Abstract
Background
There have been many attempts to develop new materials with stability and high affinity towards immunoglobulins. Some of glycolipids such as gangliosides exhibit a high affinity toward immunoglobulins. However, it is considerably difficult to develop these glycolipids into the practical separation ligand due to their limited amounts. We thus focused our attention on the feasible use of "mannosylerythritol lipid A", a yeast glycolipid biosurfactant, as an alternative ligand for immunoglobulins, and undertook the investigation on the binding between mannosylerythritol lipid A (MEL-A) and human immunoglobulin G (HIgG).
Results
In ELISA assay, MEL-A showed nearly the same binding affinity towards HIgG as that of bovine ganglioside GM1. Fab of human IgG was considered to play a more important role than Fc in the binding of HIgG by MEL-A. The bound amount of HIgG increased depending on the attached amount of MEL-A onto poly (2-hydroxyethyl methacrylate) (polyHEMA) beads, whereas the amount of human serum albumin slightly decreased. Binding-amount and -selectivity of HIgG towards MEL-A were influenced by salt species, salt concentration and pH in the buffer solution. The composite of MEL-A and polyHEMA, exhibited a significant binding constant of 1.43 × 106 (M-1) for HIgG, which is approximately 4-fold greater than that of protein A reported.
Conclusions
MEL-A shows high binding-affinity towards HIgG, and this is considered to be due to "multivalent effect" based on the binding molar ratio. This is the first report on the binding of a natural human antibody towards a yeast glycolipid.
Background
Immunoglobulins represent glycoproteins bearing carbohydrate-recognition motifs and are widely used for immunodiagnostics, epitope mapping and therapeutic applications etc. [1-3]. There have been many attempts to develop new materials possessing a stability and high affinity towards immunoglobulins, due to the high cost of protein-A or -G most commonly used as an affinity ligand for the proteins [4].
Glycolipids such as gangliosides and glycosphingolipids carry out vital functions (i.e., signal transduction, cell recognition and cell proliferation) in biomembranes through protein-carbohydrate interactions [5,6]. Some of these glycolipids exhibit a high affinity toward glycoproteins as a result of "multivalent or cluster effect" and thus are focused on as a new affinity ligand for immunoglobulins [7,8]. Gangliosides like GM1 and GD1a are known to bind to immunoglobulins [9-11], while asialo-GM1 and GD3 show a specific binding to IgM and IgG, respectively [8,12]. The possibility of developing these membrane glycolipids into the ligand, however, is far from straightforward due to their limited amounts and heterogeneity [13].
"Biosurfactants" constitute a variety of microbial extracellular lipids that are produced in large amounts from inexpensive natural sources [14,15]. We thus focused our attention on the feasible use of "glycolipid biosurfactants" as an alternative affinity ligand for IgG, the most dominant and essential immunoglobulin in mammalians.
Mannosylerythritol lipid (MEL), a yeast glycolipid biosurfactant, is abundantly produced from vegetable oils by Candida strains at a yield of 100 gl-1[16,17]. It easily makes self-assembling properties, which show binding affinity towards Concanavalin A [18]. More significantly, MEL induces the cell differentiation of HL-60 human promyelocytic leukemia cells [19], the outgrowth of neurites of PC12 rat pheochromocytoma cells [20], and the growth arrest and apoptosis of B16 mouse melanoma cells [21]. These intriguing activities of MEL are quite similar to those of gangliosides or glycosphingolipid [5,6,22], indicating that MEL may also perform the activities through a protein-carbohydrate interaction. Therefore, we undertook the investigation on the binding between the potential microbial glycolipid and human IgG (HIgG).
In this report, we describe for the first time that the yeast glycolipid shows a significant binding affinity towards a natural polyclonal HIgG. We also report the binding constant and capacity for HIgG of MEL attached onto a polymer supporting material.
Results
Binding affinity of MEL-A toward HIgG
GM1, which is a glycosphingolipid having one sialic acid, exhibits binding affinity towards IgG [9-11], and the sialic acid consisting of acetyl and glycerol groups plays an important role in carbohydrate-protein interactions [8,23-25]. The binding affinity between HIgG and MEL-A bearing acetyl and erythritol groups was thus investigated using GM1 as a proper reference.
The optical absorbance at 450 nm, which corresponds to the bound amount of HIgG, increased along with increases in the inlet amount of glycolipid, and reached to a binding-plateau (Fig. 2). This is presumably due to the limitation in the area of the glycolipid layer or in the presentation geometry [8,12].
Figure 2.
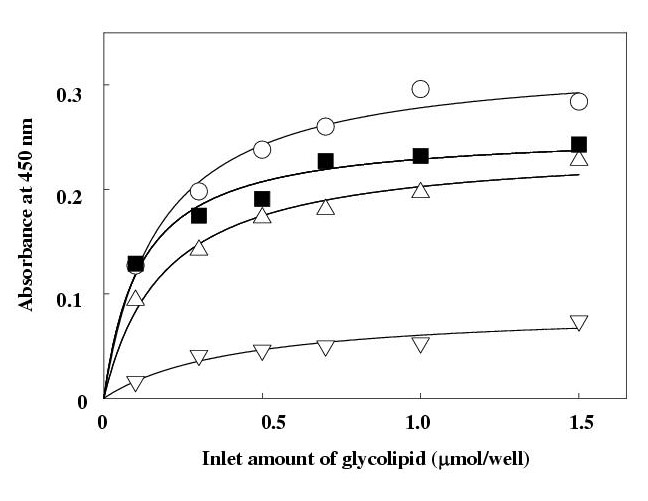
Binding assay of human IgG to mannosylerythritol lipid-A and gangliosides. The glycolipids were attached inside the wells of microtiter plate. The wells were incubated with 100 μl of human IgG solution (10 μM) of 10 m M phosphate-buffered saline (150 mM NaCl, pH 7.2) for 1 hr. After washing, bound human IgG were detected by a sandwich assay using an enzyme-labeled anti-human IgG or anti-human IgG Fab fragment or anti-human IgG Fc fragment. MEL-A (-??-) and GM1 (-??-) with anti-human IgG; MEL-A with anti-human IgG Fab fragment (-??-) and anti-human IgG Fc fragment (-??-). Each plot is the mean of triplicate. MEL-A, mannosylerythritol lipid-A
In order to elucidate the fragment of HIgG being responsible for the binding to MEL-A, Fab and Fc fragment specific anti-HIgG were then used instead of the anti-HIgG whole body as an enzyme-labeled secondary antibody. In the case of the Fc specific antibody, the observed absorbance was much higher than that of Fab, implying that mainly the Fab site of HIgG contributes to the binding to MEL-A and thus hardly interacts with the added Fab specific antibody.
No direct binding was observed between the above glycolipids and secondary antibodies, and no glycolipid was leaked from the wells during the course of the present assay.
Binding of HIgG to MEL-polyHEMA composite
The binding of HIgG to MEL-A was further confirmed using the prepared composite, considered to minimize the limitation presumed in the ELISA assay. In this experiment, the binding of HSA to the composite was also examined; HSA is the most dominant protein in human serum that is the essential source of HIgG.
The attached amount of MEL-A onto polyHEMA beads increased with increases in the charged amount of MEL-A under the conditions employed (Fig. 3). PolyHEMA itself showed no selective binding for HIgG and HSA. However, as expected from the above results, the bound amount of HIgG to the composite increased depending on the attached amount of MEL-A, whereas the amount of HSA slightly decreased (Fig. 4). The bound amount of HIgG was 2.7-fold higher than that of HSA with the composite bearing 2.99 mg MEL-A per g of polyHEMA.
Figure 3.
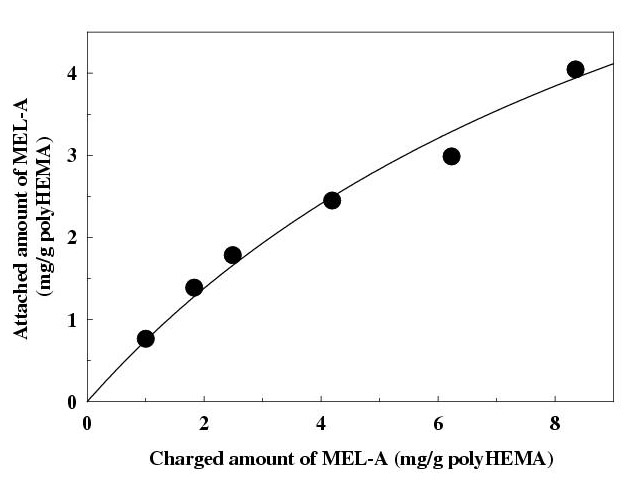
Attachment of MEL-A onto poly (2-hydroxyethyl methacrylate) beads. Different amounts of MEL-A in methanol were charged to the mixture of polyHEMA beads (0.35 ± 0.05 g) and methanol (20 ml). After the solvent was evaporated, the obtained residue was washed with water and filtrated. The amount of MEL-A attached onto the beads was determined by anthrone method after being extracted with a mixture of chloroform-methanol (2:1). Each plot is the mean of triplicate. MEL-A, mannosylerythritol lipid-A; polyHEMA, poly (2-hydroxyethyl methacrylate).
Figure 4.
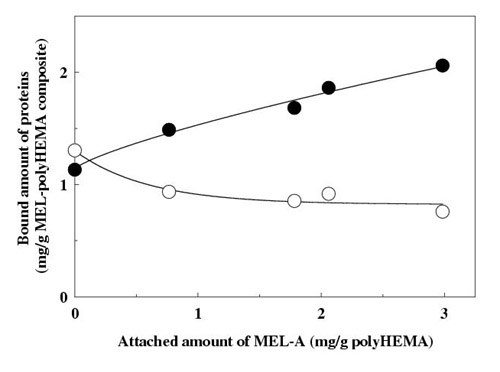
Binding assay of human IgG to mannosylerythritol-A attached onto poly (2-hydroxyethyl methacrylate) beads. Human IgG or human serum albumin (1.0 mg) was added to the mixture of MEL-polyHEMA composite (0.35±0.05 g) bearing different amounts of MEL-A and 50 mM phosphate buffer (3 ml) in a polypropylene tube. The tube was incubated for 1 hr and then centrifuged. The amount of bound protein was calculated by subtracting the unbound protein from the total added; the unbound protein was estimated by measuring the UV absorbance of the supernatant. human IgG (-??-), human serum albumin (-??-). Each plot is the mean of triplicate. MEL-A, mannosylerythritol lipid-A; polyHEMA, poly (2-hydroxyethyl methacylate).
Effects of salt and pH on the binding of protein to MEL-polyHEMA composite
The effects of salt concentration and the pH of the phosphate buffer on the binding between the composite and proteins were further examined to address thebinding mode as well as the maximum binding. Na2SO4 and NaCl were then used in this study, since these salts are well known to promote the binding of a protein to its affinity ligand [26]. The bound amount of HIgG increased with increases in the Na2SO4 and NaCl concentrations up to 1 M; the addition of Na2SO4 and NaCl into the buffer enhanced the bound amount approximately 1.4-fold and 1.1-fold, respectively, compared to that without the salt (Table 1). The effect of pH was then examined with the presence of 1 M of Na2SO4. Acidic or alkaline conditions enhanced the binding of both HIgG and HSA, but resulted in lower selectivities for the two proteins. The bound amount of HIgG was 4.7-fold higher than that of HSA with the buffer of pH 6.4 (Table 1).
Table 1.
The effects of salt concentration and the pH of phosphate buffer on the protein binding to MEL-A attached onto polyHEMA beads
| Amount of protein bound | |||
| (mg/g composite a) | |||
| Salt(M) | pH | HIgG | HSA |
| Control (0) | 7.0 | 2.06 | 0.76 |
| Na2SO4 (1.0) | 4.0 | 3.93 | 1.95 |
| 5.6 | 3.63 | 1.12 | |
| 6.4 | 3.45 | 0.73 | |
| 7.0 | 2.87 | 1.20 | |
| 7.5 | 3.73 | 1.28 | |
| NaCl (1.0) | 7.0 | 2.35 | 0.53 |
a Composite bearing 2.99 mg of MEL-A per g of polyHEMA. The protein (1.0 mg) was added to the mixture of MEL-polyHEMA composite and 50 mM phosphate buffer (3 ml) in a polypropylene tube. The tube was incubated for 1 hr and then centrifuged. The amount of bound protein was calculated by subtracting that of unbound protein from the total added; the amount of unbound protein was estimated by measuring the UV absorbance of the supernatant. Each data is the mean of triplicate. MEL-A, mannosylerythritol lipid-A; polyHEMA, poly (2-hydroxyethyl methacylate); HIgG, human immunoglobulin G; HSA, human serum albumin.
Binding constant and capacity for HIgG of MEL-polyHEMA composite
In order to deduce the binding constant and capacity for HIgG of the composite, the optimized conditions, pH 6.4 and 1 M, Na2SO4, were obtained. The binding isotherm was of the Langmuir type; from the equation for the Langmuir adsorption isotherm (Fig. 5), the binding constant and capacity were estimated to be 1.43 × 106 M-1 and 12.6 mg HIgG per g of the composite used, respectively.
Figure 5.
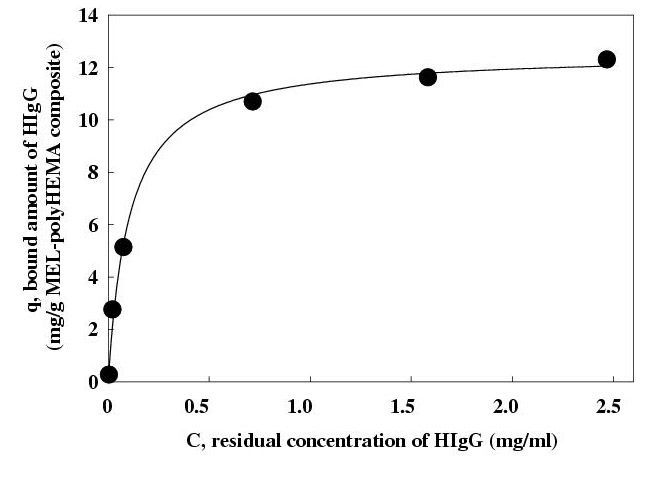
The binding isotherm for human IgG to MEL-A attached onto poly (2-hydroxyethyl methacrylate) beads. Different amounts of human IgG (0.1 to 12.0 mg) was added to the mixture of MEL-polyHEMA composite (0.35±0.05 g) bearing 4.04 mg MEL-A per g of polyHEMA and 50 mM phosphate buffer (1M of Na2SO4, pH 6.4) (3 ml) in a polypropylene tube. The tube was incubated for 1 hr and then centrifuged. The amount of bound protein was calculated by subtracting the unbound protein from the total added; the unbound protein was estimated by measuring the UV absorbance of the supernatant. The binding constant and capacity were determined from the equation for the Langmuir adsorption isotherm, q = (Qmax KaC) / (1+KaC). MEL-A, mannosylerythritol lipid-A; polyHEMA, poly (2-hydroxyethyl methacylate).
Conclusions
In this work, we demonstrated that MEL-A exhibits a significant binding affinity towards a natural HIgG. The binding affinity was nearly the same as that observed for GM1. MEL-A, however, has a different structure from that of GM1; both the hydrophilic and hydrophobic groups of the former are much smaller than those of the latter.
Some of the bindings between gangliosides and glycoproteins are enhanced by a "multivalent or cluster effect"; a simultaneous association of two or more ligands and receptors [7,8,12,27]. More importantly, the effect is considerably dependent on the density, orientation and conformation of the saccharide moieties of gangliosides [7,8]. We previously demonstrated that MELs efficiently self-assemble to form vesicles, which show a binding affinity towards Concanavalin A based on the "multivalent effect" [18]. This instantly means that MELs have a superior property on the molecular orientation and packing. Therefore, MEL-A is likely to position densely inside the plate well in a regulated manner so as to generate a "multivalent surface" leading to the interaction with HIgG. This may compensate for the small saccharide moiety and provide MEL-A with a similar binding affinity to that of GM1. Based on the observed binding capacity, the binding molar ratio between HIgG and MEL-A is approximately 1:70, supporting the binding is attributed to the "multivalent effect."
The sialic acid, especially the acetyl and glycerol side chains in it, plays an important role in the carbohydrate-protein interactions [8,23-25]. Neiser et al. reported that the binding affinity of gangliosides towards HIgG increased depending on the number of sialic acid [24]. Siebert et al. demonstrated that 9-O-acetylated GD1a bound to a natural polyclonal HIgG via the direct interaction between the acetyl group and amino acids in the binding site of the protein [25]. It is thus likely that the O-acetyl and erythritol groups in MEL-A have a critical role on the interaction with HIgG.
Interestingly, HSA exhibited a different behavior on the binding to MEL-polyHEMA composite from that of HIgG, indicating a difference in the binding mode between the two proteins. HSA is one of the representative proteins that bind to surfaces via hydrophobic-hydrophobic interactions [28]. Hence, the binding of HSA may be attribute to a interaction between the protein and the hydrophobic part of MEL-A.
It seems reasonable that the binding of HIgG to the composite was significantly enhanced by the addition of Na2SO4, because the salt more strongly affects the protein structure than NaCl in the order of the Hofmeister series [29]. Bagchi and Birbaum demonstrated that the angle of two Fab fragments of IgG considerably expanded depending on the pH; the angle was changed from "Y-shape" (arm-collapsed) into "T-shape" (arm-extended) [30]. The observed pH effect on the HIgG binding might be due to the shape change, based on the presumption that the Fab fragment of HIgG is the main binding site towards MEL-A. On the contrary, the Fc fragment of HIgG is the binding site towards protein A, which is the most representative affinity ligand for IgG [31]. The binding mode of MEL-A for HIgG, therefore, is likely to be significantly different from that of protein A, that is, a protein-protein interaction.
Teng et al. recently reported that the binding constant and capacity for HIgG of immobilized protein A (commercially available) were 3.65 × 105M-1 and 17.0 mg HIgG per g of the supporting material, respectively [4]. The binding molar ratio between HIgG and protein A was reported approximately 3:1, whereas that between HigG and MEL-A was 1:70 as described above. This also supports the multivalent effect of MEL-A toward the protein binding. In spite of these different binding mechanisms, the observed binding constant for HIgG of MEL-A is approximately 4-fold higher than that of the protein A, and the binding capacity is nearly comparable between the two ligands. The bound amount of HIgG to the composite increased with increases in the attached amount of MEL-A, and the potential selectivity for HIgG and HSA was obtained by modifying the buffer conditions. The increase of MEL-A probably enhances the density of an ordered conformation needed for the interaction with HIgG; this finally might impede the binding of HSA. Therefore, the binding capacity and selectivity of the composite would be improved with a greater attached amount of MEL-A.
Nevertheless, the detailed binding mechanism between MEL and HIgG is still obscure and remains to be clarified. The approach for the mechanisms may also contribute to a better understanding of the glycolipid activities against mammalian tumor cells. We are not aware of any existing reports on the binding of a natural human antibody to a yeast extracellular glycolipid. The yeast glycolipid, which is abundantly produced from vegetable oils, may be a potential material for human IgG separation.
Materials and Methods
Materials
Polyclonal HIgG (purity 95 %), globulin free human serum albumin (HSA), 5,5'-tetramethyl-benzidine dihydrochloride (TMB) and peroxidase-conjugated anti-HIgG Fab fragment from goat were purchased from Sigma Chemical Co. (St. Louis, USA). Peroxidase-conjugated anti-HIgG Fc fragment from goat was purchased from Jackson Immunoresearch Laboratories Inc. (West Grove, USA). GM1 from bovine brain and peroxidase-conjugated anti-HIgG from rabbit were purchased from Wako Pure Chemicals (Osaka, Japan). Other reagents were of biochemical grade and were commercially available.
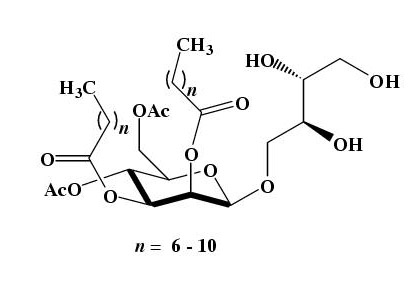
The mixture of MELs were produced from methyl tetradecanoate with a yeast strain of Candida antarctica T-34 [32], and purified by silica-gel column chromatography as reported previously [17,18]. The purified 4-O-[(4', 6'-di-O-acetyl-2', 3'-di-O-alkanoyl)-β-D-mannopyranosyl]meso-erythritol (MEL-A) (Fig. 1), which is the major component of the yeast product, was exclusively used in the following experiments. MEL-A (mean Mw: 676) is sparingly soluble in water [33].
Figure 1.
Structure of mannosylerythritol lipids produced by Candida antarctica.
Assay for HIgG-glycolipid binding by ELISA
ELISA was performed according to the "noncovalent method" used for small lipidic analytes [34]. Glycolipids and antibodies were dissolved in methanol and in 10 mM phosphate-buffered saline (PBS) (150 mM NaCl, pH 7.2), respectively. Glycolipid solutions (100 μl) of different concentrations were put into wells of microtiter plate, and the plate was dried under a nitrogen stream; glycolipids were then noncovalently bound inside the wells. Washing was performed at every step with PBS-0.05 % Tween 20.
1) The wells were blocked with 5 % bovine serum albumin for 1 hr; 2) incubated with 100 μl of HIgG solution (10 μM) for 1 hr; 3) incubated with 100 μl of the peroxidase-conjugated anti-HIgG solution (1.4 μg/ml) for 1 hr; 4) incubated with 100 μl of substrate solution (160 μM TMB in acetate buffer, pH 5.6) for 5 min.; 5) incubated with 25 μl of 1 M HCl to terminate the reaction. The optical absorbance of each well was determined at 450 nm using a microplate reader (Molecular Device, USA). In the case of peroxidase-conjugated anti-HIgG Fab and anti-HIgG Fc, the original reagent solution was used after being diluted to 1000-fold.
After each step, the washed solution was subjected to thin-layer chromatography [12] to check the glycolipid leak from the wells.
Attachment of MEL-A onto polyHEMA beads
In this study, poly (2-hydroxyethyl methacrylate) (polyHEMA), which is widely used for protein bindings due to their biocompatibility and stability [35,36], was employed as a polymer supporting material for MEL. PolyHEMA beads (diameter: 50 to 150 μm) were synthesized by the suspension polymerization method reported previously [36,37]. The weight of the beads was expressed in terms of the dry weight in the following experiments.
Different amounts of MEL-A in methanol were charged to the mixture of polyHEMA beads (0.35 ± 0.05 g) and methanol (20 ml). All the solvent were then evaporated by vigorous stirring under a nitrogen stream at room temperature. The obtained residue was washed extensively with water and filtrated to give MEL-A attached onto polyHEMA beads (designated as MEL-polyHEMA composite). The amount of MEL-A attached onto the beads was determined by the previously described anthrone method [38] after being extracted with a solvent mixture of chloroform-methanol (2:1).
Assay for protein-MEL binding using MEL-polyHEMA composite
The binding studies between proteins and the composite were conducted according to the method of Teng et al [4]. HIgG or HSA (1.0 mg) was added to the mixture of the composite (0.35 ± 0.05 g) bearing different amounts of MEL-A and 3 ml of 50 mM phosphate buffer (pH 7.0) in a polypropylene tube, unless otherwise indicated. The tube was reciprocally incubated for 1 hr at room temperature, and then centrifuged at 3,000 rpm for 20 min. The amount of bound protein to the composite was calculated by subtracting that of unbound protein from the total added; the amount of unbound protein was estimated by measuring the UV absorbance at 280 nm of the supernatant.
Determination of the binding constant and capacity for HIgG of MEL-PHEMA composite
Different amounts of HIgG (0.1 to 12.0 mg) was added to the mixture of the composite (0.35 ± 0.05 g) bearing 4.04 mg MEL-A per g of polyHEMA and 3 ml of 50 mM phosphate buffer (1 M of Na2SO4, pH 6.4) in the tube. The amounts of bound and unbound HIgG were estimated by the above method using UV measurement. The binding constant and capacity were determined from the equation for the Langmuir adsorption isotherm [4], q = (QmaxKaC) / (1+ KaC), where q is the bound amount of HIgG, C is the unbound concentration of HIgG, Ka is the binding constant and Qmax is the binding capacity.
Abbreviations
ELISA, enzyme-linked immunosorbent assay; HIgG, human immunoglobulin G; HSA, human serum albumin; IgM, immunoglobulin M; MEL, mannosylerythritol lipid; polyHEMA, poly(2-hydroxyethyl methacrylate); the designation of ganglio-series gangliosides is according to Svennerholm [39].
Acknowledgments
Acknowledgments
We are grateful to Prof. Y. Nakatani and Prof. G. Ourisson, Université Louis Pasteur, for their helpful discussion. We also thank to Ms. T. Yokoshima and Mr. A. Sasaki for their technical assistance. This work was supported by the New Energy and Industrial Technology Development Organization, Japan (Grand No. 99E-Shou-06-006-02).
Contributor Information
Jae Hong Im, Email: jaehong-im@aist.go.jp.
Takashi Nakane, Email: T.Nakane@tkc.xm.mitsui.co.jp.
Hiroshi Yanagishita, Email: yanakishita-hiroshi@aist.go.jp.
Toru Ikegami, Email: ikegami.t@aist.go.jp.
Dai Kitamoto, Email: dai-kitamoto@aist.go.jp.
References
- Nopper B, Kohen F, Wilchek M. Thiophilic adsorbent for the one-step high-performance liquid chromatography purification monoclonal antibodies. Anal Biochem. 1989;180:66–71. doi: 10.1016/0003-2697(89)90088-2. [DOI] [PubMed] [Google Scholar]
- Serres A, Muller D, Jozefonvicz J. Purification of monoclonal antibodies on dextran-coated silica support grafted by thiophilic ligand. J Chromatogr A. 1995;711:151–157. doi: 10.1016/0021-9673(95)00256-M. [DOI] [PubMed] [Google Scholar]
- Roger RD. Protein purification process engineering. New York, Marcel Dekker. 1994.
- Teng SF, Sproule K, Husain A, Lowe CR. Affinity chromatography on immobilized "biomimetic" ligands synthesis, immobilization and chromatographic assessment of an immunoglobulin G-binding ligand. J Chromatogr B. 2000;740:1–15. doi: 10.1016/S0378-4347(99)00549-6. [DOI] [PubMed] [Google Scholar]
- Hooper NM. Membrane biology; Do glycolipid microdomain really exist? Curr Biol. 1998;8:R114–116. doi: 10.1016/s0960-9822(98)70984-4. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Yamanura S, Prinetti A, Handa K, Hakomori SI. GM3-enriched microdomain involved in cell adhesion and signal transduction through carbohydrate-carbohydrate interaction in mouse melanoma B16 cells. J Biol Chem. 1998;273:9130–9138. doi: 10.1074/jbc.273.15.9130. [DOI] [PubMed] [Google Scholar]
- Boullanger P. Amphiphilic carbohydrates as a tool for molecular recognition in organized system. Top Curr Chem. 1997;187:275–312. [Google Scholar]
- Evans SV, MacKenzie CR. Characterization of protein-glycolipid recognition at the membrane bilayer. J Mol Recognit. 1999;12:155–168. doi: 10.1002/(SICI)1099-1352(199905/06)12:3<155::AID-JMR456>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Alie-Daram SJ, Fournie A, Dugoujon J-M. Gel test assay for IgG subclass detection by GM typing; application to hemolytic disease of the newborn. J Clinic Lab Anal. 2000;14:1–4. doi: 10.1002/(SICI)1098-2825(2000)14:1<1::AID-JCLA1>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabrina M, Francesco L, Mats S, Fransico P, Hans L. Multiple sclerosis is accociated with enhanced B cell responses to the ganglioside GD1a. Mult Scler. 1999;5:379–388. doi: 10.1191/135245899678846483. [DOI] [PubMed] [Google Scholar]
- Lunn MPT, Johnson LA, Fromholt SE, Itonori S, Huang J, Vyas AA, Hildreth JEK, Griffin JW, Schnaar RL, Sheikh KA. High-affinity anti-ganglioside IgG antibodies raised in complex ganglioside knockout mice: reexamination of GD1a immunolocalization. J Neurochem. 2000;75:404–412. doi: 10.1046/j.1471-4159.2000.0750404.x. [DOI] [PubMed] [Google Scholar]
- Harrison BA, MacKenzie R, Hirama T, Lee KK, Altman E. A kinetics approach to the characterization of an IgM specific for the glycolipid asialo-GM1. J Immunol Methods. 1998;212:29–39. doi: 10.1016/S0022-1759(98)00012-X. [DOI] [PubMed] [Google Scholar]
- Feizi T. Carbohydrate-mediated recognition systems in innate immunity. Immunol Rev. 2000;173:79–88. doi: 10.1034/j.1600-065X.2000.917310.x. [DOI] [PubMed] [Google Scholar]
- Banat IM, Makker RS, Cameotra SS. Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol. 2000;53:495–508. doi: 10.1007/s002530051648. [DOI] [PubMed] [Google Scholar]
- Desai JD, Banat IM. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Yoon BD, Choung DH, Oh HM, Katsuragi T, Tani Y. Characterization of a biosurfactant, mannosylerythritol lipid produced from Candida sp SY16. Appl Microbiol Biotechnol. 1999;52:713–721. doi: 10.1007/s002530051583. [DOI] [PubMed] [Google Scholar]
- Kitamoto D, Fuzishiro T, Yanagishita H, Nakane T, Nakahara T. Production of mannosylerythritol lipids as biosurfactants by resting cells of Candida antartica. Biotechnol Lett. 1992;14:305–310. [Google Scholar]
- Kitamoto D, Ghosh S, Ourisson G, Nakatani Y. Formation of giant vesicles from diacylmannosylerythritols, and their binding to concanavalin A. Chem Commun. 2000:861–862. doi: 10.1039/b000968g. [DOI] [Google Scholar]
- Isoda H, Shinmoto H, Kitamoto D, Matsumura M, Nakahara T. Differentiation of human promyelocytic leukemia cell line HL60 by microbial extracellular glycolopids. Lipids. 1997;32:263–271. doi: 10.1007/s11745-997-0033-0. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Zhao X, Jin C, Day N, Sjibahara M, Nomura N, Nakahara T, Murata T, Yokohama KK. Mannosylerythritol lipid induces characteristics of neuronal differentiation in PC12 cells through an ERK-related signal cascade. Eur J Biochemistry. 2001;268:374–384. doi: 10.1046/j.1432-1033.2001.01887.x. [DOI] [PubMed] [Google Scholar]
- Zhao X, Wakamatsu Y, Shibara M, Nomura N, Geltinger C, Nakahara T, Murata T, Yokohama KK. Mannosylerythritol lipids is a potent inducer of apoptosis and differentiation of mouse melanoma cells in culture. Cancer Res. 1999;59:482–486. [PubMed] [Google Scholar]
- Kopitz J. Glycolipids-structure and function. Glycoscience (Edited by HJ Gabius, S Gabius) Weinheim Chapman & Hall. 1997. pp. 163–189.
- Hirai M, Takizawa T, Yabuki S, Nakata Y, Arai S, Furusaka M. Structural study of protein-ganglioside interaction. Progr Colloid Polym Sci. 1997;106:237–241. [Google Scholar]
- Neisser A, Bernheimer H, Berger T, Morgan AP, Schweber B. Serum antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in Miller Fisher syndrome. Infect Immun. 1997;65:4038–4042. doi: 10.1128/iai.65.10.4038-4042.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert HC, vonder Lieth CW, Dong X, Reuter G, Schauer R, Gabius HJ, Vliegenthart JFG. Molecular dynamics-derived conformation and intramolecular interaction analysis of the N-acetyl-9-O-acertylneuraminic acid-containing G(D1a) and NMR-based analysis of its binding to a human polyclonal immunoglobulin G fraction with selectivity for O-acetylated sialic acids . Glycobiology. 1996;6:561–572. doi: 10.1093/glycob/6.6.561-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson S, Karsnäs P. Salt-promoted adsorption of proteins onto amphiphilic agarose based adsorbents II. Effects of salt and salt concentration. J Chromatogr A. 1998;803:83–93. doi: 10.1016/S0021-9673(97)01088-1. [DOI] [PubMed] [Google Scholar]
- Willison HJ, O'Hanlon GM. The immunopathogenesis of Miller Fisher syndrome. J Neuroimmunol. 1999;100:3–12. doi: 10.1016/S0165-5728(99)00213-1. [DOI] [PubMed] [Google Scholar]
- Hutchens TW, Porath J. Thiophilic adsorption-a comparison of model protein behavior. Biochemistry. 1987;26:7197–7204. doi: 10.1021/bi00396a049. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Timasheff SN. Mechanism of protein salting out by divalent cation salts; balance between hydration and salt binding. Biochem. 1984;23:5912–5923. doi: 10.1021/bi00320a004. [DOI] [PubMed] [Google Scholar]
- Bagchi P, Birbaum SM. Effect of pH on the adsorption of immunoglobulin-G on anionic poly(vinyl toluene) model latex-particle. J Colloid Interface Sci. 1981;83:460–478. [Google Scholar]
- Turková J. Oriented immobilization of biologically active proteins as a tool for revealing protein interactions and function. J Chromatogr B. 1999;722:11–31. doi: 10.1016/S0378-4347(98)00434-4. [DOI] [PubMed] [Google Scholar]
- Kitamoto D, Akiba S, Hioki T, Tabuchi T. Extracellular accumulation of mannosylerythritol lipids by a strain of Candida Antarctica. Agric Bio Chem. 1990;54:31–36. [Google Scholar]
- Kitamoto D, Yanagishita H, Shinbo T, Nakane T, Kamisawa C, Nakahara T. Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida actarctica. J Biotechnol. 1993;29:91–96. doi: 10.1016/0168-1656(93)90042-L. [DOI] [Google Scholar]
- Gervay J, McReynolds KD. Utilization of ELISA technology to measure biological activities of carbohydrates relevant in desease states. Curr Med Chem. 1999;6:129–153. [PubMed] [Google Scholar]
- Hermason GT, Mallia AK, Smith PK. Immobilized affinity ligand techniques. San Diego, Academic Press. 1992.
- Denizli A, Piskin E. DNA-immobilized polyhydroxyethylmethacrylate microbeads for affinity sorption of human immunoglobulin G and anti-DNA antibodies. J Chromatogr B. 1995;666:215–222. doi: 10.1016/0378-4347(94)00593-T. [DOI] [PubMed] [Google Scholar]
- Im JH, Jang WJ, Kim HS, Chun KY, Han HS, Joe YI. A morphological study on the preparation of polyHEMA beads. J Kor Inst Chem Eng. 1998;36:887–895. [Google Scholar]
- Kitamoto D, Haneishi K, Nakahara T, Tabuchi T. Production of mannosylerythritol lipids by Candida antarctica from vegetable oils. Agric Biol Chem. 1990;54:37–40. [Google Scholar]
- Svennerholm L. The gangliosides. J Lipid Res. 1964;5:145–162. [PubMed] [Google Scholar]