Abstract
Oxidative stress plays a key role in the pathophysiological process of uremia and its complications, particularly in cardiovascular disease. The level of oxidative stress markers is known to increase as chronic kidney disease progresses and correlates significantly with the level of renal function. Hemodialysis and peritoneal dialysis are major modes of renal replacement therapy for end-stage renal disease patients, but unfortunately they are also accompanied by increased oxidative stress. Successful kidney transplantation, however, results in near normalization of the antioxidant status and lipid metabolism by eliminating free radicals despite the surge of oxidative stress caused by the surgical procedure and ischemic injury to the organ during the operation. This success is associated with both improved renal function, reduced cardiovascular complications and overall improved morbidity and mortality. Measuring oxidative stress markers such as malondialdehyde is promising in predicting allograft survival and delayed graft function.
Keywords: antioxidants, chronic kidney disease, end-stage renal disease, kidney transplantation, oxidative stress, renal replacement therapy
Introduction
The incidence of end-stage renal disease (ESRD) continues to rise in the USA. By the year 2030, the number of patients with ESRD is projected to exceed 2.2 million. This is more than five times the current prevalence [1]. Increasing kidney disease and subsequent ESRD is related to the aging society and high morbidity due to lifestyle diseases such as diabetes, atherosclerosis and hypertension. The world’s elderly population is projected to outgrow the younger population over the next 35 years with an expected 150% growth rate compared with a growth rate that is expected to remain almost the same [2].
During the past decade, kidney transplantation has increasingly been recognized as the treatment of choice for medically suitable patients with ESRD [3]. As well as improving quality of life, successful transplantation confers major benefits by improving the morbidity and mortality of ESRD patients who receive kidney transplant over those who undergo renal replacement therapy (RRT) [4]. Cardiovascular (CV) risk reduction remains the leading cause of this improvement, although both modalities provide compatible fluid balance and glomerular filtration rates [5].
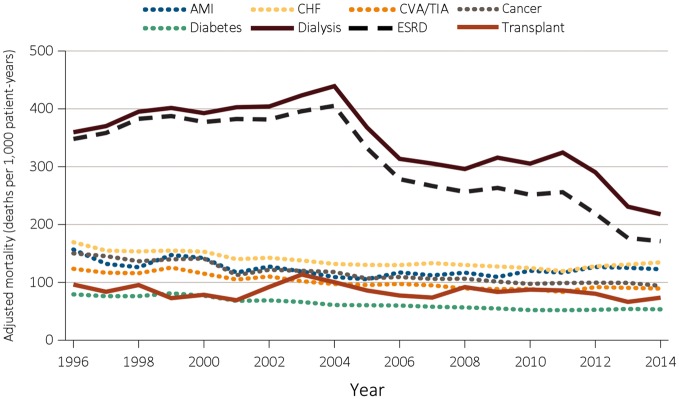
As data from the United States Renal Data System (USRDS) show, mortality improves significantly for ESRD patients who receive renal transplantation as compared with those on RRT. Figure 1 summarizes these finding.
Fig. 1.
Adjusted mortality by treatment modality and Medicare comorbidity among ESRD patients and comorbidity-specific Medicare populations ≥ 65 years of age from 1996 to 2014. Source: United States Renal Data System. 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the USA. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016.
In this article, we review kidney transplantation’s role in the reduction of oxidative stress and its effect of improved morbidity and mortality in ESRD patients.
Free radicals are unstable chemical species with a highly reactive unpaired electron. As it seeks to pair with another unpaired electron, it causes a chain reaction of free radical formation that is damaging to biological systems. The great majority of these free radicals are oxygen radicals or other reactive oxygen species (ROS) [6].
Oxidative stress occurs when ROS overwhelm the antioxidant capacity of the organism. ROS are toxic by-products of aerobic metabolism, which are disposed of by antioxidants. When the antioxidant barrier is unable to fend off the number of free radicals being generated, there is increased lipid peroxidation and oxidative stress [7]. These agents are a constant threat to living cells by severely damaging DNA, proteins and lipids.
Reactive nitrogen species (RNS) also contribute to increasing oxidative stress. RNS are derived from nitric oxide and superoxide produced via the enzymatic activity of inducible nitric oxide synthase 2 and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, respectively. Like ROS, RNS play a dual role since they can be either harmful or beneficial to living systems. ROS and RNS play an important role as regulatory mediators in signaling processes, whereas at moderate or high concentrations they are harmful for living organisms, inactivating important cellular molecules [8].
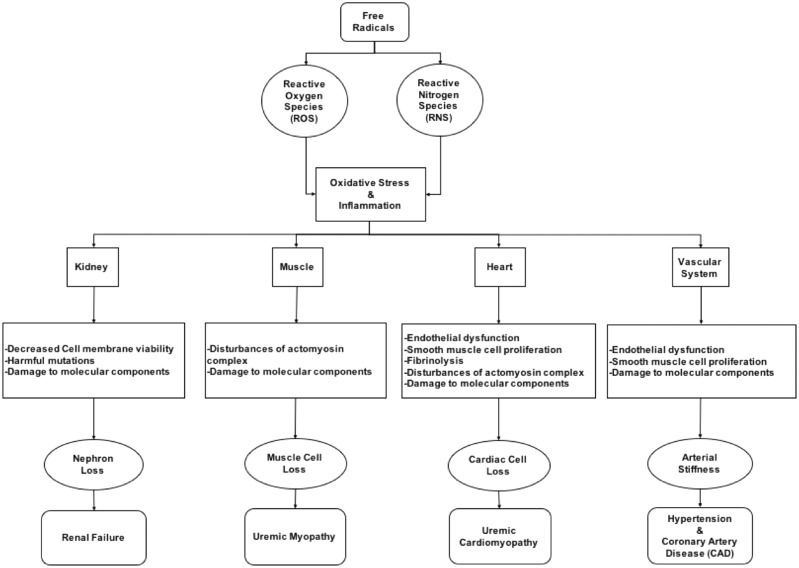
Although oxidative response and inflammation constitute a major defense against infections and regulate many physiological responses in human health, if not properly regulated they can also lead to several deleterious effects. Oxidative stress has a critical role in the pathophysiology of several kidney diseases and also expose the individual to an increased risk of hypertension, CV disease, metabolic syndrome, myopathies, cancer and an overall increased risk of morbidity and mortality [9]. Figure 2 summarizes some of these effects on different tissues.
Fig. 2.
Pathogenic effects of oxidative stress on different organs.
Renal failure and oxidative stress
Oxidative stress appears to have a key role in the pathophysiological process of uremia and its complications [10]. Patients with renal insufficiency, and particularly those on hemodialysis (HD), exhibit increased activation of oxidative and inflammatory processes.
Radicals such as superoxide and hydroxyl readily interact with the molecular components of a nephron. Radical–molecule interactions, including the oxidation of amino acids resulting in the loss of important functional properties, lipid peroxidation of cell membranes resulting in decreased membrane viability and cleavage and crosslinking of renal DNA resulting in harmful mutations, promote renal injury through damage to molecular components of the kidney. The inflammatory processes that exist to repair this radical-mediated damage may be a source of additional free radicals, resulting in further damage to renal tissue [11].
Increasing oxidative stress and inflammation may serve to promote additional damage to the kidney, as well as initial or additional damage to distal tissues, resulting in the development or progression of concomitant disease. Free radicals, along with their constant companion, inflammation, account for many of the symptoms of uremic syndrome [12]. Jun et al. [13] showed a benefit from antioxidant therapy in reducing all causes of death and the risk of CV disease in people with chronic kidney disease (CKD) [13].
Kaltsatou et al. [14] did a systemic review of the role of oxidative stress and uremic myopathy. Muscles continuously produce ROS and RNS at rest and more so during contraction. Moreover, ROS generation can acutely affect contractile function and disturbs the structural transition within the actomyosin complex, which is crucial for force generation. Reviewers found that accumulation of these oxidative stress molecules with progression of CKD is not only directly damaging to muscle cells but also affects their contractility, resulting in muscle weakness, premature fatigue, muscular atrophy and wasting and even cardiomyopathy [14].
Uremic oxidative stress might be the consequence of greater ROS production based on increases in reduced NADPH oxidase activity and expression reported in patients and models with renal insufficiency even in early CKD [15]. Oxidative stress appears to increase as CKD progresses and correlates significantly with the level of renal function. CKD patients present an altered redox status and increased signs of carbonyl stress and inflammatory activity, which all contribute to increase ROS as kidney function deteriorates [16].
Also, progressive loss of renal function is associated with functional defects in virtually all cell populations of both the innate and adaptive immune systems, but the lymphoid cell lineage is more severely affected than the myeloid cell lineage. The ESRD-related changes in the immune system resemble immunological aging in older healthy individuals, a concept known as premature immunological aging. Chronic inflammation and oxidative stress lead to this epigenetic modification [17].
RRT and oxidative stress
HD and peritoneal dialysis (PD) are two major modalities of RRT in ESRD. Unfortunately, both methods are accompanied by increased oxidative stress. The composition of the dialysis solution, the characteristics of the HD membranes, loss of antioxidants and activation of leukocytes increase the formation of ROS and promote an overall pro-inflammatory state [18].
Bacterial infection and sepsis are common complications of CKD. Furthermore, Lemesch et al. [19] showed that the type of RRT plays a role in mortality. In a cross-sectional observational study they compared endotoxemia and neutrophil function, both of which can be affected by increasing oxidative stress in CKD patients without RRT, CKD patients undergoing HD or PD and patients after kidney transplantation. Their results showed a higher endotoxin serum level and a lower neutrophil phagocytic capacity compared with all other groups in CKD patients undergoing HD, which was associated with a higher mortality rate. The findings suggest that dialysis modality and not renal function per se determine the development of neutrophil dysfunction and endotoxemia in CKD patients. HD patients are particularly prone to neutrophil dysfunction and endotoxemia, whereas neutrophil function seems to improve after kidney transplantation [19]. Differences in mortality and improvement of neutrophil phagocytic capacity after kidney transplantation can be partly due to differences in the oxidative stress level in these groups.
In a prospective cohort study by Soleymanian et al. [20] of kidney transplant candidates on HD, they measured total antioxidant capacity (TAC), total thiol molecules, lipid peroxidation, plasma catalase, superoxide dismutase, glutathione peroxidase and C-reactive protein as the biomarkers of oxidative stress and inflammation. They compared the level of these markers before and 3 months after kidney transplantation. They concluded that oxidative stress and inflammation are elevated in HD patients and could be improved significantly by restoration of kidney function after kidney transplantation [20].
It is well known that HD patients experience increased oxidative stress. In a systemic review by Poulianiti et al. [21], the authors concluded that HD therapy per se seems to exert a negative influence on systemic redox status. Retention of water and salt in kidney disease patients results in increased water-soluble toxins. In contrast, because of an abnormal balance of protein-bound blood components in the patients with CKD, there is retention of protein-bound toxins. As Gao et al. [22] reported, this increase in oxidation may alter high-density lipoprotein (HDL) function not only by chemically modifying proteins and lipids but also by altering the rate of HDL remodeling and/or shifting the population distribution among HDL subclasses. Mild oxidation in vivo may enhance HDL functions in reverse cholesterol transport, but extensive oxidation would inhibit protein distribution and lipoprotein remodeling, which would impair reverse cholesterol transport and increase oxidative stress in these patients [22].
Kidney transplant and oxidative stress
Unfortunately, the nature of the surgery and organ transfer from a donor to recipient causes inevitable renal ischemia. This renal ischemia reperfusion (RIR) injury during transplantation is the major cause of renal transplant dysfunction. It causes an oxidative burst that triggers inflammation and tubular cell injury. In a recent study, Tennankore et al. [23] showed a correlation between prolonged warm ischemia and mortality and graft failure. Evidences suggests that NADPH oxidase is activated during RIR injury and may play a potential role in the pathogenesis of progressive renal damage in this setting. Nicholson et al. [24] performed a double-blind randomized clinical trial (RCT) regarding remote ischemic conditioning and were unable to show an improvement in renal function at 1 and 3 months posttransplant [24]. Kumar et al. [25], through in vitro studies on immortalized human renal proximal tubular epithelial cells, suggested the role of antioxidants such as N-acetylcysteine (NAC) in decreasing RIR and thus improving mortality.
Also, most of the currently accepted immunosuppressive medication, such as corticosteroids, calcineurin inhibitors (CNIs) and mammalian target of rapamycin inhibitors, which are the mainstay of posttransplant antirejection medications, are all known to increase the risks of developing new-onset diabetes after transplant (NODAT) as well as metabolic syndrome, which by itself can translate to increased oxidative stress.
In contrast, kidney transplantation restores, at least partially, the fundamental processes of glomerular filtration, which eliminates toxic solutes, including from oxidative stress. To determine the net effect of the oxidative stress level after successful kidney transplantation, Antolini et al. [26] did a cross-sectional study to determine the levels of several different glycoxidative stress-related parameters after kidney transplantation. They measured glycoxidative stress markers such as albumin-bound and free pentosidine, low molecular weight advanced glycation end products (LMW-AGEs), advanced oxidation protein products (AOPPs) and low molecular weight carbonyls (LMW-Cs). TAC was monitored by measuring both the ferric reducing/antioxidant power (FRAP) and oxygen radical absorbance capacity (ORAC). Antolini et al. showed that transplant patients with normal kidney function had levels of these compounds that were comparable to normal controls, except for the LMW-AGEs, which were higher. Levels of LMW-AGEs, pentosidine, LMW-Cs and AOPPs were inversely correlated with creatinine clearance. The TAC was paradoxically higher in transplant patients than in controls, regardless of kidney function. These findings indicate that kidney transplantation seems to restore a nearly normal level of glycoxidative stress markers and an increase of TAC, but complete remission is only possible when the renal function is normal [26].
It is very important to keep in mind that TAC is a comprehensive index of antioxidant capacity and simplifying that normalization of kidney function is the only factor contributing to this drastic improvement of TAC after kidney transplantation is not realistic. Decreasing ROS after successful kidney transplantation results in breaking the vicious cycle of cell damage, inflammation and distal organ damage. This means recovery from many uremic syndrome sequelae, such as myopathy, cardiomyopathy and a decrease in appetite. Clinically, this improvement translates to more effective cardiac output, better nutrition and more muscle mass, which can all contribute to better metabolism and less production of oxidative stress and improvement in TAC after successful kidney transplantation.
CKD patients present an altered redox status and increased signs of carbonyl stress and inflammatory activity as kidney function deteriorates, which was partially but significantly improved after renal transplantation [17]. In the study conducted by Vostálová et al. [27], the restoration of kidney function after kidney transplantation led to a reduction in metabolic abnormalities and elimination of oxidative stress. Time-dependent changes in oxidative stress–related markers and kidney function and metabolic parameters were evaluated for 3 months in patients before and after kidney transplantation. In particular, TAC, AOPPs, lipid peroxidation as thiobarbituric acid–reactive substances (TBARS) and reduced glutathione (GSH); activities of glutathione peroxidase, catalase and superoxide dismutase and kidney function markers were measured. AOPPs, TAC and TBARS were significantly decreased, whereas GSH was significantly increased after kidney transplantation. Antioxidant enzyme activities were not significantly changed during the monitored period. Kidney function markers and glomerular filtration rate significantly increased and the creatinine level significantly decreased after transplantation. They also found that after transplantation there is a trend toward increased HDL cholesterol. These results show that successful kidney transplantation contributes to normalization of antioxidant status and lipid metabolism that is associated with both improved renal function and reduced CV complications [27].
Sulfatide is a major component of glycosphingolipids in lipoproteins. Wang et al. [28] reported that a low serum level of sulfatide in HD patients might be related to the high incidence of CV disease. The recovery of serum sulfatide derives from the attenuation of systemic oxidative stress. The normal level of serum sulfatide in kidney transplant recipients affects platelet function and contributes to a reduction in the incidence of CV disease [28].
Malondialdehyde (MDA) and serum sulfatide are two markers of oxidative stress, which were investigated by Kamijo et al. [29] in post–kidney transplant patients [29]. The high level of MDA in the kidney transplant recipients decreased dramatically, but was still high, 1 year after kidney transplant. MDA levels decreased further and reached near normal levels in about 3 years.
To determine the effect of immunosuppression choices in this drastic reduction of oxidative stress after kidney transplant, Vostálová et al. [30] followed 70 patients after kidney transplant. The patients were divided into two groups: those receiving cyclosporine and those receiving tacrolimus. Oxidative stress–related markers such as TAC, AOPPs and lipid peroxidation products all decreased. There was no significant difference between these two CNIs in any of the measured parameters. Improved renal function after kidney transplant is linked to a reduction in oxidative stress, but independent of immunosuppressive therapy.
Interestingly, the reduction in oxidative stress markers can be used as a prognostic factor after kidney transplantation. Increased MDA levels on the first day after kidney transplant might be an early prognostic indicator of delayed graft function (DGF), and levels on Day 7 might represent a useful predictor of 1-year graft function [31]. DNA fragmentation is one of the typical features of apoptosis, frequently induced by oxidative stress. Low levels of oxidation and apoptosis at 6 months after transplantation correlate with a better recovery of renal function in kidney allografts [32].
CV disease after kidney transplant
CV disease is the leading cause of death in patients with renal failure. Although improved, the morbidity and mortality rates are still higher after kidney transplantation than in the general population and many renal transplant recipients die with functional grafts. With the advent of improved immunosuppression and surgical technique, deaths resulting from CV disease have become an increasingly important cause of graft loss, particularly after the first posttransplantation year.
One of the major factors in the pathogenesis of CV complications is the imbalance between the formation and clearance of ROS by the antioxidative system. This disparity can cause endothelial dysfunction and impairment of the regulatory functions of endothelium for vasodilatation. It is also the foundation of smooth muscle cell proliferation and fibrinolysis, which play a pivotal role in the pathogenesis of CV events. Also, as mentioned above, this can lead to disturbances in contractility, and resulting in cardiomyopathy [14].
In a recent study, Hornum et al. [33] investigated the effect of kidney transplantation on vascular hemodynamics by measuring arterial function at baseline and 12 months after kidney transplantation. Arterial function was estimated by the pulse wave velocity of the carotid–femoral pulse wave, aortic augmentation index and flow-mediated (endothelium-dependent) and nitroglycerin-induced vasodilatation (endothelium independent) of the brachial artery. They found that arterial function improved 1 year after kidney transplantation and was also associated with a decline in blood pressure. These seemingly beneficial changes in arterial hemodynamics had occurred despite increased insulin resistance and unchanged high levels of cholesterol and triglyceride [33]. This shows the importance of a healthy endothelium, which can be achieved by reducing ROS after successful kidney transplantation.
In another study by Yilmaz et al. [34], they measured plasma asymmetric dimethyl arginine (ADMA) levels before transplantation and on Days 1, 3, 7, 14 and 28. We know that ADMA is an endogenous modulator of endothelial function and oxidative stress and increased levels of this molecule have been reported in metabolic disorders and CV diseases. In this study, they measured brachial artery flow-mediated dilatation as a marker of endothelium function before transplantation and on Day 28. Their finding indicates that ADMA is associated with flow-mediated dilatation in CKD, both before and after kidney transplantation. Endothelial functions improve at the very beginning of the posttransplantation period with an accompanying reduction in ADMA levels.
Kidney transplantation has shown promise in decreasing oxidative stress. By alleviating oxidative stress, modifying the immunologic response, improving endothelial function, suppressing platelet aggregation and decreasing arterial stiffness, kidney transplantation leads to a decrease in CV morbidity and mortality.
But it is very important not to forget that although oxidative stress is a major risk factor in CV disease, the increased risk of CV disease after kidney transplant can be also related to a high prevalence and accumulation of other atherogenic risk factors. Hypertension, NODAT and hyperlipidemia are well-recognized risk factors for the development of CV events after renal transplantation and are strongly associated with immunosuppressive therapy. The elevated risk may also be caused by nontraditional risk factors such as anemia, adhesion molecules, hyperhomocysteinemia, a microinflammatory state and abnormal coagulation [35]. Moreover, the contribution of some CV risk factors to renal allograft dysfunction has been well demonstrated. This relation causes a vicious cycle, since progressive renal dysfunction may also influence the risk of CV complications after renal transplantation.
Conclusion
Oxidative stress has a key role in the pathophysiological process of uremia and its complications, including CV disease. The level of these markers correlates with the severity of CKD and the level of renal function. Unfortunately, both modalities of RRT (HD and PD) increase the level of oxidative stress.
Although the surgical procedure of transplantation and ischemic injury to the organ during the procurement and transplantation can also cause increases oxidative stress, it seems that successful kidney transplant results in near normalization of the antioxidant status and lipid metabolism by eliminating free radicals and decreasing oxidative stress. Achieving any level of kidney function after successful kidney transplantation will decrease the oxidative stress level. This success is connected with both improved renal function and reduced CV complications and eventually improving morbidity and mortality in these patients. It is important to remember that the degree of renal function after transplantation is one of the key factors of eliminating these radicals.
Measuring oxidative stress markers such as MDA is also promising in predicting allograft survival and the possibility of DGF. Unfortunately, most of the studies in this regard have been very limited. They all have small sample size and are mainly observational or cohort studies. There are many unresolved issues regarding the role of oxidative stress and the net balance of these toxins after kidney transplantation that need to be further studied.
It is the hope of the authors that this review has identified the key literature surrounding this important discussion and that it may be used as a step for further research in this arena.
Conflict of interest statement
None declared.
References
- 1. Collins AJ, Foley RN, Chavers B. et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis, 2014; 63: A7. [DOI] [PubMed] [Google Scholar]
- 2. He W, Goodkind D, Kowal P.. An Aging World: 2015. International Population Reports P95/16-1. Washington, DC: U.S. Census Bureau, 2016
- 3. Wolfe R, Ashby V, Milford E. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 4. Gaston R, Alveranga D, Becker B. et al. Kidney and pancreas transplantation. Am J Transplant 2003; 3: 64–77 [DOI] [PubMed] [Google Scholar]
- 5. Stoumpos S, Jardine A, Mark P.. Cardiovascular morbidity and mortality after kidney transplantation. Transpl Int 2015; 28: 10–21 [DOI] [PubMed] [Google Scholar]
- 6. Ozbek E. Induction of oxidative stress in kidney. Int J Nephrol 2012; 2012: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campise M, Bamonti F, Novembrino C. et al. Oxidative stress in kidney transplant patients. Transplantation 2003; 76: 1474–1478 [DOI] [PubMed] [Google Scholar]
- 8. Di Meo S, Reed TT, Venditti P.. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev 2016; 2016: 1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libetta C, Sepe V, Esposito P. et al. Oxidative stress and inflammation: Implications in uremia and hemodialysis. Clin Biochem 2011; 44: 1189–1198 [DOI] [PubMed] [Google Scholar]
- 10. Dounousi E, Papavasiliou E, Makedou A. et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis 2006; 48: 752–760 [DOI] [PubMed] [Google Scholar]
- 11. Tucker PS, Scanlan AT, Dalbo VJ.. Chronic kidney disease influences multiple systems: describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid Med Cell Longev 2015; 2015: 806358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nafar M, Sahraei Z, Salamzadeh J. et al. Oxidative stress in kidney transplantation causes, consequences, and potential treatment. Iran J Kidney Dis 2011; 5: 357–372 [PubMed] [Google Scholar]
- 13. Jun M, Venkataraman V, Razavian M. et al. Antioxidants for chronic kidney disease. Cochrane Database Syst Rev 2012; 10: CD008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaltsatou A, Sakkas GK, Poulianiti KP. et al. Uremic myopathy: is oxidative stress implicated in muscle dysfunction in uremia? Front Physiol 2015; 6: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fortuño A, Beloqui O, San José G. et al. Increased phagocytic nicotinamide adenine dinucleotide phosphate oxidase-dependent superoxide production in patients with early chronic kidney disease. Kidney Int 2005; 68(Suppl 99): S71–S75 [DOI] [PubMed] [Google Scholar]
- 16. Aveles P, Criminácio C, Gonçalves S. et al. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin Pract 2010; 116: 294–299 [DOI] [PubMed] [Google Scholar]
- 17. Betjes M, Meijers R, Litjens N.. Loss of renal function causes premature aging of the immune system. Blood Purif 2013; 36: 173–178 [DOI] [PubMed] [Google Scholar]
- 18. Vostálová J, Galandáková A, Strebl P. et al. Oxidative stress in patients on regular hemodialysis and peritoneal dialysis. Vnitr Lek 2012; 58: 466–472 [PubMed] [Google Scholar]
- 19. Lemesch S, Ribitsch W, Schilcher G. et al. Mode of renal replacement therapy determines endotoxemia and neutrophil dysfunction in chronic kidney disease. Sci Rep 2016; 6: 34534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soleymanian T, Ranjbar A, Alipour M. et al. Impact of kidney transplantation on biomarkers of oxidative stress and inflammation. Iran J Kidney Dis 2015; 9: 400–405 [PubMed] [Google Scholar]
- 21. Scholze A, Jankowski J, Pedraza-Chaverri J. et al. Oxidative stress in chronic kidney disease. Oxid Med Cell Longev 2016; 2016: 8375186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kimak E, Halabis M, Baranowicz-Gaszczyk I. et al. Association between moderately oxidized low-density lipoprotein and high-density lipoprotein particle subclass distribution in hemodialyzed and post-renal transplant patients. J Zhejiang Univ Sci B 2011; 12: 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tennankore K, Kim S, Alwayn I. et al. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int 2016; 89: 648–658 [DOI] [PubMed] [Google Scholar]
- 24. Nicholson M, Pattenden C, Barlow A. et al. A double blind randomized clinical trial of remote ischemic conditioning in live donor renal transplantation. Medicine (Baltimore) 2015; 94: e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar A, Shalmanova L, Hammad A. et al. Induction of IL-8(CXCL8) and MCP-1(CCL2) with oxidative stress and its inhibition with N-acetyl cysteine (NAC) in cell culture model using HK-2 cell. Transpl Immunol 2016; 35: 40–46 [DOI] [PubMed] [Google Scholar]
- 26. Antolini F, Valente F, Ricciardi D, et al. ; Normalization of oxidative stress parameters after kidney transplant is secondary to full recovery of renal function. Clin Nephrol 2005; 62: 131–137 [DOI] [PubMed] [Google Scholar]
- 27. Vostálová J, Galandáková A, Svobodová AR. et al. Time-course evaluation of oxidative stress-related biomarkers after renal transplantation. Ren Fail 2012; 34: 413–419 [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Kamijo Y, Matsumoto A. et al. Kidney transplantation recovers the reduction level of serum sulfatide in ESRD patients via processes correlated to oxidative stress and platelet count. Glycoconj J 2011; 28: 125–135 [DOI] [PubMed] [Google Scholar]
- 29. Kamijo Y, Wang L, Matsumoto A. et al. Long-term improvement of oxidative stress via kidney transplantation ameliorates serum sulfatide levels. Clin Exp Nephrol 2012; 16: 959–967 [DOI] [PubMed] [Google Scholar]
- 30. Vostálová J, Galandáková A, Svobodová AR. et al. Stabilization of oxidative stress 1 year after kidney transplantation: effect of calcineurin immunosuppressives. Ren Fail 2012; 34: 952–959 [DOI] [PubMed] [Google Scholar]
- 31. Fonseca I, Reguengo H, Almeida M. et al. Oxidative stress in kidney transplantation: malondialdehyde is an early predictive marker of graft dysfunction. Transplantation 2014; 97: 1058–1065 [DOI] [PubMed] [Google Scholar]
- 32. La Manna G, Lanci N, Della Bella E. et al. Reduction of oxidative damage reflects a better kidney transplantation outcome. Am J Nephrol 2011; 34: 496–504 [DOI] [PubMed] [Google Scholar]
- 33. Hornum M, Clausen P, Idorn T. et al. Kidney transplantation improves arterial function measured by pulse wave analysis and endothelium-independent dilatation in uraemic patients despite deterioration of glucose metabolism. Nephrol Dial Transplant 2011; 26: 2370–2377 [DOI] [PubMed] [Google Scholar]
- 34. Yilmaz M, Saglam M, Caglar K. et al. Endothelial functions improve with decrease in asymmetric dimethylarginine (ADMA) levels after renal transplantation. Transplantation 2005; 80: 1660–1666 [DOI] [PubMed] [Google Scholar]
- 35. Montanaro D, Gropuzzo M, Tulissi P. et al. Cardiovascular disease after renal transplantation. G Ital Nefrol 2004; 21(Suppl 26): S53–S66 [PubMed] [Google Scholar]