Abstract
We report the case of a 59-year-old male patient suffering from locked-in syndrome (LIS) following basilar artery thrombosis despite an attempt of thrombolysis. Neurological examination showed quadriplegia and aphonia and a state of coma requiring mechanical ventilation was diagnosed. The use of 18F-fluorodeoxyglucose (18F-FDG)-positron emission tomography (PET) allowed to detect a normal 18F-FDG uptake in the main cerebral cortical areas and a significant reduction of 18F-FDG uptake in both cerebellar hemispheres, compatible with a functional deafferentation, helping confirming the clinical suspicion of LIS. The diagnosis of LIS, according to literature, is based on the clinical assessment and the utilization of scores as the Coma Recovery Scale-Revised. The standard neuroimaging techniques, although recognize the site of injury, are not able to differentiate the different conditions affecting a state of altered consciousness. Performing 18F-FDG-PET in patients with LIS might help addressing the correct diagnosis and prompting subsequent appropriate treatment, and therefore, ultimately improving the patient outcome. Therefore, 18F-FDG-PET should be taken into account in the early clinical assessment of doubtful cases.
Keywords: 18F-fluorodeoxyglucose-positron emission tomography/computed tomography, consciousness disorders, locked-in syndrome, statistical parametric mapping, vegetative state
Introduction
Locked-in syndrome (LIS) is a neurological state commonly due to traumatic injury, stroke, or anoxic damage on the ventral part of the pons and is characterized by aphonia, quadriplegia, and a “fail-soft” communication mode and palsy of all voluntary muscles except for those controlling eye movements; the main difference with patients affected by vegetative state (VS) is the self-awareness.[1,2,3,4] Distinguishing LIS from VS can be troublesome due to subtle clinical differences undetected by conventional diagnostic tools at early stage, delaying diagnosis to an average of more than 2.5 months.[5,6] It is known that 18F-fluorodeoxyglucose (18F-FDG)-positron emission tomography (PET) is an important tool for the assessment of cerebral metabolism in several neurological diseases such as Alzheimer's disease, other forms of dementia, amyotrophic lateral sclerosis and epilepsy, showing a different metabolic pattern that supports both an early diagnosis and a neurological disability evaluation.[7] Being few studies available on the use of 18F-FDG-PET in assessing the cortical functions in patients with LIS, we present a case of LIS where 18F-FDG-PET has been used for proving the preserved cortical regions and cognitive functions and ultimately helping addressing the correct diagnosis.
Case Report
A 59-year-old male was brought to the emergency area after a right hemiplegia was complicated in coma. Besides significant smoking, medical history was unremarkable. A brain computed tomography (CT) scan revealed a basilar artery thrombosis. Therefore, the patient underwent a thrombolysis procedure and subsequently an embolectomy without any significant clinical improvement. The patient was then admitted to the department of intensive care in severe coma. Neurological examination showed quadriplegia, inability to speak, spontaneous eye opening and sphincter incontinence.
A magnetic resonance imaging (MRI) scan with T1- and T2-weighted, fluid-attenuated inversion recovery, and diffusion-weighted imaging sequences revealed ischemic lesions with perilesional subacute edema at the level of the pons, midbrain, cerebellar hemispheres, vermis, and the left occipital lobe without sign of hemorrhage. The electroencephalography (EEG) findings were within the normal range, and somatosensory evoked potentials showed the conservation of cortical components; instead in VS, the EEG shows increased delta power but decreased alpha power and demonstrates a lower connectivity in the theta and alpha bands. A possible diagnosis of LIS was made as a consequence of basilar artery thrombosis with failed thrombolysis and mechanical treatment (embolectomy).
Subsequently, the patient underwent intubation and mechanical ventilation. Bronchial lavage sample was positive for Haemophilus influenzae and Streptococcus pneumoniae. The patient was treated with antibiotic therapy and underwent tracheostomy. After an eye tracker communicator was applied, the patient was able to communicate with vertical eye movements.
Since in contrast to the subjects with VS, supratentorial brain functions – including the cognitive ones – are preserved in LIS as and in healthy subjects, we aimed to assess cerebral metabolism by means of 18F-FDG-PET.
The PET scan was acquired on a Discovery STE PET/CT scanner (General Electric Medical Systems, Milwaukee, WI, USA) with an axial resolution of 5.2-mm FWHM in three-dimensional mode. The brain PET scan was followed by statistical parametric mapping analysis using Statistical Parametric Mapping 2 (SPM2) (Welcome Department Cognitive Neurology, London, UK) implemented in Matlab R2012 (Mathworks, Natick, MA, USA), using 40 normal participants as controls and registering the SPM coordinates to the Talairach coordinates [Figure 1]. A P = 0.05 corrected for multiple comparisons with the false discovery rate option was used to explore SPM t-maps at the voxel level. The PET scan and SPM analysis demonstrated a significant reduction of glucose metabolism in both cerebellar hemispheres in comparison with controls. No FDG uptake abnormalities were found in basal ganglia, cingulate structures, cortical, subcortical, or infratentorial regions suggesting that no metabolic alterations had occurred in these regions.
Figure 1.
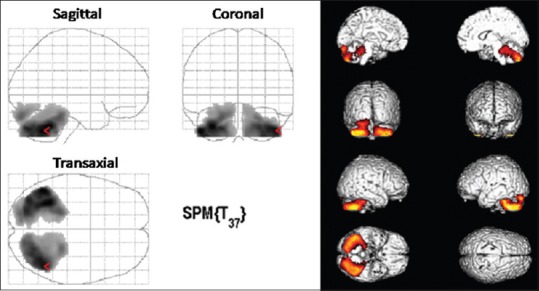
Transaxial, coronal, and sagittal statistical parametric mapping results (p=0.05 false discovery rate) confirming the hypometabolism in the cerebellar lobes
Discussion
FDG-PET has been rarely used to investigate brain metabolism in patients with clinical suspicion of LIS. This application can provide brain metabolic mapping to assess different disorders of consciousness, support a clinical suspicion of LIS and predict outcome.[8] Therefore, the role of PET in highlighting abnormality of FDG distribution, mainly decrease in frontoparietal and frontomedial regions in vegetative patients compared to normal participants, might be crucial to reach the correct diagnosis in unclear cases.[9,10] functional MRI and PET may be complementary in highlighting pathological brain networks.[10]
Less than thirty cases of LIS evaluated by 18F-FDG-PET have been described in literature, whose findings generally demonstrate normal cortical metabolism in the supratentorial brain structures but decreased metabolism in the brainstem.[11,12,13,14] All these studies agree on the fact that clinical assessment and classical neuroimaging are not sufficient in establishing the correct diagnosis and prognosis in patients with LIS, irrispective of the nature of the damage. Consequently, advanced neuroimaging techniques are essential to identifying the neuropathological substrate of the disorder. Considering the crucial role of prompt treatments to prevent massive neuronal loss and worsening of symptoms in acute vascular diseases, 18F-FDG-PET in such complicated patients might become a fundamental tool in future if our findings will be replicated in other studies.[12,13,14,15]
However 18F-FDG-PET hypometabolic patterns are not totally specific. causes of cerebral unilateral and bilateral FDG-PET hypometabolism include neurodegenerative diseases (Parkinson's disease, fronto-occipital dementia, and Alzheimer's disease), metabolic and toxic events, cerebrovascular diseases, infections, tumors, and trauma.
Conclusions
brain 18F-FDG-PET may be useful as an additional imaging tool in LIS to confirm and elucidate whether the damaged cortical areas, as detected by conventional imaging techniques, have determined functional changes. The introduction of 18F-FDG-PET in the diagnostic workup could help addressing the correct diagnosis in patients with LIS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Giacino JT. The vegetative and minimally conscious states: Consensus-based criteria for establishing diagnosis and prognosis. NeuroRehabilitation. 2004;19:293–8. [PubMed] [Google Scholar]
- 2.Bodart O, Laureys S, Gosseries O. Coma and disorders of consciousness: Scientific advances and practical considerations for clinicians. Semin Neurol. 2013;33:83–90. doi: 10.1055/s-0033-1348965. [DOI] [PubMed] [Google Scholar]
- 3.Barbic D, Levine Z, Tampieri D, Teitelbau J. Locked-in syndrome: A critical and time-dependent diagnosis. CJEM. 2012;14:317–20. [PubMed] [Google Scholar]
- 4.Pistoia F, Carolei A. The role of neuroimaging in the diagnosis, prognosis and management of disorders of consciousness and LIS. Open Neuroimag J. 2016;10:20–2. doi: 10.2174/1874440001610010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laureys S, Pellas F, Van Eeckhout P, Ghorbel S, Schnakers C, Perrin F, et al. The locked-in syndrome: What is it like to be conscious but paralyzed and voiceless? Prog Brain Res. 2005;150:495–511. doi: 10.1016/S0079-6123(05)50034-7. [DOI] [PubMed] [Google Scholar]
- 6.Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state: Definition and diagnostic criteria. Neurology. 2002;58:349–53. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 7.Cistaro A, Cuccurullo V, Quartuccio N, Pagani M, Valentini MC, Mansi L, et al. Role of PET and SPECT in the study of amyotrophic lateral sclerosis. Biomed Res Int. 2014;2014:237437. doi: 10.1155/2014/237437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips CL, Bruno MA, Maquet P, Boly M, Noirhomme Q, Schnakers C, et al. “Relevance vector machine” consciousness classifier applied to cerebral metabolism of vegetative and locked-in patients. Neuroimage. 2011;56:797–808. doi: 10.1016/j.neuroimage.2010.05.083. [DOI] [PubMed] [Google Scholar]
- 9.Tengvar C, Johansson B, Sorensen J. Frontal lobe and cingulate cortical metabolic dysfunction in acquired akinetic mutism: A PET study of the interval form of carbon monoxide poisoning. Brain Inj. 2004;18:615–25. doi: 10.1080/02699050310001622806. [DOI] [PubMed] [Google Scholar]
- 10.Soddu A, Vanhaudenhuyse A, Demertzi A, Bruno MA, Tshibanda L, Di H, et al. Resting state activity in patients with disorders of consciousness. Funct Neurol. 2011;26:37–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Bodart O, Gosseries O, Wannez S, Thibaut A, Annen J, Boly M, et al. Measures of metabolism and complexity in the brain of patients with disorders of consciousness. Neuroimage Clin. 2017;14:354–62. doi: 10.1016/j.nicl.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soddu A, Gómez F, Heine L, Di Perri C, Bahri MA, Voss HU, et al. Correlation between resting state fMRI total neuronal activity and PET metabolism in healthy controls and patients with disorders of consciousness. Brain Behav. 2016;6:e00424. doi: 10.1002/brb3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stender J, Gosseries O, Bruno MA, Charland-Verville V, Vanhaudenhuyse A, Demertzi A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet. 2014;384:514–22. doi: 10.1016/S0140-6736(14)60042-8. [DOI] [PubMed] [Google Scholar]
- 14.Heiss WD. PET in coma and in vegetative state. Eur J Neurol. 2012;19:207–11. doi: 10.1111/j.1468-1331.2011.03489.x. [DOI] [PubMed] [Google Scholar]
- 15.Hannawi Y, Lindquist MA, Caffo BS, Sair HI, Stevens RD. Resting brain activity in disorders of consciousness: A systematic review and meta-analysis. Neurology. 2015;84:1272–80. doi: 10.1212/WNL.0000000000001404. [DOI] [PMC free article] [PubMed] [Google Scholar]


