Abstract
Background
The prevalence of depressive affect is not well defined in the incident hemodialysis (HD) population. We investigated the prevalence of and associated risk factors and hospitalization rates for depressive affect in incident HD patients.
Methods
We performed a prospective investigation using the Patient Health Questionnaire 2 (PHQ2) depressive affect assessment. From January to July of 2013 at 108 in-center clinics randomly selected across tertiles of baseline quality measures, we contacted 577 and 543 patients by telephone for depressive affect screening. PHQ2 test scores range from 0 to 6 (scores ≥3 suggest the presence of depressive affect). The prevalence of depressive affect was measured at 1–30 and 121–150 days after initiating HD; depressive affect risk factors and hospitalization rates by depressive affect status at 1–30 days after starting HD were computed.
Results
Of 1120 contacted patients, 340 completed the PHQ2. In patients screened at 1–30 or 121–150 days after starting HD, depressive affect prevalence was 20.2% and 18.5%, respectively (unpaired t-test, P = 0.7). In 35 patients screened at both time points, there were trends for lower prevalence of depressive affect at the end of incident HD, with 20.0% and 5.7% of patients positive for depressive affect at 1–30 and 121–150 days, respectively (paired t-test, P = 0.1). Hospitalization rates were higher in patients with depressive affect during the first 30 days, exhibiting 1.5 more admissions (P < 0.001) and 10.5 additional hospital days (P = 0.008) per patient-year. Females were at higher risk for depressive affect at 1–30 days (P = 0.01).
Conclusions
The prevalence of depressive affect in HD patients is high throughout the incident period. Rates of hospital admissions and hospital days are increased in incident HD patients with depressive affect.
Keywords: depression, hospital admission, hospital days, incident dialysis, patient health questionnaire 2
Introduction
Clinical depression and symptoms of depressive affect are common in hemodialysis (HD) patients but have not been well characterized in the incident HD population early after initiation of dialysis and through the incident period [1–6]. In the incident HD population, the prevalence of depressive affect with or without a diagnosis of depression has been reported to be between 8.9% and 45% within 1 –120 days after initiation of dialysis [1–6]. In comparison, in the general population depressive affect with or without a diagnosis of depression has been reported to have a prevalence of 2–10% [7]; the depressive affect prevalence in the prevalent dialysis population with or without a diagnosis of depression has been documented to be between 20% and 55% [4, 8–10].
Identification of depressive affect does not discretely diagnose clinical depression but does indicate a high probability of any depressive disorder without a precise degree of severity. Clinical depression must be diagnosed by a physician through medical assessment of the patient’s psychological status and clinical symptoms of depressive affect. In the end-stage renal disease (ESRD) population, depressive affect has been assessed using several survey methods that include the Patient Health Questionnaires [4, 11], Beck Depression Inventory (BDI) [11, 12], Center for Epidemiologic Studies Depression Screening Index [13], Hospital Anxiety and Depression Scale [14] and the two questions associated with depressive symptoms within the 36-Item Short Form Health Survey (SF-36) [3, 15].
Depressive affect with or without a diagnosis of depression has been observed in incident dialysis patients to be linked to increases in withdrawing from HD in the first 3 months of treatment [3], as well as increases in risk for mortality in the first year of dialysis [3, 16, 17]. It has been identified in prevalent dialysis patients that depressive affect is associated with risks of decreases in quality of life and increases in morbidities, cardiovascular events, hospitalizations and mortality [9, 18–20]. In a recent large observational study of incident HD patients within 120 days after starting dialysis, depressive affect was found to be associated with increased rates of hospital admissions and hospital days [1].
The aims of this study were to characterize the prevalence of depressive affect early after the initiation of dialysis and at the end of the incident period, determine the risk factors for depressive affect and investigate hospitalization rates related to depressive affect in incident HD patients.
Materials and methods
This study was a prospective investigation performed at 108 outpatient HD centers in the USA and conducted as a component of the Fresenius Medical Care North America (FMCNA) RightStart program [21]. From January to July 2013, 577 patients within 1–30 days after initiation of HD and 543 patients at 121–150 days after initiating HD were contacted by telephone for depressive affect screening. Upon determination of a positive depressive affect screening score, the patient’s clinical care staff was notified for further assessment and/or intervention.
For this investigation, depressive affect was assessed by telephone using the Patient Health Questionnaire 2 (PHQ2). Survey operators were trained in survey processes, observed during training and provided with written training materials. The PHQ2 surveys were administered to patients in either English or Spanish by bilingual survey operators depending on the patient’s native language; non-English-or non-Spanish-speaking patients were excluded. The PHQ2 is a two-question assessment that can screen patients for the presence of depressed mood and anhedonia occurring during the previous 2 weeks [22, 23]. The questions of the PHQ2 are as follows: ‘Over the last 2 weeks, how often have you been bothered by any of the following problems?’ (i) ‘Little interest or pleasure in doing things’ and (ii) ‘Feeling down, depressed, or hopeless’. Each question of the PHQ2 has four possible responses with scores ranging from 0 to 3 and include (i) ‘Not at all’ (score = 0), (ii) ‘Several days’ (score = 1), (iii) ‘More than half the days’ (score = 2) and (iv) ‘Nearly every day’ (score = 3). The overall scoring for the PHQ2 test ranges from 0 to 6; scores ≥3 are suggestive of depressive affect occurring during the prior 2 weeks [22]. PHQ2 scores of ≥3 have a 75% probability of identifying the presence of ‘any depressive disorder’ in the prior 2 weeks but only a 38% probability of a ‘major depressive disorder’ [22].
The PHQ2 has been validated and shown to be effective in screening for depressive affect in primary care, hospital outpatient and coronary heart disease patients [22–24]. The PHQ2 test has not been validated specifically in the dialysis population; however, it has been used to screen for depressive affect in incident dialysis patients [4] and appears to be a simple and effective method to determine the occurrence of depressive affect. Notably, identification of the presence of depressive affect using the PHQ2 cannot determine the discrete magnitude of severity for depressive symptoms, which can be further evaluated by the clinical judgment of the treating physician.
The in-center HD clinics utilized for this study were randomly selected to equally represent each FMCNA division and were distributed across tertiles of quality levels at baseline. FMCNA dialysis clinic quality is determined by UltraScores, which, at the time, were based on levels of albumin, hemoglobin, phosphorous, estimated Kt/V, as well as rates of catheter use and mortality. Small clinics (HD census <50 patients) or those that had limitations in the ability to translate for language barriers were excluded from the study.
All patients who completed a depressive affect screening by PHQ2 during 1–30 days or 121–150 days after initiation of HD were included in the analyses. For this investigation, data for the PHQ2 scores was recorded by telephone survey operators and patient demographics, clinical parameters and rates of hospital admissions and hospital days were captured from patient data available in the FMCNA Knowledge Center Data Warehouse. Depressive affect scores during 1–30 and 121–150 days and hospitalization rates 1–150 days after initiating HD were utilized for comparisons. Additionally, clinical and laboratory parameters were collected up to the first 30 and 150 days of HD.
Analyses included descriptive statistics of patient demographics, clinical characteristics and PHQ2 scores. The mean clinical and demographic parameters in patients assessed for depressive affect at either 1–30 and/or 121–150 days after starting HD were compared using an unpaired Student’s t-test for continuous variables and chi-square test for categorical variables. Comparisons in a select group of the same patients screened for depressive affect at both time points were made using a paired Student’s t-test for continuous variables and a McNemar test for categorical variables. Associations between depressive affect at the time of assessment and demographic and clinical characteristics were studied using t-tests and chi-square tests. A Poisson regression analysis was utilized for comparisons of rates of hospital admissions and hospital days. Unpaired Student’s t-tests were performed for comparison of the prevalence of depressive affect at 1–30 days versus 121–150 days after initiation of HD in patients assessed at one of the time points; paired Student’s t-tests were used for analyzing comparisons in a select group of the same patients screened for depressive affect at both time points. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Of the overall population of 1120 incident HD patients who contacted for telephonic depressive affect screening, 340 PHQ2 assessments were completed by 305 unique patients. The overall depressive affect screening response rate was 30.4% and there was a mean of 1.6 calls performed to complete the PHQ2 survey. During 1–30 days after initiation of HD, there were 213 responders to depressive affect screening and at 121–150 days there were 127 responders; there was a 36.9% and 23.4% response rate at 1–30 and 121–150 days, respectively (Supplementary data, Table S1). Among patients who were contacted and asked to complete a PHQ2 assessment, 89.4% agreed to perform the screening and 10.6% refused.
The clinical and demographic characteristics of the 270 unique patient responders to the depressive affect screening at one of the two time points are shown in Table 1. The population of patients responding to depressive affect screening at either 1–30 days or 121–150 days after starting HD was mostly similar in clinical and demographic characteristics, with the exception of more non-Hispanics and higher online clearance (OLC) at the later time point as well as the anticipated changes happening in incident patients, such as increases in albumin, higher log of creatinine, higher percent of interdialytic weight gain (IDWG) and lower catheter utilization rates at 121–150 days after starting HD.
Table 1.
Demographics and clinical characteristics for patients surveyed at one of the time points
| Parameters | 1–30 days after incident HD | 121–150 days incident HD | P-value |
|---|---|---|---|
| Number of patients (total = 270) | 178 | 92 | NA |
| Average age, years (±SD) | 64.1 (±16.3) | 63.7 (±13.6) | 0.829 |
| Female (%) | 39.9 | 41.3 | 0.822 |
| Diabetes (%) | 67.1 | 67.9 | 0.909 |
| Hypothyroidism (%) | 8.4 | 8.7 | 0.940 |
| Race, White (%) | 74.2 | 63.0 | 0.058 |
| Ethnicity, not Hispanic (%) | 80.9 | 95.7 | 0.001 |
| BMI, kg/m2 (±SD) | 29.6 (±8.1) | 30.2 (±7.8) | 0.608 |
| Catheter access (%) | 52.7 | 15.5 | <0.0001 |
| Albumin, g/dL (±SD) | 3.5 (±0.5) | 3.8 (±0.5) | 0.001 |
| Log of creatinine (±SD) | 1.6 (±0.4) | 1.9 (±0.4) | <0.0001 |
| OLC (±SD) | 1.4 (±0.3) | 1.6 (±0.3) | <0.0001 |
| PreSBP, mmHg (±SD) | 145.0 (±20.3) | 150.3 (±20.0) | 0.058 |
| Percent IDWG (±SD) | 2.1 (±1.1) | 2.9 (±1.0) | <0.0001 |
| Average PHQ2 score | 1.2 (±2.0) | 1.2 (±1.6) | 0.705 |
| Patients with positive PHQ2 scores (≥3) (%) | 20.2 | 18.5 | 0.731 |
Summary of incident HD patient demographics, diabetes comorbidity, dialysis access characteristics and PHQ2 scores in patients that responded to the depressive affect screening at either 1–30 days or 121–150 days after initiating dialysis.
The prevalence of depressive affect was observed to be 20.2% in patients in the first 30 days after initiation of HD and 18.5% at the end of the incident period (121–150 days after initiation of HD); there were no differences between time points (P = 0.7). In a select group of 35 patients who were screened for depressive affect at both time points, the depressive affect prevalence was observed to be lower at 121–150 days (5.7% depressive affect positive) as compared with 1–30 days (20.0% depressive affect positive), albeit non-significant (P = 0.1), as shown in Table 2.
Table 2.
Demographics and clinical characteristics for the patients (n = 35)surveyed at both time points
| Parameters | 1–30 days after incident HD | 121–150 days incident HD | P-value |
|---|---|---|---|
| Average age, years (SD) | 64.2 (±13.3) | NA | |
| Female (%) | 37.1 | NA | |
| Diabetes (%) | 64.7 | NA | |
| Hypothyroidism (%) | 2.9 | NA | |
| Race, White (%) | 71.4 | NA | |
| Ethnicity, not Hispanic (%) | 85.7 | NA | |
| BMI, kg/m2 (±SD) | 29.3 (±6.5) | 29.0 (±6.4) | 0.092 |
| Catheter access (%) | 44.1 | 17.7 | 0.004 |
| Albumin, g/dL (±SD) | 3.6 (±0.5) | 3.9 (±0.4) | 0.003 |
| Log of creatinine (±SD) | 1.8 (±0.4) | 1.9 (±0.4) | 0.087 |
| OLC (±SD) | 1.3 (±0.3) | 1.5 (±0.3) | <0.0001 |
| PreSBP, mmHg (±SD) | 147.7 (±22.6) | 149.6 (±21.5) | 0.428 |
| Percent IDWG (±SD) | 2.2 (±0.90) | 2.8 (±1.0) | 0.001 |
| Average PHQ2 score | 1.3 (±2.0) | 0.7 (±1.3) | 0.091 |
| Patients with positive PHQ2 scores (≥3) (%) | 20.0 | 5.7 | 0.125 |
Summary of incident HD patient demographics, diabetes comorbidity, dialysis access characteristics and PHQ2 assessment parameters for patients assessed for depressive affect at both the study time points.
As detailed in Table 3, the analysis of risk factors in incident HD patients showed that females have a higher risk for depressive affect 1–30 days after initiation of HD (P = 0.01), but there were no differences between sexes for 121–150 days after starting HD (P = 0.8). Also, there were trending risks for depressive affect in patients with higher BMI 121–150 days after initiating HD (P = 0.054). No other clinical and demographic characteristics were identified to be associated with depressive affect in incident HD patients.
Table 3.
Depressive affect status and patient demographics, characteristics and PHQ2 scores
| Parameters of responders to depressive affect screening | 1–30 days depressive affect negative | 1–30 days depressive affect positive | P-value | 121–150 days depressive affect negative | 121–150 days depressive affect positive | P-value |
|---|---|---|---|---|---|---|
| Number of patients | 170 | 43 | NA | 108 | 19 | NA |
| Average age, years (SD) | 64.5 (±15.6) | 62.8 (±16.8) | 0.537 | 64.5 (±14.0) | 60.0 (±9.4) | 0.176 |
| Female (%) | 35.3 | 55.8 | 0.014 | 39.8 | 42.1 | 0.851 |
| Diabetes (%) | 66.7 | 66.7 | 1.000 | 64.0 | 84.2 | 0.085 |
| Race, White (%) | 75.9 | 65.1 | 0.152 | 67.6 | 52.6 | 0.206 |
| Ethnicity, not Hispanic (%) | 82.4 | 79.1 | 0.619 | 91.7 | 100.0 | 0.192 |
| BMI, kg/m2 (±SD) | 29.4 (±7.7) | 30.2 (±8.3) | 0.547 | 29.4 (±7.3) | 33.0 (±7.4) | 0.054 |
| Catheter access (%) | 51.4 | 50.0 | 0.882 | 17.0 | 10.5 | 0.480 |
| Albumin, g/dL (±SD) | 3.5 (±0.5) | 3.5 (±0.5) | 0.8892 | 3.8 (±0.5) | 3.7 (±0.3) | 0.337 |
| Average PHQ2 score | 0.3 (±0.7) | 4.8 (±1.3) | NA | 0.5 (±0.8) | 4.1 (±1.0) | NA |
Risk factor analysis of demographic and clinical patient parameters associated with depressive affect.
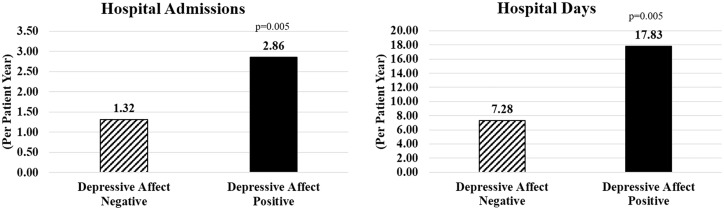
Over the 150-day period after initiation of HD, the hospital admission rate for depressive affect–positive patients at the 1–30 day time point was 2.86 admissions per patient-year (ppy) and for depressive affect–negative patients was 1.32 admissions ppy (Figure 1). Depressive affect–positive patients spent 17.8 days in hospitals ppy and depressive affect–negative patients spent 7.3 days in hospitals ppy (Figure 1). A Poisson regression model with adjustment for factors commonly associated with hospitalization rates showed significantly higher rate of admission [relative rate (RR) 2.08; P = 0.005; Table 4] and hospital days (RR = 1.87; P = 0.005; Table 5) in DA positive patients, as compared with depressive affect–negative patients. The outcomes were adjusted for the covariates of age, sex, diabetes, access type, ethnicity, race, body mass index (BMI), albumin, log of creatinine, OLC, predialysis systolic blood pressure (preSBP) and IDWG.
Figure 1.
Hospital admission rates ppy in the first 150 days of HD in patients positive for depressive affect (solid black column) versus negative for depressive affect (slash line column).
Table 4.
Associations of 150-day hospital admission rates and depressive affect in incident HD patients
| Parameter | Estimate | Standard error | 95% LCL | 95% UCL | Wald chi-square | P-value |
|---|---|---|---|---|---|---|
| Intercept | 3.314 | 1.998 | −0.603 | 7.230 | 2.75 | 0.097 |
| Depressive affect positive at 1–30 days after incident HD | 0.730 | 0.259 | 0.221 | 1.238 | 7.91 | 0.005 |
| Age | −0.004 | 0.009 | −0.022 | 0.014 | 0.19 | 0.664 |
| Male | 0.197 | 0.283 | −0.358 | 0.752 | 0.48 | 0.486 |
| Diabetes | 0.212 | 0.366 | −0.506 | 0.930 | 0.34 | 0.562 |
| Race, White | 0.508 | 0.451 | −0.377 | 1.392 | 1.27 | 0.261 |
| Ethnicity, not Hispanic | 0.433 | 0.342 | −0.237 | 1.102 | 1.6 | 0.205 |
| BMI | −0.001 | 0.020 | −0.041 | 0.038 | 0 | 0.957 |
| Catheter access at 30 days after incident HD | −0.001 | 0.246 | −0.483 | 0.482 | 0 | 0.998 |
| Albumin | −0.060 | 0.245 | −0.540 | 0.419 | 0.06 | 0.805 |
| Log of creatinine | −0.362 | 0.312 | −0.973 | 0.248 | 1.35 | 0.245 |
| OLC | −0.113 | 0.439 | −0.974 | 0.749 | 0.07 | 0.798 |
| PreSBP | −0.004 | 0.006 | −0.016 | 0.008 | 0.43 | 0.513 |
| Percent IDWG | 0.120 | 0.119 | −0.114 | 0.354 | 1.01 | 0.315 |
Poisson regression analysis of rates of hospital admissions in patients who were depressive affect–positive at 1–30 days after initiation of HD. LCL, lower confidence limit; UCL, upper confidence limit.
Table 5.
Associations of 150-day hospitalization rates and depressive affect in incident HD patients
| Parameter | Estimate | Standard error | 95% LCL | 95% UCL | Wald chi-square | P-value |
|---|---|---|---|---|---|---|
| Intercept | 3.299 | 1.767 | −0.164 | 6.762 | 3.49 | 0.062 |
| Depressive affect positive at 1–30 days after incident HD | 0.626 | 0.223 | 0.189 | 1.062 | 7.88 | 0.005 |
| Age | −0.025 | 0.008 | −0.042 | −0.009 | 8.93 | 0.003 |
| Male | −0.722 | 0.232 | −1.177 | −0.268 | 9.71 | 0.002 |
| Diabetes | 1.061 | 0.329 | 0.416 | 1.705 | 10.4 | 0.001 |
| Race, White | 1.061 | 0.328 | 0.418 | 1.703 | 10.46 | 0.001 |
| Ethnicity, not Hispanic | 0.452 | 0.284 | −0.105 | 1.008 | 2.53 | 0.112 |
| BMI | −0.041 | 0.017 | −0.074 | −0.009 | 6.33 | 0.012 |
| Catheter access at 30 days after incident HD | 0.506 | 0.221 | 0.074 | 0.939 | 5.27 | 0.022 |
| Albumin | −0.213 | 0.240 | −0.683 | 0.258 | 0.79 | 0.375 |
| Log of creatinine | −0.269 | 0.285 | −0.827 | 0.289 | 0.9 | 0.344 |
| OLC | −0.529 | 0.355 | −1.226 | 0.167 | 2.22 | 0.136 |
| PreSBP | −0.003 | 0.005 | −0.014 | 0.007 | 0.39 | 0.531 |
| Percent IDWG | 0.098 | 0.102 | −0.102 | 0.297 | 0.92 | 0.337 |
Poisson regression analysis of rates of hospital days in patients who were depressive affect positive at 1–30 days after initiation of HD. LCL, lower confidence limit; UCL, upper confidence limit.
Discussion
This study investigated the prevalence of depressive affect in incident HD patients determined by telephonic PHQ2 assessments during the first month of HD and at the end of the incident period. Our findings identified that depressive affect is common and unchanged throughout the incident period, generally affecting 19–20% of incident HD patients, and is associated with increases in rates of hospital admissions and days consistent with other findings in the incident [1] and prevalent [15] dialysis populations. Female sex was identified to be a risk factor associated with depressive affect occurrence 1–30 days after initiation of HD in this cohort of patients, which is consistent with our previous interim findings [25, 26] and other reports in the literature [13].
We observed that the prevalence of depressive affect in incident HD patients is high throughout the incident period, with 20.2% of patients screening positive for depressive affect in the first month of HD and 18.5% at the end of the incident period. These findings are very consistent with other reports that identified depressive affect using the mental health domain items in the SF-36; of 6415 US patients during 10–90 days after initiating HD, 20.8% were positive for depressive affect [3], and in the US Dialysis Outcomes and Practice Patterns Study (DOPPS) cohort, depressive affect was identified in 17.5% of 2562 patients during the first 3 months of HD [15]. Additionally, a previous investigation of depressive affect based on PHQ2 assessment in 585 US patients at 60–90 days after starting HD or peritoneal dialysis found that 12.1–32.8% of patients were positive for depressive affect dependent on employment status [4].
Contrary to the findings noted above, other reports in the literature have found the prevalence of depressive affect in incident patients utilizing the SF-36 to be at 8.9% in Dutch patients at 90 days after starting dialysis [2], 41% in US patients at 1–120 days after starting dialysis [1] and 45% in US patients [6] at 60–90 days after starting dialysis. These differences are likely influenced by decreasing rates of catheter use in studies that utilize longer incident periods, as well as differences in geography, cultural habits and treatment patterns between the Dutch and US health care systems, which requires further studies. Notably, the Dutch study did include incident HD and peritoneal dialysis patients, which may be a factor associated with the lower prevalence of depressive affect observed as compared with the US studies. Another report identified that depressive affect determined by assessment with the BDI was present in 44% of patients during the first 10 days after starting dialysis [5]. Although the observed differences in the prevalence of depressive affect in dialysis patients could be related in part to depressive affect assessment methods, patient geography, dialysis modality, timeframe of the study and cohort size, the findings by Lacson et al. in 2012 and 2014 using the SF-36 in similar large cohorts of HD patients from the same dialysis provider and during concurrent periods during 2006 reveals a 20.2% variance, with depressive affect in 20.8% of 6415 patients at 10–90 days after HD [3] and 41% of 8776 patients at 1–120 days after HD [1]. Overall, the prevalence of depressive affect is very high in incident HD patients and appears to have noteworthy differences in observed prevalence in the literature due to possible selection bias. Based on our findings, the prevalence of depressive affect is not significantly changed from the beginning to the end of the incident HD period and the timing of depressive affect assessment during the incident HD period is not expected to significantly alter the rates of depressive affect in the incident HD population. However, the results of our study were performed in a limited number of patients and need to be further investigated and confirmed.
In a subanalysis of a small number of paired patients screened for depressive affect at the initiation of and end of the incident period, the occurrence of depressive affect was found to be reduced from 20.0% in the first month of dialysis to 5.7% at the end of the incident period, albeit non-significant (P = 0.1). While adjustment to HD and patient selection might have contributed to this finding, early identification of depressive affect and subsequent interventions by dialysis care teams could be a factor. In this cohort there was a 26.4% decrease in catheter use from the first 30 days of dialysis to the end of the incident period (P = 0.004), which may be influencing these findings (Table 2). In the overall study population, rates of catheter use were similar between patients with and without depressive affect 1–30 days after starting dialysis. At 121–150 days after starting dialysis there were 6.5% fewer patients using a catheter who were positive for depressive affect compared with those with a permanent dialysis access; these findings were not significant (P = 0.48; Table 3). Further investigations are warranted to evaluate whether early identification of depressive affect leads to interventions and improved outcomes. Future studies could include analysis of follow-up depressive affect assessments with higher specificity and sensitivity than the PHQ2, physician-based diagnosis of clinical depression and the specific interventions performed, such as pharmaceutical and psychosocial therapies.
Our examination of associations in depressive affect and patient demographics and clinical parameters in this group of incident HD patients identified that females have a higher risk for experiencing depressive affect 1–30 days after starting HD as compared with males; however, we did not observe differences 121–150 days after the start of HD. While we only found sex to be a significant risk factor for depressive affect and BMI to be a trending risk factor at the end of the incident period, other studies have reported that depressive affect is associated with several risk factors, including age [3], race [5], lower albumin levels [3], diabetes and catheter use [27]. In the general population, comprehensive reviews have identified that the presence of diabetes is associated with an up to 2-fold increase in the risk of depression [28]. Conversely, patients in the general population with depression have been shown to exhibit a 10% higher incidence of diabetes [29]. Despite these findings, a recent investigation in HD patients did not find any difference in the prevalence of depressive affect in those with and without the presence of diabetes [30]. Additional studies investigating the risk factors associated with depressive affect in incident HD patients should be performed in an attempt to identify potential predictors of depressive affect in this population.
We found that incident HD patients positive for depressive affect during the first month of dialysis exhibited an ∼2-fold increase in rates of hospital admissions (RR 2.08; P = 0.005) and hospital days (RR 1.87; P = 0.005); this is consistent with the findings by Lacson et al. [1] in 2014 using a large study cohort of 8776 patients at 1–120 days after HD. These findings further identify that from the initiation of dialysis, depressive affect in the HD population is related to worsened outcomes that could potentially be modifiable through interventions. Whether enhanced early identification of depressive affect and treatment of depressive symptoms in incident HD patients has the potential to improve patient hospitalization outcomes has not been determined.
There is consensus in the literature that improvements are needed in screening for depressive affect and clinical depression in the ESRD population [1, 4, 5, 18, 19, 31]. The striking prevalence of depressive affect observed in incident and prevalent dialysis patients represents a population with notable risk for worsened outcomes that could potentially be improved through treatment of depressive symptoms. Treatment options that have been reported to be associated with improvements in the symptoms of depression in ESRD patients include antidepressant pharmaceuticals [32], cognitive behavioral therapy [33], exercise training programs [9] and music therapy [9]. In the US DOPPS cohort, it was shown that antidepressant medications were only prescribed for 38.9% of HD patients with clinical depression diagnosed by a physician and 28.9% of HD patients with depressive affect [13]. It was found in an investigation of 98 HD patients assessed for clinical depression that only 54% of depressed patients identified in the study were diagnosed with the disease previously during routine care and 23% had an antidepressant medication prescribed [18]. Additionally, in a study of 123 incident dialysis patients by Watnick et al. [5], it was shown that only 16% of patients with depressive affect received an antidepressant treatment. Despite the fact that major improvements in care are apparently needed, the ability to improve clinical outcomes via identification and treatment of depressive affect and clinical depression has not been firmly established in the dialysis population and needs to be elucidated [9].
This study did have some key limitations that include a potential selection bias secondary to the design that assessed patients at both the 1–30 day and 121–150 day time points; however, this did not yield a notable cohort of patients who repeated the survey at both time points. Additionally, the study included only 305 unique patients and further studies are needed that include larger patient numbers. Another limitation is that patients were assessed using telephone interviews and it has not been determined if this method is as accurate as in-person depression screening methods. Furthermore, this analysis did not assess the impacts of antidepressant use on PHQ2 scores or assess the number of patients identified with depressive affect who might have been prescribed antidepressant therapies by their physicians.
In conclusion, depressive affect is common in incident HD patients and did not change significantly throughout the incident period. Increases in rates of hospital admissions and hospital days are significantly associated with depressive affect in incident HD patients, and this further identifies the imperative need for improved screening and treatment. It is evident that the development of enhanced interventional paradigms for the treatment of depressive affect and clinical depression in dialysis patients will be essential in reducing the worsened outcomes observed in this population.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Supplementary Material
Acknowledgements
We would like to thank and acknowledge Eduardo K. Lacson, Jr, MD, for his notable contributions to the study design, as well as the staff and physicians who assisted in conducting the RightStart program at participating dialysis clinics.
FUNDING
This study was funded by Fresenius Medical Care North America through the RightStart quality improvement initiative.
Conflict of interest statement
Company: The RightStart quality improvement initiative is a registered trademark program of Fresenius Medical Care North America.
Authors: K.A.M.: employed by Fresenius Medical Care North America. J.W.L.: employed by Fresenius Medical Care North America. R.L.W.: employed by Fresenius Medical Care North America; stock ownership in Fresenius Medical Care. S.R.: employed by Fresenius Medical Care North America. Y.J.: employed by Fresenius Medical Care North America. L.M.: employed by Fresenius Medical Care North America. L.A.U.: employed by Fresenius Medical Care North America; stock ownership in Fresenius Medical Care. F.W.M.: employed by Fresenius Medical Care North America; stock ownership in Fresenius Medical Care.
Other non-financial conflicts of interest: F.W.M.: directorships in American National Bank & Trust, Mid-Atlantic Renal Coalition, Specialty Care Inc and Sound Physicians; chairman Pacific Care Renal Foundation 501(c)(3) nonprofit; chairr Kidney Care Partners; founder Scholarships Expanding Education 501(c)(3) non-profit.
Support: This research was conducted as part of the RightStart quality improvement program. K.A.M., J.W.L., R.L.W., Y.J., S.R., L.M., L.A.U. and F.W.M. are employed by Fresenius Medical Care North America. All authors, as employees of Fresenius Medical Care North America, assisted in the study and the analytical design, medical writing of the study-related documents and composition of the article.
References
- 1. Lacson E Jr, Bruce L, Li NC. et al. Depressive affect and hospitalization risk in incident hemodialysis patients. Clin J Am Soc Nephrol 2014; 9: 1713–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Dijk S, van den Beukel TO, Kaptein AA. et al. How baseline, new-onset, and persistent depressive symptoms are associated with cardiovascular and non-cardiovascular mortality in incident patients on chronic dialysis. J Psychosom Res 2013; 74: 511–517 [DOI] [PubMed] [Google Scholar]
- 3. Lacson E Jr, Li NC, Guerra-Dean S. et al. Depressive symptoms associate with high mortality risk and dialysis withdrawal in incident hemodialysis patients. Nephrol Dial Transplant 2012; 27: 2921–2928 [DOI] [PubMed] [Google Scholar]
- 4. Kutner NG, Zhang R, Huang Y. et al. Depressed mood, usual activity level, and continued employment after starting dialysis. Clin J Am Soc Nephrol 2010; 5: 2040–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Watnick S, Kirwin P, Mahnensmith R. et al. The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis 2003; 41: 105–110 [DOI] [PubMed] [Google Scholar]
- 6. Walters BA, Hays RD, Spritzer KL. et al. Health-related quality of life, depressive symptoms, anemia, and malnutrition at hemodialysis initiation. Am J Kidney Dis 2002; 40: 1185–1194 [DOI] [PubMed] [Google Scholar]
- 7. Kessler RC, Berglund P, Demler O. et al. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289: 3095–3105 [DOI] [PubMed] [Google Scholar]
- 8. Rosenthal Asher D, Ver Halen N, Cukor D.. Depression and nonadherence predict mortality in hemodialysis treated end-stage renal disease patients. Hemodial Int 2012; 16: 387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hedayati SS, Yalamanchili V, Finkelstein FO.. A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int 2012; 81: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zalai D, Szeifert L, Novak M.. Psychological distress and depression in patients with chronic kidney disease. Semin Dial 2012; 25: 428–438 [DOI] [PubMed] [Google Scholar]
- 11. Watnick S, Wang PL, Demadura T. et al. Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis 2005; 46: 919–924 [DOI] [PubMed] [Google Scholar]
- 12. Kimmel PL, Peterson RA, Weihs KL. et al. Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int 2000; 57: 2093–2098 [DOI] [PubMed] [Google Scholar]
- 13. Lopes AA, Albert JM, Young EW. et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int 2004; 66: 2047–2053 [DOI] [PubMed] [Google Scholar]
- 14. Riezebos RK, Nauta KJ, Honig A. et al. The association of depressive symptoms with survival in a Dutch cohort of patients with end-stage renal disease. Nephrol Dial Transplant 2010; 25: 231–236 [DOI] [PubMed] [Google Scholar]
- 15. Lopes AA, Bragg J, Young E. et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int 2002; 62: 199–207 [DOI] [PubMed] [Google Scholar]
- 16. Chilcot J, Davenport A, Wellsted D. et al. An association between depressive symptoms and survival in incident dialysis patients. Nephrol Dial Transplant 2011; 26: 1628–1634 [DOI] [PubMed] [Google Scholar]
- 17. Boulware LE, Liu Y, Fink NE. et al. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: contribution of reverse causality. Clin J Am Soc Nephrol 2006; 1: 496–504 [DOI] [PubMed] [Google Scholar]
- 18. Hedayati SS, Bosworth HB, Briley LP. et al. Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int 2008; 74: 930–936 [DOI] [PubMed] [Google Scholar]
- 19. Chilcot J, Norton S, Wellsted D. et al. Distinct depression symptom trajectories over the first year of dialysis: associations with illness perceptions. Ann Behav Med 2013; 45: 78–88 [DOI] [PubMed] [Google Scholar]
- 20. Drayer RA, Piraino B, Reynolds CF 3rd. et al. Characteristics of depression in hemodialysis patients: symptoms, quality of life and mortality risk. Gen Hosp Psychiatry 2006; 28: 306–312 [DOI] [PubMed] [Google Scholar]
- 21. Wingard RL, Chan KE, Lazarus JM. et al. The ‘right’ of passage: surviving the first year of dialysis. Clin J Am Soc Nephrol 2009; 4(Suppl 1): S114–S120 [DOI] [PubMed] [Google Scholar]
- 22. Kroenke K, Spitzer RL, Williams JB.. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41: 1284–1292 [DOI] [PubMed] [Google Scholar]
- 23. Arroll B, Goodyear-Smith F, Crengle S. et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med 2010; 8: 348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilbody S, Richards D, Brealey S. et al. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med 2007; 22: 1596–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larkin JW, McDougall KA, Wingard RL. et al. Risk factors associated with depressive affect in incident hemodialysis patients [ASN abstract SA-PO1043]. J Am Soc Nephrol 2014; 25: 883A [Google Scholar]
- 26. Larkin JW, McDougall KA, Usvyat LA. et al. Depressive affect in incident hemodialysis patients: prevalence and risk factors [ASN abstract TH-PO539]. J Am Soc Nephrol 2013; 24: 225A [Google Scholar]
- 27. Nowak L, Adamczak M, Więcek A.. Is inflammation a new risk factor of depression in haemodialysis patients? Int Urol Nephrol 2013; 45: 1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lustman PJ, Clouse RE.. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications 2005; 19: 113–122 [DOI] [PubMed] [Google Scholar]
- 29. Nichols GA, Moler EJ.. Cardiovascular disease, heart failure, chronic kidney disease and depression independently increase the risk of incident diabetes. Diabetologia 2011; 54: 523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoong RK, Mooppil N, Khoo EY. et al. Prevalence and determinants of anxiety and depression in end stage renal disease (ESRD). A comparison between ESRD patients with and without coexisting diabetes mellitus. J Psychosom Res 2017; 94: 68–72 [DOI] [PubMed] [Google Scholar]
- 31. Abdel-Kader K, Unruh ML, Weisbord SD.. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 2009; 4: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hedayati S, Finkelstein FO.. Epidemiology, diagnosis, and management of depression in patients with CKD. Am J Kidney Dis 2009; 54: 741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duarte PS, Miyazaki MC, Blay SL. et al. Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int 2009; 76: 414–421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.