Abstract
Microalgae could provide a sustainable alternative to fish oil as a source for the omega-3 polyunsaturated fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). However, growing microalgae on a large-scale is still more cost-intensive than fish oil production, and outdoor productivities vary greatly with reactor type, geographic location, climate conditions and microalgae species or even strains. The diatom Phaeodactylum tricornutum has been intensively investigated for its potential in large-scale production, due to its robustness and comparatively high growth rates and EPA content. Yet, most research have been performed in southern countries and with a single commercial P. tricornutum strain, while information about productivities at higher latitudes and of local strains is scarce. We examined the potential of the climate conditions in Bergen, western Norway for outdoor cultivation of P. tricornutum in flat panel photobioreactors and cultivated three different strains simultaneously, one commercial strain from Spain (Fito) and two local isolates (M28 and B58), to assess and compare their biomass and EPA productivities, and fatty acid (FA) profiles. The three strains possessed similar biomass productivities (average volumetric productivities of 0.20, 0.18, and 0.21 g L− 1 d− 1), that were lower compared to productivities reported from southern latitudes. However, EPA productivities differed between the strains (average volumetric productivities of 9.8, 5.7 and 6.9 mg L− 1 d− 1), due to differing EPA contents (average of 4.4, 3.2 and 3.1% of dry weight), and were comparable to results from Italy. The EPA content of strain Fito of 4.4% is higher than earlier reported for P. tricornutum (2.6–3.1%) and was only apparent under outdoor conditions. A principal component analysis (PCA) of the relative FA composition revealed strain-specific profiles. However, including data from laboratory experiments, revealed more significant differences between outdoor and laboratory-grown cultures than between the strains, and higher EPA contents in outdoor grown cultures.
Highlights
-
•
Three P. tricornutum strains were grown 6 months in outdoor-reactors in West-Norway.
-
•
Biomass productivities were similar between the three different strains.
-
•
One strain had higher eicosapentaenoic acid (EPA) productivity due to increased EPA content of its biomass.
-
•
EPA content was more dependent on the strain chosen than on the season.
-
•
Higher EPA content was found under outdoor than indoor conditions.
1. Introduction
Microalgae are suggested to be a promising and sustainable feedstock for various food and non-food products. They are fast growing, rich in oils, proteins and carbohydrates, and can be cultivated in seawater and on non-arable land, and may therefore be grown in regions unsuitable for agriculture [1]. Microalgae compounds of particular interest are the omega-3 long-chained polyunsaturated fatty acids (LC-PUFAs), eicosapentaenoic acid (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6). Both are recognized as being essential for human health by helping prevent cardiovascular and inflammatory diseases [2]. Marine fish are the major EPA and DHA source, obtaining these PUFAs themselves predominantly via the marine food chain from EPA- and DHA-synthesizing microalgae. The market for marine fish oil has been increasing in recent years, dominated by the aquaculture industries that use fish oil as an ingredient in aqua-feeds in order to meet the desired EPA and DHA content in cultivated fish [3]. Additionally, there is also an increasing demand for using fish oil for EPA- and DHA-enriched products for direct human consumption [4], [5]. As the fish oil derives from wild fish stocks, its production and application has raised economic, ethical and environmental concerns, together with considerations on its purity, taste and quality, as fatty fish have been associated with the risk of contamination with environmental pollutants [3], [6].
Many marine microalgae species naturally produce EPA and DHA as components of the polar membrane lipids [7], and could, thus, provide a sustainable alternative source for the two PUFAs. However, growing microalgae on a large-scale is still more cost intensive compared to fish oil production [8]. Thus, in the past decades, efforts have been made to improve the microalgae large-scale production, and different microalgae species and photobioreactor systems have been investigated and evaluated [9]. The majority of studies on microalgal outdoor productivity have been performed in temperate countries like Spain or Australia with high irradiance, that promote microalgae production. In contrast to this, only limited studies are available from higher latitudes, where outdoor grown microalgae face strong seasonal fluctuations in temperature, irradiance and photoperiod [10]. As a result of the increased interest in microalgae-based products, long-term investigations from higher latitudes are considered important in order to evaluate the potential of different locations for microalgal outdoor production and for its impact on fatty acid (FA) content and composition.
The diatom Phaeodactylum tricornutum has been intensively studied for its potential for large-scale production, as it is easy to cultivate, fast growing and comprises a comparatively high EPA content. Moreover, its low silica requirement makes it more attractive in terms of costs of growth media compared to other diatoms [11]. As this species is common in coastal brackish and marine waters worldwide, numerous isolates exist in different culture collections [12]. However, many studies on large-scale outdoor productivity have been performed with the same commercial P. tricornutum strain (UTEX 640).
In this study, three different P. tricornutum strains were grown simultaneously as repeated batch cultures during a six-months period, in individual outdoor flat panel photobioreactors (35 L) at northern latitudes in Bergen, western Norway, to evaluate the potential of the local climate conditions for large-scale outdoor cultivation and EPA production. The increased photoperiod during spring and summer at higher latitudes (19:5 h light:dark [L:D] around mid-summer in Western Norway) might be expected to positively affect growth rates and, thus, productivities. By comparing three different strains (two local isolates and one commercial strain), we sought to assess and compare strain-specific responses to changing environmental conditions in terms of productivity and FA profile; which are important selection criteria for outdoor cultivation.
2. Methods
2.1. Strains, stock cultures and inoculum
Three different strains of P. tricornutum were used in the experiment. Two strains (B58, M28) were isolated from the fjord next to the cultivation site and have been maintained in the laboratory since 1997 (B58 as ND58 [13]) and 2014 [14], respectively. The third strain (Fito) was obtained from the Microalgae Culture collection of Fitoplancton Marino, SL Cadiz, Spain (strain CCFM 06, isolated from local marine habitat) where it is being grown on a commercial scale, and the strain has been maintained in our laboratory since 2014. Stock cultures of the three strains were kept in 50 mL Erlenmeyer flasks in sterile Walne's medium ([15], prepared with seawater [SW; from 90 m depth in the fjord] and distilled water [80:20, v:v], salinity 29), at 15 °C and an irradiance of 50 μmol m− 2 s− 1 with a L:D cycle of 16:8 h. For inocula, biomass was up-scaled to 10 L flat-bottom glass flasks in modified F2 medium [16]. The media was prepared with SW and distilled water (80:20, v:v, salinity 29) and an increased macronutrient concentration to avoid nutrient limitation (NaNO3, 29 mM, 2.5 g L− 1; NaH2PO4·H2O, 2.9 mM, 0.4 g L− 1; Na2SiO3·5H2O, 0.14 mM, 30 mg L− 1). Cultures were kept at room temperature (15–20 °C) and continuous illumination between 100 and 200 μmol m− 2 s− 1. For carbon supply and culture mixing, 0.2 μm filtered air, enriched with 1% CO2, was bubbled through glass capillaries into the bottom of each flat-bottom glass flask, and the cultures were additionally stirred with a magnet stirrer to prevent settling and biofilm formation. Cultures reached dry weights (DW) of approximately 1 g L− 1 before they were inoculated into the outdoor reactors. Backup cultures were maintained during the whole experiment in 10 L flat-bottom glass flasks under the above-mentioned conditions by monthly dilution with fresh medium.
2.2. Photobioreactor
The photobioreactor used in this study comprised three individual modules of Green Wall Panels (GWP®-III, Fotosintetica & Microbiologica S.r.l, Florence, Italy) connected with a HMI (human machine interface) control unit. Each panel consisted of a metal frame (120 cm × 78 cm) encompassing a disposable bag (flexible and PAR transparent [> 90%] LDPE film), with a culture volume capacity of between 30 and 38 L (a volume of 35 L was used for calculations) and an optical path of approximately 3.5 cm. The panels were placed in parallel, and unshaded and south facing, on the roof of the Department of Biology in Bergen, Norway (60°22′49.7″N, 5°19′54.3″E). The inclination of each panel could be varied between 50 and 105° (panel back side with reference to the horizontal) and was adjusted to 50° during the day (04 am–24 pm) for maximum exploitation of solar irradiance and at 105° during the night (24 pm–04 am) for a better mixing of the cultures. At 50°, the ground area occupied by one panel of the photobioreactor was 1.2 m2. Each panel was equipped with a pH- and temperature sensor, and a quantum irradiance sensor (LP PAR 03, Delta Ohm, Padova, Italy) was attached on top of the second panel, facing the same direction as the panel surface. Ambient air was pumped through perforated pipes into the bottom of the bags to ensure culture mixing, and carbon was supplied by pH-controlled injection of pure CO2 into the cultures. Temperature was controlled by an internal stainless steel coil with circulating cold tap water. Temperature, pH and photon flux density (PFD, μmol m− 2 s− 1) of photosynthetically active radiation (PAR, 400–700 nm) data were recorded and stored on a PC every thirty seconds.
2.3. Outdoor cultivation
Prior to inoculation, cultivation bags were filled with tap water and sterilized with 1 mL L− 1 sodium hypochlorite (sodium hypochlorite solution, 10–15%, Sigma-Aldrich, St. Louis, MO, USA) for at least 24 h. The sodium hypochlorite was thereafter neutralized with 1 mL L− 1 sodium thiosulfate (sodium thiosulfate pentahydrate 15%, Sigma-Aldrich, St. Louis, MO, USA), and the panels were emptied and refilled with fresh and filter-sterilized medium and the inoculum. Modified F2 medium with increased macronutrient concentration (as described in 2.1), was prepared in 100 L tanks with seawater and tap water (80:20, v:v, salinity 29), and was pumped with a peristaltic pump (Masterflex Easy-Load, Millipore, Billerica, Massachusetts, USA) through 0.45 μm filters (Durapore Membrane Filters, Merck Millipore Billerica, Massachusetts, USA) into the culture bags. The three P. tricornutum strains were grown as repeated-batch cultures for a period of six months (25.04.2016–28.10.2016) in the individual GWP®-III panels (35 L), by discharging a defined volume of culture that was backfilled with fresh medium every 7–14 d to maintain a biomass concentration between approximately 0.4 and 2.5 g L− 1. Every interval between two dilutions is referred to as a “batch” with consecutive numbering. Cultures were mixed by aeration at 15–20 L min− 1, and pure CO2 was injected into the cultures when the pH exceeded 8.1 and until the pH reached 7.9 again. Temperatures were kept between 5 and 25 °C with cold tap water (~ 9 °C), circulating through the internal coil whenever the temperature settings were underrun or exceeded, respectively. Culture volumes were topped with ultra-pure water (Milli-Q) in the morning when required, to counter eventual evaporations. The position of the strains in the panels was rotated twice (06.07.16 and 13.09.16), allowing each strain to grow in each panel for a certain period, in order to exclude any influence of panel position or material on the performance of the cultures. To prevent mixing of the three strains, panels were cleaned and sterilized prior to position change as described above.
2.4. Sampling and analytical procedures
A sample volume between 50 and 100 mL was taken for each culture three times weekly between 9:00 and 10:00 am and daily for a period of two weeks (22.07.16–29.07.16 and 07.10.16–14.10.16) at 01:00 pm to measure DW, optical density (OD), and maximum quantum yield (QY) in triplicate, and take quadruplicate samples for FA analysis. To monitor strain morphology and contamination, cultures were regularly observed under the microscope. When cultures were diluted, samples were taken before and after the dilution, and a volume of 10 mL was stored at − 20 °C for phylogenetic analysis. Nitrate and phosphate concentrations were measured regularly using colour-indication-stripes (Quantofix, Macherey-Nagel GmbH & Co. KG, Düren, Germany). For batches one, two and three in the spring season, FA samples were only taken at the end of the batch, before dilution. In order to also calculate FA productivities for batches two and three, their FA starting concentrations were calculated from the former FA end-concentrations and their respective DW. From the second day of batch number four, FA were sampled at the same time intervals as the other parameters.
Optical densities were measured with a spectrophotometer (UV-2401 PC, Shimadzu Corporation, Kyoto, Japan) at 750 nm. If necessary, samples were diluted to give an attenuation of between 0.2 and 0.7. Dry weights were determined as described by Zhu and Lee [17] using 0.5 M ammonium formate as a washing buffer and are expressed as weight of the dried biomass (g) per volume (L). The QY was measured with Aqua Pen (AquaPen-C, AP-C 100, Photon System Instruments, Brno, Czech Republic) after initial dark incubation (10–60 min). For FA analysis, microalgae biomass was harvested by centrifugation (10 min at 2264 × g) into 10 mL glass tubes (PYREX), the supernatant discarded, and the pellet covered with N2 gas and kept at − 20 °C until analysis. Fatty acid extraction, methylation and analysis on gas chromatograph (GC) was performed as described in Steinrücken et al. [14]. The concentration of internal standard (23:0 FAME dissolved in isooctane) was adjusted for the respective DW of the samples, with 100, 240, 380, and 480 μg for < 1, 1–2, 2–3 and > 3 g L− 1, respectively.
2.5. Phylogenetic identification of strains
For each strain, one sample from stock culture and four samples from different time points during the cultivation period were used for phylogenetic analyses. DNA was isolated from pelleted samples using the EZNA plant kit (stock cultures and samples from 25.04.16 and 17.08.16) or the MoBio PowerWater® DNA isolation kit (samples from 13.09.16 and 28.10.16) according to instructions from the manufacturers. PCR was performed using primers GF (5′-GGGATCCGTTTCCGTAGGTGAACCTGC-3′) and GR (5′-GGGATCCATATGCTTAAGTTCAGCGGGT-3′), which target ~ 820 bp of the variable region, ITS1-5.8S-ITS2, of the genome [18]. The PCR mastermix contained (per 50 μL reaction); 25 μL Hotstart PCR mix, 2 μL 10% BSA, 1.2 μL 100% DMSO, 1 μL 10 μM primer GF, 1 μL 10 μM primer GR, 17.8 μL dH2O, and 2 μL template DNA. PCR conditions were: 15 min at 95 °C followed by 30 cycles of 1 min at 95 °C, 1 min at 55 °C and 1 min at 72 °C, and a final elongation at 72 °C for 7 min. Size of PCR products was confirmed using gel electrophoresis. PCR products were subsequently purified using ExoProStar™ (GE Healthcare, Chicago, Illinois, USA) according to the manufacturer's instructions, and prepared for sequencing using BigDye v.3.1 Kit (ThermoFisher Scientific, Watham, MA, USA) and the PCR GF/GR primer combination. Sanger sequencing was performed by the sequencing facility at the University of Bergen (http://www.uib.no/en/seqlab). Sequencing chromatograms were examined, and forward and reverse sequences were assembled and aligned using BioEdit (v.7.2.5) [19]. A minimal evolution tree of these sequences and their closest relatives obtained from Genbank was constructed using Mega6 [20].
2.6. Calculations
Average daily biomass, total fatty acid (TFA) and EPA productivities (per volume Eq. (1), panel surface-area Eq. (2) and panel ground-area Eq. (3)), and yields on light Eq. (4) were calculated for each batch by taking start and end points of its linear slope. A volume of 35 L and a surface area of 0.95 m2, and a ground area of 1.2 m2 were used for calculating the panel surface-areal and panel ground-areal productivities, respectively.
| (1) |
| (2) |
| (3) |
| (4) |
Pb,vol: volumetric productivity during a batch (DW [g L− 1 d− 1], TFA, EPA [mg L− 1 d− 1]), c: concentration (DW [g L− 1], TFA, EPA [mg L− 1]), x0 and x1: defined start and end time point of the linear slope of a batch, t: time (day), Pb,panel: panel surface-areal productivity during a batch (DW [g m− 2 d− 1], TFA, EPA [mg m− 2 d− 1]), 35: panel volume (L), 0.95: panel surface area (m2), Pb,ground: panel ground-areal productivity during a batch (DW [g m− 2 d− 1], TFA, EPA [mg m− 2 d− 1]), 1.2: panel ground area (m2), Yb,light: Yield on light during a batch (DW [g mol− 1 photon], TFA, EPA [mg mol− 1 photon]) and Eav,b: average daily PFD on the panel surface during a batch (mol m− 2 d− 1). The average daily irradiance on the panel surface during a batch (Eav,b, mol m− 2 d− 1) was calculated with Eq. [5]. The measured PFDs (μmol m− 2 s− 1) between the start and end time point of the linear slope of a batch were multiplied by 30, summed up and divided by the amount of days. E: photon flux densities (μmol m− 2 s− 1, measured every 30 s), x0 and x1: start and end time point of the linear slope of a batch, t: time (day).
| (5) |
Average seasonal (spring, summer and autumn) productivities (Pav,s), yields (Yav,s) and PFDs (Eav,s) were calculated by taking the average from of the corresponding batches.
2.7. Statistics
The three strains were grown as one biological replicate each. Optical density, QY and DW were measured in triplicates. Quadruplicate samples were taken for FA analysis, but eventually only duplicate samples were analysed due to the great amount of samples. The FA content and the biomass DW were analysed from individual subsamples. Thus the standard deviation for FA content relative to the biomass DW was calculated using the Eq. [6] with SD: standard deviation, FA: fatty acid and DW: biomass dry weight.
| (6) |
Differences in biomass and EPA productivities between the strains and between the seasons were analysed by 2-way ANOVA using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA), with p < 0.05 as a threshold for statistical significance. Principal Component Analyses (PCA) and Euclidean dendrograms of strains and their FA composition were calculated using Sirius 10.0 (Pattern Recognition Systems AS, Bergen, Norway) and edited using GraphPad Prism 6.
3. Results
3.1. Cultivation conditions
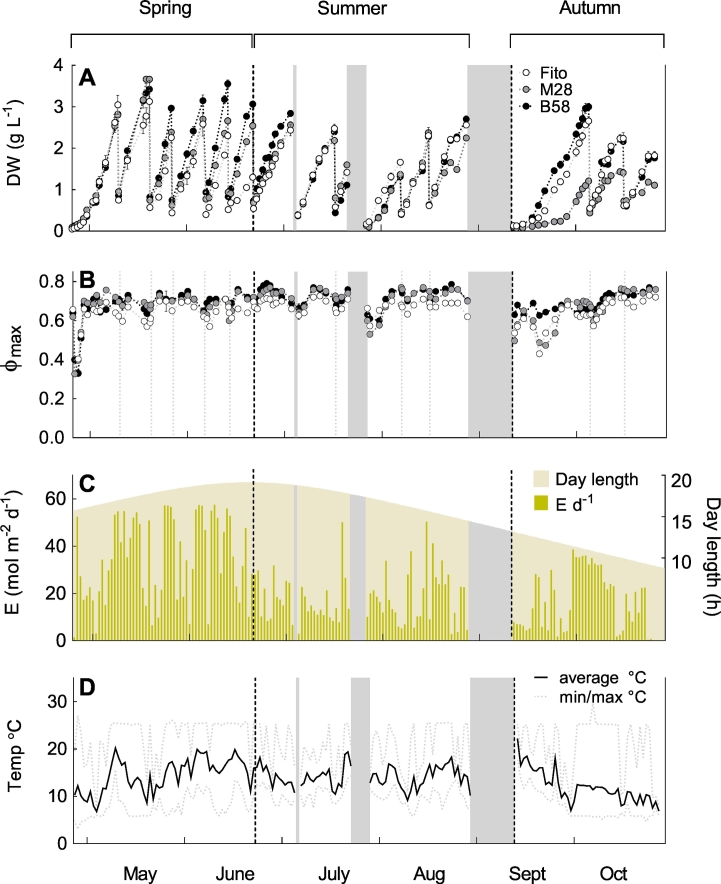
Three P. tricornutum strains (local strains M28, B58, and Spanish strain Fito) were grown as repeated-batch cultures for six months (25.04.2016–28.10.2016) in separate flat panel outdoor reactors (Green Wall III Panels, each 35 L) in Bergen, Norway. In total, fifteen repeated batches were conducted with biomass DW, on average, ranging between 0.52 and 1.94, 0.67 and 1.88, and 0.63 and 2.33 g L− 1 for strains Fito, M28 and B58, respectively, not including the starting concentrations after inoculation (Fig. 1A). The cultivation period was divided into three seasons; spring (25.04–22.06, comparatively high daily irradiance and increasing day length, batches 1–6), summer (22.06–29.08, decreasing day length and comparably low irradiance, batches 7–12) and autumn (13.09–28.10, short day length, but relatively high irradiance during the day, batches 13–15). The reactor panels had to be re-inoculated with backup cultures twice (July and September see Fig. 1), due to punctures in the cultivation bags (caused by birds) and an inappropriately connected cooling system (with a consequent lethal increase of culture temperature to > 40 °C), respectively. The starting biomass concentrations after inoculations in our study ranged between 0.05 and 0.17 g L− 1. Because of the high irradiance and low starting biomass concentration after the third inoculation, the cultures were covered with white plastic bags and a parasol until biomass concentrations reached approximately 0.2 g L− 1 to avoid photoinhibition. During the last two batches of the cultivation period, all strains appeared light-limited at earlier DW concentrations than previously, and strain M28 possessed overall lower growth rates and did not reach DW > 1.43 g L− 1 during autumn season. The maximum quantum yield was reasonably stable and varied between 0.57 and 0.73 (Fito), 0.60 and 0.77 (M28), and 0.63 and 0.79 (B58) during the cultivation period, and was generally higher for strains M28 and B58, with average value of 0.71, than for strain Fito (0.66). Only after inoculations, QY dropped below the average range with minimum values of 0.35 for strain Fito, and 0.33 for M28 and B58, but recovered after two to three days (Fig. 1B).
Fig. 1.
Overview of culture parameters during six-months repeated-batch cultivation of three Phaeodactylum tricornutum strains (Fito, M28, B58) in flat panel outdoor bioreactors in Bergen, western Norway. A: Biomass dry weight (DW) and B: Photosystem II efficiency of dark-adapted samples (Φmax, maximum quantum yield) during fifteen repeated batches. Data show average and standard deviation of measurement replicates (n = 3) from one biological batch. C: Daily-integrated irradiance (E) of photosynthetically active radiation (PAR) measured on the reactor surface, and day length. D: Daily average, minimum and maximum temperature inside the cultivation chambers. The three grey bars indicate interruptions of the cultivation process. After interruption (1) and (3), the position of the strains was rotated, and after (2) and (3), the panels were re-inoculated with backup cultures. Grey dotted vertical lines in (B) indicate dilution of the cultures with consequent decrease in biomass concentration (A). Black dotted lines mark the division of the cultivation period into three seasons; spring, summer and autumn.
The daily irradiances during the cultivation period, together with the day lengths are illustrated in Fig. 1C. Day length increased from the start of the culture period (15 h 36 min) until summer solstice (20.06.2016, 19 h 01 min), and then decreased towards the end of the cultivation period (8 h 55 min). The daily-integrated irradiance varied strongly during the cultivation season depending on the weather conditions and day length, and ranged between 0.1 and 57 mol m− 2 d− 1, whereas short-term maximum irradiance during a day varied between 41 and 2555 μmol m− 2 s− 1 (data not shown).
The culture temperature varied significantly between night and day during the whole cultivation period (Fig. 1D). It was connected to the irradiance with higher temperatures (up to 25 °C before being cooled) at high irradiance, and lower temperatures at low irradiance and during the nights. Daily average values fluctuated between 6.5 and 19.8 °C, daily minimum between 3 and 14 °C, and the daily maximum between 8.1 and 27.3 °C. Greatest observed fluctuations during 24 h were between 6 °C at night and 25 °C during the day. Average temperatures during the three seasons were 15, 14, and 11 °C for spring, summer and autumn, respectively.
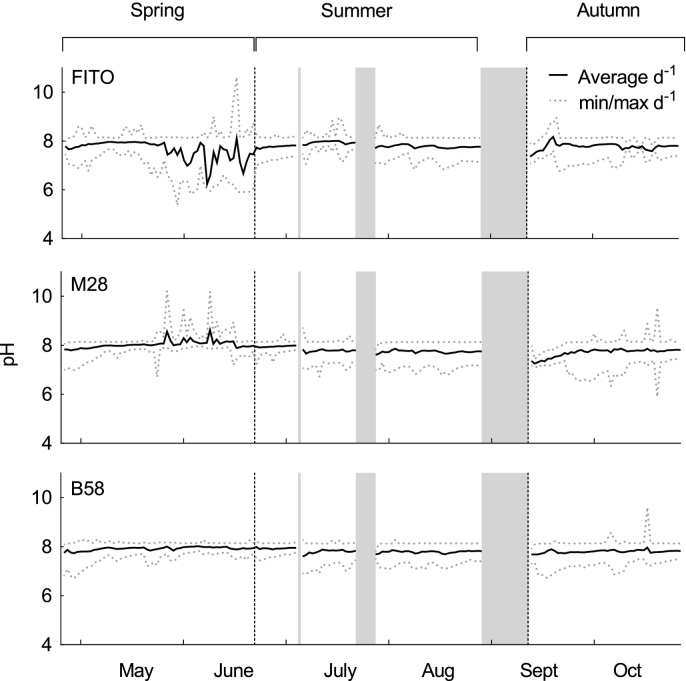
An average pH of approximately 7.8 was maintained in the cultures during the experiment (Appendix, Fig. 1). However, due to a time delay between CO2 injection and pH registration by the sensor, the pH typically varied between 7.5 and 8.1 during a day, but was also found to drop down to pH of 6 for a short time. A defect in the CO2 solenoid-valve during batch number six led to a continuous injection of CO2 into panel 1 containing strain Fito culture, resulting in a significant decrease in the daily average pH (pH 6.2–7.5), and a consequent decreased growth rate (Fig. 1A) and biomass production (0.09 g L− 1 d− 1). Therefore, this batch was excluded for productivity calculations for strain Fito.
3.2. Strain morphology and discrimination
P. tricornutum is pleiomorphic and can exist in at least three different forms (oval, fusiform or triradiate) whereas only little information is available on transformation processes [21]. The cells of the three strains were all predominantly fusiform-shaped, but differed in their form and cell size, and could, thus, be distinguished under the microscope (Fig. 2). The cells of strain Fito were exclusively found to be fusiform, and were noticeably shorter than the cells of the other two strains with an average length of approximately 20 μm (Fig. 2A). Strain M28 cells were predominantly fusiform, elongated (20–40 μm) and sickle-shaped (Fig. 2B), but also triradiate cells were present in the culture and clusters of oval morphotypes were found occasionally. The cells of strain B58 possessed different morphologies under laboratory cultivations (oval or fusiform), but only the fusiform morphotypes (and very rarely triradiate forms) were observed during the outdoor experiment. They were on average 30 μm long, and straighter and thicker than strain M28 cells. During the outdoor cultivation period, no changes in either shape or size of the cells were observed for strains Fito and M28, but approximately 40% of the cells of strain B58 became smaller after half of the cultivation period, resembling the shape of strain Fito cells.
Fig. 2.
Microscope photo of the three Phaeodactylum tricornutum strains Fito (A), M28 (B) and B58 (C). A Zeiss Axio Imager Z1 microscope (1000 × magnification with immersion oil) and an AxioCam MR3 (Carl Zeiss, Göttingen, Germany) were used, and photos were edited with GIMP 2.8 (www.gimp.org).
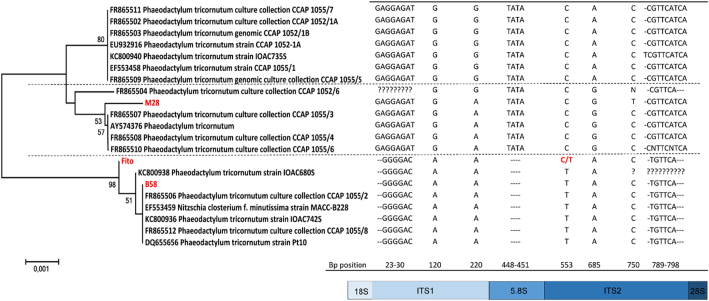
Sequencing the ITS1-5.8S-ITS2 region of the genomes confirmed the separation of the three strains and revealed that strain M28 diverged from strains Fito and B58 at several positions in the genome (Fig. 3). Strains Fito and B58 had identical sequences, with the exception of one bp (position 553, 5′-3′), where strain B58 displayed thymine, whereas the sequencing chromatograms indicated that strain Fito displayed equal amounts of cytosine and thymine. This observation was consistent throughout the experimental period, and the results, thus, exclude any mixing of the three strains.
Fig. 3.
Minimal evolution tree displaying the evolutionary relationships in the ITS1-5.8S-ITS2 region of the genome of the three Phaeodactylum tricornutum strains (Fito, M28 and B58) used in the experiment and closest related strains obtained from Genbank. The evolutionary history was inferred using the Minimum Evolution method [22]. The optimal tree with the sum of branch length = 0,01 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [23]. The evolutionary distances were computed using the Maximum Composite Likelihood method [24] and are in the units of the number of base substitutions per site. Evolutionary analyses were conducted in MEGA6 [20].
3.3. Seasonal productivities of cultures
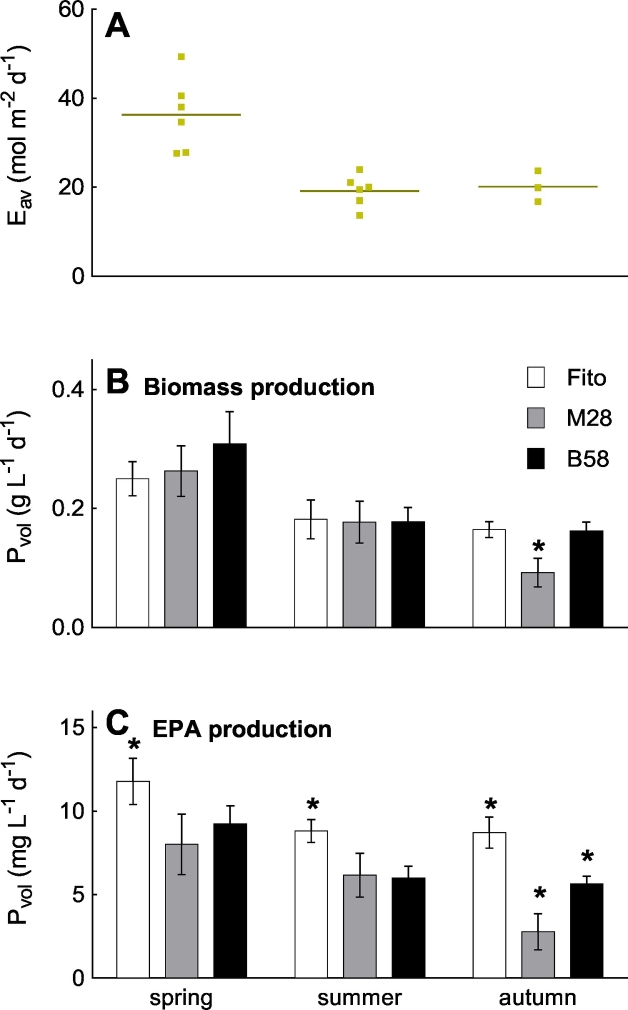
The average volumetric biomass and EPA productivities for the three strains and three seasons (spring [batches 1–6 for biomass and 2–6 for EPA], summer [batches 7–12] and autumn [batches 13–15]) are shown in Fig. 4 together with the average daily irradiance. Highest average irradiance occurred during spring season (36.3 ± 8.2 mol m− 2 d− 1), whereas the irradiance was equally low during the summer and autumn seasons (19.2 ± 3.5 and 20.1 ± 3.4 mol m− 2 d− 1, respectively, Fig. 4A).
Fig. 4.
Average daily irradiance (Eav) and average biomass and EPA productivities for three strains of Phaeodactylum tricornutum (Fito, M28 and B58) for three seasons (spring, summer and autumn), during six-months repeated-batch cultivation in flat panel outdoor bioreactors in Bergen, western Norway. A: Dots display average daily irradiance (mol m− 2 d− 1) during a batch (Eav,b) and lines are the mean value of the seasons (Eav,s). Values for biomass (B) and EPA production (C) are average and standard deviation from the respective batches of each season (biomass: n = 6 [5 for Fito], 6 and 3, and EPA: n = 5 [4 for Fito], 6 and 3 for spring, summer and autumn, respectively). Asterisks indicate significant difference to the other two strains.
Corresponding to this, the volumetric biomass productivities for all three strains were significantly higher in spring (p < 0.05) with 0.25, 0.26 and 0.30 g L− 1 d− 1 for Fito, M28 and B58, respectively, compared to summer (0.18, 0.18 and 0.18 g L− 1 d− 1) and autumn (0.16, 0.09 and 0.16 g L− 1 d− 1) (Fig. 4B). Strain M28 additionally revealed a significant decrease from summer to autumn. Differences in biomass productivity were also found between the three strains, but were not consistent during all three seasons. In spring, biomass productivity of strain B58 was significantly higher than biomass productivity of strain Fito, and in autumn strain M28 had a significant lower biomass productivity compared to strains Fito and B58.
The volumetric EPA productivities were also significantly higher during spring for all strains (11.8, 8.0 and 9.2 mg L− 1 d− 1 for Fito, M28 and B58, respectively) than in summer (8.8, 6.2 and 6.0 mg L− 1 d− 1) and autumn (8.7, 2.8 and 5.6 mg L− 1 d− 1) (Fig. 4C). In contrast to the biomass productivities, EPA productivities were significantly higher for strain Fito compared to the other two strains in all three seasons. EPA productivities of strains M28 and B58 showed no significant differences in spring and summer, but strain M28 had a significantly lower EPA productivity in autumn. An overview for productivities (volumetric, panel surface-area and ground-area), and the yield of light for biomass, TFA and EPA is summarized in Table 1.
Table 1.
Biomass, total fatty acid (TFA) and eicosapentaenoic acid (EPA) productivities per volume (Pvol), panel area (Parea, panel) and ground area (Parea, ground) and the yields on light for the three Phaeodactylum tricornutum strains (Fito, M28 and B58) for the three seasons (spring, summer and autumn) during a six-months repeated-batch cultivation in flat panel outdoor bioreactors in Bergen, western Norway. Values are average and standard deviation of the respective batch values of each season (biomass: n = 6 [5 for Fito], 6 and 3, TFA, EPA: n = 5 [4 for Fito], 6 and 3 for spring, summer and autumn, respectively).
| Spring |
Summer |
Autumn |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomass | Fito | M28 | B58 | Fito | M28 | B58 | Fito | M28 | B58 |
| Pvol (g L− 1 d− 1) | 0.25 ± 0.03 | 0.26 ± 0.04 | 0.30 ± 0.06 | 0.18 ± 0.03 | 0.18 ± 0.03 | 0.18 ± 0.02 | 0.16 ± 0.01 | 0.09 ± 0.02 | 0.16 ± 0.01 |
| Parea, panel (g m− 2 d− 1) | 9.3 ± 0.9 | 9.5 ± 1.5 | 10.9 ± 2.1 | 6.7 ± 1.2 | 6.5 ± 1.3 | 6.5 ± 0.9 | 6.1 ± 0.5 | 3.4 ± 0.9 | 6.0 ± 0.5 |
| Parea, ground (g m− 2 d− 1) | 7.3 ± 0.7 | 7.5 ± 1.2 | 8.7 ± 1.7 | 5.3 ± 1.0 | 5.2 ± 1.0 | 5.2 ± 0.7 | 4.8 ± 0.4 | 2.7 ± 0.7 | 4.7 ± 0.4 |
| Yield light (g mol− 1 photon) | 0.25 ± 0.05 | 0.27 ± 0.04 | 0.31 ± 0.05 | 0.36 ± 0.08 | 0.35 ± 0.09 | 0.35 ± 0.07 | 0.31 ± 0.06 | 0.16 ± 0.01 | 0.30 ± 0.04 |
| TFA | |||||||||
| Pvol (mg L− 1 d− 1) | 38.7 ± 4.5 | 31.5 ± 5.0 | 43.8 ± 7.9 | 26.7 ± 2.7 | 21.5 ± 3.9 | 24.0 ± 3.0 | 25.7 ± 1.3 | 10.2 ± 3.3 | 22.3 ± 1.5 |
| Parea, panel (mg m− 2 d− 1) | 1426 ± 167 | 1162 ± 185 | 1613 ± 293 | 985 ± 99 | 791 ± 143 | 883 ± 109 | 947 ± 49 | 375 ± 123 | 821 ± 55 |
| Parea, ground (mg m− 2 d− 1) | 1129 ± 132 | 920 ± 146 | 1277 ± 232 | 780 ± 78 | 626 ± 113 | 699 ± 87 | 750 ± 38 | 297 ± 97 | 650 ± 44 |
| Yield light (mg mol− 1 photon) | 35.3 ± 2.9 | 31.3 ± 5.5 | 43.3 ± 7.7 | 52.7 ± 10.1 | 43.5 ± 13.2 | 47.9 ± 9.4 | 48.1 ± 9.6 | 17.3 ± 3.2 | 41.3 ± 5.2 |
| EPA | |||||||||
| Pvol (mg L− 1 d− 1) | 11.8 ± 1.4 | 8.0 ± 1.8 | 9.2 ± 1.1 | 8.8 ± 0.7 | 6.2 ± 1.3 | 6.0 ± 0.7 | 8.7 ± 0.9 | 2.8 ± 1.1 | 5.6 ± 0.5 |
| Parea, panel (mg m− 2 d− 1) | 434 ± 51 | 295 ± 67 | 340 ± 40 | 325 ± 25 | 227 ± 48 | 221 ± 27 | 321 ± 34 | 102 ± 40 | 208 ± 17 |
| Parea, ground (mg m− 2 d− 1) | 344 ± 40 | 234 ± 53 | 269 ± 32 | 257 ± 20 | 180 ± 38 | 175 ± 21 | 254 ± 27 | 81 ± 32 | 164 ± 14 |
| Yield light (mg mol− 1 photon) | 10.7 ± 0.8 | 8.0 ± 2.2 | 9.2 ± 1.9 | 17.3 ± 2.7 | 12.5 ± 4.1 | 12.0 ± 2.5 | 16.2 ± 2.4 | 4.7 ± 1.2 | 10.5 ± 1.8 |
3.4. Total fatty acid (TFA) and eicosapentaenoic acid (EPA) content
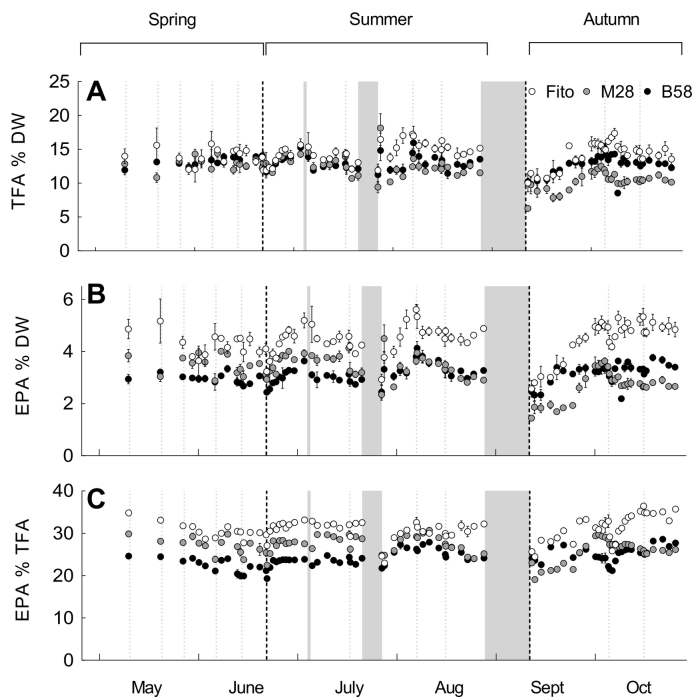
The TFA and EPA content showed moderate variations during the cultivation period, but no obvious pattern was apparent (Fig. 5). TFA content was most of the time slightly higher for strain Fito, and varied between 10.0 and 17.4, 6.3 and 18.1, and 8.5 and 16.0% DW for strains Fito, M28 and B58, respectively (Fig. 5A). The EPA content relative to DW varied between 2.6 and 5.6, 1.4 and 4.5, and 2.2 and 4.1% (Fig. 5B) and relative to TFA between 23.0 and 36.4, 19.1 and 30.7, and 19.3 and 28.9% (Fig. 5C), for strains Fito, M28 and B58, respectively. EPA content was predominantly higher for strain Fito, both relative to DW and relative to TFA throughout the cultivation period. After re-inoculation, TFA and EPA contents were considerably lower, and increased to higher levels with cultivation time in all three strains; this was particularly noticeable for the EPA content in strain Fito.
Fig. 5.
Fatty acid values of three Phaeodactylum tricornutum strains during six-months repeated-batch cultivation in flat panel outdoor bioreactors in Bergen, western Norway, with total fatty acid (TFA) content relative to the dry weight (DW) (A), eicosapentaenoic acid (EPA) content relative to DW (B) and EPA content relative to TFA (C). Values are average and standard deviation of measurement replicates (n = 2 and 3 for FA and DW values respectively). The three grey bars indicate interruptions of the cultivation process. After interruption (1) and (3), the position of the strains was rotated, and after (2) and (3), the panels were re-inoculated with backup cultures. Grey dotted, vertical lines indicate dilution of the cultures and black dotted lines mark the division of the cultivation period into three seasons; spring, summer and autumn.
3.5. Total fatty acid (TFA) composition
The same thirty-four FA were detected in the GC for the three stains in all samples analysed, from which 30 could be identified. In total ten FA (14:0, 16:0, 16:1 n-7, 16:2 n-4, 16:3 n-4, 16:4 n-1, 18:2 n-6, 20:4 n-6, 20:5 n-3, 24:0) accounted (on average) for > 2% TFA. EPA (20:5 n-3) and palmitoleic acid (16:1 n-7) were the two major FA, together accounting for approximately 50% TFA, followed by palmitic acid (16:0) with an average of 12% TFA and myristic acid (14:0) with on average 7% TFA. However, the relative TFA content differed between the strains and varied during the cultivation period within the strains (Table 2).
Table 2.
Percent major fatty acids (> 2% TFA) identified for the three Phaeodactylum tricornutum strains during six-months repeated-batch cultivation in flat panel outdoor bioreactors in Bergen, western Norway. Values show average and standard deviation of all samples of the respective seasons (n = 30, 72 and 60 for spring, summer and autumn, respectively).
| Strain | Season | 14:0 Myristic |
16:0 Palmitic |
16:1 n-7 Palmitoleic |
16:2 n-4 Hexadeca-dienoic |
16:3 n-4 Hexadeca-trienoic |
16:4 n-1 Hexadeca-tetraenoic |
18:2 n-6 Linoleic |
20:4 n-6 Arachidonic | 20:5 n-3 EPA |
24:0 Lignoceric |
Rest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fito | Spring | 8.0 ± 0.6 | 10.9 ± 0.9 | 18.7 ± 1.9 | 4.3 ± 0.8 | 6.7 ± 1.5 | 4.5 ± 1.2 | 2.0 ± 0.6 | 1.5 ± 0.6 | 30.6 ± 1.8 | 2.0 ± 0.3 | 10.8 |
| Summer | 7.9 ± 0.5 | 11.7 ± 1.6 | 18.9 ± 2.8 | 5.7 ± 0.8 | 5.6 ± 2.4 | 4.2 ± 1.2 | 2.0 ± 0.6 | 0.8 ± 0.5 | 30.8 ± 2.2 | 2.0 ± 0.3 | 10.4 | |
| Autumn | 8.0 ± 0.4 | 12.4 ± 2.1 | 17.4 ± 2.6 | 6.1 ± 1.3 | 3.4 ± 1.5 | 3.9 ± 1.4 | 3.0 ± 0.7 | 0.8 ± 0.4 | 31.4 ± 3.3 | 2.3 ± 0.4 | 11.3 | |
| M28 | Spring | 8.9 ± 0.4 | 10.2 ± 0.8 | 24.7 ± 2.0 | 1.8 ± 0.6 | 2.4 ± 0.7 | 4.8 ± 0.6 | 3.0 ± 0.8 | 2.1 ± 0.8 | 27.1 ± 1.9 | 2.4 ± 0.3 | 12.6 |
| Summer | 7.5 ± 1.0 | 10.5 ± 1.2 | 24.5 ± 2.4 | 3.2 ± 1.1 | 3.1 ± 1.3 | 4.4 ± 1.0 | 2.7 ± 0.9 | 0.7 ± 0.5 | 27.5 ± 1.9 | 2.7 ± 0.5 | 13.2 | |
| Autumn | 5.0 ± 0.5 | 11.5 ± 1.5 | 25.1 ± 2.9 | 4.5 ± 1.4 | 2.9 ± 0.9 | 4.9 ± 1.2 | 1.9 ± 0.6 | 0.2 ± 0.1 | 25.7 ± 2.8 | 3.1 ± 0.5 | 15.2 | |
| B58 | Spring | 6.7 ± 0.2 | 11.7 ± 0.7 | 24.3 ± 1.7 | 3.7 ± 0.6 | 6.9 ± 1.2 | 2.6 ± 0.7 | 2.8 ± 0.7 | 6.5 ± 1.1 | 22.4 ± 1.7 | 1.9 ± 0.2 | 10.5 |
| Summer | 6.0 ± 0.3 | 12.1 ± 1.1 | 23.2 ± 2.0 | 6.3 ± 1.8 | 6.2 ± 2.5 | 2.2 ± 0.7 | 2.9 ± 0.8 | 4.0 ± 1.6 | 24.2 ± 1.8 | 2.1 ± 0.3 | 10.8 | |
| Autumn | 6.2 ± 0.3 | 12.6 ± 1.7 | 22.1 ± 2.8 | 5.8 ± 0.9 | 5.8 ± 1.8 | 2.0 ± 1.2 | 3.4 ± 0.7 | 3.3 ± 1.4 | 25.2 ± 2.0 | 2.3 ± 0.3 | 11.3 |
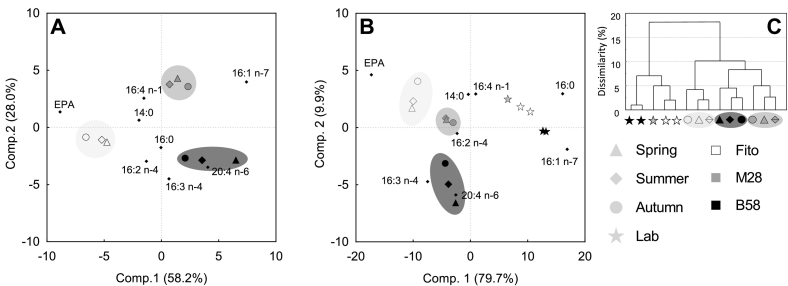
A PCA on the seasonal average of the relative FA content (% TFA) for the three strains revealed strain-specific grouping (Fig. 6A). All three seasons clustered closely together for each strain, whereas the three strains were clearly separated. The FA that predominantly contributed to the distribution of the three strains were EPA, palmitoleic acid, arachidonic acid (20:4 n-6), hexadecatetraenoic acid (16:4 n-1), hexadecatrienoic acid (16:3 n-4) and hexadecadienoic acid (16:2 n-4), at which EPA correlated positively with strain Fito, hexadecatetraenoic acid with M28, and arachidonic acid with strain B58. Palmitoleic acid was negatively correlated to strain Fito, hexadecadienoic and hexadecatrienoic acids to strain M28, and hexadecatetraenoic acid to strain B58. Palmitic acid and myristic acid contributed to only a minor extent in the grouping.
Fig. 6.
Principal component analysis (A and B) and Euclidian Dendrogram (C) of the relative fatty acids composition (% TFA) for three Phaeodactylum tricornutum strains (Fito, M28 and B58). A: Nine objects representing the three strains at the three seasons (spring, summer, autumn) during six-months repeated-batch cultivation in flat panel outdoor bioreactors in Bergen, western Norway, and eight variables, representing the fatty acids with highest impact on the distributions. Values are average of all samples of the respective seasons (n = 30, 72 and 60 for spring, summer and autumn, respectively). B: Same variables and objects as in (A) including additional data from laboratory experiments (average of two measurement replicates) for Fito, B58 (two biological replicates) and M28 (one biological replicate). C: Dendrogram showing dissimilarities between the nine outdoor and five laboratory objects. Laboratory data are from exponential growth phase during batch experiments with experimental setup described for M28 in Steinrücken et al. [14].
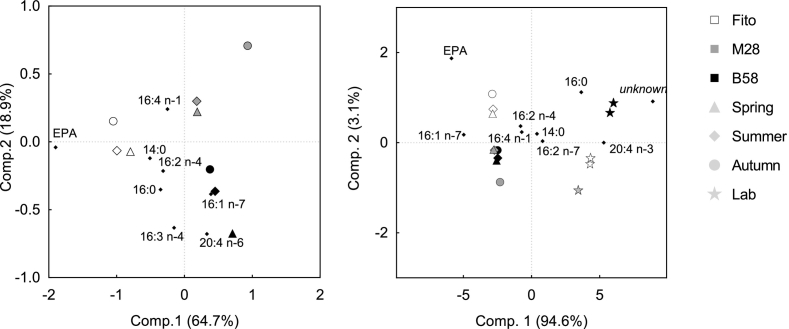
Including FA composition data from the same strains from laboratory experiments into the PCA revealed strong differences in the relative FA composition between outdoor and laboratory cultures for each strain (Fig. 6B). Laboratory FA data derived from exponential growth phase during a batch experiment (15 °C, continuous irradiance of 120 μmol m− 2 s− 1 and aeration with 1% CO2 enriched air. Experimental set up and data analysis are described for M28 in Steinrücken et al. [14]). The laboratory strains did not group together with their respective outdoor strains, but were clearly separated along Component 1. The FA EPA, palmitoleic, palmitic, hexadecatrienoic and arachidonic acid were primarily responsible for this separation. EPA, hexadecatrienoic acid and arachidonic acid correlated negatively with the laboratory strains, whereas palmitoleic acid and palmitic acid were positively correlated to the laboratory strains, and negatively to the outdoor grown ones. A Euclidean dendrogram (Fig. 6C) of the outdoor and laboratory data additionally illustrates the relationships between the strains, seasons and laboratory experiments in terms of similarities in their relative FA composition, and confirms that the laboratory strains group together, clearly separated from the outdoor cultures. The same clustering pattern was obtained in PCA analyses using FA content relative to DW. However, the contribution of FA to the distribution of the objects differed slightly (Appendix, Fig. 2).
4. Discussion
4.1. Production potential
Biomass productivities for strains Fito, M28 and B58 were maximal in spring when irradiance was highest (average of 39 mol m− 2 d− 1) and lower during summer and autumn when irradiances, on average, decreased by 45%. Although the day lengths were comparable during spring and summer seasons, the exceptionally bad weather conditions in summer of 2016 resulted in daily irradiances that were far below normal for the experimental site (ten years average around mid-summer is 42 mol m− 2 d− 1 [own unpublished data]). Contrariwise, high irradiances during the day were measured in autumn, but concurrently day length was becoming progressively shorter and, thus, the average daily irradiances were similar to those found during summer. The results showed that outdoor production of P. tricornutum is certainly feasible during at least six months of the year at high latitude locations, even though weather conditions were inclement.
When compared with studies from southern latitudes, the average volumetric biomass productivities of the three strains were generally lower (Fig. 4B). Several studies on outdoor production of P. tricornutum in photobioreactors from Almería, Spain (36°50′N, 2°27′W) revealed up to five times higher biomass productivities with 1.5, 1.25 and 0.3 g L− 1 d− 1 for cultures grown in a helical tubular PBR (3 cm internal diameter [i.d.]) [25], a horizontal tubular PBR (2.6 cm i.d.) [26] and vertical column PBRs (19 cm i.d.) [27], respectively. Additionally, a two to three times higher EPA productivity of 21 mg L− 1 d− 1 was reported from the horizontal tubular PBR [26]. More similar to our findings (Table 1) were results from P. tricornutum cultures grown in a horizontal tubular PBR (4.85 cm i.d.) during summer in Florence, Italy (43°46′N, 11°15′E) with a mean areal biomass productivity of 13.1 g m− 2 d− 1 (equivalent to a volumetric productivity of 0.26 g L− 1 d− 1) [11]. Generally, highest productivities in these studies were achieved in PBRs with smaller optical paths. However, the different reactor types, operation modes and cultivation conditions used in these studies make empirical comparisons difficult. The same GWP®-III reactor panels were used by Rodolfi et al. [28] for cultivation of P. tricornutum during spring and summer in Florence, Italy. They reported higher panel-areal biomass productivities of 12.3 and 17.2–17.8 g m− 2 d− 1 for nutrient replete cultures during spring and summer, respectively, but similar EPA productivities as found for our strains (0.29 and 0.35–0.38 g m− 2 d− 1 in spring and summer, respectively) due to a lower EPA content (2.06–2.38% DW) of their P. tricornutum strain. When recalculating the panel-areal productivities for our strains with the volume (37–43 L) and panel-area (0.78 m2) used in Rodolfi et al. [28], higher EPA productivities were achieved for strain Fito in all seasons (0.60, 0.45, and 0.45 g m− 2 d− 1) and for strain M28 and B58 in spring (0.41 and 0.47 g m− 2 d− 1, respectively).
Extrapolating the biomass that could be produced in one year (assuming 180 d of operation) per hectare (using eight GWP®-II1250 reactors, www.femonline.it/products) at our location, suggested an amount of 10.7 t (10.8, 9.7 and 11.7 t ha− 1 yr− 1 on average for strains Fito, M28 and B58, and 14.8, 9.8 and 7.5 t ha− 1 yr− 1 on average for spring, summer and autumn, respectively). Tredici et al. [29] calculated an annual biomass productivity for the green algae Tetraselmis suecica of 36 t ha− 1 yr− 1 in Tuscany, Italy (240 operation days) and 66 t ha− 1 yr− 1 when placing the reactors in North Africa (330 operation days). In regions of lower latitudes, productivities are increased due to higher irradiance and more operational days during a year. The longer day length during spring and summer that was assumed to promote productivity at higher latitudes could not compensate for this. However, the higher temperatures in southern regions demand intensive cooling of cultures. In Italy, cooling is necessary for 5–6 months of the year and corresponds to 11% of the energy input for plant operation [29]. Those inputs are likely to be reduced at higher latitudes, as cooling at our location was necessary only during days with high irradiance, and cost-efficient tap water could be used.
Although the lower productivities in our study were most likely connected to the limited irradiance at our cultivation site, alterations in cultivation conditions and operation modes could potentially increase productivities at the local site, suggesting the operation of the reactors in continuous mode by using either chemostat or turbidostat operation [30]. The light that is available for the microalgae in the culture strongly depends on the prevailing irradiance, but also on reactor design and biomass concentration of the culture. When applying repeated batch cultures, the constantly changing biomass concentrations, together with the fluctuating solar irradiances, make it difficult to ensure optimal irradiance available for cultures. Too high or too low irradiance can result in suboptimal operation, due to incomplete light absorption and photo-inhibition, or dark zones that reduce the productivity, respectively [31], [32]. Keeping the DW between approximately 0.4 and 2.5 g L− 1 turned out to be a suitable range for maintaining the strains at linear growth during spring and summer. However, during autumn, all strains appeared to become light-limited progressively earlier at DW below 2.5 g L− 1, probably as a result of the gradually decreasing day length and solar azimuth, as average daily irradiances were similar as in summer.
Maintaining nutrient replete conditions is important to measure biomass and EPA production potential accurately. Periodically-measured nitrate and phosphate concentrations in the media revealed that these nutrients were at no time point limiting to culture growth (data not shown). The silica concentration was not monitored as P. tricornutum cells usually have little or no silica requirements [2], and Zhao et al. found that silicon influenced the growth rate only under conditions of low temperature and green light [33]. Nutrient replete growth was also confirmed by the TFA content of the strains during the cultivation period. An increase in TFA due to accumulation of storage lipids is a typical response to nutrient starvation and an increase of TFA of up to 35% DW has been reported for nutrient-depleted P. tricornutum cultures [34]. Even though TFA content varied (10.0–17.4, 6.3–18.1 and 8.5–16.0% DW for Fito, M28 and B58, respectively) throughout the cultivation period, such an accumulation was not observed in our study. Similar TFA values (between 8.4 and 10% DW) were found for outdoor cultures of P. tricornutum by Sánchez Míron et al. [27]. The moderate variation of the TFA content observed in our study might reflect changing physiological processes in the cells during variations in the cultivation conditions. Moderate variations during the cultivation period were also observed for the EPA content in all three strains. Several studies have revealed that environmental factors such as nutrient availability, temperature, light, salinity, pH and cell density influence the microalgal lipid content and composition and thus EPA content [7], [35], [36], [37], [38], [39], [40], [41], [42], [43]. Generally, the TFA and EPA contents were considerably lower after re-inoculation, and increased to higher levels with increasing cultivation time in all three strains; this was especially noticeable for the EPA content in strain Fito.
4.2. Strain specific characteristics
Our investigations revealed a number of dissimilarities between the three P. tricornutum strains including morphology, phylogenetic relationship, productivity, EPA content, and relative FA composition. However, no correlation between biogeography and the other factors was observed. Even though the two local strains M28 and B58 were isolated from the same location (but at different times), strain B58 was phylogenetically closer to strain Fito than to strain M28. Strain M28 diverged from strains Fito and B58 at several positions in the ITS1-5.8S-ITS2 region of the genome, whereas strains Fito and B58 had identical sequences, with the exception of a single bp (position 553, 5′-3′), where strain B58 displayed thymine, whereas the sequencing chromatograms indicated that strain Fito displayed equal amounts of cytosine and thymine. There are several possible explanations for the divergence observed in strain Fito: (1) Strain Fito is composed of two strains, and has been since the beginning of the experiment. (2) Within strain Fito there has been a point mutation, which has been inherited and is now present in approximately half of the culture. (3) Strain Fito has two copies of the ITS1-5.8S-ITS2 gene complex with one bp difference between the two copies, resulting in the observed differences. The consistency of the genomic differences observed throughout the experimental period confirmed that strains were not mixed or substituted during the experiment. However, in terms of morphology, the two local strains M28 and B58 were more similar to each other than to strain Fito. Strain Fito exhibited only fusiform cells that were noticeably smaller than cells of the others two strains, whereas all three morphotypes were eventually detected for strains M28 and B58, both dominated by cells with fusiform morphology. This seems to indicate that polymorphism within the species P. tricornutum is strongly strain specific, and different studies have shown that strains vary in their tendency for pleiomorphy [44]. However, the conditions that promote growth and maintenance of a specific morphotype are still poorly understood. Often a specific morphotype predominates within a strain, whereby the fusiform morphotype is the most prevalent form [21].
Biomass productivity was similar for all three strains in spring and in summer with B58 having slightly higher productivities in spring. However, in autumn, after the third inoculation, strain M28 revealed a reduced biomass (and therewith EPA) productivity compared to the other strains, but at the same time the QY remained on the same level as during the previous cultivation (aside from a short drop after inoculation). This indicates that the physiological condition of the cells was not diminished, and that the reduced productivity might have been a strain-specific response to the progressively shorter day length. In that case, M28 would be an inferior candidate for outdoor cultivation at the given locations. In accordance with the biomass productivity, the EPA productivity was maximal in spring and lower in summer and autumn for all three strains. However, a significant difference between the strains was observed, with strain Fito revealing highest EPA productivities during the entire cultivation period. This was due to the high EPA content of the biomass in strain Fito (average of 4.4% DW) compared to M28 and B58 (average of 3.2 and 3.1%). Such a high EPA content has, to our knowledge, not been reported before for P. tricornutum, and compensates somewhat for the low biomass productivities. Thus, strain Fito was the most promising strain regarding EPA productivity at our location.
The same FA were detected for all thee strains with EPA, palmitoleic acid, palmitic acid, and myristic acid representing the major fraction of TFA. Similar relative FA composition for P. tricornutum were found by others [27], [45]. However, although the FA composition and contribution to TFA were similar among the three strains, a principal component analysis revealed strain-specific differences. The average FA composition of the three seasons for each strain were very similar and grouped closely together, whereas the three strains were clearly separated. Thus, differences between the strains were greater than differences between the seasons for a strain, and the three P. tricornutum strains could easily be distinguished by their relative FA composition. The FA that were primarily responsible for the clustering, were EPA, palmitoleic acid, arachidonic acid and hexadecatrienoic acid. EPA correlated positively with strain Fito, thus, reflecting the predominantly higher amount of EPA in this strain compared to M28 and B58, whereas palmitoleic acid and arachidonic acid were negatively correlated to strain Fito, indicating lower relative amounts of these FA. However, comparing the FA composition with data from laboratory cultures from the same three strains, revealed a significantly different relative FA composition between indoor and outdoor strains. The respective indoor and outdoor strains did not cluster together, but distinct from each other, and the indoor strains were more similar to each other than to the corresponding outdoor strains. These data indicate that all strains changed their relative FA composition from indoor to outdoor conditions, but responses were different between the strains. Thus, a particular FA composition only accounted for the conditions of this outdoor experiment, and did not allow for general strain identification outside this experimental setup. Interestingly, indoor/outdoor cultivations had a particular influence on the EPA content, that was noticeably lower for all strains under laboratory conditions. This correlates well with the decreased EPA contents that were observed in the outdoor cultures just after inoculation. Significant differences in the lipid fractions between laboratory- and outdoor-grown P. tricornutum cultures were previously shown by López Alonso et al. [46], who found EPA content increasing within four different lipid classes from indoor to outdoor conditions, with an increase from 31 to 40% in monogalactosyldiacylglycerols.
The key regulatory factors accounting for these different responses are difficult to identify, but are presumably related to the different cultivation conditions used in laboratory and outdoor cultures, including culture densities, pH, culture media, and daily irradiance and temperature conditions. Laboratory cultures had much lower DW concentration (0.1–0.2 g L− 1), and constant temperature, irradiance and pH conditions (15 °C, 120 μmol m− 2 s− 1 for 24 h d− 1 and pH 7.4). In contrast to this, outdoor grown strains had greater variation in DW concentrations (0.05–3 g L− 1), and were exposed to diurnal and seasonal changes in irradiance (intensity and duration) and temperatures, and a less stable and higher pH (average pH 7.8). Furthermore, the media for outdoor cultures had a considerably higher macronutrient concentration than media used for indoor cultures. Further laboratory experiments with alteration of settings of the different environmental factors, together with additional outdoor experiments might help identify which factor or combination of factors exert the primary influence on the dramatic change in FA profile. However, our results are significant in terms of strain selection for outdoor cultivation. A strain that might appear promising under laboratory conditions does not necessarily perform best under outdoor conditions. The same is true conversely, as strain Fito had the lowest EPA content under laboratory conditions and the highest content under outdoor conditions, and thus the highest EPA productivities of all strains.
Growing P. tricornutum on a large scale is realizable under the local climate conditions. However, the EPA productivity strongly depends on the strain chosen, and it can be further optimized by improving photobioreactor operation. To what extent large-scale cultivation is manageable in a sustainable and economic way, needs to be evaluated further.
5. Conclusion
Outdoor production of three different P. tricornutum strains was feasible during at least six months of the year at the climate conditions in Bergen, western Norway and productivities were maximal in spring (Biomass: 0.25, 0.26 and 0.30 g L− 1 d− 1, EPA: 11.8, 8.0 and 9.2 mg L− 1 d− 1, for Fito, M28 and B58, respectively) when irradiance was highest (mean of 36.3 mol m− 2 d− 1). In summer and autumn, average irradiances were reduced by 45% and productivities decreased by approximately 30–45% in all strains, and by 65% in strain M28 in autumn. Strain Fito revealed highest EPA productivities during the entire cultivation period due to an exceptionally high EPA content of the biomass (average of 4.4% DW compared to 3.2 and 3.1 for M28 and B58), that has to our knowledge, not been reported before for P. tricornutum. When related to studies from lower latitudes, biomass productivities of the three strains were lower, most probably due to the reduced irradiances at the given location. However, the comparatively high EPA content of our strains under outdoor conditions could partially compensate for the lower biomass productivities, and similar EPA productivities were obtained as found from studies in Italy. Microalgae cultivation at higher latitudes might be further enhanced by improving cultivation conditions, like maintaining more constant biomass concentrations, and a more accurate temperature and pH control.
The same FA were identified for the three strains, but a PCA revealed different relative abundances, allowing for discrimination between the three strains by their FA profiles, whereas the changing seasons had only little influence on the FA content. The FA that predominantly contributed to the distribution of the three strains were EPA, palmitoleic acid, arachidonic acid (20:4 n-6), hexadecatetraenoic acid (16:4 n-1), hexadecatrienoic acid (16:3 n-4) and hexadecadienoic acid (16:2 n-4); EPA correlated positively with strain Fito. Comparing the relative FA composition from the outdoor cultures with their respective FA profiles from laboratory experiments revealed stronger differences between outdoor- and laboratory-grown cultures, than between strains. Hence, the increased EPA content in strain Fito that resulted in a significantly higher EPA productivity was only observed during outdoor conditions, but not in laboratory experiments. Our results demonstrate the importance for empirical comparison of different strains at a given location to achieve maximal EPA productivities.
Declarations of interest
None.
No conflicts, informed consent, human or animal rights applicable.
Acknowledgements
This work was supported by EU MIRACLES project and has received funding from the European Union's Seventh Framework Programme for research, technological development and demonstration under grant agreement No 613588, http://www.miraclesproject.eu. We thank Fitoplancton Marino in Cadiz, Spain, for providing their P. tricornutum strain, referred to as “Fito” in this study. Big thanks to Bryan Wilson at Marine Microbiology, University of Bergen for revising and improving the language quality of this paper.
Appendix A.
Fig. 1.
Daily average, minimum and maximum pH during six-months repeated-batch cultivation of three Phaeodactylum tricornutum strains (Fito, M28, B58) in flat panel outdoor bioreactors in Bergen, western Norway. The three grey bars indicate interruptions of the cultivation process. After interruption (1) and (3), the position of the strains was rotated, and after (2) and (3), the panels were re-inoculated with backup cultures. Black dotted lines mark the division of the cultivation period into three seasons; spring, summer and autumn.
Fig. 2.
Principal component analysis of the fatty acids composition (% DW) for three Phaeodactylum tricornutum strains (Fito, M28 and B58). A: Nine objects representing the three strains at the three seasons (spring, summer, autumn) during six-months repeated-batch cultivation in flat panel outdoor bioreactors in Bergen, western Norway, and eight variables, representing the fatty acids (FA) with highest impact on the distributions. Values are average of all samples of the respective seasons (n = 30, 72 and 60 for spring, summer and autumn, respectively). B: Nine variables and same objects as in (A) including additional data from laboratory experiments (average of three measurement replicates) for Fito, B58 (two biological replicates) and M28 (one biological replicate). Laboratory data are from exponential growth phase during batch experiments with experimental setup and analyses described for M28 in Steinrücken et al. [14].
References
- 1.Draaisma R.B., Wijffels R.H., Ellen Slegers P.M., Brentner L.B., Roy A., Barbosa M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013;24:169–177. doi: 10.1016/j.copbio.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Yongmanitchai W., Ward O.P. Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl. Environ. Microbiol. 1991;57:419–425. doi: 10.1128/aem.57.2.419-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martins D.A., Custódio L., Barreira L., Pereira H., Ben-Hamadou R., Varela J., Abu-Salah K.M. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs. 2013;11:2259–2281. doi: 10.3390/md11072259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauton M.S., Reitan K.I., Norsker N.H., Tveterås R., Kleivdal H.T. A techno-economic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: research challenges and possibilities. Aquaculture. 2015;436:95–103. [Google Scholar]
- 5.Boelen P., van Dijk R., Sinninghe Damsté J.S., Rijpstra W.I.C., Buma A.G.J. On the potential application of polar and temperate marine microalgae for EPA and DHA production. AMB Express. 2013;3:26. doi: 10.1186/2191-0855-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubio-Rodríguez N., Beltrán S., Jaime I., de Diego S.M., Sanz M.T., Carballido J.R. Production of omega-3 polyunsaturated fatty acid concentrates: a review. Innovative Food Sci. Emerg. Technol. 2010;11:1–12. [Google Scholar]
- 7.Olofsson M., Lamela T., Nilsson E., Bergé J.P., del Pino V., Uronen P., Legrand C. Seasonal variation of lipids and fatty acids of the microalgae Nannochloropsis oculata grown in outdoor large-scale photobioreactors. Energies. 2012;5:1577–1592. [Google Scholar]
- 8.Patil V., Reitan K.I., Knutsen G., Mortensen L.M., Källqvist T., Olsen E., Vogt G., Gislerød H.R. Microalgae as source of polyunsaturated fatty acids for aquaculture. Curr. Top. Plant Biol. 2005;6:57–65. [Google Scholar]
- 9.Meiser A., Schmid-Staiger U., Trösch W. Optimization of eicosapentaenoic acid production by Phaeodactylum tricornutum in the flat panel airlift (FPA) reactor. J. Appl. Phycol. 2004;16:215–225. [Google Scholar]
- 10.Hulatt C.J., Thomas D.N. Energy efficiency of an outdoor microalgal photobioreactor sited at mid-temperate latitude. Bioresour. Technol. 2011;102:6687–6695. doi: 10.1016/j.biortech.2011.03.098. [DOI] [PubMed] [Google Scholar]
- 11.Silva Benavides A.M., Torzillo G., Kopecký J., Masojídek J. Productivity and biochemical composition of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown outdoors in tubular photobioreactors and open ponds. Biomass Bioenergy. 2013;54:115–122. [Google Scholar]
- 12.Kräbs G., Büchel C. Temperature and salinity tolerances of geographically separated Phaeodactylum tricornutum Böhlin strains: maximum quantum yield of primary photochemistry, pigmentation, proline content and growth. Bot. Mar. 2011;54:231–241. [Google Scholar]
- 13.Prestegard S.K., Oftedal L., Coyne R.T., Nygaard G., Skjærven K.H., Knutsen G., Døskeland S.O., Herfindal L. Marine benthic diatoms contain compounds able to induce leukemia cell death and modulate blood platelet activity. Mar. Drugs. 2009;7:605–623. doi: 10.3390/md7040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinrücken P., Erga S.R., Mjøs S.A., Kleivdal H., Prestegard S.K. Bioprospecting North Atlantic microalgae with fast growth and high polyunsaturated fatty acid (PUFA) content for microalgae-based technologies. Algal Res. 2017 doi: 10.1016/j.algal.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walne P.R. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus, Fish. Invest. Lond. Ser. 1970;2(26):1–62. [Google Scholar]
- 16.Guillard R.R.L. Culture of phytoplankton for feeding marine invertebrates. Cult. Mar. Invertebr. Anim. 1975:26–60. [Google Scholar]
- 17.Zhu C.J., Lee Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997;9:189–194. [Google Scholar]
- 18.C.M. Gachon, U.E. Achilles-Day, C. Rad-Menéndez, J.G. Day, Molecular Barcoding of Protists, n.d. https://www.ccap.ac.uk/documents/Barcoding_protocol.pdf.
- 19.Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 20.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tesson B., Gaillard C., Martin-Jézéquel V. Insights into the polymorphism of the diatom Phaeodactylum tricornutum Bohlin. Bot. Mar. 2009;52:104–116. [Google Scholar]
- 22.Rzhetsky A., Nei M. A simple method for estimating and testing minimum-evolution trees. Mol. Biol. Evol. 1992;9:945–967. [Google Scholar]
- 23.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution (NY) 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 24.Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acién Fernández F.G., Hall D.O., Cañizares Guerrero E., Krishna Rao K., Molina Grima E. Outdoor production of Phaeodactylum tricornutum biomass in a helical reactor. J. Biotechnol. 2003;103:137–152. doi: 10.1016/s0168-1656(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 26.Molina Grima E., Sánchez Pérez J.A., Garcìa Camacho F., Fernández Sevilla J.M., Acién Fernández F.G., Urda Cardona J. Biomass and icosapentaenoic acid productivities from an ourdoor batch culture of Phaeodactylum tricornutum in an airlift photobioreactor. Appl. Microbiol. Biotechnol. 1995;42:658–663. [Google Scholar]
- 27.Sánchez Mirón A., Cerón García M.C., Contreras Gómez A., García Camacho F., Molina Grima E., Chisti Y. Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem. Eng. J. 2003;16:287–297. [Google Scholar]
- 28.Rodolfi L., Biondi N., Guccione A., Bassi N., D'Ottavio M., Arganaraz G., Tredici M.R. Oil and eicosapentaenoic acid production by the diatom Phaeodactylum tricornutum cultivated outdoors in Green Wall Panel (GWP®) reactors. Biotechnol. Bioeng. 2017 doi: 10.1002/bit.26353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tredici M.R., Bassi N., Prussi M., Biondi N., Rodolfi L., Chini Zittelli G., Sampietro G. Energy balance of algal biomass production in a 1-ha “Green Wall Panel” plant: how to produce algal biomass in a closed reactor achieving a high net energy ratio. Appl. Energy. 2015;154:1103–1111. [Google Scholar]
- 30.Carvalho A.P., Malcata F.X. Optimization of ω-3 fatty acid production by microalgae: crossover effects of CO2 and light intensity under batch and continuous cultivation modes. Mar. Biotechnol. 2005;7:381–388. doi: 10.1007/s10126-004-4047-4. [DOI] [PubMed] [Google Scholar]
- 31.de Vree J.H., Bosma R., Wieggers R., Gegic S., Janssen M., Barbosa M.J., Wijffels R.H. Turbidostat operation of outdoor pilot-scale photobioreactors. Algal Res. 2016;18:198–208. [Google Scholar]
- 32.Torzillo G., Faraloni C., Silva A.M., Kopecký J., Pilný J., Masojidek J. Photoacclimation of Phaeodactylum tricornutum (bacillariophyceae) cultures grown outdoors in photobioreactors and open ponds. Eur. J. Phycol. 2012;47:169–181. [Google Scholar]
- 33.Zhao P., Gu W., Wu S., Huang A., He L., Xie X., Gao S., Zhang B., Niu J., Lin P., Wang G. Silicon enhances the growth of Phaeodactylum tricornutum Bohlin under green light and low temperature. Sci. Rep. 2014;4:3958. doi: 10.1038/srep03958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prestegard S.K., Erga S.R., Steinrücken P., Mjøs S.A., Knutsen G., Rohloff J. Specific metabolites in a Phaeodactylum tricornutum strain isolated from western Norwegian fjord water. Mar. Drugs. 2015;14:9. doi: 10.3390/md14010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu C., Rao K., Hall D., Vonshak A. Production of eicosapentaenoic acid (EPA) in Monodus subterraneus grown in a helical tubular photobioreactor as affected by cell density and light intensity. J. Appl. Phycol. 2001;13:517–522. [Google Scholar]
- 36.Pal D., Khozin-Goldberg I., Cohen Z., Boussiba S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011;90:1429–1441. doi: 10.1007/s00253-011-3170-1. [DOI] [PubMed] [Google Scholar]
- 37.Solovchenko A.E. Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ. J. Plant Physiol. 2012;59:167–176. [Google Scholar]
- 38.Cepák V., Přibyl P., Kohoutková J., Kaštánek P. Optimization of cultivation conditions for fatty acid composition and EPA production in the eustigmatophycean microalga Trachydiscus minutus. J. Appl. Phycol. 2013;26:181–190. [Google Scholar]
- 39.Seto A., Wang H.L., Hesseltine C.W. Culture conditions affect eicosapentaenoic acid content of Chlorella minutissima. J. Am. Oil Chem. Soc. 1984;61:892–894. [Google Scholar]
- 40.Van Wagenen J., Miller T.W., Hobbs S., Hook P., Crowe B., Huesemann M. Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies. 2012;5:731–740. [Google Scholar]
- 41.Hu H., Gaol K. Optimization of growth and fatty acid composition of a unicellular marine picoplankton, Nannochloropsis sp., with enriched carbon sources. Biotechnol. Lett. 2003;25:421–425. doi: 10.1023/a:1022489108980. [DOI] [PubMed] [Google Scholar]
- 42.Tatsuzawa H., Takizawa E. Changes in lipid and fatty acid composition of Pavlova lutheri. Phytochemistry. 1995;40:397–400. [Google Scholar]
- 43.Jiang H., Gao K. Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae) J. Phycol. 2004;40:651–654. [Google Scholar]
- 44.De Martino A., Meichenin A., Shi J., Pan K., Bowler C. Genetic and phenotypic characterization of Phaeodactylum tricornutum (Bacillariophyceae) accessions. J. Phycol. 2007;43:992–1009. [Google Scholar]
- 45.López Alonso D., Belarbi E.-H., Fernández-Sevilla J.M., Rodriguez-Ruiz J., Molina Grima E. Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry. 2000;54:461–471. doi: 10.1016/s0031-9422(00)00084-4. [DOI] [PubMed] [Google Scholar]
- 46.López Alonso D., Belarbi E.H., Rodríguez-Ruiz J., Segura C.I., Giménez A. Acyl lipids of three microalgae. Phytochemistry. 1998;47:1473–1481. [Google Scholar]