Abstract
We present the case of a patient presenting with embolic stroke secondary to aortic valve endocarditis, additionally complicated by splenic abscess, successfully treated by emergent valve replacement followed by delayed, robotic splenectomy.
INTRODUCTION
Infective endocarditis (IE) complicated by septic emboli is a challenging clinical problem. Embolism to the spleen can result in splenic infarction in 40% of patients, with splenic abscess occurring in 5% of these patients [1]. Optimally, splenectomy prior to valve replacement (VR) is the treatment of choice to avoid infection of the implanted valve [2].
Cerebral complications can occur in 55% of patients with IE [3] and acute neurologic deficit secondary to septic emboli may be the presenting symptom in 20% of cases [4]. Although these patients have a poor prognosis [3], the risk of hemorrhagic conversion may be mitigated the longer VR surgery can be delayed [5], and it is recommended to delay VR up to a month after ischemic stroke, if possible [4]. When valve surgery must be done emergently, it can be done safely earlier [6], but requires complex coordination of care perioperatively. We report the case of a high-risk patient with IE cerebral infarcts and splenic abscess, who required emergent VR followed by splenectomy.
CASE REPORT
A 75-year-old male presented to clinic with a decline in mental status following 6 weeks of progressive weakness. His past medical history included hypertension, coronary artery disease, stable chronic lymphoid leukemia (CLL) and newly diagnosed congestive heart failure (CHF) with moderate aortic valve insufficiency seen on outpatient echocardiogram 3 weeks earlier. Vital signs were within normal limits, and physical exam revealed a non-focal neurologic examination, diastolic heart murmur and diffuse mild abdominal pain. Admission laboratories were notable for WBC of 18 500/mm3 (slightly increased above CLL baseline), with neutrophil predominance. Brain MRI (Fig. 1) and trans-esophageal echocardiography revealed multiple bilateral areas of restricted diffusion consistent with ischemic stroke, as well as a 2.2 cm aortic valve vegetation with severe aortic regurgitation, but no intra-cardiac abscess. Blood cultures confirmed Enterococcus faecalis endocarditis. Abdominal CT scan (Fig. 2) revealed an 8.1 cm splenic lesion consistent with splenic abscess.
Figure 1:
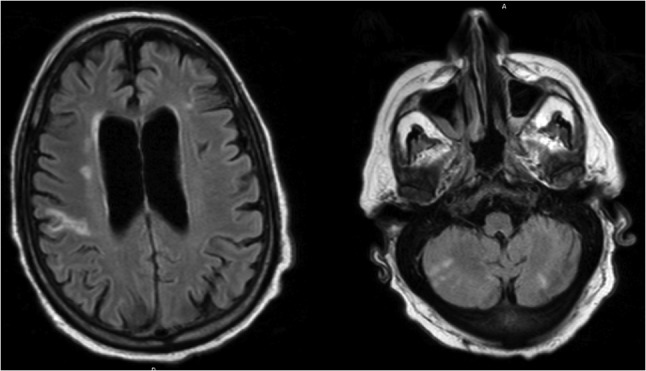
Brain MRI demonstrating multiple bilateral areas of restricted diffusion consistent with ischemic stroke.
Figure 2:
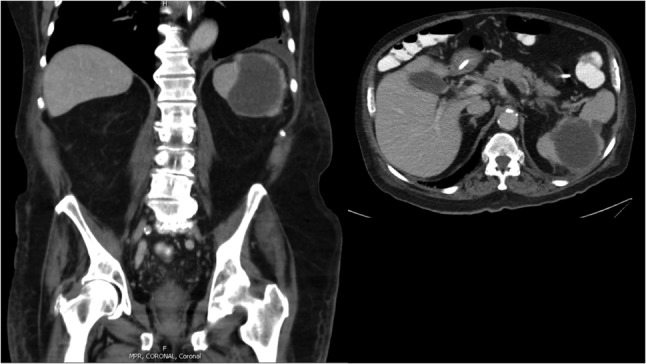
Abdominal CT scan demonstrating an 8.1 cm splenic lesion consistent with splenic abscess.
Though hemodynamically stable, his neurologic status deteriorated despite antibiotic therapy, with signs and symptoms of worsening CHF. A multi-disciplinary team determined emergent aortic VR (AVR) was necessary given his cardiac decompensation and ongoing neurologic decline. Simultaneous splenectomy and AVR were not considered a viable option. Instead, the splenic abscess was percutaneously drained and the patient underwent successful AVR with a 25 mm Magna bioprosthetic valve and two-vessel coronary artery bypass grafting. His post-operative course was largely unremarkable—however, severe deconditioning and malnutrition prevented interval splenectomy. His drain was removed and he discharged to a transitional care unit (TCU) with IV antibiotics.
After 12 weeks of intravenous antibiotic treatment, repeat CT revealed a persistent 7-cm splenic fluid collection. Definitive splenectomy was recommended to prevent re-infection of the aortic valve, and he underwent an uncomplicated robotic-assisted (da Vinci) splenectomy. His post-operative course was uneventful, and he discharged to TCU in stable condition. At 2-year follow-up, patient is doing well living independently at home.
DISCUSSION
Although outcomes for IE have improved with prompt diagnosis and tailored antibiotic treatment [7], mortality approaches 60% when patients require ICU admission [3]. Septic embolization from IE remains a common problem, occurring in up to 50% of patients [1].
Cerebrovascular accident due to septic emboli is a well-recognized complication of left-sided IE that presents additional challenges. Up to 40% of patients with active IE may develop neurologic complications [1], most commonly ischemic stroke [3]. In patients with neurologic complications requiring VR surgery for IE it has been advocated to delay surgery for at least 4 weeks [4]. This approach decreases further neurologic complications from peri-operative hypotension and/or hemorrhagic conversion following cardiopulmonary bypass from a peak of 50% in the first 2 weeks following CVA from IE to <1% at 1 month [5]. However, in patients at high risk for further complications without immediate surgical intervention, early VR has been shown to be safe and effective [6].
When surgery is indicated, identification and treatment of systemic emboli is of paramount importance to prevent re-infection of the affected valve. Failure to identify splenic abscess can result in recurrent IE and rarely can lead to splenic rupture—a potentially fatal complication [1]. Definitive treatment includes splenectomy, as high failure rates have been reported with medical therapy alone [2]. Ideally, splenectomy prior to VR is considered preferable to remove extra-cardiac foci of infection and prevent re-infection of the implanted valve [1, 2]. Recently, minimally invasive splenectomy has been shown to be safe and effective for splenic abscess secondary to IE [2], providing an alternative to laparotomy.
Our patient was at high risk for ongoing ischemic events from septic emboli if AVR was not immediately considered, with full neurologic recovery possible if no further injury occurred. In addition, CHF in the setting of IE necessitated immediate attention. The presence of CHF is well-recognized as having the biggest impact on survival in the setting of IE [1], and despite increasing the operative risk, VR in CHF patients with IE reduces long-term mortality compared to medical therapy [1].
In our patient, the emergent nature of his condition precluded the preferred approach of splenectomy preceding AVR; severe malnutrition and deconditioning also prevented immediate splenectomy. In this situation, few guidelines exist regarding treatment of the splenic abscess. A less invasive approach that has shown promise as an alternative to splenectomy involves percutaneous drainage of the abscess and intravenous antibiotic therapy, with the largest series by Chou et al. [8] reporting resolution of splenic abscess in all 21 patients treated in this manner.
We successfully bridged our patient to splenectomy with percutaneous drainage and intravenous antibiotic therapy, allowing recovery from AVR and improvement in nutritional and functional status. A minimally invasive approach to splenectomy, through the use of a da Vinci-assisted splenectomy, minimized the morbidity of a second major surgery on a patient with multiple co-morbidities.
In conclusion, IE with CVA and splenic abscess remains a complex and unusual clinical scenario. We advocate a multi-disciplinary approach to optimally determine the timing of surgical intervention. Although treatment of extra-cardiac infectious sources prior to VR is preferred, we feel an individualized approach is necessary, with percutaneous drainage, antibiotics and delayed splenectomy reasonable in patients requiring emergent cardiac surgery.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Baddour LM, Wilson WR, Bayer AS, Fowler VG, Bolger AF, Levison ME, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association. Circulation 2005;111:e394–434. [DOI] [PubMed] [Google Scholar]
- 2. Simsir SA, Cheeseman SH, Lancey RA, Vander Salm TJ, Gammie JS. Staged laparoscopic splenectomy and valve replacement in splenic abscess and infective endocarditis. Ann Thorac Surg 2003;75:1635–7. [DOI] [PubMed] [Google Scholar]
- 3. Sonneville R, Mirabel M, Hajage D, Tubach F, Vignon P, Perez P, et al. Neurologic complications and outcomes of infective endocarditis in critically ill patients: the ENDOcardite en REAnimation prospective multicenter study. Crit Care Med 2011;39:1474–81. [DOI] [PubMed] [Google Scholar]
- 4. Byrne JG, Rezai K, Sanchez JA, Bernstein RA, Okum E, Leacche M, et al. Surgical management of endocarditis: the society of thoracic surgeons clinical practice guideline. Ann Thorac Surg 2011;91:2012–9. [DOI] [PubMed] [Google Scholar]
- 5. Angstwurm K, Borges AC, Halle E, Schielke E, Einhaupl KM, Weber JR. Timing the valve replacement in infective endocarditis involving the brain. J Neurol 2004;251:1220–6. [DOI] [PubMed] [Google Scholar]
- 6. Cooper HA, Thompson EC, Laureno R, Fuisz A, Mark AS, Lin M, et al. Subclinical brain embolization in left-sided infective endocarditis: results from the evaluation by MRI of the brains of patients with left-sided intracardiac solid masses (EMBOLISM) pilot study. Circulation 2009;120:585–91. [DOI] [PubMed] [Google Scholar]
- 7. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chou YH, Tiu CM, Chiou HJ, Hsu CC, Chiang JH, Yu C. Ultrasound-guided interventional procedures in splenic abscesses. Eur J Radiol 1998;28:167–70. [DOI] [PubMed] [Google Scholar]


