article] [PubMed] [Google Scholar]
- 20.Imperatore F, Cuzzocrea S, De Lucia D, Sessa M, Rinaldi B, Capuano A, et al. Hyperbaric oxygen therapy prevents coagulation disorders in an experimental model of multiple organ failure syndrome. Intensive Care Med. 2006;32:1881–8. doi: 10.1007/s00134-006-0367-3. doi: 10.1007/s00134-006-0367-3. [DOI] [PubMed] [Google Scholar]
- 21.Cooper JS, Allinson P, Keim L, Sisson J, Schuller D, Sippel J, et al. Hyperbaric oxygen: A useful adjunct for purpura fulminans: Case report and review of the literature. Undersea Hyperb Med. 2014;41:51–7. [PubMed] [Google Scholar]
- 22.Wang QH, Yang YJ, Shen CF. Effect of different courses hyperbaric oxygen treatment on proliferation and differentiation of neural stem cells in hypoxic-ischemic brain damage rats (in Chinese) J Appl Clin Pediatr. 2009;24:538–41. [Google Scholar]


