Abstract
Objective
To estimate the prevalence of diabetic retinopathy among patients with type 2 diabetes mellitus (DM) who attended the National Center of Diabetes, Endocrinology and Genetics (NCDEG) in Jordan, and to determine the relationship between duration of DM, hyperglycemia, smoking, hypertension, age, gender, body mass index (BMI) and diabetic retinopathy.
Methods
This is a cross-sectional study that investigates a sample of 1000 diabetic patients suffering from type 2 DM who attended the NCDEG between September 2006 and January 2007. Eye examination by an ophthalmologist under adequate dilatation was performed in all patients. Socio-demographic, clinical and laboratory data were obtained. Diabetic Retinopathy was defined according to the International Clinical Diabetic Retinopathy Severity Scale adopted by American Academy of Ophthalmology (AAO) and the International Council of Ophthalmology (ICO). Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS, version 11.5).
Results
Out of 1000 patients; 51 percent were male, 49 percent were female. The mean age and duration of diabetes were 57.8 and 9.6 years, respectively. The prevalence of diabetic retinopathy in patients was 34.1 percent. Non-proliferative diabetic retinopathy was documented in 24.5 percent, while 9.6 percent had proliferative diabetic retinopathy. Duration of DM and hyperglycemia, as measured by HbA1C, were statistically significantly associated with diabetic retinopathy.
Conclusion
Diabetic retinopathy is highly prevalent among Jordanian patients with type 2 DM. Serious national efforts should be directed towards increasing primary prevention through regular ophthalmic examinations and strict glycemic control in patients with type 2 DM.
Introduction
Diabetes Mellitus (DM) is considered to be a major health problem that is predicted to turn into a global epidemic. The adult prevalence of DM in developed countries is about 5 percent.1 The actual number may be twice as high considering all the cases which have not yet been diagnosed.2 The prevalence of diabetes in developing countries, such as the Arab countries, varies from 3 to 35 percent.3 In a recent study in Jordan, the age-standardized prevalence of DM and impaired fasting glucose tolerance were 17.1 percent and 7.8 percent respectively, confirming that the prevalence of DM in Jordan is high and increasing.4
Diabetic patients are prone to develop diabetic retinopathy (DR). During the first two decades of the disease, nearly all patients with type 1 diabetes and 60 percent of those with type 2 diabetes develop retinopathy.5 Blindness due to diabetes is becoming an ever greater problem in developing countries.6 In a hospital based study from Jordan, DR was the leading cause of blindness.7 Another study indicated that the prevalence of DR among type 2 diabetics was 50 percent,8 while a more recent study in 2005 reported that the overall prevalence of DR among Jordanian diabetics was 64.1 percent.9
The present study was conducted at the National Center for Diabetes Endocrinology and Genetics (NCDEG) to estimate the prevalence of DR among patients with type 2 DM and to describe the stages of DR among the study group. The second aim of the study was to determine if there is any correlation between DR and a number of independent variables including duration of DM, hyperglycemia as measured by HbA1C level, smoking, hypertension, age, gender and body mass index.
Materials and Methods
A cross-sectional study format was adopted. It included 1000 patients with type 2 DM who attended the diabetic clinic at NCDEG located in Amman, Jordan between September 2006 and January 2007. The study sample was selected randomly. Pregnant women were excluded. The sample size was sufficient to provide 95 percent assurance that the margin of error in estimating the prevalence of DR would not exceed 3 percent, assuming the most conservative estimate of prevalence is 50 percent.
All patients underwent detailed eye examination by an ophthalmologist utilizing slit-lamp biomicroscopy including fundus examination under adequate pupillary dilatation with tropicamide 1 percent. Phenylephrine 10 percent was used in patients who did not dilate with tropicamide. For the sake of staging DR, we considered the eye with the most advanced stage. Data obtained included: duration of DM, HbA1C level, smoking, hypertension, age, gender, body mass index, and ophthalmic examination findings.
Ethical considerations
The study was approved by the NCDEG ethics committee. Informed consent was obtained from all patients included in the study.
Definition of variables
Diabetic retinopathy (DR)
The international clinical diabetic retinopathy severity scale adopted by the American Academy of Ophthalmology (AAO)10 and the International Council of Ophthalmology (ICO)11 were used to classify patients into non-proliferative diabetic retinopathy (NPDR) versus proliferative diabetic retinopathy (PDR).
NPDR was subdivided into:
Mild NPDR: micro-aneurysms only;
Moderate NPDR: more than just microaneurysms but less than severe;
Severe NPDR: consisting of any of the following intra-retinal hemorrhage in each of the four quadrants, venous beading in two or more quadrants, or intraretinal microvascular abnormalities (IRMAs) in one or more quadrants AND no signs of proliferative retinopathy.
PDR was defined as neovascularization at the optic disc (NVD) or elsewhere (NVE).
Diabetic macular edema (DME) was present when any retinal thickening or hard exudates were present in the macula.
Duration of DM
Duration of DM was defined as the time period between the age at diagnosis and the time of examination. They were categorized into the following groups: Group 1 <5 years, Group 2 5–10 years, Group 3 11–15 years, and Group 4 >15 years.
Smoking
The WHO guidelines were used as follows:
Current smoker: a person who smokes cigarettes daily or occasionally;
Past-smoker: a person who smoked in the past daily or at occasionally but quit completely;
Non Smoker: a person who had never smoked or who had smoked very little in the past.12
HbA1c
Levels of HbA1C were assayed using high performance liquid chromatography (HPLC) method (Bio-Rad). Glycemic control as measured by HbA1c was divided into two groups according to the ADA (2007):
Group 1: controlled if HbA1c <7% and
Group 2: uncontrolled if HbA1c ≥7%.13
Hypertension
Blood pressure was measured using standardized sphygmomanometer EN 1060 (RIESTER) with a cuff circumference of 24–32 cm to cover 80 percent of the upper arm. For obese patients, a larger cuff circumference was used (42–50 cm). A trained nurse measured the blood pressure while the subject was in a sitting position with the arm at the level of the heart and after 5 minutes rest.
Subjects were categorized into two groups:
Group 1: hypertensive, if systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg or if the patient is currently using prescribed antihypertensive drugs;13
Group 2: normotensive, if systolic blood pressure <130 mm Hg or diastolic blood pressure <85 mm Hg.
Age/years
The participants’ age was categorized into groups for reasons of comparison. The categorization was as follows: Group 1 <45 years, Group 2 45–55 years, Group 3 56–65 years, and Group 4 >65 years.
Body mass index (BMI)
Subjects were categorized as normal if BMI was less than 25 kg/m2, overweight if BMI was between 25–29.9 kg/m2, and obese if BMI was greater than or equal to 30 kg/m2.14
Data management and statistical analysis
Statistical analysis was carried out using Statistical Package for Social Sciences (SPSS, version 11.5). Frequencies were utilized for categorical variables; means (± standard deviations) were obtained for continuous variables. Chi-square statistics were used to assess associations. Logistic regression analyses were performed to estimate the odds ratio (OR) and 95 percent confidence interval (CI) of selected variables on diabetic retinopathy and its related subdivisions. Separate regression models were used for non-proliferative DR, proliferative DR, and maculopathy. A p-value of less than 0.005 was considered statistically significant.
Results
Demographic and clinical characteristics of the study population
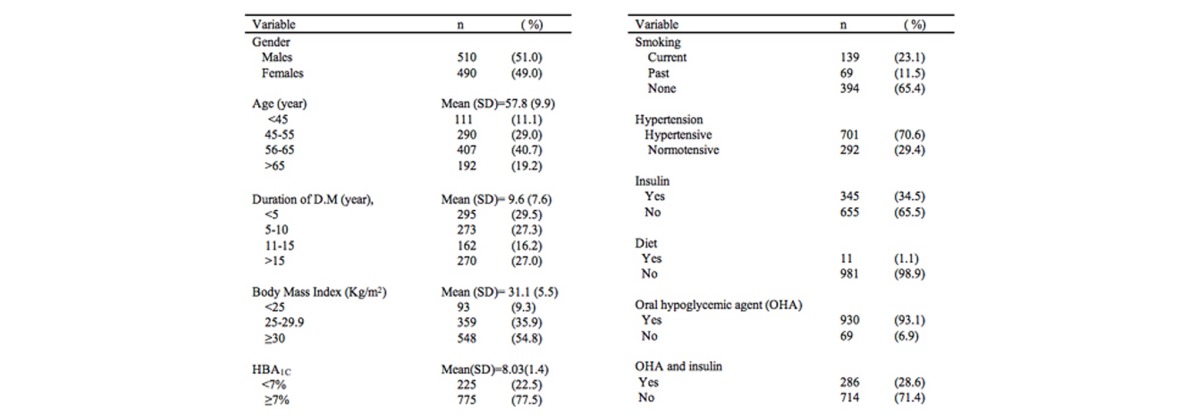
This study included 1000 patients (510 males, 490 females) with type 2 DM. The mean age was 57.8 years (SD ± 9.9), ranging from 27 to 97. Over 40 percent of patients were within the age group of 56 to 65 years of age. The mean duration of DM was 9.6 years (SD ± 7.6). Only 22.5 percent had HbA1C <7.0 percent. About 23 percent were current smokers and 70.6 percent were hypertensive. More than half of the subjects (56.8 percent) had DM for duration of less than 10 years. Fifty-four and eight tenths percent were obese, and 35.9 percent were overweight.
Demographic and clinical characteristics of all participants are depicted in Table 1. In this study, 1.1 percent of patients were treated with diet alone, 34.5 percent used insulin, 93.1 percent were on oral hypoglycemic drugs, and 28.6 percent were on a combination therapy of oral hypoglycemic drugs and insulin.
Table 1.
Demographic and important characteristics of 1000 patients with Diabetes Mellitus attending the National Center for Diabetes Endocrinology and Genetics (NCDEG).
|
Prevalence of retinopathy
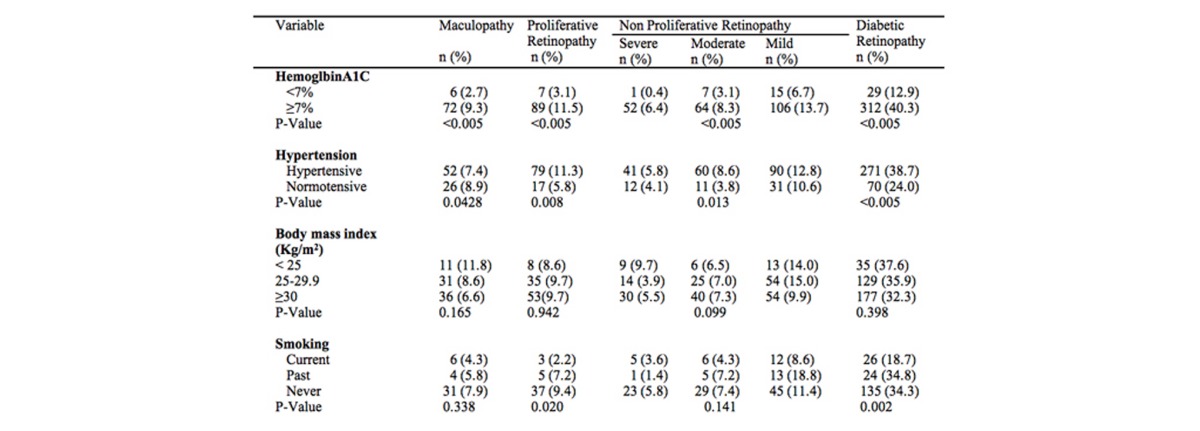
As shown in Table 2a and 2b, 34.1 percent (341) had some form of DR. Of the total number of patients, 24.5 percent (245) had NPDR, and the type of NPDR was distributed as follows: mild occurred in 12.1 percent (121), moderate in 7.1 percent (71), and severe in 5.3 percent.53 Seven and eight-tenths percent (78) had maculopathy. There were no significant differences by gender in prevalence of any diabetic retinopathy and maculopathy.
Table 2A.
Prevalence of diabetic retinopathy among Jordanian diabetics by demographic, clinical, other important characteristics.

|
Table 2B.
Prevalence of diabetic retinopathy among Jordanian diabetics by demographic, clinical, other important characteristics.
|
As shown in Figure 1, the prevalence of DR increased with age. Almost half of patients with DM who were >65 years of age had diabetic retinopathy. Figure 2 shows the prevalence of all forms of NPDR according to age and gender. Patients younger than 45 years old did not have the severe category of non-proliferative diabetic retinopathy. Figure 3 shows the prevalence of proliferative diabetic retinopathy according to age and gender. Proliferative diabetic retinopathy was not noticed before the age of 45 years. For both men and women, PDR increased with age. The prevalence of maculopathy was higher in men than women for all age groups as shown in Figure 4.
Figure 1.
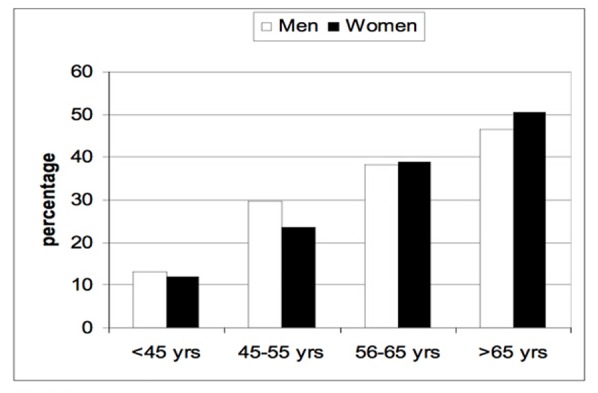
The prevalence of diabetic retinopathy according to age and gender. (yrs - years)
Figure 2.
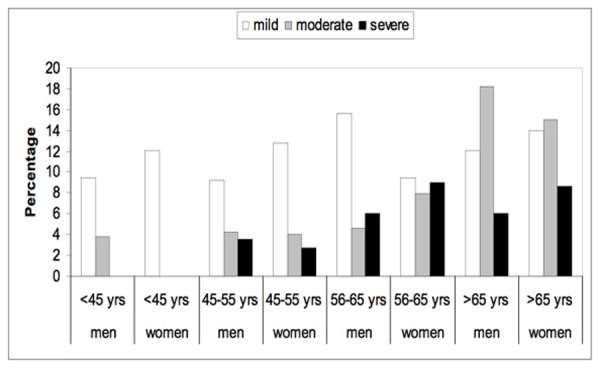
The prevalence of all kinds of non-proliferative diabetic retinopathy according to age and gender.
Figure 3.
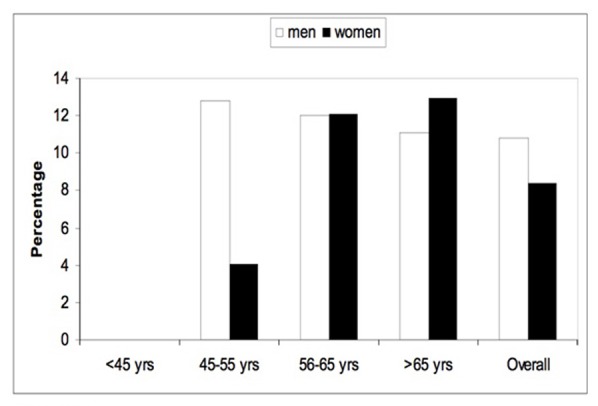
The prevalence of proliferative diabetic retinopathy according to age and gender.
Figure 4.
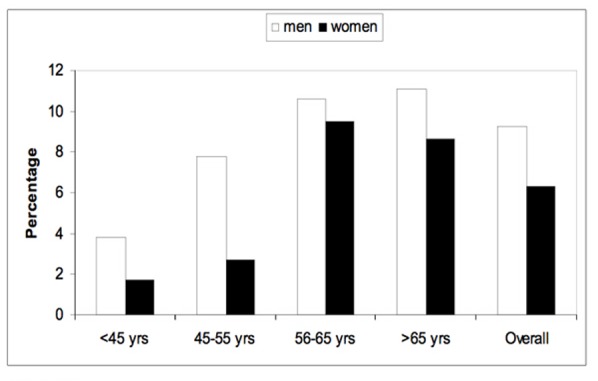
The prevalence of maculopathy according to age and gender.
Factors associated with diabetic retinopathy
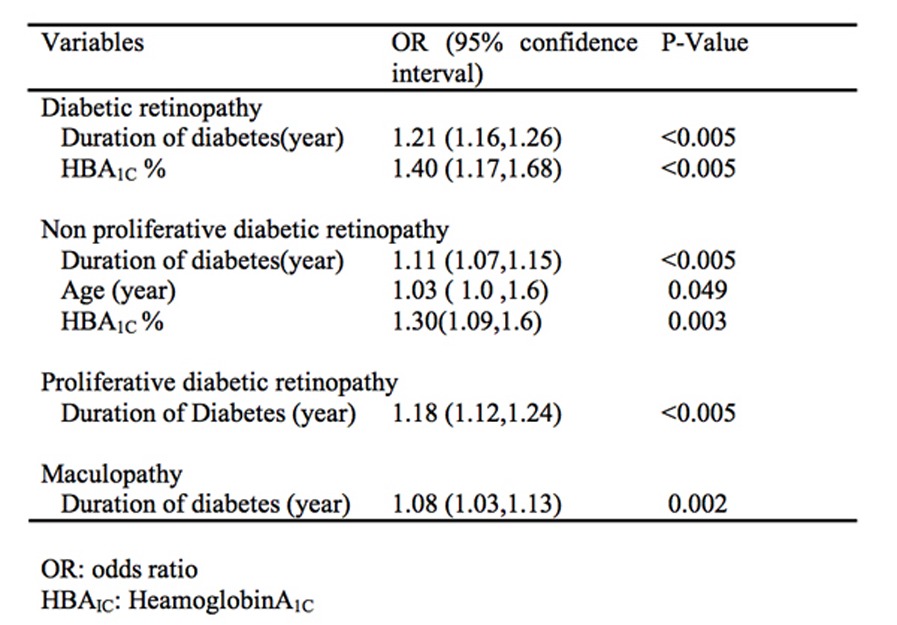
Logistic regression analysis of factors associated with DR, NPDR, PDR and maculopathy after adjustment for other confounders are shown in Table 3. Diabetic retinopathy was significantly associated with duration of DM (OR=1.21 (95% CI: 1.16, 1.26)) and HbA1C (OR=1.40 (95% CI: 1.17, 1.68)). The odds of having DR increased by 21% for an increase of one year in the duration of DM and by 40 percent for each 1 percent increase in HbA1C. Non–proliferative diabetic retinopathy was significantly associated with duration of DM (OR=1.11 (95% CI:1.07, 1.15)), age (OR=1.03 (95% CI:1.0, 1.6)) and HbA1C (OR=1.40 (95% CI:1.09, 1.6)). The odds of having NPDR increased by 11 percent for an increase of one year in the duration of DM, 3 percent for each one year increase in age and 30 percent for each one unit increase in HbA1C. Proliferative diabetic retinopathy was significantly associated with duration of DM (OR=1.18 ((95% CI: 1.12, 1.24)). The odds of having PDR increased by 18 percent for an increase of one year in the duration of DM. Maculopathy was significantly associated with the duration of diabetes mellitus (OR=1.08 (95% CI: 1.03, 1.13)). The odds of having maculopathy increased by 8 percent for every increase of one year in the duration of DM.
Table 3.
Logistic regression analysis of factors associated with diabetic retinopathy, non proliferative diabetic retinopathy, proliferative retinopathy and maculopathy after adjusting for other factors.
|
Discussion
The prevalence of DR in patients from the NCDEG using (AAO)10 and (ICO)11 definitions was 34.1 percent. NPDR, PDR, and maculopathy had a prevalence of 24.5 percent, 9.6 percent, and 7.8 percent, respectively, of the total number of patients studied. Comparison among studies is difficult because the prevalence of retinopathy has varied widely depending on the methodology and populations. The prevalence of DR in this study is comparable to the WESDR.15 In contrast, Sparrow et al. (1993)16 found a higher overall rate of retinopathy (52 percent versus 34 percent in our study) but a similar prevalence of maculopathy (10 percent versus 7.8 percent in our study). Other studies also have shown similar prevalence of DR.17,18,19,20 On the other hand, several studies show a lower prevalence of DR21,22 including two earlier studies from Jordan.8,9 In 1999, Salem et al. found that the prevalence of diabetic retinopathy, non-proliferative diabetic retinopathy, proliferative diabetic retinopathy, and maculopathy among type 2 diabetics was 50, 38.8, 11.2 and 17 percent, respectively.8 The second study by Al Till et al. in 2005 reported the following numbers: 64.1, 54.8, 9.3 and 30.8 percent, respectively.9 Our study shows lower prevalence of DR, NPDR, PDR and maculopathy of 34.1, 24.5, 9.6 and 7.8 percent, respectively. After comparison with the 2005 study, the differences can be explained by the following possibilities:
A difference in the mean duration of DM (12 years in the 2005 study versus 9.5 years in our study);
The exclusion of patients with DM diagnosed less than three years prior in the 2005 study;
The inclusion of type 1 diabetics who are known to have a high incidence of DR in the 2005 study.
The quality of referral may also impact the above numbers as more patients from lower socioeconomic areas were included in the 2005 study.
The duration of the disease in the present study was strongly associated with the frequency of retinopathy, as well as the occurrence of mild, moderate, and severe non-proliferative diabetic retinopathy and of proliferative diabetic retinopathy. The prevalence is related primarily to the duration of the disease. Aiello et al.5 reported that after 20 year of diabetes, more than 60 percent of type 2 DM will have retinopathy regardless of diabetic control. This has been confirmed by numerous other studies.8,9,15,16,18–24
Hyperglycemia (as measured by HbA1C) is considered an important risk factor associated with DR, and it was significantly associated with retinopathy in our study. This is consistent with other studies.7,9,15–25 Many studies have clearly demonstrated the benefits of improving glycemic control by reducing HbA1C concentration, in decreasing the complication rate. The UKPDS reported that patients with intensive glucose control had a reduction in retinopathy, a 25 percent drop in the overall microvascular complications, and for every 1 percent decrease in HbA1C, a 35 percent reduction in risk for microvascular complications was observed. The authors concluded that slowing the progression of retinopathy with intensive control resulted in preservation of sight, decreased morbidity and fewer interventions.25
Previous studies differ in relation to the importance of smoking as risk factors. Multivariate analysis of smoking has not identified it as a risk factor in our sample. This is similar to WESRD results.26 On the other hand, some studies indicated that smoking has been causally related to DR.27,28
Most studies concerning diabetic changes within the eye show that high blood pressure is significantly associated with diabetic retinopathy and consider hypertension as an established risk factor for macular edema.17,18,20,34,35 Similarly, major epidemiological studies like WESDR II15 and UKPDS25 report that high systolic blood pressure is significantly associated with retinopathy. In this study, hypertension showed a significant association with DR (chi-square test, p-value < 0.005, see Table 2). However, the regression model failed to sustain this significant association after multivariate adjustment (see Table 3) bearing in mind that we depended on the new criteria which considers systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg.13 Thus, our results are more in line with the HDS study, which reported that the frequency of retinopathy was not significant between hypertensive and normotensive.36
The age of the patient was also related to the occurrence of non-proliferative diabetic retinopathy. Concerning proliferative diabetic retinopathy and maculopathy, the significance was not maintained after adjusting for the duration of DM. However, some studies reported a significant association between DR and age.8,15 Other studies reported the opposite.20,28
Gender was not identified as a risk factor in our study, which agrees with a study conducted by Janghorbani et al.30 On the other hand, WESDR concluded that the severity of DR was related to male sex.15
In the present study, BMI showed no significant association with DR and macular edema. On the other hand, in a population-based cross sectional study by Van Leiden et al. (2005),31 the prevalence of DR was positively associated with BMI. However, other studies reported that the severity of DR was inversely correlated with BMI, with patients with DR having lower BMIs (32–35 kg/m2).
Limitations
A cross sectional design precludes definitive causal associations; thus, diabetic retinopathy must be interpreted with caution. Mortality and survival bias is likely, and a specialized center such as the NCDEG may over estimate the true population prevalence.
Strengths
Nevertheless, the large database available at NCDEG provides complete data to study this important problem at minimum time and cost. Using these data, we answered all questions posed in the objectives.
Conclusion
This is a center-based study exploring the prevalence and risk factors of diabetic retinopathy among patients attending the NCDEG, a tertiary diabetic center with referrals from all over Jordan. Though this is not a national-based study, it highlights the size of the problem. In light of high prevalence of DR, serious national efforts to reduce DR and its associated vision threatening complications are needed. These efforts are best directed toward increasing the awareness of the necessity for regular eye examinations in patients with DM, early detection of DR and strict glycemic control.
Acknowledgments
I would like to express my thanks to Professor Kamel Al-Ajlouni for his guidance, support and above all him being a guardian to this study. He was a mentor indeed. Special thanks go to Dr. Al-Bdour and Dr.Yousef Al-Ka’ood for supervising this study; it would not have been the same without their patience and their great belief in me. And I owe the team of Dr. Moawea Al-Bdour, especially Dr. Nakhleh Elias and Dr. Samer Malas, for their cooperation in the ocular examinations for the patients and the great work they have done which was one of the main reasons I performed this study.
Sincere thanks go to all staff in the National Center of Diabetes, Endocrinology and Genetics.
I am grateful to all individuals who participated in this study. I truly wish that the priceless gift of sight they have won’t be affected by diabetes. Thank you, indeed because you have been the basis for this study.
References
- 1.Shaw JE, Zimmet PZ, McCarty D, de Courten M. Type 2 diabetes world wide according to the new classification and criteria. Diabetes care. 2000;23(Suppl 2):B5–10. [PubMed] [Google Scholar]
- 2.Taylor HR, Keeffe JE. World blindness: a 21st century perspective. Br J Ophthalmol. 2001;85:261–6. doi: 10.1136/bjo.85.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linda A, Morton B, Adnan H, Sandra N, Nowak P, Qian Z, Anisa G. Epidemiology of Diabetes among Arab Americans. Diabetes Care. 2003;26:308–13. doi: 10.2337/diacare.26.2.308. [DOI] [PubMed] [Google Scholar]
- 4.Ajlouni K, Khader Y, Batieha A, Ajluni H, EL-Khateeb M. An increase prevalence of diabetes mellitus in Jordan during ten years. Accepted for publication at the Journal of diabetes and its complications. [DOI] [PubMed]
- 5.Aiello LP, Gardiner TW, king GL, et al. Diabetic Retinopathy (technical review) Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 6.Tabbara K, Ross-Dogan D. blindness in Saudi Arabia. JAMA. 1986;255:3378–3384. [PubMed] [Google Scholar]
- 7.Al Bdour M, Al Till M, Abu Khader I. Causes of blindness among adult Jordanian, A hospital-based study. Euro J Ophthalmol. 2002;12(1):5–10. doi: 10.1177/112067210201200102. [DOI] [PubMed] [Google Scholar]
- 8.Salem M, Ajlouni K. Diabtic Retinopathy among Jordanians: Its Patterns, Severity, and Some Associated Risk Factors. Diabbetologia Croatica. 1999;28(1):17–23. [Google Scholar]
- 9.Al-Till MI, Al-Bdour MD, Ajlouni KM. Prevalence of blindness and visual impairment among Jordanian diabetics. Eur J Ophthalmol. 2005;15(1):62–8. doi: 10.1177/112067210501500110. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Ophthalmology International Clinical Classification of Diabetic Retinopathy Severity of Diabetic Macular Edema. Available at: http://www.icoph.org/pdf/Macular-Edema-Detail.pdf. Accessed January 11, 2006.
- 11.International Councile of Ophthalmology ICO international clinical guideline: diabetic retinopathy (initial and follow up evaluation). Available as at: http//www.icoph.org/:/guide/guidedi.htmlat. Accessed March 15, 2006.
- 12.Guideline for controlling and monitoring: the tobacco epidemic. Geneva: World Health Organization; 1998. [Google Scholar]
- 13.American Diabetes Association Standards of Medical care in Diabetes 2007. Diabetes care. 2007;30(suppl 1):S4–S40. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 14.World health organization . Physical status: The use and interpretation of anthropometry: report of WHO expert committee. Geneva: WHO; 1995. Technical report series 854. [PubMed] [Google Scholar]
- 15.Klein R, Klein BEK, Moss SE, et al. The Wisconsin epidemiolgic study of diabetic retinopathy prevalence and risk of diabetic retinopathy when age at diagnosis less than 30 years. Arch Ophthalmol. 1984;102:520. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 16.Sparrow JM, McCleod BK, Smith TDW, Birch M, Rosenthal R. The prevalence of diabetic retinopathy and maculopathy and their risk factors in the non insulin treated diabetic patients of an English town. Eye. 1993;7:158–63. doi: 10.1038/eye.1993.34. [DOI] [PubMed] [Google Scholar]
- 17.Kayani H, Rehan N, Ullah N. Frequency of retinopathy among diabetics admitted in teaching hospital of Lahore. Ayub Med Coll Abbottabad. 2003;15(4):53–6. [PubMed] [Google Scholar]
- 18.Aghadoost D, Sadr F. Prevalence and risk factor for diabetic retinopathy in diabetic patients of Kashan Diabetic Center in 2002–2003. Feyz. Spring. 2005;33(13):115–20. [Google Scholar]
- 19.Abu El Asrar AM, Al-Rubeaan KA, Al-Amro SA, Kangave D, Moharram OA. Risk factors for diabetic retinopathy among Saudi diabetics. Int Ophthalmol. 1999;22:155–61. doi: 10.1023/a:1006240928938. [DOI] [PubMed] [Google Scholar]
- 20.El Haddad OA, Saad MK. Prevelance and risk factors for Diabetic Retinopathy among Omani Diabetics. Br J Ophthalmol. 1998;82:901–6. doi: 10.1136/bjo.82.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robyn J, Jonathan E, Alex H, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian Population. Diabetes Care. 2003;26(6):1731–37. doi: 10.2337/diacare.26.6.1731. [DOI] [PubMed] [Google Scholar]
- 22.Al-Shammari F, Al-Meraghi O, Alfred N, Al-Otaibi S. The prevalence of retinopathy and associated risk factors in type 2 diabetes Mellitus in Al-Naeem area: Kuwait. Middle East Journal of Family Medicine. 2005;3(2):42–48. [Google Scholar]
- 23.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of Diabetes on the development and progression of long-term complications in Insulin-dependent DM. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 24.Segato T, Midena E, Grigoletto F, et al. the epedimiology and prevalence of diabetic retinopathy in the Veneto region of North East Italy. Veneto group for diabetic retinopathy. Diabet Med. 1991;8:511–6. doi: 10.1111/j.1464-5491.1991.tb02149.x. [DOI] [PubMed] [Google Scholar]
- 25.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. erratum 1999;38:29. [PMC free article] [PubMed] [Google Scholar]
- 26.Moss SE, Klein R, Klein BEK. Aassociation of cigarette smoking with diabetic retinopathy. Diabetes Care. 1991(14):119. doi: 10.2337/diacare.14.2.119. [DOI] [PubMed] [Google Scholar]
- 27.Ballard DJ, Melton LJ, Dwyer MS, et al. Risk factor for diabetic retinopathy: A population – based study in Rochester, Minnesota. Diabetes Care. 1986(9):334–42. doi: 10.2337/diacare.9.4.334. [DOI] [PubMed] [Google Scholar]
- 28.Paetkau ME, Boyd TAS, Winship B, Grace M. Cigarette smoking and diabetic retinopathy. Diabetes. 1977;26:46–49. doi: 10.2337/diab.26.1.46. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal S, Raman R, Kumari R, et al. Diabetic retinopathy in type II diabetics detected by targeted screening versus newly diagnosed in general practice. Ann Acad Med Singapore. 2006;35(8):531–5. [PubMed] [Google Scholar]
- 30.Janghorbani M, Amini M, Ghanbari H, Safaice H. Incidence of and risk factors for diabetic retinopathy in Isfahan, Iran. Ophthalmic Epidemiol. 2003;10:81–95. doi: 10.1076/opep.10.2.81.13893. [DOI] [PubMed] [Google Scholar]
- 31.Van leiden HA, Dekker JM, Moll AC, Nijpels G, Heine R, Bouter L, et al. Blood pressure, lipid and obesity are associated with retinopathy: the hoom study. Diabetes care. 2002;25(8):1320–5. doi: 10.2337/diacare.25.8.1320. [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Klein BEK, Moss SE, Davis M, Demetes D. The Wisconsin epidemiologic study of diabetic retinopathy II. Prevalence and risk of diabetic retinopathy when age at diagnosis 30 or more years. Arch Ophthalmol. 1984;102:527–32. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 33.Teruel MC, Frenandez-Real JM, Ricart W, Valent Ferrer R, Valles Prats M. Prevalence of diabetic retinopathy in the region of Girona study of related factors. Arch Soc ESP Oftalmol. 2005;80(2):85–91. doi: 10.4321/s0365-66912005000200006. [DOI] [PubMed] [Google Scholar]
- 34.Metrreveli DS, Sudkhanishvili MZ, Margvelashvili MZ. The prevalence of diabetic retinopathy in subjects with type 2 diabetes of one year and less duration. Georgian Med News. 2006;132:64–8. [PubMed] [Google Scholar]
- 35.Leske MC, Wu SY, Hennis A, Hyman L, Nemesure B, Yang L, et al. Hyperglycemia, blood Pressure, and the nine year incidence of diabetic retinopathy: the Barbados eye studies. Ophthalmology. 2005;112(5):799–805. doi: 10.1016/j.ophtha.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 36.Hypertension in Diabetes Study (HDS) I Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications: The Hypertension in Diabetes Study Group. J Hypertension. 1993;11:309–317. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]


