Abstract
Secondary hyperparathyroidism (SHPT) is associated with increased bone turnover, risk of fractures, vascular calcifications, and cardiovascular and all-cause mortality. The classical treatment for SHPT includes active vitamin D compounds and phosphate binders. However, achieving the optimal laboratory targets is often difficult because vitamin D sterols suppress parathyroid hormone (PTH) secretion, while also promoting calcium and phosphate intestinal absorption. Calcimimetics increase the sensitivity of the calcium-sensing receptor, so that even with lower levels of extracellular calcium a signal can still exist, leading to a decrease of the set-point for systemic calcium homeostasis. This enables a decrease in plasma PTH levels and, consequently, of calcium levels. Cinacalcet was the first calcimimetic to be approved for clinical use. More than 10 years since its approval, cinacalcet has been demonstrated to effectively reduce PTH and improve biochemical control of mineral and bone disorders in chronic kidney patients. Three randomized controlled trials have analysed the effects of treatment with cinacalcet on hard clinical outcomes such as vascular calcification, bone histology and cardiovascular mortality and morbidity. However, a final conclusion on the effect of cinacalcet on hard outcomes remains elusive. Etelcalcetide is a new second-generation calcimimetic with a pharmacokinetic profile that allows thrice-weekly dosing at the time of haemodialysis. It was recently approved in Europe, and is regarded as a second opportunity to improve outcomes by optimizing treatment for SHPT. In this review, we summarize the impact of cinacalcet with regard to biochemical and clinical outcomes. We also discuss the possible implications of the new calcimimetic etelcalcetide in the quest to improve outcomes.
Keywords: calcimimetic agents, chronic kidney disease, cinacalcet, etelcalcetide, secondary hyperparathyroidism
Introduction
Chronic kidney disease (CKD) is rapidly becoming a public health issue, with increasing incidence and estimated worldwide prevalence of 8–16% [1]. During the course of CKD, the decline in renal function is accompanied by the development of disorders of calcium and phosphate metabolism, leading to the well-known condition ‘Mineral Bone Disease associated with CKD’ (CKD-MBD) [2]. This condition develops due to the inability of the kidney to excrete phosphate properly, leading to its retention and accumulation, which stimulates fibroblast growth factor 23 (FGF-23) and serum parathyroid hormone (PTH) [3]. FGF-23, a peptide hormone produced mainly in the osteocytes, is able to reduce phosphate levels using three different pathways: increased renal excretion, PTH stimulation and calcitriol synthesis inhibition. The latter promotes decreased gastrointestinal absorption of phosphate and calcium, leading to hypocalcaemia. The low calcitriol levels and hypocalcaemia also stimulate PTH secretion in the parathyroid glands and induce the development of parathyroid gland hyperplasia and secondary hyperparathyroidism (SHPT).
SHPT is associated with increased bone turnover, risk of fractures, vascular calcifications and, most importantly, risk of cardiovascular and all-cause mortality [4]. Recent observational data indicate that PTH >600 pg/mL is associated with a higher risk of cardiovascular mortality as well as all-cause cardiovascular hospitalization [5]. It is interesting to note that patients have better outcomes the longer the time that they have PTH within recommended levels. Indeed, consistent control of bone metabolism parameters, including PTH, within published recommended targets is a strong predictor of survival in haemodialysis patients [6].
The classical treatment for SHPT includes active vitamin D compounds and phosphate binders (to limit its gastrointestinal phosphate absorption) [7]. However, achievement of optimal laboratory targets is often difficult because vitamin D sterols suppress PTH secretion, but also promote calcium and phosphate intestinal absorption.
Calcium-sensing receptor (CaSR) is essential for the maintenance of systemic calcium homeostasis, and has become an excellent target for the treatment of bone and mineral disorders. Its ligands are called calcimimetics and can be classified as Type 1 (agonists), such as ionized calcium and other divalent anions that directly stimulate CaSR, or Type 2 (positive allosteric modulators), which bind to a site that is distinct from the physiological ligand and increase the sensitivity of CaSR to ionized calcium, leading to the decrease of the set-point for systemic calcium homeostasis (homeostasis is achieved with lower concentrations of ionized calcium). [8]. This enables a decrease in plasma PTH levels, and consequently of calcium levels (Table 1). Additionally, lower levels of phosphorus and calcium × phosphorus are also seen [9], demonstrating the capability of calcimimetics to improve the four critical disease biomarkers associated with SHPT (the phosphorus- and calcium-lowering effect distinguishes calcimimetics from active vitamin D) [10].
Table 1.
Effect of PTH-suppressive therapies on biochemical parameters
| PTH | Calcium | Phosphorus | FGF-23 | |
|---|---|---|---|---|
| Vitamin D analogues | ↓ | ↑ | ↑ | ↑ |
| Cinacalcet | ↓ | ↓ | ↓ | ↓ |
| Etelcalcetide | ↓↓ | ↓↓ | ↓ | ↓↓ |
First-generation compounds include phenylalkylamines R-567 and R-568, which were tested in haemodialysis patients, but pharmacokinetics issues halted further clinical development [7]. Second-generation calcimimetic drugs include cinacalcet and others that have never achieved clinical use, such as calindol and AC-265347. Cinacalcet hydrochloride was the first Type 2 calcimimetic to be approved for clinical use [11].
Treatment with cinacalcet effectively reduces PTH, calcium and phosphorus [12]. More than 10 years since its approval, cinacalcet has been demonstrated to effectively reduce PTH and improve biochemical control of CKD-MBD.
Etelcalcetide is a new second-generation calcimimetic. As a novel intravenous formulation with a pharmacokinetic profile that allows thrice-weekly dosing (at the time of haemodialysis), etelcalcetide was developed to improve efficacy and adherence, and reduce gastrointestinal adverse effects relative to cinacalcet. It was recently approved in Europe, and is regarded as a second opportunity to improve outcomes in CKD-MBD patients by optimizing treatment for SHPT using this promising new calcimimetic [13].
In this review, we summarize the impact of cinacalcet in biochemical and relevant clinical outcomes such as cardiovascular mortality, vascular calcifications and fractures. We also discuss the possible implications of the new calcimimetic etelcalcetide in the quest to improve outcomes for CKD patients.
Cinacalcet effectively controls SHPT
Cinacalcet’s efficacy and safety has been tested in several randomized controlled trials (RCTs) [11, 12, 14]. Cinacalcet treatment in addition to active vitamin D in patients with SHPT that is inadequately controlled despite standard therapy effectively decreases PTH levels, while also reducing serum calcium and phosphorus. Post hoc analysis has demonstrated that treatment with cinacalcet improves the achievement of biochemical targets recomended by international societies [15]. In a meta-analysis [16] involving eight trials (1429 patients) comparing cinacalcet treatment plus standard therapy with placebo plus standard therapy, end-of-treatment values for PTH [−290.49 pg/mL, 95% confidence interval (CI) −359.91 to −221.07], calcium (−0.85 mg/dL, 95% CI −1.14 to −0.56), phosphorus (−0.29 mg/dL, 95% CI −0.50 to −0.08) and calcium × phosphorus product (−7.90 mg2/dL2, 95% CI −10.25 to −5.54) were significantly lower with cinacalcet compared with placebo.
To allow an objective assessment of the effect of cinacalcet on lowering PTH, the vitamin D activator receptor agonist (VDRag) dose was kept constant. However, subsequent clinical studies have confirmed the PTH-lowering effect of cinacalcet used in varying or constant doses of VDRags. This is an important finding considering that, in clinical practice, cinacalcet is used often with VDRags [17]. Adverse effects of cinacalcet are described in Table 2.
Table 2.
Lateral effects of calcimimetic agents and suggested actions to take
| Side effect | Frequency | Proposed action |
|---|---|---|
| Gastrointestinal events | ||
| Nausea | Very common | Give cinacalcet with main meal after dialysis/in the eveningDecrease or fractionate the dose if symptoms appear after a dose increase Caution is advised with anti-emetics, including metoclopramide (QT prolongation) |
| Vomiting | Very common | |
| Diarrhoea and dyspepsia | Uncommon | |
| Anorexia | Common | |
| Hypocalcaemia and nervous system disorders | ||
| Hypocalcaemia | Common | Withhold or reduce cinacalcet until serum calcium levels reach 8 mg/dL or symptoms have resolvedUse of calcium-based phosphate binders, vitamin D sterols or adjustments of dialysis fluid calcium have been suggested by some authors, according to clinical judgement |
| Dizziness and paraesthesia | Common | |
| Seizures | Uncommon | |
| Others | ||
| Skin and cutaneous disorders, rash | Common | Seek other causes; consider discontinuing drug |
| Musculoskeletal, connective tissue and bone disorders, myalgia | Common | Seek other causes; consider discontinuing drug |
| Immune system disorders, hypersensitivity reactions | Uncommon | Seek other causes; consider discontinuing drug |
Besides its suppressive effect on PTH levels, data suggest that cinacalcet treatment can induce a volume reduction of the enlarged parathyroid glands with nodular hyperplasia seen in SHPT patients [18].
Cinacalcet reduces FGF-23 levels
FGF-23 levels have been associated with adverse clinical outcomes [19–24] such as progression of CKD, arterial calcification, left ventricle hypertrophy, cardiovascular events and increased mortality. Pharmacologic intervention capable of reducing FGF-23 would hold the promise of having a beneficial effect on these important clinical outcomes.
CUPID (Cinacalcet Study for Peritoneal Dialysis Patients in Double Arm On the Lowing Effect of iPTH level), a prospective RCT that evaluated the effect of cinacalcet on FGF-23 levels [25], enrolled patients that had been on peritoneal dialysis for >3 months and had PTH >300 pg/mL. Patients were randomized to cinacalcet therapy or vitamin D. The cinacalcet group had a reduction in FGF-23 levels (3960–2325 RU/mL), whereas the control group had an increase in FGF-23 concentration (2085–2415 RU/mL). ACHIEVE (ACHIEVE: Optimizing the Treatment of SHPT: A Comparison of Sensipar and Low Dose Vitamin D vs Escalating Doses of Vitamin D Alone)—a Phase 4, open-label, placebo-controlled, multicentre, RCT—was designed to compare treatment results with escalating doses of cinacalcet plus fixed low-dose calcitriol (cinacalcet-D group) versus calcitriol alone (Flex-D group) [26]. Using the data of 91 subjects from this study, Wetmore et al. verified that the percentage change of FGF-23 between the two groups differed significantly (P = 0.002) [27]. In the cinacalcet-D group, the percentage change decreased (−9.7 ± 18.2; P = 0.021), while an increase was found in the Flex-D group; however, the results were not significant (4.1 ± 16.5).
The mechanism underlying the ability of cinacalcet to decrease FGF-23 levels remains to be clarified. The CUPID investigators concluded that cinacalcet treatment was independently associated with FGF-23 reduction, and not related to the drug’s effects on PTH, calcium and phosphorus serum levels. Others have observed that the decrease of FGF-23 levels is concomitant with the decrease in phosphorus levels (but not to PTH or calcium levels) [28]. Wetmore et al. suggested that calcium and phosphorus are responsible for cinacalcet’s effect on FGF-23 levels [27]. Further investigation is needed in order to understand exactly how cinacalcet therapy results in a greater reduction of serum FGF-23 when compared with traditional drugs.
Recently, a post hoc evaluation of the EVOLVE (Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events) study added some more information to the FGF-23 issue [29]. When analysing the results from the cinacalcet-treated patient group (n = 1338), a decrease in FGF-23 levels was seen (5555–2255 pg/mL; P < 0.001), in contrast to the control group (n = 1264), where levels remained unchanged (5600–5580 pg/mL). Moreover, when compared with the control group, a larger proportion of patients in the cinacalcet group had a meaningful decline (≥30%) of FGF-23 (28% versus 64%; P < 0.001). This finding is relevant because the ≥30% reduction of FGF-23 levels was associated with a decreased risk of cardiovascular mortality (P < 0.001), sudden death (P < 0.001) and heart failure (P = 0.04).
Cinacalcet and fractures
Patients with CKD have increased risk of fractures compared with the general population [30, 31]. There is also a high rate of death and hospitalization following bone fracture among haemodialysis patients [32]. Abnormalities in bone structure, which affect bone quality, are observed in patients with CKD-MBD. Malluche et al. demonstrated that bone with high turnover had material and nanomechanical abnormalities such as a reduced mineral to matrix ratio [33]. Such turnover-related alterations in bone quality may contribute to the diminished mechanical competence of bone in CKD.
In their single centre cohort study, Iimori et al. [34] demonstrated a U-shaped correlation between PTH level and risk of fracture; only decreased [PTH <150 pg/mL; hazard ratio (HR) 3.27] or increased levels were related to a superior hazard of clinical fracture (PTH >300 pg/mL; HR 2.69).
To date, no RCT has been specifically designed to evaluate whether any compounds used in the treatment of SHPT (phosphate binders, vitamin D analogues or calcimimetics) decrease the risk of fracture in CKD patients. However, treatment with cinacalcet has been associated with reduced risk of fractures. A combined post hoc analysis of safety data from four Phase 3 RCTs enrolling 1184 patients with end-stage renal disease (ESRD) and uncontrolled SHPT (defined as PTH >300 pg/mL) showed that randomization to cinacalcet, in addition to conventional treatment with active vitamin D, resulted in a significant reduction in the risk of fracture [relative risk (RR) 0.46, 95% CI 0.22–0.95, P = 0.04] compared with placebo and conventional treatment [35].
In the EVOLVE trial, in the intention-to-treat (ITT) analysis, cinacalcet did not significantly reduced the risk of fractures [36]. During the study, more than two-thirds of patients in both groups discontinued the treatment, so a predetermined lag-censoring analysis (censoring time >6 months after stopping the study drug) was performed and a relative hazard for fracture of 0.72 (95% CI 0.58–0.90; P = 0.003) was obtained. When participants were censored at the time of co-interventions, such as parathyroidectomy and kidney transplant, the relative hazard was 0.71 (95% CI 0.58–0.87; P = 0.001). Moreover, when considering the risk of all clinical fractures (not only the first, but also subsequent ones) the multivariable-adjusted relative hazard was 0.83 (95% CI 0.72–0.98; P = 0.02). They concluded that, when considering events prompting discontinuation of the study drug, such as co-interventions and cumulative clinical fractures, cinacalcet reduced the rate of clinical fracture by 17–29% [36]. These data provide suggestive evidence that cinacalcet reduces the risk of fracture in patients with SHPT.
This effect of cinacalcet in reducing fractures is not a surprising one considering two reports that used bone histomorphometry from different groups to describe improved histology in patients treated with cinacalcet [37, 38]. In the BONAFIDE (Bone Biopsy Study for Dialysis Patients with SHPT of End Stage Renal Disease) study [38], dialysis patients with PTH >300 pg/mL and biopsy-proven high-turnover bone disease were treated with cinacalcet and a second bone biopsy was performed after 6–12 months of cinacalcet treatment. Bone formation and bone reabsortion indices were improved; most impressive was that the number of patients with normal bone histology increased from none at baseline to 20 at 12 months.
The role of bone mineral density (BMD) evaluation in chronic kidney patients is evolving. Unlike previous versions, the current KDIGO (Kidney Disease: Improving Global Outcomes) guidelines suggest that BMD testing is undertaken in patients with CKD G3a-G5D with CKD-MBD and/or risk factors for osteoporosis if results will impact therapeutic decisions [39]. Evidence-based information is scarce about the effect of cinacalcet on BMD in CKD patients. Some of the available data have shown a positive effect of cinacalcet on BMD, namely on the femoral neck [40] and proximal femur [41]. However, others have revealed that cinacalcet therapy shows no effect on BMD of the lumbar spine [41] or has a detrimental effect with associated bone loss on the femoral neck and lumbar spine [42]. The effect and significance of cinacalcet on BMD remains to be clarified.
Cinacalcet and vascular calcification
SHPT is associated with vascular calcification. Once established in haemodialysis patients, it generally progresses much faster than in the general population, leading to increased risk of all-cause and cardiovascular mortality [2].
In a single-centre prospective cohort study (n = 23), Nakayama et al. [43] evaluated the impact of cinacalcet on abdominal aortic calcification by calculating the aortic calcification area index (ACAI) before and after treatment (−12, 0, 12, 24 and 36 months). The mean ACAI values were not decreased during the observation period (21.4% at baseline, 23.9% at 12 months, 23.7% at 24 months and 24.3% at 36 months). In addition, Tsuruta et al. [44] compared coronary artery calcification (CAC) in cinacalcet (n = 8) and control (n = 60) groups, verifying a decrease (−0.094/year) in the CAC score when cinacalcet was used, while opposite was seen in the control group the (+0.034/year). However, the results were not statistically significant (P = 0.102).
The ADVANCE (A Randomized Study to Evaluate the Effects of Cinacalcet Plus Low Dose Vitamin D on Vascular Calcification in Subjects with CKD) study [45] compared vascular and cardiac valve calcification progression in 360 adult haemodialysis patients with SHPT, treated either with cinacalcet plus low-dose vitamin D (n = 180) or flexible doses of vitamin D alone (n = 180). The primary endpoint was a change in the Agatston Total CAC score (which uses the concept of plaque density and therefore reflects the amount of calcium deposited within a calcified lesion). The median percent increase in Agatston total CAC score was 24% for the cinacalcet plus vitamin D group and 31% for in the vitamin D group (stratified median treatment difference = −10.3%; 95% CI −22.6% to 0.8%; P = 0.073). Similarly, Agatston score changes in the thoracic aorta and mitral valve were not statistically significant. The aortic valve, on the other hand, had a stratified median treatment difference of −44.7% (95% CI −85.8% to −6.1%; P = 0.014).
The ADVANCE study had some limitations. A substantial number of patients assigned to the cinacalcet group received doses of vitamin D that were higher than specified in the protocol. A post hoc analysis [46] comparing CAC progression among protocol-adherent patients treated with cinacalcet showed that the percentage increase in CAC and aortic valve calcification was significantly slower in the cinacalcet group. Other limitations included the open-label design and short period of follow-up (12 months, which is unlikely to be sufficient for the detection of substantial changes in vascular calcification). Finally, the reduced observed calcification progression could not be solely attributed to cinacalcet; the lower doses from vitamin D sterols in the cinacalcet group also have to be considered.
Cinacalcet, cardiovascular disease and all-cause mortality
Elevated serum levels of phosphorus, calcium, PTH and FGF-23 have been linked to death and cardiovascular outcomes [47–49]. In a post hoc analysis, Cunningham et al. [35] combined data on clinical outcomes from four Phase 3 RCTs and showed that treatment with cinacalcet resulted in a significant reduction in the risk of cardiovascular hospitalization (HR 0.61; 95% CI 0.43–0.86) and a non-significant tendency to reduce all-cause mortality. Another observational study, including 19 186 haemodialysis patients from a large dialysis provider [50] receiving intravenous vitamin D analogues (as a surrogate for the diagnosis of SHPT), found that treatment with cinacalcet was associated with significant reductions in all-cause and cardiovascular mortality, with more pronounced survival benefits found in patients with SHPT of greater severity. In a prospectively observational study, Block et al. [50] described a significant survival benefit associated with cinacalcet prescription. These observations and those of others [51, 52] led to the development of a prospective RCT evaluating the effect of cinacalcet treatment on cardiovascular mortality. The EVOLVE [53] study was an RCT that enrolled 3883 haemodialysis patients with moderate to severe SHPT (median PTH 693 pg/mL) assigned to receive cinacalcet (n = 1948) or placebo (n = 1935). All patients were eligible to receive conventional treatment including phosphate binders and vitamin D sterols. The primary composite endpoint was time until death, myocardial infarction, hospitalization for unstable angina, heart failure or a peripheral vascular event. In an unadjusted ITT analysis, the primary endpoint was reached in 48.2% of patients in the cinacalcet group and 49.2% in the placebo group (relative HR in the cinacalcet group 0.93; 95% CI 0.85–1.02; P = 0.11). After adjusting for baseline characteristics, the relative HR for the primary composite endpoint was 0.88 (95% CI 0.79–0.97; P = 0.008). In fact, despite randomization, there was an unexpected 1-year difference in age between groups (median age 55 years in the cinacalcet group and 54 years in the placebo group). As age is one of the strongest predictions of death, this difference may have affected the results. Also, the statistical power of the EVOLVE study was affected by high rates of treatment crossover because of discontinuation in the cinacalcet group (dropout) and use of commercially available cinacalcet in the placebo group (drop-in). A prespecified lag-censoring analysis, in which data was censored 6 months after patients stopped cinacalcet, was performed. This analysis found a significant reduction in the risk of primary composite endpoint (HR 0.85; 95% CI 0.76–0.95; P = 0.003) and risk of death (HR 0.83; 95% CI 0.73–0.96; P = 0.009) in the cinacalcet group.
Another predetermined protocol analysis compared younger (<65 years) and older patients (≥65 years) [54]. Cinacalcet reduced the risk of death and major cardiovascular events in older but not younger patients (HR 0.70; 95% CI 0.60–0.81 for older subjects and HR 0.97; 95% CI 0.86–1.09 for younger subjects).
Although the primary analysis of the EVOLVE trial was negative, prespecified additional analysis showed significant reduction of the risk of death or cardiovascular outcomes, which suggests a potential benefit of cinacalcet.
Palmer et al. [55] have published a meta-analysis of randomized trials that evaluated the effects of calcimimetic therapy on mortality and adverse events in adults with CKD. Including 18 trials and a total of 7446 patients, they found that cinacalcet had little or no effect on all-cause mortality (RR 0.97; 95% CI 0.89–1.05) and an imprecise effect on cardiovascular mortality (RR 0.67; 95% CI 0.16–2.87). The results of this meta-analysis should be interpreted with caution. More than half of the patients were derived from the EVOLVE trial and this meta-analysis only included the results from primary analysis, with the potential setbacks discussed above. Also, all of trials included, except the EVOLVE trial, were small and not specifically designed to assess clinically relevant outcomes such as mortality or cardiovascular events.
New calcimimetics: etelcalcetide, a step forward
Etelcalcetide is a novel second-generation calcimimetic agent that was recently approved for the treatment of SHPT [13]. Etelcalcetide is a eight amino acid peptide agonist of CaSR, which binds to CaSR by a covalent disulphide bond that results in the allosteric activation of CaSR and consequently reduces the circulating levels of PTH and calcium [56]. In contrast to cinacalcet, etelcalcetide functions as a direct agonist of CaSR, slightly activating CaSR even under calcium-free conditions (Table 3). However, downstream signalling is stronger in the presence of calcium; thus, the main action of etelcalcetide is mediated through its effects as an allosteric activator [57].
Table 3.
Comparison between cinacalcet and etelcalcetide
| Cinacalcet | Etelcalcetide | |
|---|---|---|
| Class | Calcimimetic | Calcimimetic |
| Year of approval (Europe) | 2004 | 2016 |
| Mechanism of action | Interacts with membrane-spanning segments of CaSR and enhances signal transduction, thereby reducing PTH secretion | Peptide agonist of the CaSR that interacts with and activates the receptor, thereby reducing PTH secretion |
| Mode of administration | Daily oral | IV at the end of dialysis |
| Half-life | 30–40 h | >7 days |
| Excretion | Renal (80%), faecal (15%) | Renal |
| Interaction with CYPs | Metabolized by CYP3A4, and to a lesser extent CYP1A2; inhibits CYP2D6 (caution is advised when prescribing potentially interacting drugs) | No significant interactions |
| Daily dosing (starting; maximal) | 30–180 mg | 2.5–15 mg/dialysis |
| Efficacy endpoints | ||
| >30% reduction from baseline in mean serum PTH level during the EAP | 63.9 | 77.9 |
| >50% reduction from baseline in mean serum PTH during the EAP | 40 | 52 (P = 0.001) |
| Adverse effects | ||
| Nausea | 22.6 | 18.3 |
| Vomiting | 13.8 | 13.3 |
| Diarrhoea | 10.3 | 6.2 |
| Headache | 7.0 | 6.5 |
| Hypertension | 6.7 | 6.2 |
| Hypotension | 2.9 | 6.8 |
| Muscle spasms | 5.9 | 6.5 |
| Pain in extremity | 4.1 | 5.0 |
| Asymptomatic hypocalcaemia | 59.8 | 68.9 |
| Symptomatic hypocalcaemia | 2.3 | 5.0 |
Values are expressed as percentage unless indicated otherwise. EAP, efficacy assessment phase.
Etelcalcetide has a favourable pharmacokinetic profile, with a longer elimination half-life than cinacalcet, and half-life elimination that exceeds 7 days in ESRD patients [58]. It is administered intravenously at the end of a haemodialysis session; the plasma concentration of etelcalcetide decreases over time but remains relatively constant from 24 h post-dose to the next dialysis session [13]. It is a molecule that is dialysable during haemodialysis and, with the doses of etelcalcetide reaching between 2.5 and 5 mg at the end of a dialysis session, plasma concentrations of etelcalcetide reach steady-state by week 4. Although clinical experience is of course currently limited, in vitro data show that etelcalcetide is not an inhibitor, inducer or substrate of hepatic cytochrome (CYP) enzymes, nor is it an inhibitor or substrate of common efflux and uptake human transport proteins such as P-glycoprotein [59]. Thus, etelcalcetide is expected to have a low risk for CYP or transporter-mediated drug interactions.
The immunogenicity risk of etelcalcetide has been evaluated [60]. While both preexisting anti-etelcalcetide antibodies and those that developed after treatment were detected, no consequences have been reported regarding the clinical exposure, efficacy or safety of etelcalcetide.
Pivotal trials testing etelcalcetide in the treatment of SHPT were recently published [61, 62]. Two parallel Phase 3 placebo-controlled trials were conducted in 1023 haemodialysis patients with moderate to severe hyperparathyroidism. Intravenous administration of etelcalcetide (n = 503) or placebo (n = 513) after each haemodialysis session for 26 weeks was performed. Patients randomized to etelcalcetide were significantly more likely to achieve the primary efficacy endpoint (a reduction >30% in baseline PTH: 74.0–75.3% in the etelcalcetide group versus 8.3–9.6% in the placebo group). In addition, patients randomized to etelcalcetide were significantly more likely to achieve a PTH level of 300 pg/mL or lower (49.6–53.3% in the etelcalcetide group versus 4.6–5.1% in the placebo group). The median dose of etelcalcetide during the efficacy assessment phase was 5.0 and 7.1 mg, respectively. Patients randomized to etelcalcetide were more likely to experience substantial lowering of FGF-23 despite more frequent provision of calcium and vitamin D. Treatment with etelcalcetide decreased bone-specific alkaline phosphatase and collagen Type 1 cross-linked C-telopeptide. Patients randomized to etelcalcetide had more muscle spasms, nausea and vomiting than the placebo group. Hypocalcaemia occurred in 63.8% of patients, but symptomatic hypocalcaemia was reported in only 7% of patients assigned to etelcalcetide. Similar results were obtained in a placebo-controlled trial from Japan, which tested the efficacy and safety of etelcalcetide [63].
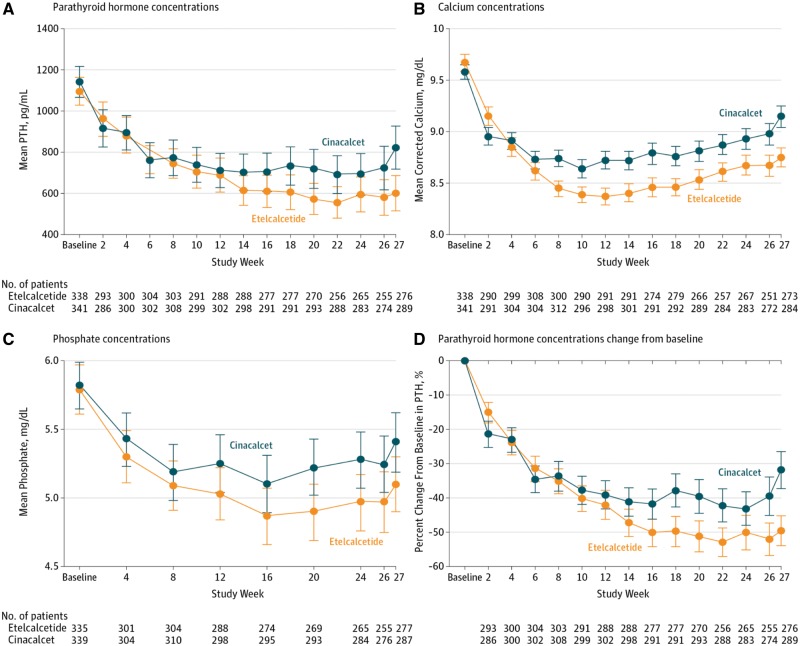
A randomized double-bind, double-dummy active clinical trial has been conducted that compared intravenous etelcalcetide versus oral placebo and oral cinacalcet versus intravenous placebo in 683 haemodialysis patients with PTH higher than 500 pg/mL [62]. The primary efficacy endpoint was non-inferiority of etelcalcetide at achieving more than a 30% reduction from baseline in mean predialysis PTH concentration, and secondary endpoints included superiority in achieving biochemical endpoints (>50% and >30% reduction in PTH) and self-reported nausea or vomiting. Etelcalcetide was not inferior to cinacalcet in reducing PTH concentration and also met superior criteria. The proportion of patients who achieved >30% PTH reduction was 68.2% in the etelcalcetide group and 57.7% in the cinacalcet group (Figure 1). There was also a significant difference in the proportion of patients who achieved >50% reduction of PTH. Hypocalcaemia was more frequent in etelcalcetide group (68.9% versus 59.8%) and the mean number of days of vomiting or nausea were not significantly different. Overall safety and tolerability between etelcalcetide and cinacalcet were similar. There was a numerically higher number of heart failure episodes in the etelcalcetide group, but overall the event rates were very low and similar to those observed in the EVOLVE trial.
Fig. 1.
Parathyroid hormone, calcium and phosphate concentrations in patients receiving cinacalcet or etelcalcetide. Reproduced with permission from Block et al. [62].
The effect of etelcalcetide on FGF-23 levels is also noteworthy. Etelcalcetide treatment yielded a more pronounced reduction in FGF-23 levels than cinacalcet. As discussed above, FGF-23 is elevated in CKD patients and has been associated with adverse outcomes such as left ventricular hypertrophy and cardiac failure. In the EVOLVE trial, a 30% reduction of FGF-23 levels was associated with significant reduction of the primary composite endpoint, heart failure and death [29]. This promising finding in the etelcalcetide group raises the possibility of a more pronounced impact on cardiovascular outcomes.
There are some important clinical aspects considering the results of the trials mentioned above that we want to highlight. Etelcalcetide is superior to cinacalcet in achieving a reduction of PTH and FGF-23 concentrations in ESRD patients; however, it also leads to more frequent episodes of hypocalcaemia. Data suggest that this hypocalcaemic effect could be more pronounced at the beginning of treatment when PTH is highest. Indeed, in a multinational placebo-controlled trial, the calcium-lowering effect of etelcalcetide was evident early after treatment initiation and reached a nadir by weeks 10–12. This calcium-lowering effect was observed despite increased use of oral calcium-containing binders and active vitamin D analogues, and increases in dialysate calcium concentration in an important proportion of patients. This observation raises legitimate concerns regarding the possible cumulative positive calcium balance. Etelcalcetide is given at the end of haemodialysis sessions, which improves medication adherence and reduces pill burden. Etelcalcetide does not seem to result in fewer gastrointestinal symptoms related to calcimimetic treatment despite intravenous administration, in contrast to what was previously anticipated. The nausea and vomiting induced by cinacalcet and etelcalcetide appears to be a systemic effect rather than a local gastrointestinal class effect.
Finally, it is tempting to speculate that the longer elimination half-life of etelcalcetide could lead to more stable control of biochemical parameters like PTH and calcium phosphate, and that such a sustained suppression of PTH could translate into improved bone turnover and metabolism, decreased vascular calcification and, ultimately, improved cardiovascular patient outcomes. Any impact on such important outcomes remains to be identified.
Conclusion
In conclusion, SHPT is associated with increased bone turnover, risk of fractures, vascular calcifications, and cardiovascular and all-cause mortality. Cinacalcet, the first calcimimetic approved for clinical use, effectively reduces PTH and improves biochemical control of mineral and bone disorders in CKD patients. However, the effect of cinacalcet on hard outcomes remains to be proved.
Etelcalcetide, a new second-generation calcimimetic, is superior to cinacalcet in achieving the reduction of PTH and FGF-23 concentrations in ESRD patients, but also leads to more frequent episodes of hypocalcaemia that could be more pronounced at beginning of treatment. Etelcalcetide is given at the end of haemodialysis sessions, which improves medication adherence and reduces pill burden. However, etelcalcetide does not seem to cause fewer gastrointestinal symptoms despite intravenous administration.
In our view, etelcalcetide represents a significant advance in the treatment of SHPT, via better control of PTH and FGF-23 levels and improved adherence. However, whether this improved biochemical control translates into improved clinical outcomes such as bone fracture rate and cardiovascular morbidity and mortality remains to be elucidated by prospective randomized trials.
Conflicts of interest statement
None declared.
References
- 1. Jha V, Garcia-Garcia G,, Iseki K. et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272 [DOI] [PubMed] [Google Scholar]
- 2. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009; 113: S1–S130 [DOI] [PubMed] [Google Scholar]
- 3. Diniz H, Frazao JM.. The role of fibroblast growth factor 23 in chronic kidney disease-mineral and bone disorder. Nefrologia 2013; 33: 835–844 [DOI] [PubMed] [Google Scholar]
- 4. Kalantar-Zadeh K, Kuwae N, Regidor DL. et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006; 70: 771–780 [DOI] [PubMed] [Google Scholar]
- 5. Tentori F, Wang M, Bieber BA. et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 2015; 10: 98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danese MD, Belozeroff V, Smirnakis K. et al. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol 2008; 3: 1423–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodriguez M, Goodman WG, Liakopoulos V. et al. The use of calcimimetics for the treatment of secondary hyperparathyroidism: a 10 year evidence review. Semin Dial 2015; 28: 497–507 [DOI] [PubMed] [Google Scholar]
- 8. Harrington PE, Fotsch C.. Calcium sensing receptor activators: calcimimetics. Curr Med Chem 2007; 14: 3027–3034 [DOI] [PubMed] [Google Scholar]
- 9. St Peter WL, Li Q, Liu J. et al. Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol 2009; 4: 354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frazao J, Rodriguez M.. Secondary hyperparathyroidism disease stabilization following calcimimetic therapy. NDT Plus 2008; 1: i12–i17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindberg JS, Culleton B, Wong G. et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol 2005; 16: 800–807 [DOI] [PubMed] [Google Scholar]
- 12. Block GA, Martin KJ, de Francisco AL. et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004; 350: 1516–1525 [DOI] [PubMed] [Google Scholar]
- 13. Blair HA. Etelcalcetide: first global approval. Drugs 2016; 76: 1787–1792 [DOI] [PubMed] [Google Scholar]
- 14. Goodman WG, Hladik GA, Turner SA. et al. The Calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol 2002; 13: 1017–1024 [DOI] [PubMed] [Google Scholar]
- 15. Moe SM, Chertow GM, Coburn JW. et al. Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 2005; 67: 760–771 [DOI] [PubMed] [Google Scholar]
- 16. Strippoli GF, Palmer S, Tong A. et al. Meta-analysis of biochemical and patient-level effects of calcimimetic therapy. Am J Kidney Dis 2005; 47: 715–726 [DOI] [PubMed] [Google Scholar]
- 17. Stubbs JR, Wetmore JB.. Does it matter how parathyroid hormone levels are suppressed in secondary hyperparathyroidism? Semin Dial 2011; 24: 298–306 [DOI] [PubMed] [Google Scholar]
- 18. Komaba H, Nakanishi S, Fujimori A. et al. Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol 2010; 5: 2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Isakova T, Xie H, Yang W. et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fliser D, Kollerits B, Neyer U. et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 2007; 18: 2600–2608 [DOI] [PubMed] [Google Scholar]
- 21. Nasrallah MM, El-Shehaby AR, Salem MM. et al. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant 2010; 25: 2679–2685 [DOI] [PubMed] [Google Scholar]
- 22. Gutierrez OM, Januzzi JL, Isakova T. et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119: 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seiler S, Reichart B, Roth D. et al. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant 2010; 25: 3983–3989 [DOI] [PubMed] [Google Scholar]
- 24. Nakano C, Hamano T, Fujii N. et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone 2012; 50: 1266–1274 [DOI] [PubMed] [Google Scholar]
- 25. Kim HJ, Kim H, Shin N. et al. Cinacalcet lowering of serum fibroblast growth factor-23 concentration may be independent from serum Ca, P, PTH and dose of active vitamin D in peritoneal dialysis patients: a randomized controlled study. BMC Nephrol 2013; 14: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fishbane S, Shapiro WB, Corry DB. et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol 2008; 3: 1718–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wetmore JB, Liu S, Krebill R. et al. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol 2010; 5: 110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuczera P, Adamczak M, Wiecek A. Cinacalcet treatment decreases plasma fibroblast growth factor 23 concentration in haemodialysed patients with chronic kidney disease and secondary hyperparathyroidism. Clin Endocrinol (Oxf) 2014; 80: 607–612 [DOI] [PubMed] [Google Scholar]
- 29. Moe SM, Chertow GM, Parfrey PS.. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the Evaluation Of Cinacalcet HCl Therapy To Lower Cardiovascular Events (EVOLVE) Trial. Circulation 2015; 132: 27–39 [DOI] [PubMed] [Google Scholar]
- 30. Nickolas TL, McMahon DJ, Shane E.. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 2006; 17: 3223–3232 [DOI] [PubMed] [Google Scholar]
- 31. Kaji H, Suzuki M, Yano S. et al. Risk factors for hip fracture in hemodialysis patients. Am J Nephrol 2002; 22: 325–331 [DOI] [PubMed] [Google Scholar]
- 32. Tentori F, McCullough K, Kilpatrick RD. et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 2014; 85: 166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malluche HH, Porter DS,, Monier-Faugere MC.. Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol 2012; 23: 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iimori S, Mori Y,, Akita W. et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant 2012; 27: 345–351 [DOI] [PubMed] [Google Scholar]
- 35. Cunningham J, Danese M., Olson K.. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int 2005; 68: 1793–1800 [DOI] [PubMed] [Google Scholar]
- 36. Moe SM, Abdalla S, Chertow GM. et al. Effects of cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE Trial. J Am Soc Nephrol 2015; 26: 1466–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malluche HH, Monier-Faugere MC, Wang G. et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol 2008; 69: 269–278 [DOI] [PubMed] [Google Scholar]
- 38. Behets GJ, Spasovski G, Sterling LR. et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int 2015; 87: 846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ketteler M, Block GA, Evenepoel P. et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int 2017; 92: 26–36 [DOI] [PubMed] [Google Scholar]
- 40. Tsuruta Y, Okano K, Kikuchi K. et al. Effects of cinacalcet on bone mineral density and bone markers in hemodialysis patients with secondary hyperparathyroidism. Clin Exp Nephrol 2013; 17: 120–126 [DOI] [PubMed] [Google Scholar]
- 41. Lien YH, Silva AL, Whittman D.. Effects of cinacalcet on bone mineral density in patients with secondary hyperparathyroidism. Nephrol Dial Transplant 2005; 20: 1232–1237 [DOI] [PubMed] [Google Scholar]
- 42. Mitsopoulos E, Ginikopoulou E, Economidou D. et al. Impact of long-term cinacalcet, ibandronate or teriparatide therapy on bone mineral density of hemodialysis patients: a pilot study. Am J Nephrol 2012; 36: 238–244 [DOI] [PubMed] [Google Scholar]
- 43. Nakayama K, Nakao K, Takatori Y. et al. Long-term effect of cinacalcet hydrochloride on abdominal aortic calcification in patients on hemodialysis with secondary hyperparathyroidism. Int J Nephrol Renovasc Dis 2013; 7: 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsuruta Y, Ohbayashi T, Fujii M. et al. Change in coronary artery calcification score due to cinacalcet hydrochloride administration. Ther Apher Dial 2008; 12: S34–S37 [DOI] [PubMed] [Google Scholar]
- 45. Raggi P, Chertow GM, Torres PU. et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011; 26: 1327–1339 [DOI] [PubMed] [Google Scholar]
- 46. Urena-Torres PA, Floege J, Hawley CM. et al. Protocol adherence and the progression of cardiovascular calcification in the ADVANCE study. Nephrol Dial Transplant 2013; 28: 146–152 [DOI] [PubMed] [Google Scholar]
- 47. Block GA, Klassen PS, Lazarus JM.. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 48. Floege J, Kim J, Ireland E. et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 2011; 26: 1948–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gutierrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Block GA, Zaun D, Smits G. et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int 2010; 78: 578–589 [DOI] [PubMed] [Google Scholar]
- 51. Akizawa T, Kurita N, Mizobuchi M. et al. PTH-dependence of the effectiveness of cinacalcet in hemodialysis patients with secondary hyperparathyroidism. Sci Rep 2016; 6: 19612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gillespie IA, Floege J, Gioni I. et al. Propensity score matching and persistence correction to reduce bias in comparative effectiveness: the effect of cinacalcet use on all-cause mortality. Pharmacoepidemiol Drug Saf 2015: 24: 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chertow GM, Block GA, Correa-Rotter R. et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012: 367: 2482–2494 [DOI] [PubMed] [Google Scholar]
- 54. Parfrey PS, Drueke TB, Block GA. et al. The effects of cinacalcet in older and younger patients on hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial. Clin J Am Soc Nephrol 2015; 10: 791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Palmer SC, Nistor I, Craig JC. et al. Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS Med 2013; 10: e1001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alexander ST, Hunter T, Walter S. et al. Critical cysteine residues in both the calcium-sensing receptor and the allosteric activator amg 416 underlie the mechanism of action. Mol Pharmacol 2015; 88: 853–865 [DOI] [PubMed] [Google Scholar]
- 57. Walter S, Baruch A, Dong J. et al. Pharmacology of AMG 416 (Velcalcetide), a novel peptide agonist of the calcium-sensing receptor, for the treatment of secondary hyperparathyroidism in hemodialysis patients. J Pharmacol Exp Ther 2013, 346: 229–240 [DOI] [PubMed] [Google Scholar]
- 58. Chen P, Melhem M, Xiao J. et al. Population pharmacokinetics analysis of AMG 416, an allosteric activator of the calcium-sensing receptor, in subjects with secondary hyperparathyroidism receiving hemodialysis. J Clin Pharmacol 2015; 55: 620–628 [DOI] [PubMed] [Google Scholar]
- 59. Subramanian R, Zhu X, Kerr SJ. et al. Nonclinical pharmacokinetics, disposition, and drug-drug interaction potential of a novel d-amino acid peptide agonist of the calcium-sensing receptor AMG 416 (etelcalcetide). Drug Metab Dispos 2016; 44: 1319–1331 [DOI] [PubMed] [Google Scholar]
- 60. Kroenke MA, Weeraratne DK, Deng H. et al. Clinical immunogenicity of the d-amino acid peptide therapeutic etelcalcetide: method development challenges and anti-drug antibody clinical impact assessments. J Immunol Methods 2017; 445: 37–44 [DOI] [PubMed] [Google Scholar]
- 61. Block GA, Bushinsky DA, Cunningham J. et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 2017; 317: 146–155 [DOI] [PubMed] [Google Scholar]
- 62. Block GA, Bushinsky DA, Cheng S. et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017; 317: 156–164 [DOI] [PubMed] [Google Scholar]
- 63. Fukagawa M, Yokoyama K, Shigematsu T. et al. A Phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of etelcalcetide (ONO-5163/AMG 416), a novel intravenous calcimimetic, for secondary hyperparathyroidism in Japanese haemodialysis patients. Nephrol Dial Transplant 2017; 32: 1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]