Abstract
Background:
Acute minor ischemic stroke (AMIS) or transient ischemic attack (TIA) is a common cerebrovascular event with a considerable high recurrence. Prior research demonstrated the effectiveness of regular long-term remote ischemic conditioning (RIC) in secondary stroke prevention in patients with intracranial stenosis. We hypothesized that RIC can serve as an effective adjunctive therapy to pharmacotherapy in preventing ischemic events in patients with AMIS/TIA. This study aimed to investigate the feasibility, safety, and preliminary efficacy of daily RIC in inhibiting cerebrovascular/cardiovascular events after AMIS/TIA.
Methods:
This is a single-arm, open-label, multicenter Phase IIa futility study with a sample size of 165. Patients with AMIS/TIA receive RIC as an additional therapy to secondary stroke prevention regimen. RIC consists of five cycles of 5-min inflation (200 mmHg) and 5-min deflation of cuffs on bilateral upper limbs twice a day for 90 days. The antiplatelet strategy is based on individual physician's best practice: aspirin alone, clopidogrel alone, or combination of aspirin and clopidogrel. We will assess the recurrence rate of ischemic stroke/TIA within 3 months as the primary outcomes.
Conclusions:
The data gathered from the study will be used to determine whether a further large-scale, multicenter randomized controlled Phase II trial is warranted in patients with AMIS/TIA.
Trial Registration:
ClinicalTrials.gov, NCT03004820; https://www.clinicaltrials.gov/ct2/show/NCT03004820.
Keywords: Ischemic Preconditioning, Secondary Prevention, Stroke, Transient Ischemic Attack
INTRODUCTION
Stroke is the number one cause of death in China. The incidence rate of stroke was around 136–485 per 100,000 population.[1] Nondisabling cerebrovascular events consist of acute minor ischemic stroke (AMIS) and high-risk transient ischemic attack (TIA). The most commonly used definition of AMIS is a score of National Institutes of Health Stroke Scale (NIHSS) <4 at the time of the event.[2] The combination of aspirin and clopidogrel within 24 h of an AMIS/TIA and continuation for 21 days was recommended according to the latest Chinese and American guidelines for the secondary prevention of stroke.[3,4]
In a large-scale clinical trial among patients with AMIS/TIA (the CHANCE study), the recurrence rates of stroke/TIA within 3 months were still as high as 9.4% even the patients received dual antiplatelets within 24 h.[5] There are considerable proportional of patients who cannot arrive at the emergency rooms within 24 h to receive thrombolytic therapy or receive dual antiplatelet therapy on time in the real-world practice in China.[6] In addition, the published data demonstrating whether the guideline has changed the real-world clinical practice or not in China are rare.
Remote ischemic conditioning (RIC) involves repetitive and brief inflation of a cuff around the limb to pressures above systolic blood pressure and deflation, in order to protect distant organs such as the heart, kidney, or brain.[7] Potential protective mechanisms involved in RIC include amelioration of oxidative damage, prevention of mitochondria-dependent cell death pathways, suppression of inflammation, and immune responses.[8,9] Long-term regular (twice a day for at least 90 days) RIC showed protective effects in reducing stroke recurrence in symptomatic intracranial artery stenosis in small sample-size clinical studies.[10,11] However, the feasibility and safety of applying long-term regular RIC have not been systematically studied, and the prior study on investigating the protective effect of RIC to patients who suffered AMIS/TIA is limited.
Considering all of the factors above, it is a logical step to design a single-arm study to assess the feasibility and safety of such intervention in this subgroup of stroke patients with AMIS/TIA before launching an expensive Phase II two-arm efficacy comparison study. We hypothesized that adjunctive twice-daily RIC for 3 months is feasible, safe, and can further reduce the cerebrovascular events in patients with AMIS/TIA.
METHODS
Ethical approval
The study is being conducted at four sites in China. The study protocol was approved by Institutional Review Board (IRB) at each study site. IRB meets regularly and monitors the progress of Preventing Ischemic Cerebrovascular events in high-risk patients with acute Non-disabling Ischemic Cerebrovascular events using RIC (PICNIC-One) study to ensure that research ethics are met and every participant receives the highest standard of human protection.
Study design
PICNIC-One is a single-arm, open-label, multicenter Phase IIa futility study. The antiplatelet strategy is based on physician's best judgment: aspirin alone, clopidogrel alone, or a combination of aspirin and clopidogrel. RIC is done by 5 cycles of 5-min inflation and 5-min deflation of bilateral upper-arm blood pressure cuffs to 200 mmHg twice daily for 90 days (45 min, twice a day). The primary objective is to explore the feasibility, safety, and preliminary efficacy of 3-month regular RIC in preventing recurrent ischemic stroke or TIA in patients with AMIS/TIA.
Study organization
PICNIC-One was designed by a team of researchers from Xuanwu Hospital, the Capital Medical University (China), and the Medical University of South Carolina (USA). It is being conducted at four sites in China: Xuanwu Hospital, Capital Medical University; Shengli Oilfield Center Hospital, the First Affiliated Hospital of Hainan Medical University, and Taoyuan People's Hospital. PICNIC-One Executive Committee includes project manager, research physicians/investigators, and clinical research coordinators. The project manager reviews case report forms to ensure that enrolment criteria were met, the protocol was correctly implemented, and reports were accurate each week after the intimation of enrolment. All investigators and study staffs were required to obtain certifications in NIHSS, Modified Rankin Scale (mRS), and Barthel Index through a secure website (https://www.healthcarepoint.com). In addition, they are required to attend a site training to understand the study protocol before initiating the study.
Participants
We planned to recruit adult (age ≥18 years old) patients of either gender who has AMIS/TIA with the following inclusion and exclusion criteria.
Inclusion criteria
Each participant must meet all the following criteria in order to participate in this study: (1) ≥18 years of any gender or ethnicity; (2) diagnosed with a noncardiogenic AMIS/TIA within 14 days of stroke symptoms' onset; AMIS is defined by NIHSS score ≤3 at the time of enrolment and TIA is defined as a transient episode of neurological dysfunction without acute infarction plus the moderate-to-high risk of stroke recurrence (defined as an ABCD2 [(a): age; (b): blood pressure; (c): clinical features of TIA; (d): diabetes and duration] score of ≥4 at the time of enrolment); (3) stable vital signs, normal cardiac (Class I–II in New York Heart Association Functional Classification),[12] hepatic (normal range in blood liver function tests) and renal functions (normal range in blood renal function tests); (4) able to consent by himself/herself or by a legally authorized representative; and (5) agreed to conduct regular RIC by himself/herself or others.
Exclusion criteria
Individuals who meet any of the following criteria will be excluded from the study: (1) diagnosis of brain hemorrhage or other pathologies, such as vascular malformation, tumor, abscess, or other nonvascular diseases, based on brain computed tomography (CT) or magnetic resonance imaging (MRI); (2) mRS score >2 before the indexed event; (3) received intravenous thrombolytic therapy (alteplase or urokinase) or endovascular treatment for the indexed event; (4) contradiction to aspirin or clopidogrel (known allergy, severe asthma, or heart failure, etc.); (5) indication for anticoagulation therapy (cardiac source of embolus); (6) hemorrhagic tendency of any reason (including but not limited to hemostatic disorder, platelet count <100 × 109/L, a history of hepatic dysfunction, etc.); (7) any hemorrhagic transformation on brain scan (MRI or CT); (8) gastrointestinal bleed or major surgery within 3 months before the indexed event; (9) stroke or TIA due to medical procedure or other iatrogenic cause; (10) any upper extremity soft-tissue disease, vascular injury, or peripheral blood vessel disease which is contraindication for RIC; (11) hypertension with systolic blood pressure ≥200 mmHg despite medical treatment at the time of enrolment; (12) planned revascularization (any angioplasty or vascular surgery) within the next 3 months; (13) scheduled for surgery or intervention within the next 3 months that may affect the study procedure; (14) life expectancy ≤6 months; (15) pregnancy; and (16) currently receiving an investigational drug or device by other studies.
Study procedures and treatments
Potential participants will be identified from inpatient service or stroke emergency center. A research physician will confirm the diagnosis of AMIS (NIHSS score ≤3) or TIA with ABCD2 score ≥4. If the patient meets the criteria and gives a written informed consent, he/she will be instructed how to do RIC by himself/herself or family member. Patient will use an electric autocontrol device (patent number: ZL200820123637.X, China) for the procedure. RIC consists of five cycles of 5-min inflation at 200 mmHg and 5-min deflation of cuffs on bilateral upper limbs twice a day (45 min, twice a day). Medication strategy is based on physician's best judgment: aspirin alone (100 mg to 300 mg daily), clopidogrel alone (75 mg daily), or a combination of aspirin and clopidogrel. Study visits are scheduled on the day of enrolment (day 1), on day 30 ± 7, and on day 90 ± 14 [Table 1].
Table 1.
Event table of PICNIC-one study procedure
| Event | Enrolment | 1-month visit | 3-month visit |
|---|---|---|---|
| Day 1 | Day 30 ± 7 | Day 90 ± 14 | |
| Informed consent | X | O | O |
| Clinico-demographics | X | O | O |
| Vital signs | X | X | X |
| NIHSS | X | X | X |
| mRS | O | X | X |
| Barthel Index | O | X | X |
| Handgrip | X | X | X |
| Biochemistry test | X | (X) | X |
| Brain MRI/CT | X | (X) | (X) |
| Outcome assessments | O | X | X |
| Adverse events | O | X | X |
PICNIC: Preventing Ischemic Cerebrovascular events in high-risk patients with acute Non-disabling Ischemic Cerebrovascular events using Remote Ischemic Conditioning; MRI: Magnetic resonance imaging; CT: Computed tomography; X: Scheduled procedure by the study protocol; (X): The procedure is not required by the study protocol, but would be needed if necessary; O: The procedure is not applicable; NIHSS: National Institutes of Health Stroke Scale; mRS: Modified Rankin Scale.
Study outcomes
The primary outcome is the number (percentage) of patients who have a recurrent ischemic stroke or TIA within 90 days after the indexed event. Ischemic stroke is defined as an acute focal infarction of the brain or retina with one of the following: (1) sudden onset of a new focal neurologic deficit lasting 24 h or more, with clinical or imaging evidence of infarction and not attributable to a nonischemic cause; or (2) a new focal neurologic deficit lasting for <24 h but with neuroimaging evidence of new brain infarction and not attributable to a nonischemic cause; or (3) rapid worsening of an existing focal neurologic deficit and not attributable to a nonischemic cause, with evidence of new ischemic changes on MRI or CT of the brain and clearly distinct from the index ischemic event.[5]
Secondary outcome measures include: (1) The number (percentage) of patients who have a second ischemic stroke or TIA within 1 month after the indexed event; (2) the number (percentage) of patients with new cerebrovascular and coronary artery events within 1 and 3 months, which include hemorrhagic stroke, myocardial infarction, death from cardiovascular causes, and death from all causes, from the indexed event; (3) NIHSS score change (continuous) from the baseline to 1 and 3 months; (4) mRS score (continuous) and dichotomized at percentage with score ≤1 versus ≥2 at 1 and 3 months; (4) Barthel Index score (continuous) and dichotomized at percentage with score ≥95 versus <95 at 1 and 3 months; and (5) handgrip strength change (continuous) on the affected side in patients with upper-limb motor deficit from baseline to 1 and 3 months.
Compliance will be assessed with the simultaneous records, which are delivered by the electric autocontrol device through 4G signals. It will be used for feasibility assessment: the number (percentage) of patients who complete ≥50% and <50% of the long-term regular 45-min complete RIC tasks as well as incomplete RIC tasks (≤45 min).
Safety end points are risks of expected treatment-related local or systemic adverse events, including the number (percentage) of patients having pain with arms assessed by visual analog scale, redness or swelling of arms, skin petechiae on arms, palpitation, and dizziness. Any new condition (symptom, injury, or significant abnormal laboratory value) that is not present at the beginning of the study will be documented as an unexpected adverse event. Whether the unexpected adverse event is associated with the RIC device will be adjudicated by research physicians. Serious adverse events (SAEs) will be reported to the local IRB within 24 h. SAEs include death, life-threatening events, inpatient hospitalization or prolongation of existing hospitalization, requirements of medical/surgical intervention to prevent permanent impairment or damage, and other serious medical events.
Statistical analysis
The null hypothesis is that the recurrence rate of ischemic stroke/TIA within 3 months is greater than the largest regression probability of recurrence (P0); the alternative hypothesis is that the recurrence rate of ischemic stroke/TIA within 3 months is less than the smallest regression probability of recurrence (PA).[13]
The CHANCE study is a large-scale (5170 patients) randomized controlled clinical trial investigating the superior medication strategy in secondary prevention in patients with AMIS/TIA in a Chinese population.[5] In the CHANCE study, the recurrence rate of ischemic stroke/TIA within 3 months was 9.4% on the combination of clopidogrel and aspirin group and 13.2% on the aspirin-only group.[5] The recurrence rate after AMIS/TIA was around 10–20% according to epidemiology studies.[14,15,16,17,18,19] With α as 0.95 and power as 0.80, we assume that P0= 12% and PA= 6% in the study, thus a minimally required sample size is 150 [Figure 1].[13] If the attrition rate can be kept at no higher than 10%, a sample size of 165 should be adequate.
Figure 1.
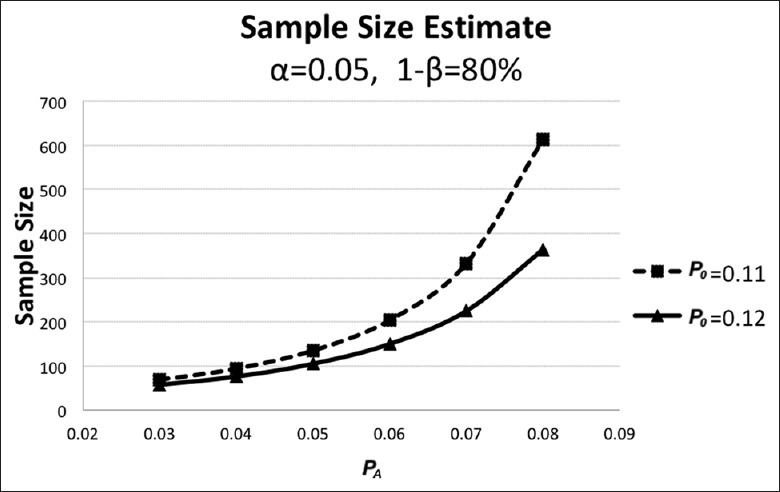
Sample size calculation according to one-sample single-stage procedure for the Phase II clinical trial. With α as 0.95 and power as 0.80, we assume that P0 = 12%, and PA = 6% in the study, a minimally required sample size is 150.
Baseline categorical variables were listed as number (percentage). Baseline normally distributed continuous variables were reported as mean ± standard deviation (SD), while nonnormally distributed continuous variables were reported in median (interquartile range). The primary analysis is per-protocol analysis as this is an open, single-arm study.
We use multivariate logistic regression models to assess the association between odds ratio of having recurrent ischemic stroke/TIA with compliance rate and other covariates. We also use repeated measures analysis with mixed models to analyze the changes in NIHSS score and handgrip strength between compliance rate and other covariates. A value of P < 0.05 was considered statistically significant.
DISCUSSION
PICNIC-One study is a single-arm Phase IIa futility study aiming to investigate the feasibility, safety, and preliminary efficacy of regular long-term RIC in preventing recurrent stroke and other vascular events in patients with AMIS/TIA. The study will provide evidence whether or not to initiate a large-scale and multicenter randomized controlled clinical study or to avoid futile, expensive, and unnecessary Phase II trials.
PICNIC-One is expected to make several unique contributions in understanding the potential role of RIC in secondary stroke prevention. First, PICNIC-One will provide preliminary evidence of the efficacy of RIC as an adjunctive therapy to pharmacotherapy after an AMIS/TIA in the real-world practice. Second, PICNIC-One will advance the current knowledge of feasibility of applying long-term regular RIC in this subset of stroke population. To our knowledge, only several small clinical trials investigated the long-term regular RIC in stroke patients, and three of them were carried at the same single site.[10,11,20] It remains a concern whether patients could strictly follow the study protocol to use RIC twice daily (90 min daily) for 90 days. The feasibility of applying RIC in multiple sites is also uncertain and requires a careful feasibility assessment. The study has four sites in different regions of China: two of them are academic tertiary hospitals and the other two are nonacademic community hospitals. The electric autocontrol RIC device used in this study has the capacity to upload application data simultaneously through 4G signals to allow the study team to track the compliance rate. Third, PICNIC-One is expected to provide evidence of the safety of RIC. Adverse events related to RIC were not sufficiently assessed due to inadequate sample size. Although there have been no reports of RIC-related SAEs in prior clinical studies,[10,11,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] SAEs may occur at a low rate and may be only detected with large sample size and many studies. PICNIC-One will continue to monitor the safety of long-term regular RIC in patients with an AMIS/TIA.
In summary, we designed PICNIC-One as a single-arm study without a control group because it is an early-phase futility study without prior preliminary data. The goal was to gain critical knowledge about the feasibility and safety of the long-term daily RIC in these stroke patients' population.
Financial support and sponsorship
PICNIC-One study is supported by the grants from the National Natural Science Foundation of China (No. 81325007 and No. 81620108011), the Capital Health Research and Special Development (No. 2016-4-1032), the American Stroke Association (No. 14SDG1829003), and the National Institute of Health (No. P20GM109040).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge Dr. Alexander Paroshos for English language edit.
Footnotes
Edited by: Ning-Ning Wang
REFERENCES
- 1.Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: Huge burden, significant workload, and a national priority. Stroke. 2011;42:3651–4. doi: 10.1161/STROKEAHA.111.635755. doi: 10.1161/strokeaha.111.635755. [DOI] [PubMed] [Google Scholar]
- 2.Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, De Marchis GM, et al. What is a minor stroke? Stroke. 2010;41:661–6. doi: 10.1161/STROKEAHA.109.572883. doi: 10.1161/strokeaha.109.572883. [DOI] [PubMed] [Google Scholar]
- 3.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. doi: 10.1161/STR.0000000000000024. doi: 10.1161/str.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Liu M and Pu C. 2014 chinese guidelines for secondary prevention of ischemic stroke and transient ischemic attack. International journal of stroke: Official journal of the International Stroke Society. 2017;12:302–20. doi: 10.1177/1747493017694391. doi: 10.1177/1747493017694391. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med. 2013;369:11–9. doi: 10.1056/NEJMoa1215340. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 6.Jin H, Zhu S, Wei JW, Wang J, Liu M, Wu Y, et al. Factors associated with prehospital delays in the presentation of acute stroke in urban China. Stroke. 2012;43:362–70. doi: 10.1161/STROKEAHA.111.623512. doi: 10.1161/strokeaha.111.623512. [DOI] [PubMed] [Google Scholar]
- 7.Hess DC, Blauenfeldt RA, Andersen G, Hougaard KD, Hoda MN, Ding Y, et al. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015;11:698–710. doi: 10.1038/nrneurol.2015.223. doi: 10.1038/nrneurol.2015.223. [DOI] [PubMed] [Google Scholar]
- 8.Narayanan SV, Dave KR, Perez-Pinzon MA. Ischemic preconditioning and clinical scenarios. Curr Opin Neurol. 2013;26:1–7. doi: 10.1097/WCO.0b013e32835bf200. doi: 10.1097/WCO.0b013e32835bf200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: Similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–37. doi: 10.1016/S0140-6736(03)14412-1. doi: 10.1016/s0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- 10.Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–61. doi: 10.1212/WNL.0b013e318271f76a. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 11.Meng R, Ding Y, Asmaro K, Brogan D, Meng L, Sui M, et al. Ischemic conditioning is safe and effective for Octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics. 2015;12:667–77. doi: 10.1007/s13311-015-0358-6. doi: 10.1007/s13311-015-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.9th ed. Boston, Mass: Little, Brown and Co; 1994. The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. [Google Scholar]
- 13.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–51. doi: 10.2307/2530297. [PubMed] [Google Scholar]
- 14.Wu L, Wang A, Wang X, Zhao X, Wang C, Liu L, et al. Factors for short-term outcomes in patients with a minor stroke: Results from china national stroke registry. BMC Neurol. 2015;15:253. doi: 10.1186/s12883-015-0505-z. doi: 10.1186/s12883-015-0505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–6. doi: 10.1001/jama.284.22.2901. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 16.Coull AJ, Lovett JK, Rothwell PM, Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: Implications for public education and organisation of services. BMJ. 2004;328:326. doi: 10.1136/bmj.37991.635266.44. doi: 10.1136/bmj.37991.635266.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP, et al. The high risk of stroke immediately after transient ischemic attack: A population-based study. Neurology. 2004;62:2015–20. doi: 10.1212/01.wnl.0000129482.70315.2f. doi: 10.1212/01.WNL.0000129482.70315.2F. [DOI] [PubMed] [Google Scholar]
- 18.Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–3. doi: 10.1161/01.STR.0000158917.59233.b7. doi: 10.1161/01.STR.0000158917.59233.b7. [DOI] [PubMed] [Google Scholar]
- 19.Ois A, Gomis M, Rodríguez-Campello A, Cuadrado-Godia E, Jiménez-Conde J, Pont-Sunyer C, et al. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke. 2008;39:1717–21. doi: 10.1161/STROKEAHA.107.505438. doi: 10.1161/strokeaha.107.505438. [DOI] [PubMed] [Google Scholar]
- 20.Zhao W, Meng R, Ma C, Hou B, Jiao L, Zhu F, et al. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: A Proof-of-concept, randomized controlled trial. Circulation. 2017;135:1325–35. doi: 10.1161/CIRCULATIONAHA.116.024807. doi: 10.1161/circulationaha.116.024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munk K, Andersen NH, Schmidt MR, Nielsen SS, Terkelsen CJ, Sloth E, et al. Remote ischemic conditioning in patients with myocardial infarction treated with primary angioplasty: Impact on left ventricular function assessed by comprehensive echocardiography and gated single-photon emission CT. Circ Cardiovasc Imaging. 2010;3:656–62. doi: 10.1161/CIRCIMAGING.110.957340. doi: 10.1161/circimaging.110.957340. [DOI] [PubMed] [Google Scholar]
- 22.Crimi G, Pica S, Raineri C, Bramucci E, De Ferrari GM, Klersy C, et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: A randomized controlled trial. JACC Cardiovasc Interv. 2013;6:1055–63. doi: 10.1016/j.jcin.2013.05.011. doi: 10.1016/j.jcin.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 23.White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:178–88. doi: 10.1016/j.jcin.2014.05.015. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Wagner R, Piler P, Bedanova H, Adamek P, Grodecka L, Freiberger T, et al. Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: A randomised controlled trial. Interact Cardiovasc Thorac Surg. 2010;11:758–62. doi: 10.1510/icvts.2010.243600. doi: 10.1510/icvts.2010.243600. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Luo W, Huang L, Zhang W, Gao Y, Jiang H, et al. Remote perconditioning reduces myocardial injury in adult valve replacement: A randomized controlled trial. J Surg Res. 2010;164:e21–6. doi: 10.1016/j.jss.2010.06.016. doi: 10.1016/j.jss.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Choi YS, Shim JK, Kim JC, Kang KS, Seo YH, Ahn KR, et al. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: A randomized controlled trial. J Thorac Cardiovasc Surg. 2011;142:148–54. doi: 10.1016/j.jtcvs.2010.11.018. doi: 10.1016/j.jtcvs.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: A phase ib study of safety and feasibility. Stroke. 2011;42:1387–91. doi: 10.1161/STROKEAHA.110.605840. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet. 2010;375:727–34. doi: 10.1016/S0140-6736(09)62001-8. doi: 10.1016/s0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 29.Rentoukas I, Giannopoulos G, Kaoukis A, Kossyvakis C, Raisakis K, Driva M, et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: Enhancement by opioid action. JACC Cardiovasc Interv. 2010;3:49–55. doi: 10.1016/j.jcin.2009.10.015. doi: 10.1016/j.jcin.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Prunier F, Angoulvant D, Saint Etienne C, Vermes E, Gilard M, Piot C, et al. The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res Cardiol. 2014;109:400. doi: 10.1007/s00395-013-0400-y. doi: 10.1007/s00395-013-0400-y. [DOI] [PubMed] [Google Scholar]
- 31.Manchurov V, Ryazankina N, Khmara T, Skrypnik D, Reztsov R, Vasilieva E, et al. Remote ischemic preconditioning and endothelial function in patients with acute myocardial infarction and primary PCI. Am J Med. 2014;127:670–3. doi: 10.1016/j.amjmed.2014.02.012. doi: 10.1016/j.amjmed.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–75. doi: 10.1093/eurheartj/eht369. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 33.Hougaard KD, Hjort N, Zeidler D, Sørensen L, Nørgaard A, Hansen TM, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: A randomized trial. Stroke. 2014;45:159–67. doi: 10.1161/STROKEAHA.113.001346. doi: 10.1161/strokeaha.113.001346. [DOI] [PubMed] [Google Scholar]
- 34.Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: A randomised controlled trial. Heart. 2009;95:1567–71. doi: 10.1136/hrt.2008.155770. doi: 10.1136/hrt.2008.155770. [DOI] [PubMed] [Google Scholar]
- 35.Ali N, Rizwi F, Iqbal A, Rashid A. Induced remote ischemic pre-conditioning on ischemia-reperfusion injury in patients undergoing coronary artery bypass. J Coll Physicians Surg Pak. 2010;20:427–31. doi: 07.2010/jcpsp.427431. [PubMed] [Google Scholar]
- 36.Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: From promise to disappointment? Circulation. 2010;122:S53–9. doi: 10.1161/CIRCULATIONAHA.109.926667. doi: 10.1161/circulationaha.109.926667. [DOI] [PubMed] [Google Scholar]


