Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease worldwide. The renal phenotype is characterized by progressive cystic enlargement of the kidneys leading to a decline in renal function, hypertension and often end-stage renal disease (ESRD). Supportive care with blood pressure control and management of pain, urinary infections and renal stone disease has, until recently, been the mainstay of treatment. With the recent approval of tolvaptan for use in ADPKD, the disease progression may now be targeted specifically. Algorithms that guide treatment initiation have been proposed but a more pragmatic and patient-individualized approach is often needed to make decisions regarding therapy. It is highly important to identify ADPKD patients with rapidly progressive disease who are likely to benefit most from this treatment and avoid treatment in patients that are unlikely to reach ESRD.
Methods and Results
Here we present a series of cases of ADPKD patients in whom therapy with tolvaptan has been considered and report the rationale for the treatment decisions based on available lifestyle, clinical, biochemical, radiological and genetic data.
Conclusions
These cases provide a discussion for the use of tolvaptan in ADPKD within the nephrology clinic and allow insights into the practicalities of using this therapy outside of clinical trials.
Keywords: autosomal dominant polycystic kidney disease, patient selection, PROPKD score, rapid progression, tolvaptan, total kidney volume
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited disorder that predominantly affects the kidneys, but also frequently causes abnormalities in other organs, such as the liver and the pancreas, and the cerebral vasculature [1, 2]. The hallmark of ADPKD is continuous development of renal cysts accompanied by an increase in total kidney volume (TKV), hypertension and a reduction in glomerular filtration rate, culminating in end-stage renal disease (ESRD) in the majority of patients [1, 3, 4]. ADPKD is the most common inherited renal disorder in adults; it accounts for ∼10% of ESRD cases [5], and dialysis-dependent kidney failure develops in >50% of patients by 60 years of age [6]. The prevalence of ADPKD is often stated to be between 1 in 400 and 1 in 1000 live births [1]. However, these numbers reflect the finding of cystic kidneys in autopsy studies or a general lifetime risk of ADPKD rather than point prevalence in a population [7]. A recent analysis of both population-based studies and registries revealed a point prevalence for ADPKD of ∼3–5/10 000 individuals [8]. ADPKD usually presents clinically in the third or fourth decade of life and is associated with a range of symptoms, including pain in the abdomen, flank or back, abdominal fullness, cyst haemorrhage, nephrolithiasis, cyst infection, haematuria and hypertension [1, 9, 10]. Screening programmes, usually based on renal ultrasound scanning (USS), allow early detection of ADPKD in patients with a positive family history [11, 12]. It is worth noting that using renal USS to exclude ADPKD where there is a family history of disease requires imaging up to the age of 40 years [11].
ADPKD follows an autosomal-dominant inheritance, resulting in a positive family history in the vast majority of patients, with individuals affected in every generation. However, spontaneous mutations do also occur and account for ∼5% of cases [2]. ADPKD is a consequence of mutations in two genes, PKD1 and PKD2, which encode polycystin (PC)-1 and -2, respectively [2]. Mutations in PKD1 are more common than in PKD2, accounting for 85% and 15% of cases, respectively [13]. Patients with ADPKD resulting from PKD1 mutations show faster progression of disease with more rapid cyst growth and loss of renal function [14, 15]. Both PC-1 and PC-2 localize to the primary cilium—a finding that is in line with cystic kidney diseases in general being regarded as so-called ciliopathies [16]. Mutations in either PKD1 or PKD2 lead to the perturbation of numerous intracellular signalling pathways, resulting in increased secretion and proliferation of renal epithelial cells [1]. One of the most consistent and thus probably pivotal findings is an increase seen in the levels of the secondary messenger cAMP whilst intracellular calcium levels are reduced [1].
Care of ADPKD patients was, until recently, limited to supportive measures, due to the lack of targeted therapeutic strategies. Consequently, nephrologists could only offer limited strategies regarding disease progression and treatment mainly focussed on symptoms and complications of ADPKD, including blood pressure and pain control, in addition to antibiotic treatment of cyst infections and treatment of renal stones; however, therapy for the disease itself was not available [1, 17]. One of the key strategies to slow down disease progression is the reduction of intracellular cAMP levels, which, in rodent models, can be achieved efficiently by blocking vasopressin receptor signalling [18]. In 2012, tolvaptan, a potent, highly selective, orally available, V2 receptor antagonist [19], was proven to significantly reduce both kidney growth and loss of estimated glomerular filtration rate (eGFR) in the 3-year randomized double-blinded placebo-controlled TEMPO 3:4 trial, which enrolled 1445 ADPKD patients from 129 sites [20]. Consequently, in 2015 tolvaptan was approved by the European Medicines Agency (EMA) for the treatment of adult patients with ADPKD in chronic kidney disease (CKD) stages 1–3 at treatment initiation with evidence of rapid disease progression [21]. However, use of tolvaptan is partly different in European countries due to national regulations. As an example, whilst in Germany the therapy is reimbursed for all patients that fulfil the indication as formulated by the EMA, in England, reimbursement is reserved for CKD stages 2 and 3, as use of tolvaptan for CKD stage 1 was not deemed cost-effective in a review by NICE [22]. As the first approved targeted treatment of ADPKD, V2 receptor blockade is an urgently awaited advance for both ADPKD patients and their physicians. However, a major new challenge is the selection of ADPKD patients who will benefit from this treatment, which is largely based on evidence of rapid progression.
The current consensus, as well as the approval criteria of the EMA, is that patients in whom tolvaptan is initiated should have evidence of rapid progression; however, it should be remembered that the inclusion criteria in TEMPO 3:4 study that documented the effect of tolvaptan did not require explicit evidence of rapid progression (although by kidney size the included patients were likely to be so). Thus, there is some discrepancy between the clinical indications for tolvaptan use and inclusion criteria used in recent clinical trials adding to the complexity of selecting the appropriate patients for treatment.
In general, only patients with a high risk of reaching ESRD are suitable candidates for tolvaptan treatment. Treatment algorithms have been published that allow physicians to follow expert guidance and look for evidence of disease progression in order to inform treatment decisions, but the real-world scenarios insist that a more practical and realistic approach must be adopted in some cases.
Quantifying rapid progression
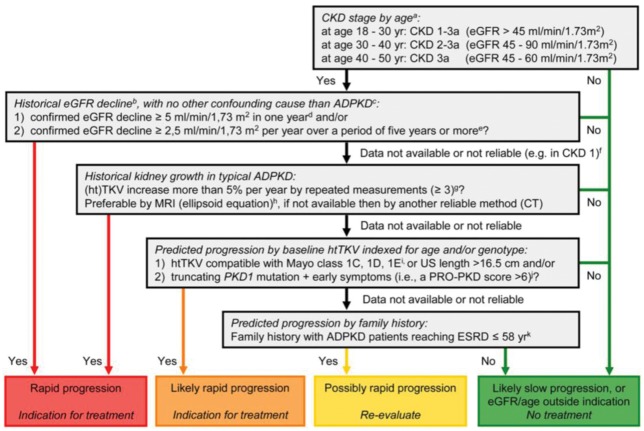
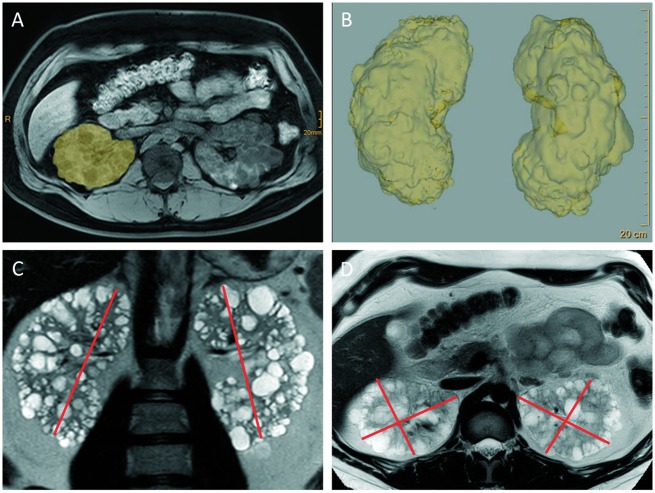
When considering rapid progression in ADPKD patients, the criteria that provide direct evidence of rapid progression (Table 1) can be separated from those that serve as predictors of future progression (Table 2). Documenting the slope of eGFR is both the easiest and most powerful indicator of disease progression and is the first step in the algorithm provided by the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice (Figure 1). When judging eGFR loss, it is crucial that potential causes of renal function loss other than ADPKD are excluded. Conversely, when taking into account the variability of creatinine values, a sufficient number of measurements should be available. A decrease of ≥5 mL/min/1.73m2 in 1 year or ≥2.5 mL/min/1.73m2 per year over 5 years has been suggested as a cut-off point for defining rapid progression [24]. Where this evidence is lacking, consideration of markers of risk of progression can help treatment decisions to be made. Younger patients in CKD stage 1 cannot be judged solely based on eGFR loss, whereas CKD stage 1 in a patient >50 years of age would exclude rapidly progressive disease. Whilst eGFR in ADPKD patients may be maintained at a stable range for decades through glomerular hyperfiltration [3], the disease progresses, as evidenced by the continuous increase in TKV [20, 35]. Repetitive measurements of TKV could consequently be used to measure the speed of kidney growth; this has shown efficacy in clinical trials, with magnetic resonance imaging (MRI)-based stereology and manual planimetry used to accurately quantify kidney volume [4, 27, 36] (Figure 2). However, in our experience, this strategy is impeded by the lack of access to repetitive MRI volumetry and the variation in volume between measurements in the practical setting.
Table 1.
Direct evidence of rapid disease progression in ADPKD
Table 2.
Indirect evidence/predictors of rapid disease progression in ADPKD
| Parameters | Key indication/prediction |
|---|---|
| Genetics | Truncating PKD1 mutations are associated with more rapid progression than non-truncating PKD1 mutations [26] |
| PKD2 mutations are associated with the slowest progression [27] | |
| Kidney size/Mayo classification | Kidney length >16.5 cm by ultrasound may indicate risk of progression [28], but also consider patient age |
| htTKV>600 mL/m indicates rapid progression [29] | |
| Mayo classification allows for prediction of eGFR loss based on a one-time htTKV measurement in an age-dependent fashion [30] | |
| PROPKD score | Incorporates genetics, early onset of urological complications and hypertension, as well as gender into a model predicting disease progression [31] |
| PROPKD score of >6 predicts reaching ESRD before 60 years of age [31] | |
| Hypertension | Hypertension has been linked to an increased risk of CKD progression [32] |
| Early onset of hypertension at <35 years old indicates earlier development of ESRD versus patients who are normotensive until >35 years old [31, 33] | |
| Gender | Male ADPKD patients have been linked with decreased renal function and earlier onset of ESRD versus female patients (52 versus 56 years old, respectively) [31, 33, 34] |
| Urological complications | Onset of urological complications (flank pain, gross hematuria, cyst hemorrhages, cyst infections) before the age of 35 is associated with rapid progression [31] |
| Age-adjusted eGFR loss | If no sufficient eGFR measurements are available to evaluate the slope, the current eGFR can be used to estimate whether a patient has undergone rapid progression in the past. For example, an old patient (e.g., >45 years) who has not lost kidney function will not be a rapid progressor whilst a young patient (e.g., 25 years, CKD 2) showing a reduced eGFR due to ADPKD is very likely a rapid progressor [24] |
Fig. 1.
Algorithm to assess indications for initiation of treatment in ADPKD. The diagram aims to define rapid progression, and thus allow the identification of patients eligible for treatment. It is based on the assumption that GFR for age, or historical changes in GFR, provides more information on disease progression than changes in TKV or risk prediction scores based on (ht)TKV or PKD gene mutation analysis in conjunction with clinical signs. Patients identified as showing ‘rapid progression’ or ‘likely rapid progression’ may be considered for treatment with tolvaptan. Patients with ‘possible rapid progression’ should be re-evaluated during follow-up visits. Besides assessing the indication for treatment, contraindications to and special warnings for tolvaptan use in ADPKD should be considered. Notes to the decision algorithm: (i) in our opinion, the indication ‘CKD stages 1–3 at initiation of treatment’ is not sufficiently specific as eGFR should be indexed for age. ADPKD patients with a high eGFR for age are unlikely to show rapid disease progression. There is currently no published evidence for the effect of tolvaptan in patients below the age of 18 or above the age of 50 years. (ii) The eGFR may vary over time in individual patients, especially when close to the normal range. To confidently define ‘rapid disease progression’, the rate of eGFR decline should be supported by multiple measurements that reliably indicate a rate of decline in eGFR. For this reason, this criterion should also be defined more strictly when historical data are available for only a short period compared with when available for a longer period. (iii) When ‘evidence of rapid disease progression’ is based on historical eGFR data, the decline in renal function should be due to ADPKD and not related to other diseases, medications or factors that may contribute (reversibly or irreversibly) to a decline in renal function (e.g., diabetes mellitus, NSAIDs, calcineurin inhibitors, dehydration or contrast agents). (iv) The criterion of decline in eGFR ≥5 mL/min/1.73 m2 in 1 year is adopted from the KDIGO CKD Guideline. (v) The criterion of decline in eGFR ≥2.5 mL/min/1.73 m2 per year over a period of 5 years is comparable to class 1C patients in the Mayo classification of ADPKD. (vi) In young ADPKD patients with CKD stage 1, the observation of ‘no change in eGFR’ in general is not considered a sensitive marker of slow disease progression, as eGFR often remains fairly stable during a prolonged period of time, whereas TKV increases steadily, suggesting disease progression. In such patients, changes in TKV and/or prediction models should be applied to assess historical or predicted disease progression. (vii) The criterion of increase in TKV ≥5% per year is likely to be conservative. It is based on the threshold defining the Mayo class 1D patients. This criterion has also been advocated by the Japanese regulatory authorities. The average rate of TKV growth in placebo-treated patients in the TEMPO 3:4 trial was 5.5% per year. (viii) The ellipsoid equation estimates TKV reliably when compared with classical volumetry. (ix) The Mayo classification of ADPKD is based on htTKV indexed for age. It predicts that patients with class 1C, 1D and 1E have more rapid disease progression. A kidney length ≥16.5 cm, as assessed by ultrasound (or MRI), can be used in patients <45 years to indicate a high likelihood of rapid disease progression. (x) The PROPKD score suggests that patients with a truncating PKD1 mutation and early onset of clinical signs (i.e., hypertension, macroscopic haematuria, cyst infection or flank pain before the age of 35 years) have rapid disease progression with start of renal replacement therapy at a relatively young age. (xi) Although there is significant variability in the age of reaching ESRD within families that share the same mutation, clinical experience as well as observational studies have shown that a detailed family history can provide important information for risk prediction. (Reproduced from Figure 3 of Gansevoort et al. [24].)
Fig. 2.
MRI-based volumetry of polycystic kidneys using stereology and planimetry. In the clinical studies, the determination of TKV was performed mostly using stereology or planimetry-based tracing (A and B). Whilst this approach yields the most accurate measurements, it is cumbersome and time-consuming and will consequently not be generally available in the everyday clinical setting. Irazabal et al. showed that estimation of TKV using the ellipsoid equation, by measuring kidney width and depth (D) in the axial and kidney length and (C) in both the sagittal and the coronal plane (with only the coronal plane being shown here), correlates well with classical volumetry and is thus sufficient to guide clinical decision making outside the setting of clinical trials [30]. Images kindly provided by Thorsten Persigehl, Department of Radiology, University of Cologne.)
Data from the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort has provided good evidence that a large, height-adjusted TKV (htTKV), as measured by MRI-based volumetry or USS-guided kidney length (>16.5 cm), is a strong indicator of a patient reaching CKD stage 3 within 8 years [28, 29, 37]. When using a single measure of TKV as an indicator of disease progression, it is important to take patient height (as achieved by using htTKV) and age into account due to continuous kidney growth in ADPKD. The Mayo classification that was established by Irazabal et al. incorporates these parameters into an efficient model predicting future eGFR loss [30]. It is important to note that the calculator is not valid for ‘atypical’ patients, which includes ADPKD kidneys where there is asymmetrical, unilateral or segmental cystic change [30]. Mayo classes 1C–E are associated with rapid progression and patients in these classes are predicted to lose eGFR at a speed of >2.4 mL/min/1.73 m2 per year. Importantly, this model demonstrated that complicated protocols of volumetry, that would not be available in most centres, are not required for clinical decision-making (in contrast to clinical trials), as measuring TKV using the ellipsoid equation showed a good correlation with stereology measurements [30].
Cornec-Le Gall et al. identified other clinical and genetic factors associated with rapid progression using data from the French Genkyst cohort, including male sex, early occurrence of urological complications or hypertension, and type of PKD mutation. These factors were used to establish the PROPKD score, which classifies patients according to their risk of progression to ESRD (with a score >6 indicating rapid progression) [31]. Even though the PROPKD score is a potentially useful tool, its use in clinical practice has been hampered by the limited availability of genetic data for the vast majority of ADPKD patients. In the event that the score cannot be calculated for this reason, the features integrated in the score (sex, urological complications and hypertension) should still be considered when assessing ADPKD patients [31]. However, a PROPKD score cannot be used in the absence of genetic data. Despite intra-familial variance in disease progression, a detailed family history documenting the age of ESRD in affected members (especially if <55 years) is informative in terms of risk prediction and would prompt imaging and genetic studies to allow an informed decision regarding treatment to be made [25, 38].
The important challenge is now to implement a strategy that allows for usage of the factors described in clinical practice to select patients as candidates for the initiation of tolvaptan treatment. The ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice (Figure 1) as well as the UK Renal Association have proposed algorithms that can be used to guide this decision and patient counselling [24, 25]. It is important, however, that each patient is considered in an individualized manner using all data on indicators of rapid progression, since no available algorithm will address all aspects of progression. Therapy has to be considered and discussed with all patients that fulfil the criteria of the EMA approval of tolvaptan, even if they do not meet all requirements of proposed decision algorithms. In general, comprehensive patient counselling on efficacy and side effects of the therapy as well as individual characteristics that indicate the expected degree of benefit are central in order to come to an informed decision. Furthermore, aspects other than rapid progression have to be taken into account when giving advice to patients, such as comorbidities and lifestyle (which may make taking a drug that increases urine volume to 4–6 L impossible), as well as the contraindications for tolvaptan treatment and potential drug interactions. The characteristics of patients enrolled in the TEMPO 3:4 study should also be considered, as all patients were ≤50 years of age with only very few patients being in CKD stage 3b (n = 39, 24 of whom were in the tolvaptan treatment group) [39].
Here, we present five case studies of patients with ADPKD who were considered for treatment with tolvaptan. We examine how the criteria of rapid progression were incorporated with other data on the patients’ history, to come to a decision, which takes into account available evidence and also realistic considerations regarding the initiation of tolvaptan. Patients reported here provided verbal and written informed consent.
Results
Clinical case histories from five individuals diagnosed with ADPKD are described. The cases involve patients with a broad age range of 19–65 years. In deciding whether to initiate tolvaptan therapy in these patients, a number of factors were taken into consideration, including: the patient’s clinical history, their family history, their current age and health, their prognosis, contraindications, potential adverse effects, as well as the patients’ own personal desires/motivations for taking the therapy.
Case 1
Case 1 was a 38-year-old male with a professional role in a large corporation. He was an ex-smoker of 14 years and had a low alcohol intake (<14 units/week). The patient’s mother and younger brother both had a clinical diagnosis of ADPKD. The mother had advanced to ESRD by the age of 61, while the brother (aged 40) had not yet reached ESRD. Due to the patient’s family history of ADPKD, the patient had undergone renal USS screening at age 14, whereupon polycystic kidneys consistent with a diagnosis of ADPKD were detected.
The patient presented with several comorbidities, including hypertension, which had been diagnosed at the age of 26 and was being controlled well using irbesartan. At the age of 15, the patient experienced an episode of severe flank pain associated with macrohaematuria. The patient’s extrarenal manifestations of ADPKD included sigmoid diverticulosis and a small intracranial aneurysm, which was managed conservatively. Echocardiography also revealed a concentric myocardial hypertrophy but without signs of valvular dysfunction.
The patient’s renal function showed an eGFR [CKD Epidemiology Collaboration (CKD-EPI)] of 41 mL/min/1.73 m2, with a documented decline from 58 mL/min/1.73 m2 2.5 years previous [eight creatinine values in between documenting the slope; (CKD-3b)]. Genetic testing had not been performed, hence a PROPKD score could not be calculated. A recent MRI had documented large kidneys with a htTKV of 1692 mL/m, putting the patient into Mayo class 1E.
The family history of ADPKD and the patient’s early onset of hypertension and urological complications, together with their documented CKD-3b disease and Mayo class 1E at age 38, put the patient at a high likelihood for rapid disease progression. Based on the fact that few patients in CKD stage 3b had been enrolled in TEMPO 3:4, the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice position statement suggests that only patients up to CKD stage 3a should be commenced on tolvaptan [24]. This fact and its implications were discussed with the patient. However, taking into account that he had just reached this threshold, as well as his young age, a decision was made to start treatment with tolvaptan, following counselling regarding possible impact on his work as a manager of a large company. The patient did not experience any major side effects and was able to plan his workday and meetings around the polyuria without any significant disruption.
Case 2
Case 2 was a 65-year-old male pensioner. Whilst the patient had low alcohol consumption (<14 units/week) and a moderate caffeine intake (1 cup/day), the patient was a heavy cigarette smoker (46 pack years smoked). The patient had been diagnosed with ADPKD during a routine clinical examination at the age of 55. The patient’s father died early without a diagnosis of ADPKD and their mother did not suffer from ADPKD. The patient’s sister, however, had been diagnosed with ADPKD and underwent unilateral nephrectomy at the age of 55. At the time of the patient’s presentation, their sister had not reached ESRD at the age of 64 years.
The patient’s comorbidities included hypertension, which was diagnosed in later life and treated with candesartan and bisoprolol, but with poor control. The patient had not experienced any urological complications. The patient had suffered various cardiovascular disorders, including hypertrophic obstructive cardiomyopathy [which had been treated with an automated implantable cardioverter defibrillator (AICD), subvalvular myectomy and mitral valve reconstruction], a recent transient ischaemic attack and atrial fibrillation. Extrarenal manifestations of ADPKD included sigmoid diverticulosis.
The patient’s renal function at presentation showed an eGFR (CKD-EPI) of 32 mL/min/1.73 m2, with a documented decline from 49 mL/min/1.73 m2 3.5 years prior (seven creatinine values in between documenting the slope; CKD-3b). Genetic testing had not been performed, so a PROPKD score could not be calculated. Also, due to the AICD, it had not been possible to perform an MRI; however, sonographic measurements revealed an htTKV (ellipsoid formula) that placed the patient into Mayo class 1D.
In this patient, tolvaptan was not recommended. Generally, as the patient showed a significant loss of renal function and fulfilled the criteria of Mayo class 1D, prescription of tolvaptan would have been possible. However, there were no data regarding the patient’s age group and little data regarding late CKD-3b. In addition, the patient’s late onset of hypertension, the poor control of hypertension, the patient’s other comorbidities and their sister’s clinical history (not having reached ESRD despite unilateral nephrectomy) indicated that these could have been alternative explanations for the patient’s loss of kidney function. All these aspects were discussed with the patient and they followed the recommendation not to commence tolvaptan therapy.
Case 3
Case 3 was a 19-year-old, non-smoking, female high-school student with low alcohol/caffeine intake. The patient had been diagnosed with ADPKD in childhood due to bilaterally enlarged cystic kidneys. The patient’s mother and grandmother both had a history of ADPKD. At the time of presentation, the patient’s mother was in CKD 4 at the age of 45. The patient’s grandmother died from ESRD at the age of 42 years.
At presentation, the patient had not experienced any comorbidities; the only known extrarenal manifestation was a single liver cyst. An echocardiogram revealed no abnormalities. The patient’s renal function showed an eGFR (CKD-EPI) of 140 mL/min/1.73 m2 and MRI volumetry showed large kidneys with an htTKV of 742 mL/m, putting the patient into Mayo class 1E. Genetic testing was not performed, so a PROPKD score could not be calculated.
Despite the lack of comorbidities and not being able to quantify the patient’s eGFR loss, tolvaptan treatment was recommended in this patient, primarily due to Mayo classification (1E). Furthermore, despite the lack of genetics the family history pointed towards a severe disease-causing mutation (such as a truncating PKD1 mutation). Treatment was commenced at a low dose after informing the patient that a safe method of contraception is required while on therapy. However, after several weeks at low dose, the patient chose to discontinue treatment due to difficulty incorporating polyuria into her everyday life. This points to the fact that certain patient groups, including very young patients that have not experienced any symptoms of the disease, may have problems with adherence and require a great deal of counselling and motivation. It was decided that the patient would be reviewed on a yearly basis to re-discuss treatment and commence tolvaptan once she felt she would be able to incorporate this treatment in everyday life. Furthermore, it was advised that a sufficient fluid intake (>3 L per day) should be aimed for, even if not taking tolvaptan, as well as low salt intake and an early initiation of antihypertensive medication once hypertension is detected.
Case 4
Case 4 was a 29-year-old, fit and active male who worked as a plumber. The patient had a known diagnosis of ADPKD, detected following family screening (using renal USS) at the age of 22 years.
The patient’s mother had ADPKD, reaching ESRD at the age of 40. The patient’s maternal grandmother was also affected, reaching ESRD at 50 years old. The patient’s maternal aunt also had ADPKD and was in CKD 4 at the age of 50. The patient’s comorbidities included hypertension, which was diagnosed at the age of 22 following a nephrology outpatient clinic review and treated with bisoprolol and perindopril with good control. The patient’s renal function showed an eGFR [Modification of Diet in Renal Disease (MDRD)] of 58 mL/min/1.73 m2, with a documented decline from 64 mL/min/1.73 m2 14 months prior. The patient had no major urological events. The investigation of the mother’s younger siblings for potential live kidney donor transplant allowed genetic testing to be performed, consistent with the current UK Genetic Testing Network guidelines [40]. This identified a truncating mutation in PKD1 as the pathogenic change leading to ADPKD in the family.
Recent imaging using renal USS had documented large kidneys (right renal length 22 cm; left renal length 23 cm) but MRI imaging had not been performed. The patient’s PROPKD score was 7 (out of a possible score of 9), suggesting that the patient was at high risk of progression to ESRD—a PROPKD score of >6 predicts ESRD by the age of 60 [31]. Using Mayo criteria, the patient’s estimated renal volume of >1000 mL/m would have given a classification of 1E.
Treatment with tolvaptan was indicated in this patient due to a risk of rapidly progressive disease, based on their family history, age and risk factors. The effect of increased diuresis was discussed in terms of its possible impact on his work as a commercial plumber. Treatment was commenced and titrated to full dose, with no major side effects. The patient was subsequently able to carry on with his work without disruption. This case demonstrates the utility of the PROPKD score in personalizing the care of ADPKD patients, based on their risk for progression to ESRD.
Case 5
Case 5 was a 49-year-old, fit and active male who worked as a chef. The patient had a known diagnosis of ADPKD, detected by renal USS following documented hypertension at the age of 25 years and an episode of loin pain and haematuria.
There was no clear documented family history of ADPKD, although his paternal grandfather had died from a subarachnoid haemorrhage aged 70 years. His hypertension was treated with lisinopril with good control. The patient’s renal function showed an eGFR (MDRD) of 54 mL/min/1.73 m2, with a documented decline from 68 mL/min/1.73 m2 over the previous 5 years. The patient had no new major urological events or other noted cause for a rapid loss of GFR. Genetic testing had not been performed. Updated renal imaging using MRI documented a TKV of 830 mL, a calculated htKTKV of 461 mL/m and Mayo classification 1B. Treatment with tolvaptan was discussed and initiated. It was noteworthy that the patient worked in a hot environment and was concerned over keeping up with fluid intake. The tolvaptan treatment was tolerated for just 3 months before complaints about polyuria and polydipsia necessitated a break from treatment. This case demonstrates that despite low prediction scores (via TKV) a rapid decline of GFR was sufficient evidence for treatment with tolvaptan to be initiated. It also demonstrated that work environments should be discussed in detail prior to treatment and play an important role in the ability to maintain compliance with the medication.
Discussion
The use of tolvaptan has brought about a new era for patients with ADPKD. As our selected cases have shown, there is need for an up-to-date review of family history, medical history, renal imaging and genetic findings in order to provide an assessment of disease progression risk. The clinic setting to discuss tolvaptan now becomes an important opportunity to provide a holistic approach to all aspects of disease related to ADPKD. It is our belief that such discussions are best suited to being led by a multi-disciplinary team (e.g., including a nurse specialist, renal pharmacist and a renal physician, complemented by other specialties such as a radiologist and a pain specialist in specific cases) where progression of renal disease, hypertension, renal pain and lifestyle factors can all be reassessed. Historically, ADPKD patients were treated simply with supportive measures. The advent of a new therapy allows us to approach these patients in a new and more thoughtful way. Real life cases, as presented here to provide treatment decisions for tolvaptan, often lack data such as precise genetic diagnosis and changes in renal volumes. Consequently, we suggest a more pragmatic approach that considers the individual factors as well as the patients’ lifestyle and comorbidities. Monitoring of liver function is a requirement during tolvaptan therapy but generally speaking, the drug appears to be safe and has few serious adverse effects [39].
Whilst there is a deservedly new emphasis on commencing tolvaptan therapy in patients with ADPKD, other supportive measures should not be neglected. It remains very important to consider blood pressure control, low salt intake, sufficient fluid intake, and avoidance of smoking, caffeine and non-steroidal anti-inflammatory drugs. Indeed, results from the HALT-PKD trial emphasize the importance of blood pressure control in all ADPKD patients [35], giving this factor particular attention among healthcare professionals. Supportive measures should be discussed with all patients independent from the question as to whether they take tolvaptan. As to the drug itself careful patient selection is essential to avoid treating patients that will not benefit from this therapy, but also to minimize the economic burden. We are convinced that with continued use of tolvaptan in patients with ADPKD, determination of its use will be possible in a considered rational and pragmatic approach. Appropriate guidance based upon published evidence will help nephrologists gain experience with this important novel therapeutic option in ADPKD.
Acknowledgements
The authors would like to thank Claudia Witte, Polina Todorova and Franziska Grundmann for assistance with the preparation of patient cases and Mehrdad Bahadori for help with preparing the MRI images. The authors would like to thank James Wallis and Nina C. Kennard from iS LifeScience, who under the direction of the authors provided editorial assistance throughout the manuscript development process. Patients reported here provided verbal and written informed consent. All authors were involved in critically reviewing the publication for important intellectual content and for approving the final draft prior to submission.
Funding
J.A.S. is funded by Medical Research Council (grant number MR/M012212/1), Newcastle Upon Tyne Hospitals NHS Charity, Northern Counties Kidney Research Fund and Kidney Research UK; R.-U.M. is funded by the Nachwuchsgruppen, NRW program of the Ministry of Science Northrine-Westfalia (MIWF), the German Kidney Foundation (Deutsche Nierenstiftung) and the Deutsche Forschungsgemeinschaft (MU 3629/2-1). Editorial support for the development of this manuscript was funded by Otsuka Pharmaceutical Europe Limited.
Conflicts of interest statement
R.-U.M. has received personal fees for participation in advisory boards and as an expert speaker from Otsuka, and has received research funding from Otsuka; C.S.H. has received personal fees and non-financial support from Otsuka; J.A.S. has received lecture fees and consultancy fees from Otsuka.
References
- 1. Chebib FT, Torres VE.. Autosomal dominant polycystic kidney disease: core curriculum 2016. Am J Kidney Dis 2016; 67: 792–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grantham JJ. Autosomal dominant polycystic kidney disease. N Engl J Med 2008; 359: 1477–1485 [DOI] [PubMed] [Google Scholar]
- 3. Grantham JJ, Chapman AB, Torres VE.. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol 2006; 1: 148–157 [DOI] [PubMed] [Google Scholar]
- 4. Grantham JJ, Torres VE.. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol 2016; 12: 667–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spithoven EM, Kramer A, Meijer E. et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival–an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant 2014; 29 (Suppl 4): S15–S25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spithoven EM, Kramer A, Meijer E. et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int 2014; 86: 1244–1252 [DOI] [PubMed] [Google Scholar]
- 7. Dalgaard OZ. Bilateral polycystic disease of the kidneys; a follow-up of two hundred and eighty-four patients and their families. Acta Med Scand 1957; 328: S1–S255 [PubMed] [Google Scholar]
- 8. Willey CJ, Blais JD, Hall AK. et al. Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant 2017; 32: 1356–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perrone RD, Neville J, Chapman AB. et al. Therapeutic area data standards for autosomal dominant polycystic kidney disease: a report from the polycystic kidney disease outcomes consortium (PKDOC). Am J Kidney Dis 2015; 66: 583–590 [DOI] [PubMed] [Google Scholar]
- 10. Igarashi P. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 2002; 13: 2384–2398 [DOI] [PubMed] [Google Scholar]
- 11. Pei Y, Hwang Y-H, Conklin J. et al. Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2015; 26: 746–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pei Y, Obaji J, Dupuis A. et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 2009; 20: 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossetti S, Strmecki L, Gamble V. et al. Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am J Hum Genet 2001; 68: 46–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PC, Bae KT, Rossetti S. et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2006; 17: 3013–3019 [DOI] [PubMed] [Google Scholar]
- 15. Hateboer N, v Dijk MA, Bogdanova N. et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet 2016; 353: 103–107 [DOI] [PubMed] [Google Scholar]
- 16. Patel V, Chowdhury R, Igarashi P.. NIH Public Access. Curr Opin Nephrol Hypertens 2009; 18: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Srivastava A, Patel N.. Autosomal dominant polycystic kidney disease. Am Fam Physician 2014; 90: 303–307 [PubMed] [Google Scholar]
- 18. Torres VE, Wang X, Qian Q. et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 2004; 10: 363–364 [DOI] [PubMed] [Google Scholar]
- 19. Otsuka. JINARC Summary of Product Characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002788/WC500187921.pdf
- 20. Torres VE, Chapman AB, Devuyst O. et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012; 367: 2407–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. European Medicines Agency. Public Assessment Report Jinarc. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002788/WC500187923.pdf (8 March 2017, date last accessed)
- 22. NICE. Tolvaptan for treating autosomal dominant polycystic kidney disease. 2015. www.nice.org.uk/guidance/ta358 (8 March 2017, date last accessed)
- 23. Kidney Disease: Improving Global Outcomes (KDIGO). CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 24. Gansevoort RT, Arici M, Benzing T. et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA working groups on inherited kidney disorders and European renal best practice. Nephrol Dial Transplant 2016; 31: 337–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Renal Association Working Group on Tolvaptan in ADPKD. Tolvaptan for ADPKD: Interpreting the NICE decision. http://www.renal.org/docs/default-source/default-document-library/tolvaptan-in-adpkd-nice-commentary.pdf?sfvrsn=0 (6 March 2017, date last accessed)
- 26. Cornec le Gall E, Audrézet MP, Chen JM. et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013; 24: 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grantham JJ, Torres VE, Chapman AB. et al. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354: 2122–2130 [DOI] [PubMed] [Google Scholar]
- 28. Bhutani H, Smith V, Rahbari-Oskoui F. et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int 2015; 88: 146–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chapman AB, Bost JE, Torres VE. et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2012; 7: 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Irazabal MV, Rangel LJ, Bergstralh EJ. et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol 2014; 26: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cornec-Le Gall E, Audrézet MP, Rousseau A. et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2016; 27: 942–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ozkok A, Akpinar TS, Tufan F. et al. Clinical characteristics and predictors of progression of chronic kidney disease in autosomal dominant polycystic kidney disease: a single center experience. Clin Exp Nephrol 2013; 17: 345–351 [DOI] [PubMed] [Google Scholar]
- 33. Johnson AM, Gabow PA. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol 1997; 8: 1560–1567 [DOI] [PubMed] [Google Scholar]
- 34. Gabow PA, Johnson AM, Kaehny WD. et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 1992; 41: 1311–1319 [DOI] [PubMed] [Google Scholar]
- 35. Schrier RW, Abebe KZ, Perrone RD. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 2014; 371: 2255–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen D, Ma Y, Wang X. et al. Clinical characteristics and disease predictors of a large Chinese cohort of patients with autosomal dominant polycystic kidney disease. PLoS One 2014; 9: e92232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schrier RW, Brosnahan G, Cadnapaphornchai MA. et al. Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol 2014; 25: 2399–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barua M, Cil O, Paterson AD. et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 2009; 20: 1833–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torres VE, Higashihara E, Devuyst O. et al. TEMPO 3:4 Trial Investigators. Effect of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease by CKD Stage: Results from the TEMPO 3:4 Trial. Clin J Am Soc Nephrol 2016; 11: 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. UK Genetic Testing Network. UKGTN Guide to specialised services for rare genetic disorders. https://ukgtn.nhs.uk/our-work/ukgtn-reportsguidelines/ (8 March 2017, date last accessed)