Although primary cardiac tumors are rare, cardiac metastasis of malignant tumors is not as infrequent as we might suspect. The pericardium is the most frequently involved site and myocardial involvement is less common. In this report, we present the case of a patient with a metastatic tumor on the left ventricular wall secondary to the lung cancer with sustained ventricular tachycardia (VT).
In July 2015, a 49-year-old female was admitted to Beijing Hospital complaining of palpitation and dyspnea. She had been diagnosed with pulmonary adenosquamous carcinoma in April 2014 and had undergone tumor resection, radiation, and chemotherapy with four cycles of cisplatin and gemcitabine. In February 2015, she developed dyspnea gradually and underwent an echocardiogram which revealed moderate-to-large pericardial effusion and a new mass at the apex of the left ventricle with a left ventricular ejection fraction (LVEF) of 55%. The cardiac magnetic resonance imaging showed a 5.79 cm × 2.79 cm sessile mass at the apex and free wall of the left ventricle with large pericardial effusion [Figure 1]. The bloody pericardial effusion was drained out for symptom relief. She was then given gefitinib, and drainage tube was removed. She stayed free of symptoms for 4 months. However, the follow-up imaging showed the enlargement of the heart mass and multiple pulmonary nodules.
Figure 1.
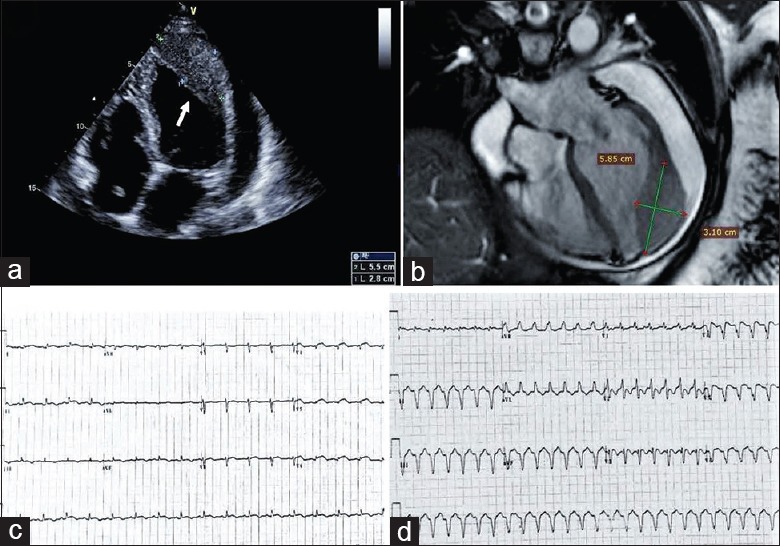
(a) Transthoracic echocardiogram showed a new mass at the apex of the left ventricle and moderate-large pericardial effusion. The arrow points to the mass. (b) Cardiac magnetic resonance showed a homogeneous slightly hyperintense mass compared with the adjacent normal myocardium in the apical and lateral wall of the left ventricle and the pericardial effusion. (c) The 12-lead ECG on admission revealed sinus tachycardia with poor R wave progression in precordial leads and ST-segment elevation in leads V4 through V6. (d) The 12-lead ECG revealed ventricular tachycardia. ECG: Electrocardiogram.
On the July 2015 admission, physical examination was unremarkable except distant heart sound and tachycardia. Hemoglobin, serum electrolytes, creatinine, and cardiac enzymes were within normal limits. NT-proBNP level was 338 pg/ml. An electrocardiogram (ECG) on presentation revealed sinus tachycardia with poor R wave progression in precordial leads and ST-segment elevation in leads V4 through V6, mimicking acute myocardial infarction [Figure 1]. An echocardiogram revealed the fixed mass at the apex and free wall of the left ventricle with moderate-sized pericardial effusion and a LVEF of 60%. On the 5th day during hospitalization, she developed sustained wide QRS complex tachycardia on telemetry monitoring. Her blood pressure was 100/70 mmHg (1 mmHg = 0.133 kPa), and she complained of palpitation. The 12-lead ECG revealed VT with a cycle length of 360 ms and right bundle branch block morphology with a left axis, consistent with origination from the anatomic location of the cardiac tumor. The VT episode sustained despite intravenous lidocaine and amiodarone therapy. The patient remained alert but her blood pressure decreased to 90/60 mmHg, and she got electrical cardioversion successfully. The intravenous amiodarone suppressed further episodes of VT and then oral amiodarone was given to prevent further ventricular arrhythmia. Defibrillator implantation and catheter ablation were considered; however, because of the patient's poor overall prognosis, these therapies were thought to be inappropriate. Since gefitinib treatment is not effective in this patient, she resumed chemotherapy and was treated with osimertinib. During 11 months of follow-up, she had no recurrences of VT on oral amiodarone. In May 2016, she suffered from another episode of amiodarone-refractory sustained VT at a rate of 160 beats/min with the same morphology as the previous VT, which was terminated by intravenous lidocaine. No more VT was recorded since then. However, the malignant tumor progressed, and the patient was repeatedly hospitalized for symptom relief and supportive care. On the last admission, the echocardiography showed very large fixed heart mass adhered to adjacent tissue and protruding into the left ventricular cavity with significantly reduced LVEF and mild pericardial effusion. The patient died due to disease progression and ventricular fibrillation in December 2016.
The survival rates of cancer have increased dramatically over the past decades. With increasing life expectancy, more patients are being seen with both cancer and heart disease. Cardiovascular complications, including cardiac metastases, are not uncommon in cancer patients with potentially profound impact on morbidity and mortality. A new discipline termed cardio-oncology has evolved to address the cardiovascular needs of cancer patients and optimize their care in a multidisciplinary approach. Chemotherapy and radiotherapy can induce cardiovascular complications including heart failure and arrhythmia. The symptoms of this patient might attribute to the cancer treatment partially. Radiation therapy can affect the heart, and pericarditis is the most frequent complication. The reported cardiotoxicities due to cisplatin and gemcitabine, which were used by this patient, include cardiomyopathy, supraventricular tachycardia, and bradycardia. Moreover, gefitinib might have a small risk of QT prolongation, which may trigger torsades de pointes. Thus, the sustained monomorphic VT episodes had little relationship with these cancer therapies, but more related to the metastatic mass, because the origin of VT was consistent with anatomic locations of cardiac metastasis. The most frequently reported malignant tumors with myocardium metastasis include lymphoma, melanoma, and lung cancer.[1,2,3] Myocardium metastases associated with ventricular arrhythmia are very rare. The ECG before and after VT episodes of this patient revealed ST-segment elevation in leads V4 through V6, mimicking acute myocardial infarction. However, the absence of cardiovascular risk factors and the normal cardiac enzymes indicated that the ECG abnormalities were not associated with acute myocardial infarction. Cates et al.[4] suggested that ECG findings of myocardial ischemia or injury, particularly localized and prolonged ST elevation, in the absence of ischemic symptoms have a high specificity for cardiac metastasis in patients with malignancy. Evidence of cardiac involvement indicates a poor prognosis.[5] Most of the reported cases with VT associated with cardiac metastases ended up in death shortly after the VT episodes. With the progression of cardiac metastasis in this patient, cardiac function was reduced, and multiple drug-refractory VT episodes recurred, and she died 1½ year later.
Cancer patients can present with a variety of cardiovascular problems not all of which are directly related to cancer therapy. Optimal treatment requires close collaboration between cardiology and oncology specialists.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient's guardians have given their consent for their images and other clinical information to be reported in the journal. The patient's guardians understood that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Cho JG, Ahn YK, Cho SH, Lee JJ, Chung IJ, Park MR, et al. Acase of secondary myocardial lymphoma presenting with ventricular tachycardia. J Korean Med Sci. 2002;17:549–51. doi: 10.3346/jkms.2002.17.4.549. doi: 10.3346/jkms.2002.17.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheldon R, Isaac D. Metastatic melanoma to the heart presenting with ventricular tachycardia. Chest. 1991;99:1296–8. doi: 10.1378/chest.99.5.1296. doi: 10.1378/chest. 99.5.1296. [DOI] [PubMed] [Google Scholar]
- 3.Lim HE, Pak HN, Jung HC, Kim JS, Shim WJ, Kim YH, et al. Electrocardiographic manifestations of cardiac metastasis in lung cancer. Pacing Clin Electrophysiol. 2006;29:1019–21. doi: 10.1111/j.1540-8159.2006.00480.x. doi: 10.1111/j. 1540-8159.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 4.Cates CU, Virmani R, Vaughn WK, Robertson RM. Electrocardiographic markers of cardiac metastasis. Am Heart J. 1986;112:1297–303. doi: 10.1016/0002-8703(86)90363-7. doi: 10.1016/0002-8703(86)90363-7. [DOI] [PubMed] [Google Scholar]
- 5.Ekmektzoglou KA, Samelis GF, Xanthos T. Heart and tumors: Location, metastasis, clinical manifestations, diagnostic approaches and therapeutic considerations. J Cardiovasc Med (Hagerstown) 2008;9:769–77. doi: 10.2459/JCM.0b013e3282f88e49. doi: 10.2459/JCM.0b013e3282f88e49. [DOI] [PubMed] [Google Scholar]


