ABSTRACT
The immune contexture, a composition of the tumor microenvironment, plays multiple important roles in cancer stem cell (CSC) and epithelial–mesenchymal transition (EMT), and hence critically influences tumor initiation, progression and patient outcome. Tumor-associated macrophages (TAMs) are abundant in immune contexture, however their roles in CSC, EMT and prognosis of colorectal cancer (CRC) have not been elucidated. In 419 colorectal carcinomas, immune cell types (CD68+ macrophages, CD3+, CD4+ or CD8+ T lymphocytes, CD20+ B lymphocytes), EMT markers (E-cadherin and Snail) as well as the stem cell marker (CD44v6) were detected in tumor center (TC) and tumor invasive front (TF) respectively by immunohistochemistry. Tumor buds, that represent EMT phenotype, were also counted. It was found CD68+ macrophages were the most infiltrating immune cells in CRC. By correlation analysis, more CD68+TF macrophages were associated with more CD44v6 expression (p < 0.001), lower SnailTF expression (p = 0.08) and fewer tumor buds (p < 0.001). More CD68+TF macrophages were significantly related to more CD3+TF T lymphocytes (p = 0.002), CD8+TF T lymphocytes (p < 0.001) and CD20+TF B lymphocytes counts (p = 0.004). Strong CD68+TF macrophages infiltration also predicted long term overall survival. CRC patients with more tumor buds had worse survival. However, strong CD68+TF macrophages infiltration could reverse the unfavorable results since patients with more tumor buds but increasing CD68+TF macrophages infiltration had the favorable outcome, similar to lower tumor buds groups. This study provided direct morphological evidence that tumor-associated macrophages in the invasive front play critical roles in fighting with the unfavorable results of tumor buds, thus resulting favorable outcomes for CRC patients.
KEYWORDS: epithelial-mesenchymal transition, prognosis, stemness, tumor associated macrophages, tumor bud
Introduction
Tumor cells that are heterogeneous, often show varied phenotypes and reside in distinct microenvironment niches.1 Tumor associated immune cells (TAIs), whose types, densities, or functional differences in the tumor microenvironment, are thought to play critical roles in tumor prevention or progression.2 Tumor associated macrophages (TAM) were the main components in the tumor microenvironment.3 Whether the TAM infiltration in CRC could benefit or harm patient outcome is still controversial.4,5 Cancer stem cells (CSCs) are characterized as the subtype of cancer cells that have self-renewal ability, clonal tumor initiation capacity, and clonal long-term repopulation potential.6 Currently, the immune milieu that helps CSCs to escape from cytotoxic insult and elimination of infiltrating inflammatory cells, reveals its coherent relation with CSC.7,8 It has been reported that CSCs interacted with tumor macrophages to promote tumor cell invasion and escape from killing by natural killer cells.9 However, exploring the complex interactions between TAMs and CSCs is still urgently needed.
Epithelial-mesenchymal transition (EMT) is a pro-metastasis process that epithelial cells lose their cell polarity and cell-cell adhesion, with the observation by reduced epithelial marker like E-cadherin (E-cad), increased mesenchymal markers like vimentin and activated transcription factor like snail.10 Tumor bud (TB), which is defined as a single cell or cell clusters composed of at most 5 de-differentiated cells at the invasive front,11 is a well validated prognostic factor in CRC.12-15 Prevailing opinion acknowledges that TB is at least a morphological characteristic of EMT.16 EMT can be induced by many factors like growth factors such as TGF-β and cytokines such as IL-6, which can be secreted not only by tumor cells but also by activated TAMs.17,18 However, it was also hypothesized that the specific immune response acts against the EMT with strong lymphocytes infiltration by targeting and then destroying TBs.19 The role of TAM, either blocking or enhancing tumor formation and/or progression is controversial, and their impact on EMT is still unclear.
In this study, we focused on CD68+ macrophages, as well as other immune cell components CD3+, CD4+ or CD8+ T lymphocytes and CD20+ B lymphocytes in the tumor center (TC) and tumor invasive front (TF) respectively, and investigated whether their behaviors affected either tumor cell stemness or the EMT as well as patient's outcome in CRC.
Results
Distribution of Tumor-associated immune cells
Immune cells infiltrated in the stroma of tumor were counted in TC and TF separately (Fig. 1B-F and Supplementary Table S2). In TC and TF, CD68+ macrophages were the most infiltrating immune cells. CD68+ macrophages ranged from 0 to 94 (mean: 25.75, median: 22.13) in TC and from 0 to 185.5 per HP (mean: 36.59, median: 30.00) in TF. In TF, the density of CD3+ T lymphocytes ranged from 0 to 230.50 per HP (mean: 26.40, median: 18.67), that of CD4+ T lymphocytes ranged from 0 to 40.25 (mean: 1.95, median: 0.13), that of CD8+ T lymphocytes ranged from 0 to 51.25 (mean: 13.25 median: 10.75), and that of CD20+ B lymphocytes ranged from 0 to 230.75 (mean: 8.34, median: 3.00). The densities of these types of lymphocyte in TC were also shown in Supplementary Table S2.
Figure 1.
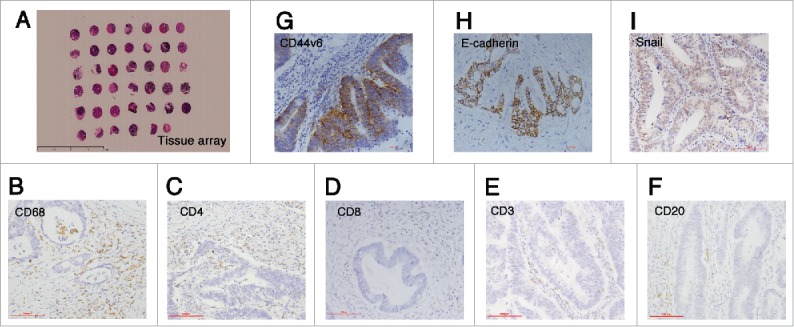
Immunohistochemistry staining results. (A) The construction of tissue array (5x), scale bar: 100 μm; (B-F) The staining of immune cells markers: CD68, CD4, CD8, CD3 and CD20 (20x); (G) The staining of cancer stemness markers: membranous CD44v6 (20x), scale bar: 100 μm; (H-I) The staining of epithelial-mesenchymal transition markers: membranous E-cadherin and nuclear Snail (20x), scale bar: 100 μm.
The association between Tumor-associated macrophages and other immune cells
We compared the consistency of CD68+ macrophages and other immune cells. In TF, more CD68+TF macrophages were significantly related to more CD3+TF T lymphocytes (r = 0.172, p = 0.002), CD8+TF T lymphocytes (r = 0.187, p < 0.001) and CD20+TF B lymphocytes (r = 0.152, p = 0.004) (Table 1). However, CD68+TC had no relation to any other immune cell types in TC (p > 0.05) (Supplementary Table S3). It revealed the particularly located CD68+ macrophages in TF could recruitment lymphocytes infiltration, especially CD8+ T lymphocytes and CD20+ B lymphocytes to the surrounded sites.
Table 1.
The correlations between density of CD68+ macrophages and other immune cells in TF (Spearman rank test).
| CD68+TF |
||||
|---|---|---|---|---|
| Variables | low | high | p (rho value) | |
| CD3+TF | low | 100 | 68 | 0.002 (0.172) |
| high | 63 | 86 | ||
| CD4+TF | low | 94 | 86 | 0.71 |
| high | 92 | 91 | ||
| CD8+TF | low | 114 | 75 | <0.001 (0.187) |
| high | 69 | 97 | ||
| CD20+TF | low | 112 | 82 | 0.004 (0.152) |
| high | 67 | 91 | ||
Tumor-associated immune cells and clinicopathological variables
The densities and locations of CD3+, CD4+, CD8+, CD20+, CD68+ cells and their relationships with the clinicopathological features of 419 CRC patients were shown in supplementary Table S4 and Table S5. Reduced CD68+TF macrophages were correlated with increased lymph node metastasis (p < 0.001, r = −0.195), higher TNM stage (p = 0.001, r = −0.178), increased vessel invasion (p = 0.001, r = −0.203) and more overall death rate (p = 0.001, r = −0.176). Similar to CD68+TF macrophages, reduced CD8+TF T lymphocytes and CD20+TF T lymphocytes also were related to higher TNM stage, increased vessel and perineural invasion and more distant metastasis (p < 0.05).
Correlation of tumor-associated macrophages with CSC marker
The CSC marker CD44v6 was distributed on the membrane (Fig. 1G). CD44v6 was detected in 139 of 367 cases (37.87%) in TC and in 97 of 289 (33.56%) in TF. Then the relations of TAM and CD44v6 were analyzed in TC and TF respectively. More CD68+TF macrophages significantly related to stronger CD44v6TF expression (r = 0.211, p < 0.001) (Table 2), while CD68+TC macrophages had no relation to CD44v6TC (p = 0.13) (supplementary Table S6).
Table 2.
Correlation between CD68+ macrophages and expression of CSC and EMT markers in TF (Spearman rank test).
| CD68+TF |
||||
|---|---|---|---|---|
| variables | low | high | p (rho value) | |
| CD44v6TF | low | 106 | 81 | <0.001 (0.211) |
| high | 33 | 63 | ||
| E-cadherinTF | low | 33 | 33 | 0.808 |
| high | 98 | 105 | ||
| snailTF | low | 141 | 144 | 0.08 (−0.096) |
| high | 28 | 16 | ||
| Tumor bud | low | 67 | 50 | <0.001 (−0.048) |
| mediate | 85 | 122 | ||
| high | 58 | 28 | ||
Correlation of tumor-associated macrophages with EMT markers and Tumor bud
Epithelial marker E-cadherin and representative EMT transcriptional factor Snail were also detected. Membranous E-cadherin and nuclear Snail were defined as positive staining (Fig. 1H-I). In TC, E-cadherinTC was highly expressed in 287 samples and lowly expressed in 54 samples, and SnailTC was stained in 62/386 samples. In TF, E-cadherinTF was highly expressed in 207 samples and lowly expressed in 68 samples, and SnailTF was stained in 44 samples and not stained in 292 samples. Then the number of tumor buds was counted and three groups were determined as low with 0–4 tumor buds (n = 120), mediate with 5–14 tumor buds (n = 209) and high with ≥15 tumor buds (n = 88).
The relations of TAM and EMT markers were analyzed. In TF, CD68+TF macrophages (r = −0.096, p = 0.08) tended to decrease SnailTF expression (Table 2). In TC, only CD68+TC macrophages (r = 0.167, p = 0.002) positively related to E-cadherinTC (Supplementary Table S6). Other immune cells had no relations to E-cadherinTC or snailTC (p > 0.05) (Supplementary Table S7). In TF, CD3+TF T lymphocytes was negatively related to SnailTF (r = −0.139, p = 0.021) (Supplementary Table S8). CD20+TF B lymphocytes had a tendency to associate with SnailTF (r = −0.103, p = 0.069) (Supplementary Table S8). Though E-cadherinTF had no correlation to CD68+TFmacrophages (p = 0.808), more CD68+TF macrophages infiltration significantly related to less TBs densities (r = −0.048, p < 0.001) (Table 2).
CD68+TF macrophages predict good prognosis
We compared prognostic values of CD68+ macrophages, CD3+, CD4+ or CD8+ T lymphocytes and CD20+ B lymphocytes in TC and TF respectively. By Kaplan-Meier survival analysis, lower CD20+TC (p = 0.028) or CD68+TF(p = 0.003) indicated unfavorable overall survival respectively (Fig. 2A/B). On the contrary, lower tumor buds indicated better overall survival (Fig. 2C, p = 0.005).
Figure 2.
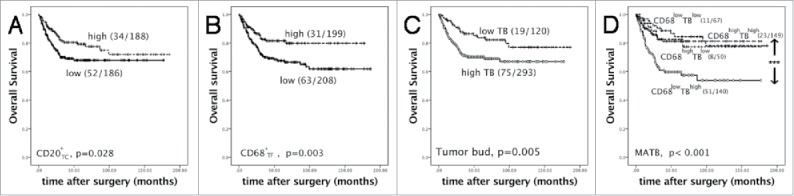
The survival analyses by Kaplan-Meier survival analysis (Log-rank test). (A-C) High CD20+TC density, high CD68+TF density and low tumor buds predicted favorable overall survival respectively (death/ total); (D) CD68+TFlow TBhigh had the worst survival (CD68+TFlow TBhigh vs CD68+TFhigh TBhigh, p < 0.001; CD68+TFlow TBhigh vs CD68+TFlow TBlow, p < 0.001; CD68+TFlow TBhigh vs CD68+TFhigh TBlow, p = 0.008). In high TB group, when CD68+TF were high, overall survival time was prolonged, which was similar to low TB group (death/ total). *** p < 0.001.
Univariate COX proportional hazard model showed CD68+TF macrophage was an indicator for outcome in CRC (HR = 1.89 (95%CI: 1.228-2.908), p = 0.004). Since CD68+TF macrophages and TB were negatively related (r = −0.167, p = 0.001). TB was merged into low (0-4 TBs) and high (≥5 TBs). We combined CD68+TF macrophages and TB, named MATB (macrophages and TB), then four groups were got: CD68+TFhigh TBhigh, CD68+TFhigh TBlow, CD68+TFlow TBhigh, CD68+TFlow TBlow. Kaplan-Meier survival analysis showed CD68+TFlow TBhigh had the worst survival (p < 0.001). In high TB group, when CD68+TF were high, overall survival time was prolonged, which was similar to low TB group (Fig. 2D). This results revealed that CD68+TF macrophages could increase CRC patients overall survival even if they had higher tumor buds. For better understanding how significant that MATB did in per TNM stage, patients were divided by TNM stage. Kaplan-Meier survival showed only in stage III that MATB significantly influenced overall survival (p = 0.009) (Supplementary Fig. S1).
In order to evaluate prognostic value for the combination of CD68+TF macrophages and TB, multivariate COX proportional hazard model was performed. Those predictors, including gender, age at diagnosis, chemotherapy, histological grade, histological type and TNM stage were entered into the analysis. Eventually, only TNM stage (p < 0.001) and MATB (p = 0.002) remained in the model. MATB was an independently prognostic predictor in addition to TNM stage (Table 3). After dividing patients by TNM stage, multivariate COX proportional hazard model showed MATB was significant only in stage III (HR = 0.337 (95%CI: 0.160-0.337), p = 0.004).
Table 3.
The results of multivariate COX proportional hazard model.
| variables | β | RR (CI 95%) | p |
|---|---|---|---|
| TNM stage | <0.001 | ||
| TNM stage(I) | |||
| TNM stage(II) | 0.205 | 1.228(0.527-2.286) | 0.634 |
| TNM stage(III) | 0.741 | 2.097(0.946-4.652) | 0.068 |
| TNM stage(IV) | 1.749 | 5.747(2.467-13.384) | <0.001 |
| CD68 and TB (MATB) | 0.002 | ||
| CD68low TBhigh | |||
| CD68high TBlow | −0.631 | 0.531(0.243-1.166) | 0.115 |
| CD68lowTBlow | −0.955 | 0.385(0.197-0.752) | 0.005 |
| CD68high TBhigh | −0.803 | 0.448(0.273-0.737) | 0.002 |
Discussion
In this study, we evaluated the components of tumor associated immune cell types and counted their densities in TC and TF respectively in 419 CRCs. The stemness marker CD44v6, and the EMT marker E-cadherin and transcriptional factor snail were also detected. We found CD68+ macrophages were the most infiltrating immune cells. By correlation analysis, TAM infiltration in TF was not only accompanied by T and B lymphocytes increasement, but also inherently linked with cancer cell stemness and EMT. More CD68+ macrophages infiltration in TF was a favorable factor for patient outcome, which could even reverse the unfavorable results caused by high TB counts. The combination of CD68+TFmacrophages and TB (MATB) was an independent prognostic predictor.
Tumor-associated immune microenvironment was significantly correlated with CSC and EMT. CSCs possess the intrinsic characteristics to evade recognition by the immune surveillance, thereby allowing transformed cells to proliferate, spread and plant.7 CSC phenotype also has a strong overlap with EMT.20 The modulation of CSC by the tumor immune microenvironment is poorly understood. An inflammatory microenvironment contributes to EMT in gastric cancer since TAIs like CD68+ macrophages could secret EMT inducers like TNF-α, TGFβ, TGF-α, and IL-6.21,22 However, whether immune cells could eliminate abnormal cells with EMT traits, or support tumor cells to more EMT-like is still complex.
As CD68+ macrophages infiltrated most in CRC, we were focusing on CD68+ macrophages. Macrophages represent the first line to defend against foreign pathogens as an innate immune defense23 by inducing phagocytosis and subsequently degrading aberrant cells or possibly tumor cells.24 Macrophages could also distinguish tumor cells and non-tumorigenic cells by specific cell membrane recognition25 and subsequently clear away malignant cells by macrophage-mediated tumor cytotoxicity or ADCC (antibody-dependent cellular cytotoxicity).26 TAMs are dynamically shifting in a balance between anti-tumor M1 and pro-tumor M2 phenotypes. CD44v6 belongs to the cell adhesion molecule CD44 family, whose expression on the membrane has been regarded as one of the CSC markers in CRC.27 CSC and TAM frequently interacted.28 Raggi et al found stem-like tumor cells could recruit circulating monocytes to the lesion and induce macrophages differentiation in Cholangiocarcinoma.28 Liang Yi et al proved that glioma-initiating cells predominantly recruited TAMs, but not adhesive glioma cells.29 CSCs inhibit cytotoxic T cell proliferation while stimulate activation of T regulatory (Treg) cells30 and promote recruitment of immunosuppressive TAMs31 as well as enhance the transition of TAMs from the antitumor (M1) to the pro-tumor (M2) phenotype.9,32 In this study, we found CD68+TF macrophages had a positive relation to CD44v6TF. It revealed that the stem-like tumor cells seemed to recruit CD68+ macrophages to the invasive front in CRC.
The interaction behaviors of TAM and EMT have been explored these years. However, most reports by now focused on M2-like macrophages phenotype, and their roles in promoting tumor metastasis and EMT.17,33 While in this study, CD68+TC macrophages increased E-cadherinTC expression, CD68+TF macrophages tended to decrease SnailTF expression. Strong CD68+TF infiltration also inhibited TB. It demonstrated that CD68+ macrophages had potential to fight against malignant cells with EMT traits.
The mechanisms behind the anti-tumor effects of TAMs have not been fully elucidated, and seem potentially be ascribed to the M1 phenotype,3 partly controlled by the adaptive immune response of CD4+ and CD8 T+ lymphocytes.34,35 In adaptive immune response, macrophages acting as antigen-presenting cells can recognize abnormal structures as dendritic cells do, subsequently stimulate Th1 by presenting MHC-II. Classically activated macrophages also regulate T lymphocytes activation by producing IL-6, IL-12, TNF-α, or CXCL9/10/15 23. Here, we also found more CD68+ macrophages infiltration, particularly located in TF, was also accompanied by more T and B lymphocytes. CD3+TF T lymphocytes were negatively related to SnailTF. CD20+TF B lymphocytes had a tendency to associated with SnailTF. Clinical parameters also showed higher CD68+TF macrophages were correlated with decreased lymph node metastasis, decreased vessel invasion, lower TNM stage. Our work highlighted that an activated immune response might impair EMT-like and CSC-like cells, especially TB. This study provided a hypothesis that tumor cells with stem-like phenotypes recruitment TAMs to the invasive front of tumor lesion and surround around tumor buds. Subsequently, TAMs induce the immune response, especially CD8+ T and CD20+ B lymphocytes, to eliminate these highly invasive tumor buds (Fig. 3). As a result, the activated immune response makes less frequency of tumor metastasis.
Figure 3.
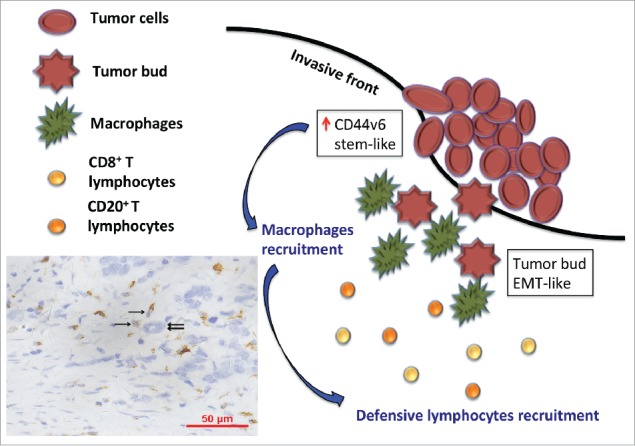
The model shows a hypothesis that tumor cells with stem-like phenotypes recruitment TAMs (brown, single sword) to the invasive front of tumor lesion and surround around tumor buds (double swords). Subsequently, TAMs induce immune response, especially CD8+T and CD20+ B lymphocytes, to eliminate these highly invasive tumor buds.
Host immune responses had an impact on survival.36 Zlobec et al 37and our work proposed high CD68+ macrophages infiltration made a good difference in CRC patient survival, while others revealed TAM mainly exhibited a M2-like phenotype, promoting tumor progression and causing bad outcome.38,39 In this study, we also found high CD68+TF macrophages group live longer than low CD68+TF macrophages group especially in TNM stage III and IV by Kaplan Meier analysis (p = 0.030, data not shown). These discrepancies between different studies may be due to the macrophage phenotypes and tumor types as well as the assessment methods. The CD68+TF macrophages anti-tumor mechanism was supposed to be that recruitment of macrophages contributes to the development of an adaptive immune response against the tumor, and the balance between antigen availability and elimination through phagocytosis and subsequent degradation of senescent or apoptotic cells,36 which was concerned with good outcome. We also assumed that high infiltration of CD68+TF macrophages also arrested EMT traits that might also contribute to the favorable outcome. Furthermore, we wanted to clarify whether CD68+TF macrophages could suppress EMT and lead to prolonged survival. Zlobec et al gave a concept of cell-to-cell contacts between tumor buds and TAMs, and high CD68+ counts predicted long term overall survival.37 We also found CD68+TF macrophages and TBs were negatively related. TB has been validated as a poor predictor in CRC.12-14 After combining CD68+TF macrophages and TBs to assess overall survival in CRC patient, Kaplan-Meier survival analysis showed higher CD68+TF macrophages infiltration reverse the unfavorable results caused by higher TB counts. Multivariate COX proportional hazard model also demonstrated the combination of CD68+TF macrophages and TB (MATB) was an independently prognostic predictor, which had great potential for further application in CRC.
Conclusion
This study provided direct morphological evidence that tumor-associated immune cells play critical roles on EMT and cancer cell stemness. CD68+TF macrophages, which impeded EMT and CSC, and induced an adaptive immune response, had a good influence on overall survival. CD68+TF macrophages could even reverse the bad survival results of tumor buds. CD68+TFmacrophages and TB (MATB) was an independent predictor in colorectal carcinoma.
Materials and methods
Case materials
In all 419 patients with sporadic colorectal carcinoma who underwent radical surgery but no chemotherapy or radiotherapy before the operation, there were 227 males and 192 females. The age ranged from 24 to 91 years (median, 64 years). According to patient's records, 217 cases were from colon and 202 cases were from rectum. Of all patients, 230 received regular chemotherapy based on 5-Fu after radical surgery, 186 never received chemotherapy, and 3 were unclear. Eighty-one patients were classified as TNM I stage, 127 as stage II, 170 as stage III, and 41 as stage IV.
Three hundred and forty-five specimens were adenocarcinoma, 46 were mucinous adenocarcinoma, and 28 were signet ring cell carcinoma or undifferentiated carcinoma. Histological differentiation were graded into low grade (gland formation≥50%, 299 specimens) and high grade (gland formation<50%, 120 specimens). At the end of follow-up, 327 were dead and 92 were alive. Follow-up periods ranged from 1–60 months (median: 33 months, mean: 35 months).
Construction of tissue array
Tissue microarrays of 419 CRC specimens were constructed (Fig. 1A). Using a tissue cylinder in diameter of 1 cm, three tissue punches were taken from formalin-fixed, paraffin-embedded blocks. In brief, each case had multiple tissue punches taken from formalin-fixed, paraffin-embedded blocks. Then punches were transferred into one recipient paraffin block (6 × 7 punches) with a semi-automated tissue arrayer. Tissues were obtained from the TC, TF, and normal mucosa, which were verified from corresponding H&E slides. The TC area was determined as at least a distance of 20 × fields from the border of normal mucosa. The TF area was determined as a 20 × field within the most distal tumor cells. Finally, recipient paraffin blocks were cut into 4μm thick slices and mounted on APES coated slides.
Immunohistochemical staining
Five types of immune cell (CD3, CD4, and CD8T lymphocytes, CD20 B lymphocyte, CD68 macrophages), stem cell marker (CD44v6), and 2 EMT markers (E-cadherin and Snail) were investigated in this study. The information of antibodies and staining pattern was summarized in Supplementary. Table S1. Missed data were caused by tissue falling off.
Immunohistochemical staining by two-step method (PV-9000 polymer detection system, GBI Labs, USA) was performed according to the conditions presented in Supplementary. Table S1. For blank controls, the primary antibodies were replaced with PBS solution (100 mM, PH7.4). Definitely brown granular stain was defined as positive. The numbers of immune cells were counted in four hotspots (20 ×, 545 × 577 μm2) using a computer-automated method (Image-pro plus 6.0, Media Cybernetics Inc.) and the density of immune cells was defined as average counts per high-power field (HP, 20 ×).
As regards the stem cell markers and EMT markers, the percentages of positive cells were scored using the following scale: 0 = no staining or less than 5%; 1 = 5-25%; 2 = 26-50%; 3 = 51-75%; 4 = more than 75%, and staining intensity (0: negative, 1: weak, 2: moderate, and 3: strong) were scored. The final score was achieved when the score of positive cells percentage was multiplied by the score of staining intensity. Subsequently, CD44v6 was defined as low expression when the final score was less than 6, and high expression when the final score was more than or equal to 6, E-cadherin was defined as negative (final score ≤ 2) and positive (final score≧3), and Snail was defined as negative (final score = 0) and positive (final score ≥ 1) separately. Then the number of tumor buds was counted in TF and mean densities in an area of 500 μm × 2500 μm were calculated. Three groups were determined as low with 0–4 tumor buds, mediate with 5–14 tumor buds and high with ≥15 tumor buds.
Statistical
IBM SPSS Statistics 20.0 (New York, NY, USA) was used for statistical analysis. Correlations were analyzed by Spearman correlation. Univariate survival analyses were performed and survival curves were drawn using Kaplan-Meier method. The differences between curves were tested by the log-rank test. Cumulative survival rate was calculated by life-table method. Multivariate analysis was performed using the COX proportional hazard model and a forward stepwise method was used to bring variables into the model. A significant difference was identified if the P-value was less than 0.05.
Study approval
This study was approved by the Ethics Committee of Biomedicine, Zhejiang University, China.
Supplementary Material
Financial support
The research is supported by the grants of Key Project for Science and Technology of Zhejiang Province under Grant 2012C13014-3, Programme of Introducing Talents of Discipline to Universities under Grant B13026, National Natural Science Foundation of China under Grant 81472215.
References
- 1.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119-50. doi: 10.1146/annurev.pathol.1.110304.100224 [DOI] [PubMed] [Google Scholar]
- 2.Kiraz Y, Baran Y, Nalbant A. T cells in tumor microenvironment. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:39-45. doi: 10.1007/s13277-015-4241-1 [DOI] [PubMed] [Google Scholar]
- 3.Edin S, Wikberg ML, Oldenborg PA, Palmqvist R. Macrophages Good guys in colorectal cancer. Oncoimmunology. 2013;2(2):e23038. doi: 10.4161/onci.23038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JC, Chen JS, Lee CH, Chang JJ, Shieh YS. Intratumoral macrophage counts correlate with tumor progression in colorectal cancer. J Surg Oncol. 2010;102:242-8. doi: 10.1002/jso.21617 [DOI] [PubMed] [Google Scholar]
- 5.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res: an official journal of the American Association for Cancer Research. 2007;13:1472-9. doi: 10.1158/1078-0432.CCR-06-2073 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133-43 [DOI] [PubMed] [Google Scholar]
- 7.Bruttel VS, Wischhusen J. Cancer stem cell immunology: key to understanding tumorigenesis and tumor immune escape? Front immunol. 2014;5:360. doi: 10.3389/fimmu.2014.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kise K, Kinugasa-Katayama Y, Takakura N. Tumor microenvironment for cancer stem cells. Adv Drug Deliv Rev. 2016;99:197-205. doi: 10.1016/j.addr.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 9.Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al.. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat cell biol. 2015;17:170-82. doi: 10.1038/ncb3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-8. doi: 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol: an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:1315-25. doi: 10.1038/modpathol.2012.94 [DOI] [PubMed] [Google Scholar]
- 12.Prall F, Nizze H, Barten M. Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology. 2005;47:17-24. doi: 10.1111/j.1365-2559.2005.02161.x [DOI] [PubMed] [Google Scholar]
- 13.Rieger G, Koelzer VH, Dawson HE, Berger MD, Hadrich M, Inderbitzin D, Lugli A, Zlobec I. Comprehensive assessment of tumour budding by cytokeratin staining in colorectal cancer. Histopathology. 2017;70(7):1044-51. doi: 10.1111/his.13164 [DOI] [PubMed] [Google Scholar]
- 14.van Wyk HC Park JH, Edwards J, Horgan PG, McMillan DC, Going JJ. The relationship between tumour budding, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Br J Cancer. 2016;115:156-63. doi: 10.1038/bjc.2016.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karayannopoulou G, Euvrard S, Kanitakis J. Tumour Budding Correlates with Aggressiveness of Cutaneous Squamous-cell Carcinoma. Anticancer Res. 2016;36:4781-5. doi: 10.21873/anticanres.11036 [DOI] [PubMed] [Google Scholar]
- 16.Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: Tumor budding as oncotarget. Oncotarget. 2010;1:651-61. doi: 10.18632/oncotarget.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Che DH, Pan B, Tian Q, Shang LH, Liu F, Jin S, Jingyan C, Yan Y, Guangbin W, Zhenjun Y, et al.. Enhanced invasion of lung adenocarcinoma cells after co-culture with THP-1-derived macrophages via the induction of EMT by IL-6. Immunol Lett. 2014;160:1-10. doi: 10.1016/j.imlet.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Shankar BS, Sainis KB. TGF-beta 1-ROS-ATM-CREB signaling axis in macrophage mediated migration of human breast cancer MCF7 cells. Cell Signal. 2014;26:1604-15. doi: 10.1016/j.cellsig.2014.03.028 [DOI] [PubMed] [Google Scholar]
- 19.Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol. 2007;212:260-8. doi: 10.1002/path.2164 [DOI] [PubMed] [Google Scholar]
- 20.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396-403. doi: 10.1016/j.semcancer.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma HY, Liu XZ, Liang CM. Inflammatory microenvironment contributes to epithelial-mesenchymal transition in gastric cancer. World J Gastroenterol. 2016;22:6619-28. doi: 10.3748/wjg.v22.i29.6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuxe J, Karlsson MC. TGF-beta-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Semin Cancer Biol. 2012;22:455-61. doi: 10.1016/j.semcancer.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 23.Narendra BL, Reddy KE, Shantikumar S, Ramakrishna S. 5 Immune system: a double-edged sword in cancer. Inflamm Res. 2013;62:823-34. doi: 10.1007/s00011-013-0645-9 [DOI] [PubMed] [Google Scholar]
- 24.Hill AA, Bolus WR, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262:134-52. doi: 10.1111/imr.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elnemr A, Ohta T, Yachie A, Fushida S, Ninomiya I, Nishimura GI, Yamamoto M, Ohkuma S, Miwa K. N-ethylmaleimide-enhanced phosphatidylserine externalization of human pancreatic cancer cells and immediate phosphatidylserine-mediated phagocytosis by macrophages. Int J Oncol. 2000;16:1111-6 [DOI] [PubMed] [Google Scholar]
- 26.Gul N, van Egmond M. Antibody-Dependent Phagocytosis of Tumor Cells by Macrophages: A Potent Effector Mechanism of Monoclonal Antibody Therapy of Cancer. Cancer Res. 2015;75:5008-13. doi: 10.1158/0008-5472.CAN-15-1330 [DOI] [PubMed] [Google Scholar]
- 27.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al.. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158-63. doi: 10.1073/pnas.0703478104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, Chiorino G, Forti E, Glaser S, Alpini G, et al.. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102-15. doi: 10.1016/j.jhep.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, Li M, Luo C, Yu S, Bian X, et al.. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. 2011;232:75-82. doi: 10.1016/j.jneuroim.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 30.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, Gumin J, Henry V, Colman H, Priebe W, et al.. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9:67-78. doi: 10.1158/1535-7163.MCT-09-0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-oncology. 2010;12:1113-25. doi: 10.1093/neuonc/noq082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar S, Doring A, Zemp FJ, Silva C, Lun X, Wang X, Kelly J, Hader W, Hamilton M, Mercier P, et al.. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Ann Neurosci. 2013;20:154. doi: 10.5214/ans.0972.7531.200407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinnakota K, Zhang Y, Selvanesan BC, Topi G, Salim T, Sand-Dejmek J, Jönsson G, Sjölander A. M2-like macrophages induce colon cancer cell invasion via matrix metalloproteinases. J Cell Physiol. 2017;232(12):3468-80. doi: 10.1002/jcp.25808 [DOI] [PubMed] [Google Scholar]
- 34.Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, et al.. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med. 2010;8:13. doi: 10.1186/1479-5876-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu Z, Tarca AL, et al.. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J Immunol. 2016;196:2476-91. doi: 10.4049/jimmunol.1502055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grizzi F, Bianchi P, Malesci A, Laghi L. Prognostic value of innate and adaptive immunity in colorectal cancer. World J Gastroenterol. 2013;19:174-84. doi: 10.3748/wjg.v19.i2.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koelzer VH, Canonica K, Dawson H, Sokol L, Karamitopoulou-Diamantis E, Lugli A, Zlobec I. Phenotyping of tumor-associated macrophages in colorectal cancer: Impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology. 2015;5(4):e1106677. doi: 10.1080/2162402X.2015.1106677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KJ, Wen XY, Yang HK, Kim WH, Kang GH. Prognostic Implication of M2 Macrophages Are Determined by the Proportional Balance of Tumor Associated Macrophages and Tumor Infiltrating Lymphocytes in Microsatellite-Unstable Gastric Carcinoma. Plos One. 2015;10(12):e0144192. doi: 10.1371/journal.pone.0144192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bostrom MM, Irjala H, Mirtti T, Taimen P, Kauko T, Aring;lgars A, Jalkanen S, Boström PJ. Tumor-Associated Macrophages Provide Significant Prognostic Information in Urothelial Bladder Cancer. Plos One. 2015;10(7):e0133552. doi: 10.1371/journal.pone.0133552 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


